Abstract
An 8-year-old, male, Alaskan malamute was evaluated for a 2-week history of lethargy, inappetence, and distended abdomen. The dog was diagnosed with severe hypothyroidism, exhibited facial myxedema and had myocardial dysfunction with ascites and pleural effusion. Myocardial function improved and facial myxedema and effusions resolved with levothyroxine supplementation.
Résumé
Amélioration d’une dysfonction myocardique chez un chien hypothyroïdien. Un Alaskan Malamute mâle âgé de 8 ans a été évalué pour des antécédents de 2 semaines d’abattement, d’inappétence et d’abdomen distendu. Le chien a été diagnostiqué avec de l’hypothyroïdisme grave, a manifesté un myxœdème facial et avait une dysfonction myocardique avec une ascite et un épanchement pleural. La fonction myocardique s’est améliorée et le myxœdème facial et les épanchements ont été résolus avec une recharge de lévothyroxine.
(Traduit par Isabelle Vallières)
An 8-year-old, intact male, Alaskan malamute was referred to the Oklahoma State University Veterinary Teaching Hospital with a 2-week history of lethargy, inappetence, and a distended abdomen. The dog had been diagnosed with hypothyroidism several years previously but thyroid hormone supplementation was discontinued 7 mo prior to presentation because a free serum thyroxine (FT4) level taken after administration of thyroid hormone was increased and it was presumed that the dog no longer needed thyroid supplementation.
Case description
The physical examination findings included obesity (53 kg, ideal body weight = 45 kg), weakness, mental dullness, rectal temperature of 38°C, heart rate of 118 beats per minute (bpm), and a respiratory rate of 40 breaths per minute. Subtle facial and submandibular nonpitting edema was evident, and abdominal palpation revealed moderate distension with hepatomegaly and ascites. Weak femoral pulses with pulse deficits were present. Thoracic auscultation revealed muffled heart sounds with increased normal breath sounds and an irregular heart rhythm. Tentative differential diagnoses included congestive heart failure with an arrhythmia (atrial fibrillation), hepatic, renal, or endocrine disease (originating from the thyroid gland or adrenal gland dysfunction).
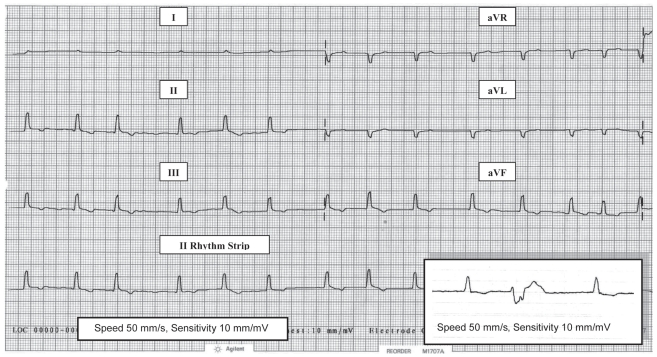
An electrocardiogram (ECG) performed in right lateral recumbency (Figure 1) confirmed atrial fibrillation with a slow ventricular response rate of 125 bpm. The ventricular complexes were small (R of 0.5 mV) and there were ≥ 20/min premature ventricular contractions (PVCs). The PVCs appeared similarly (monomorphic) and originated in the left ventricle with negative QS complexes in lead 2.
Figure 1.
Pretreatment electrocardiogram (limb leads) in right lateral recumbency from an 8-year-old male Alaskan malamute with severe hypothyroidism and dilated cardiomyopathy. There is an irregular rhythm with no visible p waves consistent with atrial fibrillation, a relatively slow rate of 125 bpm, and small ventricular complexes (R waves 0.5 mV). Inset in lead 2 (II) shows the typical PVC configuration (QS) that was seen.
Complete blood (cell) count (CBC) revealed a moderate leukocytosis [white blood (cell) count (WBC) 35.5 × 109/L; reference range: 5 to 17 × 109/L] with a mature neutrophilia (32.3 × 109/L; reference range: 3 to 12 × 109/L). A serum biochemical profile revealed increased alanine aminotransferase (ALT 241 U/L; reference range: 3 to 69 U/L) and creatine kinase (CK 5105 U/L; reference range: 22 to 491 U/L). Submission of serum for CK isoenzyme analyses to investigate the source of the elevated value was declined by the owner. A catheterized urine sample was analyzed and the results showed ≤ 5 WBC/HPF with 4+ bacteriuria but no proteinuria. Urine culture and sensitivity testing yielded an Escherichia coli sensitive to most antibiotics including ampicillin. Abdominocentesis revealed a straw-colored modified transudate with a low WBC (0.49 × 109/L) and total protein of 43 g/L.
Abdominal radiographs revealed moderate hepatomegaly with poor serosal detail attributed to the abdominal effusion. Thoracic radiographs revealed moderate left atrial and ventricular enlargement with mild pulmonary venous distension and interstitial infiltrates that were suggestive of pulmonary edema and consistent with left-sided congestive heart failure. Pleural fissure lines suggested concurrent mild pleural effusion.
