Summary
The Cystic Fibrosis Transmembrane conductance Regulator (CFTR) gene encodes a cAMP-regulated chloride channel that is important in controlling the exchange of fluid and electrolytes across epithelial cells. Mutation of CFTR can lead to Cystic Fibrosis (CF), the most common lethal genetic disease in Caucasians. CF is a systemic illness with multiple organ systems affected including pulmonary, gastrointestinal, pancreatic, immune, endocrine and reproductive systems. To understand the role of CFTR in the various tissues in which it is expressed, we generated a murine conditional null allele of Cftr (Cftrfl10) in which loxP sites were inserted around exon 10 of the Cftr gene. The Cftr conditional null allele was validated by generating constitutive Cftr null (CftrΔ10) mice using the protamine-cre system. The CftrΔ10/Δ10 mice displayed almost identical phenotypes to previously published CF mouse models, including poor growth, decreased survival, intestinal obstruction and loss of Cftr function as assessed by electrophysiology measurements on gut and nasal epithelium. Mice containing the conditional null Cftr allele will be useful in future studies to understand the role of Cftr in specific tissues and developmental time points and lead to a better understanding of CF disease.
Mutation of the Cystic Fibrosis Transmembrane conductance Regulator (CFTR) gene can lead to Cystic Fibrosis (CF), an autosomal recessive disorder which occurs in about 1 in every 3000 Caucasian live births. The CFTR protein is a chloride channel involved in the exchange of fluid and electrolytes across epithelial cells (Gadsby et al., 2006). Cystic fibrosis is a systemic disorder consistently characterized by poor growth, chronic lung infection, exocrine pancreatic insufficiency, intestinal malabsorption, reduced fertility and shortened lifespan. Many CF patients experience meconium ileus, distal intestinal obstructive syndrome, delayed puberty, liver disease and diabetes. Although these phenotypes ultimately arise as a consequence of reduced or absent CFTR function, the wide expression of CFTR throughout the body makes it difficult to establish the mechanism behind the variety of phenotypes. For example, poor growth may be due to loss of CFTR in the intestinal tract, pancreas, neuroendocrine cells or any combination thereof. CFTR is expressed in many epithelial tissues such as lung (Engelhardt et al., 1992; Trezise and Buchwald, 1991; Trezise et al., 1993), nose (Engelhardt et al., 1992), salivary glands (Manson et al., 1997; Trezise and Buchwald, 1991), intestine (Manson et al., 1997; Trezise and Buchwald, 1991), stomach (Manson et al., 1997), pancreas (Trezise and Buchwald, 1991; Trezise et al., 1993), liver (Yang et al., 1993), gall bladder (Yang et al., 1993), sweat gland (Kartner et al., 1992), kidney (Todd-Turla et al., 1996), male and female reproductive tracts (Trezise et al., 1993; Trezise et al., 1993), thyroid (Devuyst et al., 1997) and the early embryo (Ben-Chetrit et al., 2002) as well as non-epithelial tissues such as heart (Davies et al., 2004), brain (Mulberg et al., 1998), smooth muscle (Robert et al., 2004) and lymphocytes (Yoshimura et al., 1991). The identification of CFTR’s role in each of these tissues will facilitate the understanding of CF disease.
Currently available mouse models of CF were created using conventional gene targeting strategies leading to constitutive null or hypomorphic alleles of Cftr. While the lung pathology so prevalent in human CF patients is mostly absent in these mouse models, Cftr deficient mice do display multiple CF phenotypes including poor growth, intestinal obstruction, delayed puberty, infertility, pancreatic abnormalities, gall bladder abnormalities and decreased survival.(Colledge et al., 1995; Delaney et al., 1996; Grubb and Boucher, 1999; Hasty et al., 1995; Hodges et al., 2008; Jin et al., 2006; Kent et al., 1996; O'Neal et al., 1993; Ratcliff et al., 1993; Rozmahel et al., 1996; Snouwaert et al., 1992; Zeiher et al., 1995). As in the human, it is not clear how the etiology of these phenotypes relate to each other, because in these global knockouts, the physiology of the tissues involved overlap. Thus, fully understanding the effects of losing Cftr in individual tissues or organs of the mouse cannot be accomplished through conventional gene knockout approach.
To better understand the physiologic relationship of CF-affected tissues and organs, we created a conditional null Cftr allele in the mouse. Here we describe the generation of a murine Cftr allele with loxP sites flanking exon 10 allowing conditional deletion of exon 10 by directed expression of the bacterial Cre recombinase. Exon 10 of Cftr was chosen as a target due to its known importance in protein function. Not only are many exon 10 mutations associated with CF in humans (Tsui, 1992), but disruption of exon 10 in mice is known to create severe CF phenotypes (Colledge et al., 1995; Ratcliff et al., 1993; Snouwaert et al., 1992; Zeiher et al., 1995). In addition, future comparison of this model with other null or hypmorphic Cftr alleles of exon 10 should be straightforward because the same region of the gene is altered in other Cftr mouse models.
