Abstract
Given that exposure to captive wild animals at circuses or zoos can be a source of zoonotic infection, a case and control study was carried out with a collection of exotic fowl at a zoo in Bogotá, Colombia. The presence of Mycobacterium avium-II was directly related to the death of birds kept in the original enclosure, and of 50% of a group of sentinel birds. Failure to detect the organism in a control group of birds outside the enclosure indicated that the infection was limited to the original enclosed area. We demonstrated that M. gordonae-IV was disseminated in all organs from 1 bird with macroscopic granulomatous lesion, a finding which has not been reported previously. We emphasize the importance of establishing handling norms to reduce the risk of zoonotic transmission.
Résumé
Tuberculose aviaire d’importance zoonotique dans un zoo sur le plateau andéen de Bogotá (Sabana), en Colombie. Vu que l’exposition à des animaux sauvages en captivité dans les cirques ou les zoos peut devenir une source d’infection zoonotique, une étude de cas-témoin a été réalisée avec une collection d’oiseaux aquatiques exotiques à un zoo de Bogotá, en Colombie. La présence de Mycobacterium avium-II a été directement reliée à la mort d’oiseaux gardés dans un enclos original et de 50 % d’un groupe d’oiseaux sentinelles. La non-détection de l’organisme dans un groupe d’oiseaux témoins à l’extérieur de l’enclos a indiqué que l’infection s’est limitée à l’enclos original. Nous avons démontré que M. gordonae-IV a été disséminé dans tous les organes en provenance d’un oiseau avec une lésion granulomateuse macroscopique, un résultat qui n’a pas été signalé antérieurement. Nous insistons sur l’importance d’établir des normes de manipulation afin de réduire le risque de transmission zoonotique.
(Traduit par Isabelle Vallières)
Introduction
Zoonotic diseases are a worldwide challenge associated with globalization, international mobility, increase in cattle ranching, and climate change. The challenge must be met while using sustainable agro-industrial practices and maintaining the environment and free-roaming wildlife. Exposure to captive wild animals at circuses or zoos is a source of zoonotic infection, that is not often reported (1).
Avian mycobacteriosis is a bacterial disease affecting several bird species (2); it is highly contagious and chronic. The disease is characterized by granulomatous lesions (3) and a variety of clinical presentations. It is widely distributed throughout the world (4), and recently, it has been reported as being prevalent in wild and domestic fowl (5). In most countries, its distribution and incidence are unknown due to the absence of bacteriological studies (4) that identify the species that are involved. This is a weakness of many epidemiological surveillance systems.
The etiologic agent most frequently involved in avian mycobacteriosis is Mycobacteirum avium (6), although there are reports on the isolation of M. tuberculosis (7,8), M. bovis (9), and M. genavense (10–12). Mycobacteirum avium is often assigned to the M. avium-intracellulare complex (MAC), given the difficulty in differentiating members of this group through culture and biochemical methods. It is frequently present in soil and water (13), and has been reported as the 2nd most common mycobacterial species infecting both animals and human beings (14). Ubiquitous environmental exposure makes prevention difficult (15).
In humans, lymphadenitis is one of the most frequent clinical presentations of mycobacteriosis. Mycobacteirum avium is isolated in 70% to 80% of cases involving the MAC (16) and is responsible for the most prevalent disseminated infection in AIDS patients (17). There is increased interest in its epidemiology as human-to-human transmission has not been proved. It is assumed that human infection occurs through contact with birds. Nosocomial infections transmitted through water pipes have been described (18) and contaminated water is considered the principal reservoir for AIDS patients.
Nongranulomatous or atypical avian mycobacteriosis is difficult to detect at necropsy (19), as histopathologic examination of tissues stained with hematoxylin and eosin (H&E) does not detect the bacteria. This, along with the lack of effective vaccines or appropriate drug treatment programs, associated mortality, survival of the bacteria in the soil, and the absence of adequate procedures to clean and disinfect contaminated sites make it difficult to diagnose and control (20), especially in birds kept in zoos (4).
This study was conducted because of the finding of granulomatous lesions and acid-fast bacteria in H&E and Ziehl-Neelsen stained tissues, respectively, during necropsy of 3 birds that died while being kept in the same enclosure at a zoo: 2 Porphirura martinica (tinguas) and a Burhinus bistriatus (alcaraván). Conventional microbiological methods were used to isolate and identify the mycobacterial species. Morphological, biochemical, and antituberculosis drug sensitivity characteristics were determined. A polymerase chain reaction (PCR) for detection of gene hsp65 and enzymatic restriction analysis of the PCR product were used to identify the species involved.