Abdominal ultrasonography revealed a large amount of anechoic abdominal fluid, an enlarged diffusely hyperechoic liver with slightly rounded margins but with normal (not distended) hepatic veins. Echocardiography revealed moderate left ventricular and atrial dilation, and a mildly thickened mitral valve, but normal right atrial and ventricular chambers in the right parasternal long axis 2D views. Moderate mitral valve regurgitation was confirmed by color flow and pulse wave Doppler ultrasound in the 4-chambered apical view. On M-mode in the right parasternal short axis view the left ventricular end diastolic diameter (LVEDD) and left ventricular end systolic diameter (LVESD) were increased at 6.48 cm (normal 5.20 cm) and 5.60 cm (normal 3.60 cm), respectively, for a 55-kg dog (1–3). The left atrium was also dilated with a left atrial to aortic diameter ratio of 2.59 (normal ≤ 1.30) (1–3). On M-mode in right parasternal long and short axis views, the mitral valve “E” point to interventricular septal separation (EPSS), an indicator of left ventricular size (dilation) and function (dysfunction), was increased at ≥ 1.23 cm (normal < 0.6 cm) and the left ventricular fractional shortening (LVFS), an estimate of myocardial contractility, was decreased at 14% (normal 30% to 50%) (1–4). These echocardiographic findings indicated a volume overload with mitral regurgitation and myocardial systolic dysfunction (increased LVEDD and LVESD, and decreased LVFS) that is consistent with dilated cardiomyopathy (DCM).
Treatment consisted of ampicillin (Ampicillin sodium; American Pharmaceutical Partners, Schaumburg, Illinois, USA) 20 mg/kg body weight (BW), IV, q8h for the urinary tract infection (UTI); benazepril (Lotensin; Novartis Pharmaceuticals Corporation, East Hanover, New Jersey, USA) 0.3 mg/kg BW, PO, q12h to decrease systemic arterial resistance (afterload) and improve cardiac output from the apparently failing left ventricle with mitral insufficiency. Furosemide (Disal; Boehringer Ingelheim Vetmedica, St. Joseph, Missouri, USA) 2 mg/kg BW, IV, q8h was also instituted to reduce the vascular volume overload with left atrial and ventricular dilation (increased preload), pulmonary venous congestion and interstitial pulmonary edema, as well as alleviate the dog’s ascites. Abdominal fluid (5.0 L) was slowly removed by centesis to relieve pressure on the dog’s diaphragm and normalize his respiratory rate (≤ 24 bpm). Serum was submitted to the Endocrinology Animal Health Diagnostic Laboratory at Michigan State University for a thyroid hormone profile. Sera were also submitted to Oklahoma Animal Disease Diagnostic Laboratory for the first of paired antibody titers (IgG and IgM) against Toxoplasma gondii, and to Texas Veterinary Medical Diagnostic Laboratory for a Trypanosoma cruzii immunofluorescent antibody (IFA) test to rule out infectious myocarditis as a cause of the PVCs, as the history indicated there was possible exposure to these organisms.
Over the next 3 d the dog started eating on his own. Thoracic radiographs were repeated and confirmed resolution of the pulmonary edema and pleural effusion with only mild left atrial and ventricular enlargement and normal pulmonary vasculature. The dog’s heart rate remained at 100–120 bpm and atrial fibrillation persisted with less frequent PVCs (≤ 5/min) on ECG. Despite diuretic therapy, the abdominal distension from ascites persisted, his facial myxedema worsened and pitting edema of the distal limbs became evident. Since the dog was more responsive to his owners and was eating, they elected to take him home pending the results of the diagnostic tests submitted. While at home instructions were given for strict confinement, Hill’s k/d diet (Hill’s Prescription Diet k/d; Hill’s Pet Nutrition, Topeka, Kansas, USA), furosemide 2 mg/kg BW, PO, q12h, benazepril 0.3 mg/kg BW, PO, q12h, and amoxicillin/clavulanic acid (Clavamox; Pfizer, New York, New York, USA) 14 mg/kg BW, PO, q12h.
The results of the thyroid hormone profile were received 4 d after the patient was discharged and revealed a markedly decreased total thyroxine (TT4 = 0 nmol/L; reference range: 5–67 nmol/L), total triiodothyronine (TT3 = 0 nmol/L; reference range: 1–2.5 nmol/L), free thyroxine (FT4 = 2 pmol/L; reference range: 6–42 pmol/L) and free triiodothyronine (FT3 = 0.3 pmol/L; reference range: 4.5–12.0 pmol/L), with increased thyroid stimulating hormone (TSH = 41 mU/L; reference range: 0 to 37 mU/L), and a normal thyroglobulin autoantibody level (TAA = 116%; reference range: <200%). These results were consistent with severe primary hypothyroidism. Serologic tests for T. gondii and T. cruzii were negative. Levothyroxine (Soloxine; Daniels Pharmaceuticals, St. Petersburg, Florida, USA) at 0.01 mg/kg BW, PO, q12h was added to the dog’s therapeutic regimen at home and the dosage was increased 1 wk later to 0.015 mg/kg BW, PO, q12h. Benazepril was continued as previously dosed, but furosemide was administered at 2 mg/kg BW, PO, q24h (mornings) versus q12h to decrease the dog’s nocturia.