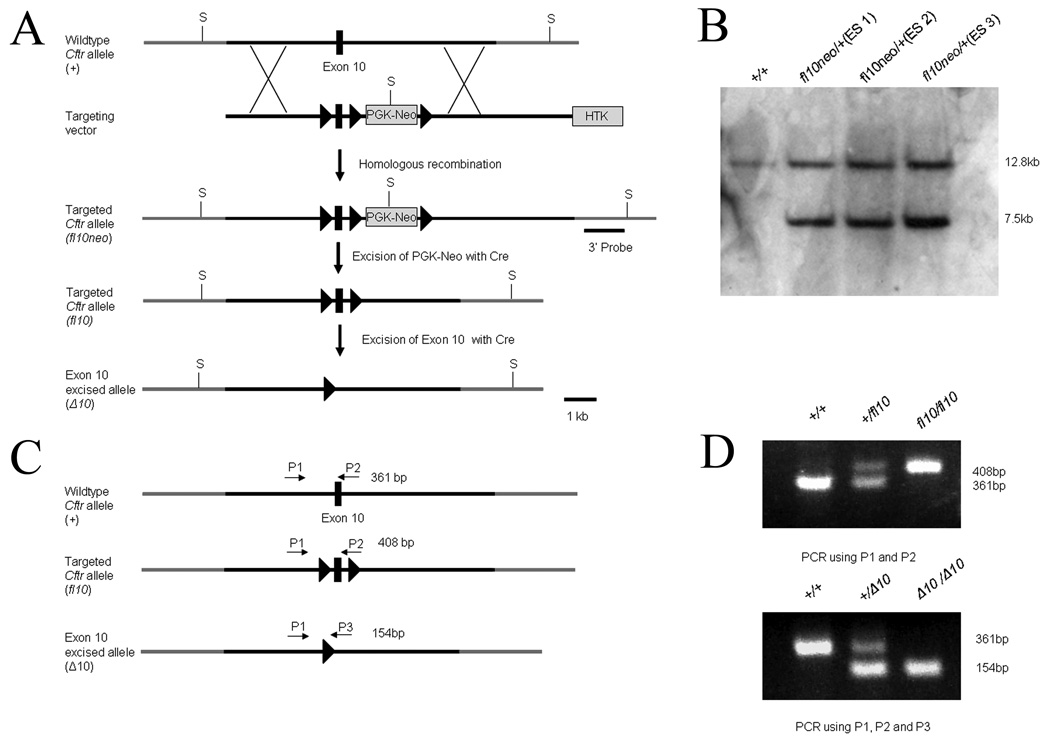
The mouse Cftr gene consists of 27 exons and spans over 150 kb. To generate a conditional null allele of Cftr, we constructed a targeting vector in which exon 10 of the Cftr gene was flanked by loxP sites (Figure 1), or “floxed”. The vector also contained selectable markers for both positive (neomycin resistance cassette) and negative (herpes thymidine kinase gene) selection of mouse embryonic stem (ES) cells. The neomycin cassette was surrounded by loxP sites allowing for its removal with Cre recombinase expression as well. R1 ES cells were electroporated with the targeting vector and neomycin resistant colonies were used for DNA blot confirmation of correct targeting of the 3’ end of the construct (Figure 1). Three ES colonies out of 201(1.5%) tested were correctly targeted. Additional PCR strategies were used to confirm correct homologous recombination on both the 5’ and 3’ ends (data not shown). Chimeras were generated from 2 of the correctly targeted ES colonies using standard procedures. Germline transmission of the conditional null allele of Cftr (Cftrfl10neo) was confirmed by both Southern and PCR analysis (Figure 1). The neomycin cassette was removed in subsequent generations of mice (referred to as Cftrfl10) using Cre recombinase to circumvent any possible complications caused by the presence of the neomycin cassette. Mice homozygous for the conditional null allele (Cftrfl10/fl10) were indistinguishable from wildtype mice in growth, survival and Cftr activity.
Figure 1.
Generation of the Cftr conditional null allele. a) The wildtype Cftr allele contains exon 10 of Cftr (black box). The targeting vector contains 3 loxP sites (triangles) that flank both exon 10 and the PGK-Neo. Both arms of the targeting vector have ∼4.5 Kb homologous sequence to Cftr. A herpes thymidine kinase selectable marker was also included for negative selection. A 3’ probe was used in conjunction with Sph1 restriction enzyme (S) to detect correct targeting events by Southern (Cftrfl10neo). Recombination of the loxP sites surrounding PGK-Neo results in a floxed exon 10 of Cftr (Cftrfl10) without PGK-Neo. Recombination of the remaining loxP sites results in the excision of exon 10 leaving one loxP site (CftrΔ10). b) Southern-blot analysis of 3 correctly targeted ES cell lines along with the wildtype R1 cell line. Correctly targeted ES cells displayed a unique 7.5kb band when compared to wildtype cells. c) PCR strategy for detecting the conditional null allele. P1 and P2 amplify 361 bp and 408 bp fragments from the wildtype (Cftr+) and conditional null allele (Cftrfl10) respectively. P1 and P3 amplify a 154 bp fragment from the exon 10 deleted allele (CftrΔ10). d) A represtative agarose gel image of the PCR results.
To validate the utility of our conditional null Cftr allele, we generated a constitutive deletion of exon 10 by crossing mice with theCftrfl10 allele to mice expressing Cre recombinase during the final stages of spermatogenesis through a protamine promoter (O'Gorman et al., 1997). Male mice heterozygous for the Cftrfl10 allele and positive for protamine Cre recombinase were mated to wildtype females resulting in a portion of offspring heterozygous for a deleted exon 10 allele (CftrΔ10/+). CftrΔ10/+ mice were mated to generate CftrΔ10/Δ10 mice which were verified by PCR analysis (Figure 1). The transmission of the CftrΔ10 allele followed mendelian inheritance proportions with 22 (24.4%) wildtype Cftr+/+, 46 (51.1%) heterozygote CftrΔ10/+ and 22 (24.4%) homozygote mutant mice CftrΔ10/Δ10.