Materials and methods
A case-control study was carried out between September, 2003 and December, 2004 at a zoo located on the Bogotá Plateau (Colombia). The birds were confined in an enclosure 3 m tall by 5 m wide and 15 m long. Its walls were constructed of cement up to 1 m from the ground, followed by glass windows, and ending with wire mesh. The roof had plastic roof tiles and the floor was earth; there was a waterer made out of cement. The various species of birds were separated by wire mesh. The research was approved by the ethics committees of both participating centers.
Population
The birds that were investigated belonged to a collection of exotic fowl at the zoo. In addition, 15 domestic fowl were introduced as part of the research. The birds were divided into 4 groups.
Group 1 consisted of the 5 birds that were kept in the same enclosure in which acid-fast positive cases had been detected. The group included a Burhinus bistriatus (alcaraván), a Speotito cunicularia (mochuelo de hoyo), 2 Ortalis motmot columbiana (guacharaca variable), and a Chamaepetes goudotii goudotii (pava maraquera). All birds in this group died during the study. Necropsies were conducted and samples of lung, spleen, intestine, and liver were taken in sterile tubes for microbiology and molecular studies. In the case of the B. bistriatus (alcaraván), muscle and bone tissue were also examined. A blood sample with EDTA for culture and molecular studies was taken from the ulnar vein of the C. g. goudotii before it died.
Group 2 (experimental control) comprised five 5-week-old Hy-line brown pullets from a traditional breeding farm. The apparently healthy animals were delivered directly from the farm and then slaughtered. Samples of spleen, liver, intestine, lung, and blood were collected and tested for infection by Mycobaterium.
Group 3 (sentinel bird group) was made up of 10 birds with the same characteristics as those of Group 2, and were taken directly from the farm to the zoo enclosure. They were kept there for 49 wk, during which 2 birds died. Ulnar vein blood samples were taken from the remaining 8 birds, and then they were slaughtered. Necropsy was carried out and samples of liver, lung, spleen, and intestine were collected. This group was set up to evaluate the transmissibility of the causal agent.
Group 4 (external dissemination control group) was made up of 12 birds kept in enclosures different from the affected one. Three of these birds died, and lung, spleen, intestine, and liver samples were taken during necropsy. The other 9 birds survived the study and blood samples were taken as before.
All tissue samples were homogenized using a disposable pellet pestle in a tissue homogenizer and were decontaminated with sodium hydroxide.
Microbiology
The following culture media were prepared in the laboratory and used to isolate mycobacteria: biphasic medium of solid Ogawa-Kudoh medium and liquid Sauton tween modified albumin (MSTA), biphasic medium Lowenstein Jensen/MSTA, and Stonebrink modified by Giraldo (ST-G) (8). The Ziehl-Neelsen stain was done on cultures to determine the presence of acid fast bacilli (AFB) and the cultures were then identified phenotypically by the Atlanta CDC standard methodology (21).
Genotyping
DNA was extracted for PCR by thermal shock (22). Samples that showed amplification of a fragment of 439 pb with the PCR that targeted gene hsp65, indicating the presence of Mycobacterium, were identified by enzymatic restriction of the amplified fragment with BstE-II and Hae-III enzymes (23). The mycobacteria species were determinined based on the sizes of the digestion fragments. The molecular weights of the bands were compared with those registered in the PRASITE (http://app.chuv.ch/prasite) data base, thus determining the specific patterns for the different species and their variants (24).
Statistical analysis
Statistical analysis was carried out by using EpiInfo 2002 (CDC, Atlanta, Georgia, USA). The proportions of positivity, according to the diagnostic tests in terms of confidence intervals (95% CI) and P-values were compared using Fisher’s exact test.
Results
The numbers of birds and samples in each group are shown in Table 1 according to the methodology that was used.
Table 1.
Number of birds and samples by group and methodology
| Methodology | Number of birds | Number of samples | Number of cultures or PCR tests | Number of isolates or genotypic positives |
|---|---|---|---|---|
| GROUP 1 | ||||
| Microbiological | 5 | 21 | 38 cultures | 10 isolates |
| Molecular | 5 | 20 | 20 PCRs | 10 genotypic identification |
| GROUP 2 | ||||
| Microbiological | 5 | 25 | 75 cultures | 0 isolates |
| Molecular | 5 | 25 | 25 PCRs | 1 genotypic identification |
| GROUP 3 | ||||
| Microbiological | 10 | 48 | 98 cultures | 8 isolates |
| Molecular | 10 | 46 | 46 PCRs | 14 genotypic identification |
| GROUP 4 | ||||
| Microbiological | 12 | 20 | 40 cultures | 0 isolates |
| Molecular | 12 | 20 | 20 PCRs | 0 PCR products |
PCR — polymerase chain reaction.