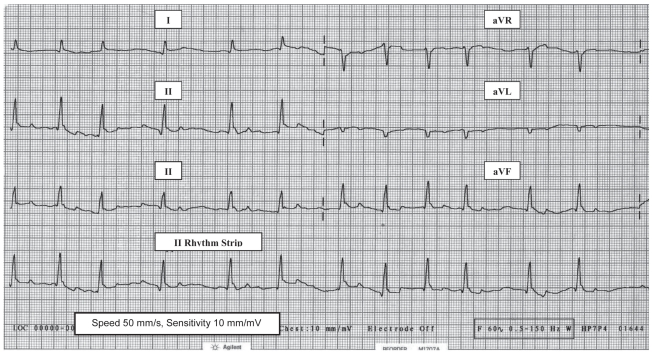
A recheck physical examination performed 1 wk after increasing the dose of levothyroxine, revealed a heart rate of 118 bpm, 5 kg of body weight loss (48 kg) and complete resolution of the distal limb edema, facial myxedema, and the abdominal distension. The owners reported that the dog had a normal appetite, was more alert, stronger, and more energetic, which required them to restrict his activity. A CBC, serum chemistry profile, and urinalysis were repeated and the results were unremarkable. Thoracic radiographs were repeated and revealed slight reduction in left atrial enlargement with no evidence of pulmonary venous distension or pulmonary edema; however, the cardiac silhouette remained enlarged primarily due to left ventricular enlargement. An ECG was performed and revealed persistent atrial fibrillation with a rate of 118 bpm but the QRS complexes were larger (R amplitude = 1.2 mV) and no PVC’s (Figure 2) were seen with continuous monitoring for more than 10 min. The right parasternal short axis view M-mode echocardiogram revealed a decrease in LVEDD (5.49 cm; normal 5.20 cm) and LVESD (4.23 cm; normal 3.60 cm), and atrial dilation with a left atrial to aortic ratio of 1.58 (normal ≤ 1.30). Right parasternal long and short axis view M-mode echocardiograms also revealed a decrease in mitral valve EPSS to 0.93 cm (normal < 0.6 cm) and an increase in the LVFS to 23% (normal 30%–50%). A thyroid hormone profile drawn 6 h after administration of thyroxine was within the normal range of values and levothyroxine was continued at 0.015 mg/kg BW, PO, q12h. Amoxicillin/clavulanic acid was continued for an additional 2 wk for treatment of the UTI. Urine culture and sensitivity testing was performed after therapy was terminated and the results were negative for bacterial growth.
Figure 2.
Electrocardiogram (limb leads) in right lateral recumbency from an 8-year-old male Alaskan malamute with severe hypothyroidism and dilated cardiomyopathy 14 d after starting oral levothyroxine replacement therapy. Atrial fibrillation persists, but the ventricular complexes are > 100% larger (R waves 1.2 mV) than prior to levothyroxine and no ventricular origin premature contractions are seen. The dog’s ventricular response rate remains relatively slow at 118 bpm.
The PVCs appeared to have resolved 2 wk after initiation of therapy and digoxin (Lanoxin; GlaxoSmithKline, Mississauga, Ontario) was prescribed at a low dose of 0.004 mg/kg BW, PO, q12h to evaluate whether a response of improved cardiac contractility (LVFS) would also result as had occurred following the initiation of thyroid replacement supplementation. The dog’s serum digoxin level (Digoxin; IDEXX Laboratories, Chicago, Illinois, USA) was rechecked a week after, then checked monthly for 2 mo; it remained in the therapeutic range between 1.8 and 1.9 nmol/L (reference range: 1.3 to 2.6 nmol/L). Although a positive inotropic response cannot always be predicted by an increase in LVFS, digoxin was withdrawn as there were no demonstrable benefits either clinically or echocardiographically (5). There was no decrease in LVFS or deterioration in the dog’s clinical condition when digoxin was discontinued. Diuretics were unnecessary and furosemide was also discontinued after 57 d. The hypothyroid dog continued to be treated with levothyroxine and oral benazepril for the mitral regurgitation.