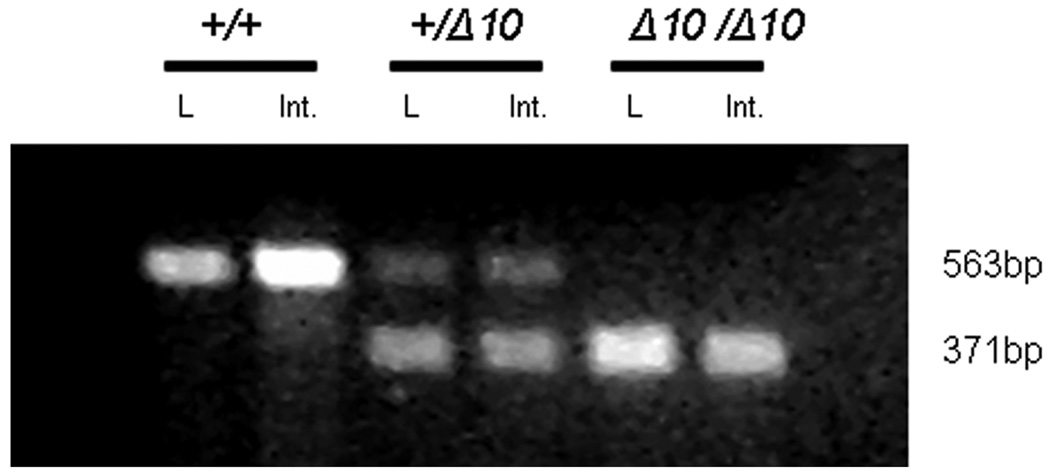
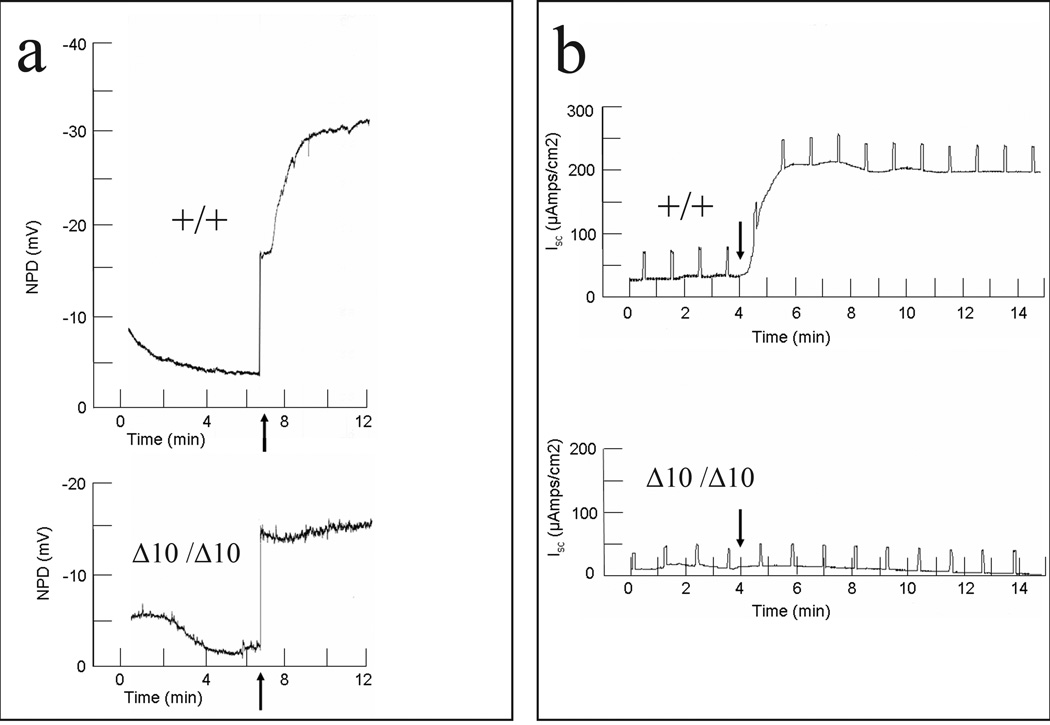
The removal of exon 10 in Cftr creates an in-frame deletion in which Cftr is transcribed but exon 10 is absent from the RNA (Figure 2). Antibodies raised to Cftr will not distinguish between Cftr containing and lacking exon 10 and so functional assays were employed to determine the effects of the allele. To test for Cftr function, we assayed Cftr’s ability to conduct chloride across the nasal and intestinal epithelium in Cftrfll0/fl10, Cftr+/+ and CftrΔ10/Δ10 mice. Nasal potential difference (NPD) across the nasal epithelium was measured before and after the addition of a chloride-free solution containing forskolin (Figure 3a). Forskolin stimulates adenylate cyclase, leading to activation of Cftr through protein kinase A-mediated phosphorylation. Challenge with a nominally chloride-free solution provides a concentration gradient in favor of chloride secretion. Non-CF animals display chloride secretion in response to this challenge, generating a lumen-negative potential difference, while CF mice do not respond (Figure 3a). After solution perfusion, Cftrfll0/fl10 animals showed a change in NPD of −15.8±5.2 mV, Cftr+/+ animals showed a change in NPD of −25.0±6.8 mV, while CftrΔ10/Δ10 animals showed a change of only −0.5±0.5 mV (p<0.01 for CftrΔ10/Δ10 vs. either control group; 3 mice per genotype). The lack of change in NPD in CftrΔ10/Δ10 mice is consistent with absence of Cftr activity.
Figure 2.
Expression of Cftr in Cftr+/+, Cftr+/Δ10 and CftrΔ10/Δ10 mice. Amplification of exons 8–11 from cDNA created from RNA from lung (L) and intestine (Int.) display a 563 bp product from the wildtype allele and a 371 bp product from the exon 10 deleted allele (the 192 bp exon 10 is absent in the CftrΔ10 allele).
Figure 3.