Microbiology
In group 1, there were 3 positive birds with 9 positive samples, 4 of which were identified as MAC, 4 as Mycobacterium sp. and 1 with MAC + M. chelonae (Table 2). In group 3, 5 positive birds were detected with 8 positive samples that phenotypically corresponded to: 2 MAC, 2 Mycobacterium sp., 3 nonpigmented M. gordonae, and 1 M. chelonae. In groups 2 and 4, there were no mycobacterial isolations and no positive birds.
Table 2.
Mycobacteria species identified by samples, group, and species of bird
| Bird
|
||||
|---|---|---|---|---|
| Group | Name | Sample | Molecular method | Microbiological method |
| 1 | Chamaepetes g. goudotii (Pava maraquera) | Spleen, liver | M. avium-II | MAC |
| Intestine, lung | M. avium-II | Mycobacterium sp. | ||
| 1 | Burhinus bistriatus (alcaraván) | Liver, muscle | M. avium-II | MAC |
| Bone | M. avium-II + | |||
| M. fortuitum-III | MAC + M. chelonae | |||
| 1 | Speotito cunicularia (mochuelo de hoyo) | Lung, liver | M. avium-II | Mycobacterium sp. |
| 2 | Gallus gallus (gallina) | Intestine | Mycobacterium sp. | No isolate |
| 3 | Gallus gallus (gallina) | Spleen | M. avium-II | MAC |
| Liver, lung | M. avium-II | No isolate | ||
| 3 | Gallus gallus (gallina) | Lung | M. avium-I | MAC |
| Spleen | M. avium-I | No isolate | ||
| 3 | Gallus gallus (gallina) | Spleen | Mycobacterium sp. | No isolate |
| 3 | Gallus gallus (gallina) | Liver | M. chelonae-I | M. chelonae |
| Spleen | M. chelonae-I | No isolate | ||
| 3 | Gallus gallus (gallina) | Intestine | Mycobacterium sp. | No isolate |
| 3 | Gallus gallus (gallina) | Spleen, liver, | M. gordonae-IV | M. gordonae |
| Intestine | M. gordonae-IV | M. gordonae | ||
| Lung | M. gordonae-IV | Mycobacterium sp. | ||
| 3 | Gallus gallus (gallina) | Lung | ND | Mycobacterium sp. |
| Spleen | Mycobacterium sp. | No isolate | ||
ND = not done.
Molecular studies
In group 1, 3 positive birds with 9 positive samples consisted of 8 M. avium-II and 1 co-infection by M. avium-II + M. fortuitum-III. In group 3, there were 7 positive birds with 14 positive samples that consisted of 3 M. avium-II, 2 M. avium-I, 4 M. gordonae-IV, 2 M. chelonae-I, and 3 Mycobacterium sp.
In group 2, 1 positive bird yielded a sample that was positive for Mycobacterium sp. and in group 4 there were no positive birds. Mycobacterium avium was the species with the widest distribution in the various samples examined by conventional microbiology as well as molecular methodology, with which 2 varieties could be differentiated. Mycobacterium avium-II was the species found in the highest proportion, above 50%, independently of the methodology, followed by M. gordonae-IV. One of the 4 birds from which M. avium-II was isolated was young; the other 3 were adults. Likewise, the bird from group 2 in which Mycobacterium sp. was reported, was also young.
The PCR showed an increase in sensitivity compared with the traditional microbiology for the spleen, intestine, and liver samples but the increase was not statistically significant (Table 3). Samples of lung and liver allowed a greater proportion of diagnoses by culture, while the spleen samples were the most efficient when evaluated through PCR.
Table 3.