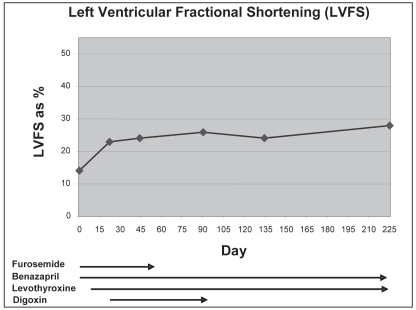
At re-examination 7½ mo (225 d) later, the dog weighed 44.5 kg and was considered to be in ideal body condition. He had become very active and appeared to have compensated clinically for the heart failure despite being in persistent atrial fibrillation. The thyroid hormone profile values after administration of thyroxine remained within normal ranges. Although there was some additional improvement in the dog’s LVFS to 28% on the M-mode right parasternal view, the echocardiogram revealed left ventricular dilation with LVEDD 5.78 cm (normal 5.00 cm). The LVESD was 4.14 cm (normal 3.60 cm) with an EPSS 0.88 cm (normal < 0.6 cm) and left atrial dilation with left atrial to aortic ratio of 1.86 (normal < 1.3); these were still consistent with DCM. A comparison of the LVFS values on serial echocardiograms from days 0 to 225 from this dog with treatment for hypothyroidism which started on day 8 are presented in Figure 3.
Figure 3.
Left ventricular fractional shortenings from M mode echocardiographic measurements of an 8-year-old male Alaskan malamute during treatment for hypothyroidism with improvement in cardiac systolic function.
Future reevaluations at 10, 14, and 18 mo confirmed that the dog remained clinically compensated and thyroid profile values after administration of thyroxine were within normal ranges. Although atrial fibrillation persisted on ECGs, the dog’s heart rate remained normal (between 100–140 bpm) and the improvement in LVFS was sustained at ≥ 28% on echocardiograms despite persistence of cardiac dysfunction consistent with DCM (increased LVEDD, EPSS, and left atrial to aortic diameter ratio). However, at 22 mo after diagnosis, the abdominal effusion recurred and the dog’s thyroid hormone profile levels were again below the normal ranges. Furosemide was not reinstituted but the dose of levothyroxine was increased to 0.02 mg/kg BW PO from 0.015 mg/kg BW, PO, q12h; the abdominal effusion resolved within a week. The dog remained clinically compensated and was euthyroid when rechecked at 25 and 28 mo. Thyroid hormone profiles submitted annually by the dog’s regular veterinarian remained within normal ranges but the dog’s owners declined annual follow-up echocardiograms because the dog had no further signs of cardiac problems. When contacted for follow-up, the owners reported that the dog continued to be active and appeared normal 2 y after being restabilized on an increased dose of levothyroxine.
Discussion
Hypothyroidism is a commonly recognized endocrinopathy in the dog, but can be easily overlooked due to its wide variety of nonspecific clinical signs and insidious onset (6). Clinical signs, such as mental dullness, exercise intolerance, and lethargy, usually develop in middle-age and are a result of decreased cellular metabolism (7,8). Lack of adequate thyroid hormones affects the metabolic function of almost all organ systems (7). Abnormalities related to cardiovascular dysfunction are not common, but include sinus bradycardia, decreased amplitude of the P and R waves, inversion of T waves, 1st and 2nd degree atrioventricular block on electrocardiogram (ECG), and reduced left ventricular pump function on echocardiography (6,7). Hypothyroidism can have direct myocardial effects including decreased cardiac muscle myosin ATPase, decreased sarcoplasmic reticulum calcium-ATPase activity, decreased calcium channel activity, reduced sodium-potassium ATPase activity, reduced β-adrenergic receptor number and reduced myocardial norepinephrine levels that can decrease contractility and impair relaxation (7,9).
Dilated cardiomyopathy, characterized by chamber dilation with myocardial systolic and diastolic dysfunction, is one of the most common heart diseases in dogs (10,11). The etiology is seldom known in the individual case of DCM, although several theories concerning genetic, nutritional, metabolic, inflammatory, infectious, or drug- or toxin-induced myocardial disease have been discussed (10,11). Recently there have been reports of DCM caused by taurine deficiency that have improved survival times after taurine supplementation (12,13). Regardless of etiology, dogs with DCM typically have a poor long-term prognosis (14–16). This report presents the clinical findings in a dog with longstanding hypothyroidism that developed myocardial dysfunction consistent with DCM, facial myxedema, and peripheral limb edema, ascites, and pleural effusion that responded to thyroid replacement therapy and concurrent cardiac medications.
Although DCM and primary hypothyroidism are known to occur together, there is little evidence that supports a causal relationship (8,14). In the dog reported in this case, it appears that severe hypothyroidism based on thyroid profile levels and concurrent clinical signs (myxedema) was associated with this dog’s apparent heart failure and the 2 were likely not coincidental. This deduction was based on an improvement in cardiac function (contractility) and the complete resolution of myxedema, ascites, and pitting distal extremity edema after initiation of thyroid replacement therapy. There are similar reports of improved cardiac function in hypothyroid dogs after levothyroxine supplementation (17–19).