Electrophysiology in Cftr+/+ and CftrΔ10/Δ10 mice. a) NPD of Cftr+/+ and CftrΔ10/Δ10 mice. Arrow indicates the addition of forskolin (10µM) and chloride free HEPES buffered ringers solution. A bi-ionic junction potential of −13 mV was observed (step increase in voltage coincident with solution change) independent of mouse genotype and was excluded from the calculation of change in NPD. b) Intestinal short circuit current in Cftr+/+ and CftrΔ10/Δ10 mice. Arrow indicates the addition of forskolin(10µM) and IBMX(100µM). Vertical deflections (at 1 min intervals) result from voltage-clamp to non-zero values to measure transepithelial resistance. (Cftrfl10/fl10 mice were indistinguishable from Cftr+/+ mice)
Cftr function in the intestinal epithelium was assessed by measuring short circuit current across intestinal sections before and after stimulation by a cocktail of forskolin and IBMX to raise cAMP levels. This maneuver increases short circuit current in non-CF animals but not in CF animals (Figure 3b). Intestinal sections from the ileum, jejunum, duodenum, cecum and colon were tested for change in short circuit current and all showed similar differences between Cftrfll0/fl10, Cftr+/+ and CftrΔ10/Δ10 animals (Table 1). The lack of increase in short-circuit current in CftrΔ10/Δ10 mice is consistent with the absence of Cftr function in the intestinal epithelium.
Table 1.
Effect of forskolin/IBMX on short circuit current across intestinal epithelium.
| Intestinal tissue |
Cftrfl10/fl10 ΔIsc (µA/cm2)# |
Cftr+/+ ΔIsc (µA/cm2) |
CftrΔ10/ Δ10 ΔIsc (µA/cm2) |
|---|---|---|---|
| Duodenum | 87.81±20.6 | 153.66±24.6 | 1.10±1.0* |
| Jejunum | 92.17±27.8 | 121.61±22.6 | 3.47±1.5* |
| Ileum | 98.90±24.8 | 238.25±24.4 | 2.15±1.8* |
| Cecum | 155.16±37.4 | 291.57±77.6 | 0.49±0.2* |
| Colon | 131.00±42.7 | 129.49±19.1 | 0.32±0.4* |
ΔIsc (µA/cm2) is averaged from a minimum of 4 measurements per tissue and 5 mice per genotype; mean±SE
P<0.05 vs. Cftrfl10/fl10 or Cftr+/+
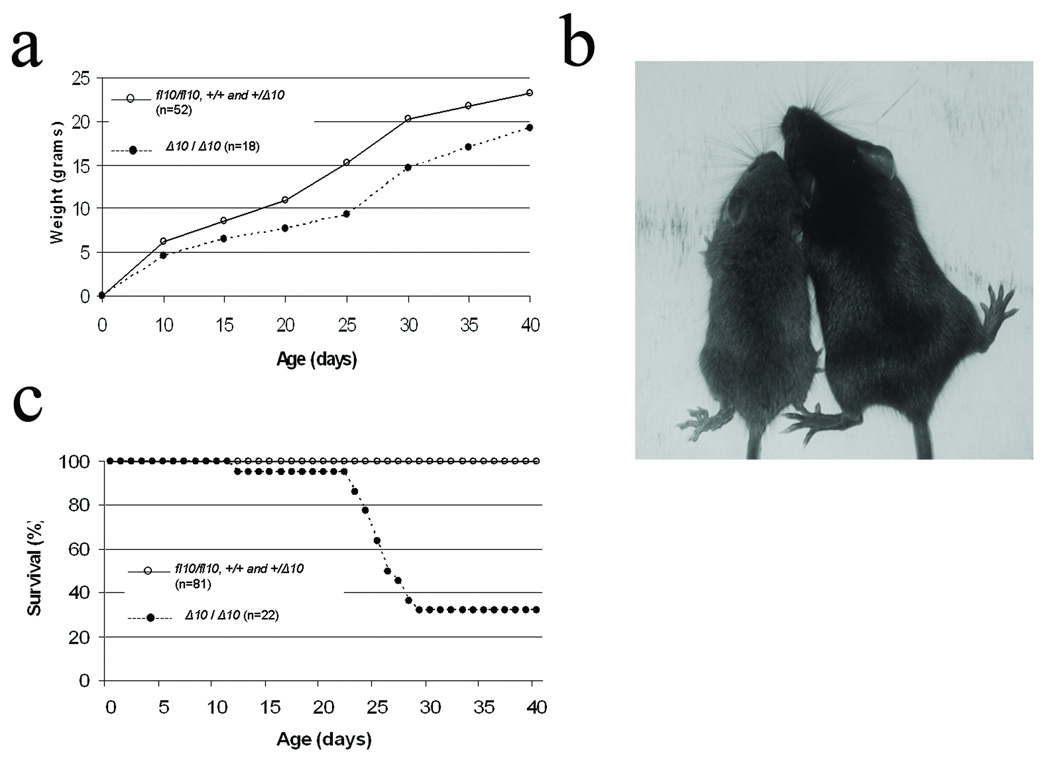
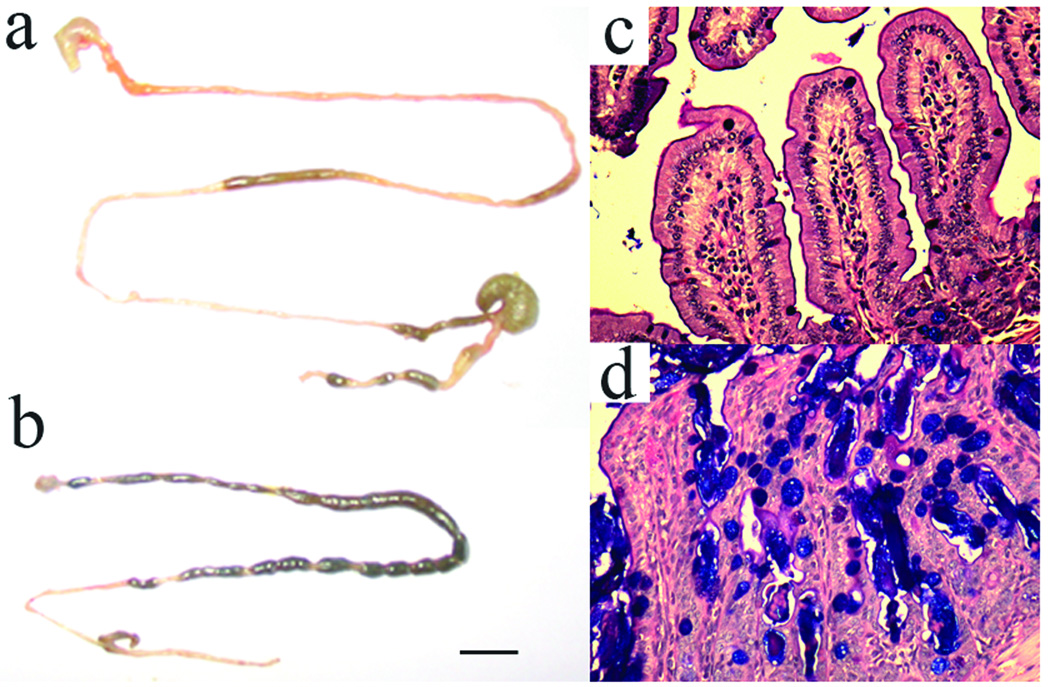
The CftrΔ10/Δ10 mice displayed decreased growth and decreased survival (Figure 4) similar to other published reports of CF mouse models (Colledge et al., 1995; Delaney et al., 1996; Hasty et al., 1995; O'Neal et al., 1993; Ratcliff et al., 1993; Rozmahel et al., 1996; Snouwaert et al., 1992; Zeiher et al., 1995). Growth was reduced in CftrΔ10/Δ10 mice by an average of 17–39% depending on the age of the animals (Figure 1) similar to the 20–50% growth reduction observed in Cftr null models. CftrΔ10/Δ10 mice also displayed decreased survival compared to littermates with only 32% surviving up to 40 days similar to the 5–40% survival observed in Cftr null models. The majority of deaths in CftrΔ10/Δ10 mice occurred shortly after weaning (20–30 days) due to intestinal obstruction (Figure 4c and Figure 5). Further examination of the intestine of CftrΔ10/Δ10 mice revealed typical intestinal histology observed in CF mouse models including increased luminal mucus accumulation and goblet cell hyperplasia (Figure 5).