Distribution of samples according to methodology and positivity
| Microbiological study
|
Molecular study
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Sample
|
Positives
|
Positives
|
||||||
| Tissue | n | Total | n | % (95% CI) | Total | n | % (95% CI) | P |
| Lung | 23 | 23 | 5 | 21.7 (3.9–63.0) | 23 | 6 | 26.1 (5.5–66.5) | 0.729 |
| Spleen | 19 | 19 | 3 | 15.8 (1.7–61.8) | 19 | 7 | 36.8 (8.8–77.0) | 0.140 |
| Liver | 23 | 23 | 5 | 21.7 (3.9–63.0) | 23 | 6 | 26.1 (5.5–66.5) | 0.729 |
| Intestine | 22 | 22 | 2 | 9.1 (0.6–25.8) | 22 | 4 | 18.2 (2.6–60.9) | 0.660 |
| Blood | 23 | 23 | 0 | 0 | 22 | 0 | 0 | |
| Muscle | 1 | 1 | 1 | 100 | 1 | 1 | 100 | |
| Bone | 1 | 1 | 1 | 100 | 1 | 1 | 100 | |
| Total | 112 | 112 | 17 | 15.2 (5.0–32.6) | 111 | 25 | 22.5 (10.4–41.0) | 0.161 |
95% CI — 95% confidence interval.
Discussion
Mycobacteria were detected in 50% of the birds in the original enclosed area and in 30.4% of the sentinel birds (group 3). A shorter duration of exposure and different species of birds may have contributed to this difference. In the control sentinel group, 50% of contaminated birds were detected by culture and 70% by PCR (P = 0.464), reflecting the ability of PCR to detect the lower concentrations of the bacteria that are present in the early stages of infection (22).
The presence of M. avium-II was directly related to the pathology observed in the birds kept in the original enclosure. Mycobacterium avium-II was also related to the pathology observed in the 50% of the sentinel birds that died. This type of M. avium, which corresponds to M. avium subsp. avium, in both the old and modern classifications (25), has been accepted as the causative agent of avian tuberculosis. It has been associated with dissemination in AIDS patients, in children with lymphadenitis, and in adolescents and elders with chronic lung tuberculosis. It is a different subspecies from the one causing infections in pigs but the question of host specificity among members of the MAC is still unclear (26). M. avium subsp. avium may have wildlife as a major reservoir with wild birds being responsible for its excretion into water and soil where it can remain for long periods of time and infect various animal species and human beings (27).
Mycobacterium avium-I was present exclusively in one of the 8 sentinel birds that were slaughtered at the end of the study, which may suggest that this Mycobacterium might not be as virulent in birds as M. avium-II.
Macroscopic granulomatous lesions were observed in the lung and spleen of only 1 of the 3 birds from group 3 in which Mycobacterium sp. was detected. This illustrates the great sensitivity of the molecular methodologies used to detect early infection by mycobacteria. A very interesting finding was the microbiological and molecular demonstration of M. gordonae-IV disseminated in all the organs studied from 1 bird with macroscopic evidence of granulomatous lesions. This had not been reported in the literature prior to this study. In humans, this mycobacterium has caused pathology and has frequently been reported in treated and untreated water (15).
In another bird of the sentinel group, M. chelonae-I was detected and isolated in association with macroscopic granulomatous lesions in the liver and spleen. This bird may have been immunosuppressed, favoring the dissemination of this potentially pathogenic mycobacterium (28). Within group 4, the external dissemination control group, mycobacteria were not detected by microbiology or PCR, indicating that the infection was likely limited to the original enclosure in which the infected birds were identified originally.
Samples of intestine showed low positivity, contrary to what might be expected in an organ where initial colonization occurs (9). This might be due to the abundant microbial flora, which would make isolation of mycobacteria difficult.
The results lead to the conclusion that the birds in the sentinel group became infected within the original enclosure and that the agent was able to infect 70% of these birds. Through this study, a definite diagnosis of avian mycobacteriosis caused by M. avium-II was established in a zoo on the Bogota Andean Plateau (Sabana).
The findings in this study highlight the importance of establishing handling norms to reduce the risk of zoonotic transmission. Since some studies (29) have shown a high proportion of mycobacteriosis by M. avium-II in immunosupressed human patients, there is a need to establish routinely the immunologic state of those handling the birds. Such procedures should be performed exclusively by immunocompetent individuals. The soil of enclosures is the biggest source of infection due to the spread of bacilli contained in the feces from birds infected with M. avium, which can remain viable in soil for more than 4 y. In the case of carcasses burned and buried 1-m deep, this time could be as long as 27 mo (4). It is therefore a priority to establish disinfection protocols of enclosures of exotic birds, which should be reviewed periodically and when the birds become infected with mycobacteria.