Myxedema is a rare sequela seen with severe hypothyroidism that is characterized by nonpitting edema, especially around the face. It is caused by the accumulation of mucopolysaccharides and hyaluronic acid in the dermis which forms tissue gel in the interstitial spaces (7). When myxedema occurs, it may be misinterpreted as edema due to right-sided congestive heart failure, nephrotic syndrome, or liver failure with ascites (20). With the exception of the ascites that resolved after initiating levothyroxine treatment, none of these other concurrent conditions were found in this hypothyroid dog. In this dog, the extent of myxedema followed by distal limb edema that occurred as his condition initially progressed was better appreciated when the clinical signs resolved on rechecks.
The small QRS complexes seen initially on the ECG were attributed to hypothyroidism and possibly to obesity, as there was no evidence of pericardial or pleural effusion, and the complexes normalized in size after thyroid replacement (6,21,22). The negative QS deflection PVCs in Lead 2 suggested an ectopic focus of electrical discharge most likely located in the dilated left ventricle (23,24). Premature ventricular contractions could also represent re-entry with a unidirectional conduction block, myocardial infarction, or myocardial failure in DCM, or less likely an infiltrative (mycotic or neoplastic) disease (23,25,26). Atrial fibrillation may occur with increased frequency in hypothyroid dogs, as adequate levels of thyroid hormones are important for normal myocardial contractility (14,25,27,28). The absence of tachycardia usually associated with atrial fibrillation was attributed to hypothyroidism which decreases the sensitivity of the beta adrenergic receptors in myocardial cells, causing a reduced response to sympathetic stimulation (14,29). Structural AV nodal disease (fibrosis or inflammation) creating a physical barrier to cardiac impulses may also have contributed to the slow ventricular rate and the emergence of PVCs, but this appears unlikely as the PVCs resolved (29).
Hepatomegaly and marked ascites characterized as a modified transudate are typical with increased venous hydrostatic pressure with right-sided congestive cardiac failure. However, there was no evidence of hepatic or post caval venous obstruction seen on the abdominal sonogram and there was no evidence to support right-sided congestive heart failure on physical examination, thoracic radiographs, or the echocardiogram (30). Hepatomegaly with a hyperechoic homogenous parenchyma and rounded liver lobes on ultrasound was suggestive of hepatic lipidosis in this moderately obese, hypothyroid dog (31). Hypercholesterolemia often associated with hypothyroidism in dogs was not seen in this dog, but myxedema was detected (7). The increased CK value found on the initial chemistry profile could reflect cellular dysfunction and cytosol leakage, hypothyroid myopathy and/or decreased clearance of CK associated with hypothyroidism (7). However, since the CK normalized prior to initiating thyroid supplementation, the source of increased CK was more likely due to the dog’s manipulation and struggling prior to presentation. While there is no proven association between hypothyroidism and the development of ascites, the resolution of the ascites with thyroid replacement therapy has lead the authors to speculate that this dog’s ascites may have been associated with cellular dysfunction in severe hypothyroidism resulting in increased vascular endothelial permeability (7,32).
Although a few cases of reversible DCM secondary to tachycardia-induced cardiomyopathy have been documented, this does not appear to be the case in this dog (33). Myocardial failure with concurrent primary hypothyroidism has been reported in dogs that showed similar improvement in cardiac function with treatment for hypothyroidism (34). A decreased LVFS of 14% was seen initially in this dog; however, when primary hypothyroidism was confirmed and levothyroxine replacement was instituted the dog’s LVFS improved within 14 d (day 22) from 14% to 23%. It is possible that the concurrent treatment with furosemide and benazepril, at least initially, contributed to this dog’s clinical improvement. However, while angiotensin-converting enzyme inhibitors (ACEI) appear effective in acute or short-term management of congestive heart failure in dogs with myxomatous mitral valve disease (MMVD), they have not been effective in delaying the disease progression and congestive heart failure (35–39). Typically, dogs with congestive heart failure secondary to their valvular disease die within a year after development of clinical signs (39–41). This dog relapsed when the dosage of oral levothyroxine was inadequate and then remained clinically compensated for over 2 y when maintained on an adequate dose. Pimobendan, a phosphodiesterase III inhibitor, was unavailable at the time for use in treating this dog but has been recently promoted for inodilation (improved contractility and diastolic relaxation) of dogs in congestive heart failure secondary to MMVD and is still under investigation (35,39,42–44). It is not known if the addition of this inotrope might have provided additional improvement in this dog’s cardiac output. Ultimately, the dog’s LVFS increased to nearly normal (30–50%) at 28% on day 225. Although the dog’s LVEDD and EPSS also approached normal values (5.0 cm and < 0.6 cm), persistently increased values suggested impairment in cardiac function that was consistent with DCM. The authors contend that decreased myocardial cellular metabolism associated with severe hypothyroidism may have contributed to the development of cardiac dysfunction in this dog (7,14,45). Cumulative data from humans and animals support this hypothesis since low thyroid function promotes atherosclerosis and myocardial fibrosis (46,47). In addition, long-standing untreated hypothyroidism in humans can cause myxedema of the heart with irreversible myocardial damage and myocardial fibrosis (48). Severe hypothyroidism with pump dysfunction in this dog may have been associated with myocardial infiltration of mucopolysaccharides, coronary arterial atherosclerosis, or both; however, without a biopsy of the myocardium this could not be confirmed (18). If this hypothesis is true, the authors speculate that effective treatment of hypothyroidism before myocardial fibronecrosis and myocyte loss occurred may have allowed pump function in this dog to improve to near normal (47).