Figure 4.
Phenotype of Cftr−/− mice created from the conditional null allele. a) Growth curve for Cftrfl10/fl10, Cftr+/+, Cftr+/Δ10 and CftrΔ10/Δ10 mice. b) Representative picture of CftrΔ10/ Δ10 (left) and Cftr+/+ (right) littermates (20 days old). c) Survival curve for Cftrfl10/fl10, Cftr+/+, Cftr+/Δ10 and CftrΔ10/Δ10 mice. (There was no difference in growth or survival among Cftrfl10/fl10, Cftr+/+, and Cftr+/Δ10 so they were combined).
Figure 5.
Characteristics of the intestine from Cftr+/+ and CftrΔ10/Δ10 mice. a) Intestines from Cftr+/+ and b) CftrΔ10/Δ10 mice. CftrΔ10/Δ10 mice display intestinal blockage (dark areas along intestine) compared to Cftr+/+ litter mate. Bar is 1 cm. c) Ileum of Cftr+/+ and d) CftrΔ10/ Δ10 mice with PAS staining. Cftr+/+ ileum displays normal histology while CftrΔ10/ Δ10 ileum displays characteristic intestinal disease including goblet cell hyperplasia (purple filled circles), excess luminal mucus and villi fusion. Ileum photomicrographs are ×200 magnification. (Cftrfl10/fl10 mice were indistinguishable from Cftr+/+ mice)
These results indicate that loss of exon 10 results in a functionally null phenotype in mice, displaying decreased growth, high incidence of intestinal obstruction, goblet cell hyperplasia and loss of normal transepithelial ion transport. As this allele can be generated in a conditional fashion using the Cftrfl10 mouse and regulated Cre expression, it provides a tool to study tissue-specific and temporal effects of losing Cftr. For example, decreased growth and intestinal obstruction in CF mice have been hypothesized to be due to loss of Cftr activity in the intestinal epithelium. This hypothesis can be tested by crossing the Cftrfl10 mouse with a mouse in which Cre recombinase expression is driven by the villin promoter so that Cre is expressed specifically in the intestinal epithelium (Madison et al., 2002). Delayed growth in CF mice may also have a neuroendocrine origin given the multiple reports of Cftr expression in the brain (Mulberg et al., 1998; Weyler et al., 1999). Possible Cftr function in the nervous system could be examined by crossing the Cftrfl10 mouse with a mouse in which Cre expression is driven by the nestin promoter so that Cre is expressed specifically in the central and peripheral nervous system (Tronche et al., 1999). Finally, one can test the importance of Cftr activity on the development of the mouse (e.g., in utero, postnatal, pubertal and adult) by deleting Cftr function in the whole mouse at different time points by crossing the Cftrfl10 mouse with a mouse in which Cre expression is driven by an inducible promoter (e.g,. Badea et al., 2003). The ability to delete Cftr in a tissue or time-dependent manner in the mouse will not only help understand how Cftr function contributes to the function and development of each tissue but how the absence of CFTR in these same tissues leads to CF disease in humans.
Methods and Materials
Generation of the Cftr floxed allele
Genomic DNA was isolated from R1 cells. The targeting vector consisted of amplified fragments of Cftr that included 4.4 kb of intron 9 (5’ arm), 4.5 kb of intron 10 (3’ arm) and 0.5 kb consisting of exon 10 and ∼150 bp of intron 9 and 10 on each side. These fragments were subcloned into pBluescript. Two loxP sites surrounding the PGK-neocassette (phosphoglycerol kinase promoter attached to a neomycin-resistance gene) were inserted downstream of exon 10 while the third loxP site was inserted upstream of exon 10. A herpes thymidine kinase (HTK) cassette was inserted downstream of intron 10. A schematic of the targeting construct is shown in Figure 1. The linearized targeting vector was transfected into R1 ES cells and neomycin resistant colonies were screened by southern blot using a 3’ external probe with SphI digested genomic DNA. Three colonies out of 201(1.5%) were correctly targeted. Correctly targeted ES cells were microinjected into C57BL/6 blastocysts and transferred to a pseudopregnant mouse. Eight chimeric males were bred to C57BL/6 females and F1 agouti offspring were analyzed by Southern blot and PCR genotyping to confirm germ line transmission of the conditional Cftr null allele.
PCR
Genotyping was completed by PCR analysis using DNA extracts from ear biopsies. To detect the Cftrfl10 (or Cftrtm1Cwr) allele (408 bp), the Cftr+ allele (361 bp) and the CftrΔ10 allele (154 bp), primers P1 (5’-GTAGGGGCTCGCTCTTCTTT-3’) P2 (5’ GTACCCGGCATAATCCAAGA-3’) and P3 (5’-AGCCCCTCGAGGGACCTAAT-3’) were used (Figure 1 C and D). PCR reactions were completed for 40 cycles of 95°C for 30 seconds, 58°C for 1 min and 72°C for 1 minute.
Lungs and intestines were collected from Cftr +/+, +/− and −/− animals and total RNA was isolated using Trizol (Invitrogen) per the manufacturer’s instructions. One ug of RNA was reverse transcribed into cDNA with MMLV. cDNA was used in PCR analysis to amplify Cftr exons 8–11 to detect wildtype allele(563 bp) and excised exon 10 allele (371 bp) expression. PCR reactions were completed for 40 cycles of 95°C for 30 seconds, 55°C for 30 seconds and 72°C for 1 minute with primers (5’-CCACAGGCATAATCATGGAA-3’) and (5’-TGTGACTCCACCTTCTCCAA-3’).
Histology
Mouse intestines were isolated and fixed in 10% formalin, embedded in paraffin and sectioned every 5µm. Periodic acid schiff (PAS) staining of these sections were completed to detect mucus accumulation.
Bioelectric measurements
The potential difference (PD) across the nasal epithelium of the mice was measured according to procedures previously described elsewhere (Brady et al., 2001; Kelley et al., 1997), with some modifications. Mice were anesthetized with 8.5 mg/ml ketamine, 1.7 mg/ml xylazine, and 0.3 mg/ml acepromazine in sterile saline. Animals were dosed intraperitoneally with 0.012 ml/g mouse weight. A PE-10 tube stretched to one-half the original diameter was inserted 2 mm into the mouse nostril and placed against the septum. With the use of a syringe pump (model A-99; Razel Scientific Instruments) and 3-ml syringes, N-2-hydroxyethylpiperazine-N’-2-ethanesulfonic acid (HEPES)-buffered Ringer solution perfused into the nostril at a rate of 5 ml/min. Valves controlled which solution entered the nostril, with a 15-s delay until the new solution reached the nasal epithelium. Readings were taken until a steady-state value was reached before perfusing the nasal epithelium with a new solution. Paper wicks were used to absorb excess liquid from the mouth and opposite nostril of the mouse being tested. A trachea tube consisting of an Angiocath IV Catheter was inserted into the mouse trachea to facilitate breathing. Bridges (4% agar) connected the tubing to calomel electrodes, and each bridge was made in the same solution as the perfusate it measured (HEPES-buffered Ringer or chloridefree HEPES-buffered Ringer). A needle containing 4% agar in HEPES-buffered Ringer was placed subcutaneously in the mouse’s back and connected to another calomel electrode to serve as a reference. The measuring and reference calomel electrodes were situated in a KCl bath in which one end of the agar bridge was also immersed. The other end of the measuring electrode’s bridge was in contact with the perfusate 20 cm from the mouse nose. The transepithelial difference was measured with a voltmeter (model ISO-DAM-D; World Precision Instruments), and the signal was recorded on a chart recorder (model BD112; Kipp and Zonen). The PD measurements were corrected for junction potentials, and changes in PD values were calculated by subtracting the value after 4 minutes of perfusion with chloride free ringers containing forskolin from the value when the perfusion began. Before measurements, the system was zeroed by adjusting the voltmeter after connecting the reference needle to the tube normally placed in the mouse nose to complete the circuit.
Electrolyte transport was investigated in the intestine of animals 8–12 weeks old that were fasted overnight. Mice were rendered unconscious by CO2 asphyxiation and killed by exsanguination. The large and small intestine was removed and immediately placed in ice-cold N-2-hydroxy-ethylpiperazine-N’-2-ethanesulfonic acid (HEPES)-buffered Ringer solution (in mM: 138 NaCl, 5 KCl, 2.5 Na2HPO4, 1.8 CaCl2, 1.0 MgSO4, and 10 HEPES-NaOH, pH 7.4) for dissection. The intestine was cut longitudinally, and the contents were removed with a stream of Ringers solution. The tissue was stretched and pinned in an Ussing chamber with an aperature of 0.125 cm2. Tissues were bathed on both sides by 6–8 ml of mammalian Krebs-Ringer bicarbonate solution (in mM: 115 NaCl, 25 NaHCO3, 5 KCl, 2.5 Na2HPO4, 1.8 CaCl2, 1.0 MgSO4, and 10 glucose, pH 7.4 [mannitol replaced glucose in the apical bathing solution), which was warmed to 37°C and circulated with 95% O2-5% CO2 through gas lifts. Transepithelial electrical voltage difference (Vms) was measured between two Ringer-agar bridges. Calomel cells connected the bridges to a high-impedance voltmeter (DVC 1000 Voltage/Current Clamp, World Precision Instruments). Current from an external DC source was passed by silver-silver chloride electrodes and Ringer-agar bridges to clamp the spontaneous Vms to zero. The current required (short-circuit current,Isc) was corrected for solution resistance between the tips of the voltage-sensing electrodes and recorded. Tissues were maintained with Vms clamped to zero (short-circuit conditions). At 60-s intervals, Vms was clamped to +2 mV for 3 s to calculate transepithelial resistance (Rms). Tissues were mounted and observed until the Isc stabilized (generally 10–15 min), at which time experimental maneuvers were begun.