There is controversy as to whether thyroid supplement should be started at standard replacement dosages of levothyroxine or at a lower dose and then gradually increased to avoid cardiac decompensation (7,28). In this dog, slowly increasing levothyroxine to a maintenance dose was efficacious based on the clinical response and follow-up thyroid hormone profiles. At the last recheck, at 36 mo after being stabilized on oral benazepril and levothyroxine, this dog remained euthyroid and, except for persistent atrial fibrillation, was clinically normal.
Longstanding hypothyroidism with vascular dysfunction may have resulted in increased permeability and could have contributed to this dog’s cardiac systolic dysfunction. Hypothyroidism was considered to be the cause of facial myxedema, and may also be associated with the atrial fibrillation with slow ventricular response rate (heart rate) and bicavitary effusion. Based on the clinical findings of the dog, we recommend performing a thyroid hormone profile in large breed dogs that have clinical signs of congestive heart failure with systolic failure attributed to DCM; especially when there is concurrent non-pitting facial edema, an inappropriately normal to slow heart rate with atrial fibrillation, and small ventricular complexes on ECG. If concurrent primary hypothyroidism is confirmed, and hormone replacement therapy is instituted before any associated myocardial changes become irreversible, myocardial contractility and cardiac output may increase thereby improving the prognosis of hypothyroid dogs with cardiac systolic dysfunction. Additional studies are needed to investigate the role that hypothyroidism may play in the development of cardiac systolic dysfunction consistent with that seen in dogs with DCM.
Footnotes
Reprints will not be available from the authors.
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office ( hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Boon JA, Wingfield WE, Miller CW. Echocardiographic indices in the normal dog. Vet Radiol. 1983;24:214–221. [Google Scholar]
- 2.Lombard CW. Normal values of the canine M-mode echocardiogram. Am J Vet Res. 1984;45:2015–2027. [PubMed] [Google Scholar]
- 3.Kittleson MD, Kienle RD. Small Animal Cardiovascular Medicine. St. Louis: Mosby; 1998. Echocardiography; pp. 95–117. [Google Scholar]
- 4.Fox PR, Moise NS. Echocardiography and doppler imaging. In: Fox PR, Sisson D, Moise NS, editors. Textbook of Canine and Feline Cardiology. 2nd ed. Philadelphia: WB Saunders; 1999. pp. 130–171. [Google Scholar]
- 5.Kittleson MD, Eyster GE, Knowlen GG, Olivier NB, Anderson LK. Efficacy of digoxin administration in dogs with idiopathic congestive cardiomyopathy. J Am Vet Med Assoc. 1985;186:162–165. [PubMed] [Google Scholar]
- 6.Catherine J, Scott-Moncrieff R, Guptill-Yoran L. Hypothyroidism. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine: Diseases of the Dog and Cat. 6th ed. Philadelphia: WB Saunders; 2005. pp. 1535–1544. [Google Scholar]
- 7.Feldman EC, Nelson RW. Canine and Feline Endocrinology and Reproduction. 3rd ed. Philadelphia: WB Saunders; 2004. Hypothyroidism; pp. 86–151. [Google Scholar]
- 8.Kittleson MD, Kienle RD. Small Animal Cardiovascular Medicine. St. Louis: Mosby; 1998. The effects of systemic disease on the cardiovascular system; pp. 552–560. [Google Scholar]
- 9.Panciera DL. Conditions associated with canine hypothyroidism. Vet Clin North Am Small Anim Pract. 2001;31:935–950. [PubMed] [Google Scholar]
- 10.Tidholm A, Haggstrom J, Borgarelli M, Tarducci A. Canine idiopathic dilated cardiomyopathy. Part I: Aetiology, clinical characteristics, epidemiology and pathology. Vet J. 2001;162:92–107. doi: 10.1053/tvjl.2001.0571. [DOI] [PubMed] [Google Scholar]
- 11.Meurs KM. Primary myocardial disease in the dog. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine: Diseases of the Dog and Cat. 6th ed. Philadelphia: WB Saunders; 2005. pp. 1077–1082. [Google Scholar]
- 12.Kittleson MD, Keene B, Pion P. Results of the multicenter spaniel trial (MUST): Taurine- and carnitine- responsive dilated cardiomyopathy in American cocker spaniels with decreased plasma taurine concentration. J Vet Intern Med. 1997;11:204–211. doi: 10.1111/j.1939-1676.1997.tb00092.x. [DOI] [PubMed] [Google Scholar]