Acknowledgements
We thank Nicole Brown and Christine Zhang for assistance with the bioelectric measurements and Bill Marcus for assistance with genotyping. Correspondence and requests for materials should be addressed to mitchell.drumm@case.edu.
References
- Badea TC, Wang Y, Nathans J. A noninvasive genetic/pharmacologic strategy for visualizing cell morphology and clonal relationships in the mouse. J Neurosci. 2003;23:2314–2322. doi: 10.1523/JNEUROSCI.23-06-02314.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Chetrit A, Antenos M, Jurisicova A, Pasyk EA, Chitayat D, Foskett JK, Casper RF. Expression of cystic fibrosis transmembrane conductance regulator during early human embryo development. Mol Hum Reprod. 2002;8:758–764. doi: 10.1093/molehr/8.8.758. [DOI] [PubMed] [Google Scholar]
- Brady KG, Kelley TJ, Drumm ML. Examining basal chloride transport using the nasal potential difference response in a murine model. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1173–L1179. doi: 10.1152/ajplung.2001.281.5.L1173. [DOI] [PubMed] [Google Scholar]
- Colledge WH, Abella BS, Southern KW, Ratcliff R, Jiang C, Cheng SH, MacVinish LJ, Anderson JR, Cuthbert AW, Evans MJ. Generation and characterization of a delta F508 cystic fibrosis mouse model. Nat Genet. 1995;10:445–452. doi: 10.1038/ng0895-445. [DOI] [PubMed] [Google Scholar]
- Davies WL, Vandenberg JI, Sayeed RA, Trezise AE. Cardiac expression of the cystic fibrosis transmembrane conductance regulator involves novel exon 1 usage to produce a unique amino-terminal protein. J Biol Chem. 2004;279:15877–15887. doi: 10.1074/jbc.M313628200. [DOI] [PubMed] [Google Scholar]
- Delaney SJ, Alton EW, Smith SN, Lunn DP, Farley R, Lovelock PK, Thomson SA, Hume DA, Lamb D, Porteous DJ, Dorin JR, Wainwright BJ. Cystic fibrosis mice carrying the missense mutation G551D replicate human genotype-phenotype correlations. Embo J. 1996;15:955–963. [PMC free article] [PubMed] [Google Scholar]
- Devuyst O, Golstein PE, Sanches MV, Piontek K, Wilson PD, Guggino WB, Dumont JE, Beauwens R. Expression of CFTR in human and bovine thyroid epithelium. Am J Physiol. 1997;272:C1299–C1308. doi: 10.1152/ajpcell.1997.272.4.C1299. [DOI] [PubMed] [Google Scholar]
- Engelhardt JF, Yankaskas JR, Ernst SA, Yang Y, Marino CR, Boucher RC, Cohn JA, Wilson JM. Submucosal glands are the predominant site of CFTR expression in the human bronchus. Nat Genet. 1992;2:240–248. doi: 10.1038/ng1192-240. [DOI] [PubMed] [Google Scholar]
- Gadsby DC, Vergani P, Csanady L. The ABC protein turned chloride channel whose failure causes cystic fibrosis. Nature. 2006;440:477–483. doi: 10.1038/nature04712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb BR, Boucher RC. Pathophysiology of gene-targeted mouse models for cystic fibrosis. Physiol Rev. 1999;79:S193–S214. doi: 10.1152/physrev.1999.79.1.S193. [DOI] [PubMed] [Google Scholar]
- Hasty P, O'Neal WK, Liu KQ, Morris AP, Bebok Z, Shumyatsky GB, Jilling T, Sorscher EJ, Bradley A, Beaudet AL. Severe phenotype in mice with termination mutation in exon 2 of cystic fibrosis gene. Somat Cell Mol Genet. 1995;21:177–187. doi: 10.1007/BF02254769. [DOI] [PubMed] [Google Scholar]
- Hodges CA, Palmert MR, Drumm ML. Infertility in Females with Cystic Fibrosis is Multifactorial: Evidence from Mouse Models. Endocrinology. 2008 doi: 10.1210/en.2007-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R, Hodges CA, Drumm ML, Palmert MR. The cystic fibrosis transmembrane conductance regulator (Cftr) modulates the timing of puberty in mice. J Med Genet. 2006;43:e29. doi: 10.1136/jmg.2005.032839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartner N, Augustinas O, Jensen TJ, Naismith AL, Riordan JR. Mislocalization of delta F508 CFTR in cystic fibrosis sweat gland. Nat Genet. 1992;1:321–327. doi: 10.1038/ng0892-321. [DOI] [PubMed] [Google Scholar]
- Kelley TJ, Thomas K, Milgram LJ, Drumm ML. In vivo activation of the cystic fibrosis transmembrane conductance regulator mutant deltaF508 in murine nasal epithelium. Proc Natl Acad Sci U S A. 1997;94:2604–2608. doi: 10.1073/pnas.94.6.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent G, Oliver M, Foskett JK, Frndova H, Durie P, Forstner J, Forstner GG, Riordan JR, Percy D, Buchwald M. Phenotypic abnormalities in long-term surviving cystic fibrosis mice. Pediatr Res. 1996;40:233–241. doi: 10.1203/00006450-199608000-00008. [DOI] [PubMed] [Google Scholar]
- Madison BB, Dunbar L, Qiao XT, Braunstein K, Braunstein E, Gumucio DL. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem. 2002;277:33275–33283. doi: 10.1074/jbc.M204935200. [DOI] [PubMed] [Google Scholar]
- Manson AL, Trezise AE, MacVinish LJ, Kasschau KD, Birchall N, Episkopou V, Vassaux G, Evans MJ, Colledge WH, Cuthbert AW, Huxley C. Complementation of null CF mice with a human CFTR YAC transgene. Embo J. 1997;16:4238–4249. doi: 10.1093/emboj/16.14.4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulberg AE, Weyler RT, Altschuler SM, Hyde TM. Cystic fibrosis transmembrane conductance regulator expression in human hypothalamus. Neuroreport. 1998;9:141–144. doi: 10.1097/00001756-199801050-00028. [DOI] [PubMed] [Google Scholar]
- O'Gorman S, Dagenais NA, Qian M, Marchuk Y. Protamine-Cre recombinase transgenes efficiently recombine target sequences in the male germ line of mice, but not in embryonic stem cells. Proc Natl Acad Sci U S A. 1997;94:14602–14607. doi: 10.1073/pnas.94.26.14602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neal WK, Hasty P, McCray PB, Jr, Casey B, Rivera-Perez J, Welsh MJ, Beaudet AL, Bradley A. A severe phenotype in mice with a duplication of exon 3 in the cystic fibrosis locus. Hum Mol Genet. 1993;2:1561–1569. doi: 10.1093/hmg/2.10.1561. [DOI] [PubMed] [Google Scholar]
- Ratcliff R, Evans MJ, Cuthbert AW, MacVinish LJ, Foster D, Anderson JR, Colledge WH. Production of a severe cystic fibrosis mutation in mice by gene targeting. Nat Genet. 1993;4:35–41. doi: 10.1038/ng0593-35. [DOI] [PubMed] [Google Scholar]
- Robert R, Thoreau V, Norez C, Cantereau A, Kitzis A, Mettey Y, Rogier C, Becq F. Regulation of the cystic fibrosis transmembrane conductance regulator channel by beta-adrenergic agonists and vasoactive intestinal peptide in rat smooth muscle cells and its role in vasorelaxation. J Biol Chem. 2004;279:21160–21168. doi: 10.1074/jbc.M312199200. [DOI] [PubMed] [Google Scholar]
- Rozmahel R, Wilschanski M, Matin A, Plyte S, Oliver M, Auerbach W, Moore A, Forstner J, Durie P, Nadeau J, Bear C, Tsui LC. Modulation of disease severity in cystic fibrosis transmembrane conductance regulator deficient mice by a secondary genetic factor. Nat Genet. 1996;12:280–287. doi: 10.1038/ng0396-280. [DOI] [PubMed] [Google Scholar]
- Snouwaert JN, Brigman KK, Latour AM, Malouf NN, Boucher RC, Smithies O, Koller BH. An animal model for cystic fibrosis made by gene targeting. Science. 1992;257:1083–1088. doi: 10.1126/science.257.5073.1083. [DOI] [PubMed] [Google Scholar]
- Todd-Turla KM, Rusvai E, Naray-Fejes-Toth A, Fejes-Toth G. CFTR expression in cortical collecting duct cells. Am J Physiol. 1996;270:F237–F244. doi: 10.1152/ajprenal.1996.270.1.F237. [DOI] [PubMed] [Google Scholar]
- Trezise AE, Buchwald M. In vivo cell-specific expression of the cystic fibrosis transmembrane conductance regulator. Nature. 1991;353:434–437. doi: 10.1038/353434a0. [DOI] [PubMed] [Google Scholar]
- Trezise AE, Chambers JA, Wardle CJ, Gould S, Harris A. Expression of the cystic fibrosis gene in human foetal tissues. Hum Mol Genet. 1993;2:213–218. doi: 10.1093/hmg/2.3.213. [DOI] [PubMed] [Google Scholar]
- Trezise AE, Linder CC, Grieger D, Thompson EW, Meunier H, Griswold MD, Buchwald M. CFTR expression is regulated during both the cycle of the seminiferous epithelium and the oestrous cycle of rodents. Nat Genet. 1993;3:157–164. doi: 10.1038/ng0293-157. [DOI] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schutz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- Tsui LC. The spectrum of cystic fibrosis mutations. Trends Genet. 1992;8:392–398. doi: 10.1016/0168-9525(92)90301-j. [DOI] [PubMed] [Google Scholar]
- Weyler RT, Yurko-Mauro KA, Rubenstein R, Kollen WJ, Reenstra W, Altschuler SM, Egan M, Mulberg AE. CFTR is functionally active in GnRH-expressing GT1-7 hypothalamic neurons. Am J Physiol. 1999;277:C563–C571. doi: 10.1152/ajpcell.1999.277.3.C563. [DOI] [PubMed] [Google Scholar]
- Yang Y, Raper SE, Cohn JA, Engelhardt JF, Wilson JM. An approach for treating the hepatobiliary disease of cystic fibrosis by somatic gene transfer. Proc Natl Acad Sci U S A. 1993;90:4601–4605. doi: 10.1073/pnas.90.10.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura K, Nakamura H, Trapnell BC, Chu CS, Dalemans W, Pavirani A, Lecocq JP, Crystal RG. Expression of the cystic fibrosis transmembrane conductance regulator gene in cells of non-epithelial origin. Nucleic Acids Res. 1991;19:5417–5423. doi: 10.1093/nar/19.19.5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiher BG, Eichwald E, Zabner J, Smith JJ, Puga AP, McCray PB, Jr, Capecchi MR, Welsh MJ, Thomas KR. A mouse model for the delta F508 allele of cystic fibrosis. J Clin Invest. 1995;96:2051–2064. doi: 10.1172/JCI118253. [DOI] [PMC free article] [PubMed] [Google Scholar]