- 13.Fascetti AJ, Reed JR, Rogers QR, Backus RC. Taurine deficiency in dogs with dilated cardiomyopathy: 12 cases (1997–2001) J Am Vet Med Assoc. 2003;223:1137–1141. doi: 10.2460/javma.2003.223.1137. [DOI] [PubMed] [Google Scholar]
- 14.Kienle RD, Bruyette D, Pion PD. Effects of thyroid hormone and thyroid dysfunction on the cardiovascular system. Vet Clin North Am Small Anim Pract. 1994;24:495–507. doi: 10.1016/s0195-5616(94)50055-x. [DOI] [PubMed] [Google Scholar]
- 15.Tidholm A, Svensson H, Sylven C. Survival and prognostic factors in 189 dogs with dilated cardiomyopathy. J Am Anim Hosp Assoc. 1997;33:364–368. doi: 10.5326/15473317-33-4-364. [DOI] [PubMed] [Google Scholar]
- 16.Kittleson MD, Kienle RD. Small Animal Cardiovascular Medicine. St. Louis: Mosby; 1998. Primary myocardial disease leading to chronic myocardial failure (dilated cardiomyopathy and related diseases) pp. 319–346. [Google Scholar]
- 17.Panciera DL. M-mode echocardiographic and electrocardiographic findings before and after levothyroxine supplement in hypothyroid dogs. J Vet Intern Med. 1993;7:115. [Google Scholar]
- 18.Panciera DL. An echocardiographic and electrocardiographic study of cardiovascular function in hypothyroid dogs. J Am Vet Med Assoc. 1994;205:996–1000. [PubMed] [Google Scholar]
- 19.Stephan I, Nolte I, Hoppen HO. The effect of hypothyroidism on cardiac function in dogs. Dtsh Tierarztl Wochenschar. 2003;110:231–239. [PubMed] [Google Scholar]
- 20.Nicoloff JT. Myxedema coma. In: Hershman JM, Bray GA, editors. The Thyroid — Physiology and Treatment of Disease. Elmsford: Pergamon; Pr: 1979. pp. 401–411. [Google Scholar]
- 21.Tobias AH. Pericardial disorders. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine: Diseases of the Dog and Cat. 6th ed. Philadelphia: WB Saunders; 2005. pp. 1104–1118. [Google Scholar]
- 22.Tilley LP. Essentials of Canine and Feline Electrocardiography. 3rd ed. Malvern: Lea & Febiger; 1992. Analysis of canine P-QRS-T deflections; pp. 59–99. [Google Scholar]
- 23.Kittleson MD, Kienle RD. Small Animal Cardiovascular Medicine. St. Louis: Mosby; 1998. Diagnosis and treatment of arrhythmias (dysrhythmias) pp. 449–494. [Google Scholar]
- 24.Carr AP, Tilley LP, Miller MS. Treatment of cardiac arrhythmias and conduction disturbances. In: Tilley LP, Goodwin J-K, editors. Manual of Canine and Feline Cardiology. 3rd ed. Philadelphia: WB Saunders; 2001. pp. 371–405. [Google Scholar]
- 25.Miller MS, Tilley LP, Smith FWK, Fox PR. Electrocardiography. In: Fox PR, Sisson D, Moise NS, editors. Textbook of Canine and Feline Cardiology. 2nd ed. Philadelphia: WB Saunders; 1999. pp. 67–105. [Google Scholar]
- 26.Sisson D, O’Grady MR, Calvert CA. Myocardial diseases of dogs. In: Fox PR, Sisson D, Moise NS, editors. Textbook of Canine and Feline Cardiology. 2nd ed. Philadelphia: WB Saunders; 1999. pp. 581–619. [Google Scholar]
- 27.Gerritsen RJ, van den Brom WE, Stokhof AA. Relationship between atrial fibrillation and primary hypothyroidism in the dog. Vet Q. 1996;18:49–51. doi: 10.1080/01652176.1996.9694614. [DOI] [PubMed] [Google Scholar]
- 28.Panciera DL. Cardiovascular complications of thyroid disease. In: Bonagura JD, editor. Kirk’s Current Veterinary Therapy XIII Small Animal Practice. Philadelphia: WB Saunders; 2000. pp. 716–719. [Google Scholar]
- 29.Adin DB, France MK. ECG of the month. J Am Vet Med Assoc. 2004;224:1258–1260. doi: 10.2460/javma.2004.224.1258. [DOI] [PubMed] [Google Scholar]
- 30.Autran de Morais H, Schwartz DS. Pathophysiology of heart failure. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine: Diseases of the Dog and Cat. 6th ed. Philadelphia: WB Saunders; 2005. pp. 914–940. [Google Scholar]
- 31.Kealy JK, McAllister H. Diagnostic Radiology and Ultrasonography of the Dog and Cat. 4th ed. St. Louis: Elsevier Saunders; 2005. The abdomen; pp. 21–171. [Google Scholar]
- 32.Stephenson RB. Capillaries and fluid exchange. In: Cunningham JG, editor. Textbook of Veterinary Physiology. 3rd ed. Philadelphia: WB Saunders; 2002. pp. 181–191. [Google Scholar]
- 33.Wright KN, Mehdirad AA, Giacobbe P, Grubb T, Maxson T. Radiofrequency catheter ablation of atrioventricular accessory pathways in 3 dogs with subsequent resolution of tachycardia-induced cardiomyopathy. J Vet Intern Med. 1999;13:361–371. doi: 10.1892/0891-6640(1999)013<0361:rcaoaa>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 34.Phillips DE, Harkin KR. Hypothyroidism and myocardial failure in two Great Danes. J Am Anim Hosp Assoc. 2003;39:133–137. doi: 10.5326/0390133. [DOI] [PubMed] [Google Scholar]
- 35.Pion PD, Rishniw M. Canine chronic mitral valve disease — the search for the silver bullet. Proc Am Coll Vet Intern Med. 2008:98–100. [Google Scholar]
- 36.Kvart C, Haggstrom J, Pedersen HD, et al. Efficacy of enalapril for prevention of congestive heart failure in dogs with myxomatous valve disease and asymptomatic mitral regurgitation. J Vet Intern Med. 2002;16:80–88. [PubMed] [Google Scholar]
- 37.Atkins CE, Keene BW, Brown WA, et al. Results of the veterinary enalapril trial to prove reduction in onset of heart failure in dogs chronically treated with enalapril alone for compensated, naturally occurring mitral valve insufficiency. J Am Vet Med Assoc. 2007;231:1061–1069. doi: 10.2460/javma.231.7.1061. [DOI] [PubMed] [Google Scholar]
- 38.Haggstrom J, Hansson K, Karlberg BE, Kvart C, Madej A, Olsson K. Effects of long-term treatment with enalapril or hydralazine on the renin-angiotensin-aldosterone system and fluid balance in dogs with naturally acquired mitral valve regurgitation. Am J Vet Res. 1996;57:1645–1652. [PubMed] [Google Scholar]
- 39.Haggstrom J, Boswood A, O’Grady M, Jons O. Effect of pimobendan on survival in dogs with congestive heart failure due to myxomatous mitral valve disease. Proc Am Coll Vet Intern Med. 2008:90–91. doi: 10.1111/j.1939-1676.2008.0150.x. [DOI] [PubMed] [Google Scholar]
- 40.Bench Study Group. The effect of benazepril on survival times and clinical signs of dogs with congestive heart failure: Results of a multicenter, prospective, randomized, double-blinded, placebo-controlled, long-term clinical trial. J Vet Cardiol. 1999;1:7–18. doi: 10.1016/S1760-2734(06)70025-X. [DOI] [PubMed] [Google Scholar]
- 41.Ettinger SJ, Benitz AM, Ericsson GF, et al. Effects of enalapril maleate on survival of dogs with naturally acquired heart failure. The long-term investigation of veterinary enalapril (LIVE) study group. J Am Vet Med Assoc. 1998;213:1573–1577. [PubMed] [Google Scholar]
- 42.Smith PJ, French AT, Van Isral N, et al. Efficacy and safety of pimobendan in canine heart failure caused by myxomatous mitral valve disease. J Small Anim Pract. 2005;46:121–130. doi: 10.1111/j.1748-5827.2005.tb00302.x. [DOI] [PubMed] [Google Scholar]
- 43.Lombard CE, Jons O, Bussadori CM. Clinical efficacy of pimobendan versus benazepril for treatment of acquired atrioventricular valvular disease in dogs. J Am An Hosp Assoc. 2006;42:249–261. doi: 10.5326/0420249. [DOI] [PubMed] [Google Scholar]
- 44.Chetboul V, Lefebvre HP, Sampedrano CC, et al. Comparative adverse cardiac effects of pimobendan and benazepril monotherapy in dogs with mild degenerative mitral valve disease: A prospective, controlled, blinded, and randomized study. J Vet Intern Med. 2007;21:742–753. doi: 10.1892/0891-6640(2007)21[742:caceop]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 45.Klein I. Thyroid hormone and cardiac contractility. Am J Cardiol. 2003;91:1331–1332. doi: 10.1016/s0002-9149(03)00433-8. [DOI] [PubMed] [Google Scholar]
- 46.Liu SK, Tilley LP, Tappe JP, Fox PR. Clinical and pathologic findings in dogs with atherosclerosis: 21 cases (1970–1983) J Am Vet Med Assoc. 1986;189:227–232. [PubMed] [Google Scholar]
- 47.Khalife WI, Tang YD, Kuzman JA, et al. Treatment of sub-clinical hypothyroidism reverses ischemia and prevents myocyte loss and progressive LV dysfunction in hamsters with dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2005;289:2409–2415. doi: 10.1152/ajpheart.00483.2005. [DOI] [PubMed] [Google Scholar]
- 48.Okabe M, Kubara K, Kawaguchi H, et al. A case of myxedema with diffuse myocardial fibrosis proven by endomyocardial biopsy. Kokyu To Junkan. 1990;38:1159–1163. [PubMed] [Google Scholar]