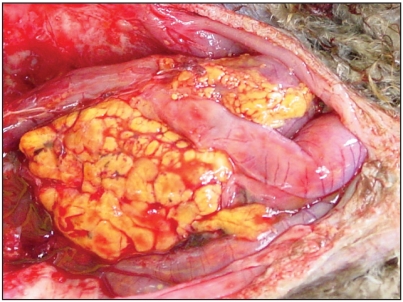
Figure 1.
Granulomatous lesion in the peritoneum, caused by M. gordonae-IV.
Acknowledgments
We are grateful to PATOMOL’s members from Quindío University, and to Tania Porras and Claudia Castro from the Mycobacteria Group, Sub direction of Research, National Health Institute in Bogotá, Colombia, for their assistance. The authors acknowledge the support of The La Salle University, College of Veterinary Medicine, project Code P-29-031210-29 COI and the Mycobacteria Group, Subdirection of Research, National Health Institute in Bogotá, Colombia. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office ( hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Stewart WC, Pollock KGJ, Browning LM, Young D, Smith Palmer A, Reilly WJ. Survey of zoonoses recorded in Scotland between 1993 and 2002. Vet Rec. 2005;157:697–702. doi: 10.1136/vr.157.22.697. [DOI] [PubMed] [Google Scholar]
- 2.Tell LA, Foley J, Needham ML, Walker RL. Diagnosis of avian mycobacteriosis: Comparison of culture, acid-fast stains, and polymerase chain reaction for the Identification of Mycobacterium avium in experimentally inoculated Japanese quail (Coturnix coturnix japonica) Avian Diseases. 2003;47:444–452. doi: 10.1637/0005-2086(2003)047[0444:DOAMCO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 3.Hermoso J. Género Mycobacterium. In: Vadillo S, Píriz S, Mateos E, editors. Manual de Microbiología Veterinaria Espana. McGraw-Hill; Interamericana: 2002. pp. 507–518. [Google Scholar]
- 4.Thoen CO. Tuberculosis. In: Calneck J, Barnes C, Beard L, McDougald, Saif Y, editors. Enfermedades de las aves. 10a ed. México: Manual Moderno; 2003. pp. 167–177. [Google Scholar]
- 5.Cooper J. Diagnostic pathology of selected diseases in wildlife. Rev Sci Tech Off Int Epiz. 2005;21:77–89. doi: 10.20506/rst.21.1.1320. [DOI] [PubMed] [Google Scholar]
- 6.Dorrestein G. Bacteriology. In: Altman R, Clubb S, Dorrestein G, Quesenberry K, editors. Avian Medicine and Surgery. Philadelphia: WB Saunders; 1997. pp. 255–280. [Google Scholar]
- 7.Fernández J, Ferrer L, Ramis A, Böttger E. Granulomatous dermatitis caused by Mycobacterium genavense in two psittacine birds. Proceedings American Association of Zoo Veterinarians. Annual conference; 1996; Nov 3–8; Puerto Vallarta, México. 1996. pp. 175–177. [Google Scholar]
- 8.Instituto Nacional de Salud. Manual de Procedimientos Bacteriología del Mycobacterium tuberculosis y de MNT. 1a ed. Bogotá: Instituto Nacional de Salud; 2001. [Google Scholar]
- 9.Carpenter JW, Gentz EJ. Zoonotic diseases of avian origin. In: Altman R, Clubb S, Dorrestein G, Quesenberry K, editors. Avian Medicine and Surgery. Philadelphia: WB Saunders; 1997. pp. 350–361. [Google Scholar]
- 10.Hoop RK, Böttger EC, Pfyffer GE. Etiological agents of mycobacteriosis in pet birds between 1986 and 1995. J Clin Microbiol. 1996;334:991–992. doi: 10.1128/jcm.34.4.991-992.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoop RK, Böttger EC, Ossent P, Salfinger M. Mycobacteriosis due to Mycobacterium genavense in six pet birds. J Clin Microbiol. 1993;31:990–993. doi: 10.1128/jcm.31.4.990-993.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thoen CO. Mycobacterium avium infections in animals. Res Microbiol. 1994;145:169–172. doi: 10.1016/0923-2508(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 13.Caminero J UICTER. Guía de tuberculosis para médicos especialistas. Paris, France: Compogravure Impresión; 2003. Enfermedades producidas por micobacterias ambientales; pp. 370–389. [Google Scholar]
- 14.Maslow J. Tuberculosis and other Mycobacteria as Zoonoses. Proceedings American Association of Zoo Veterinarians Annual Conference. 1997:110–115. [Google Scholar]
- 15.Pedley S, Bartram J, Rees G, Dufour A, Cotruvo JA, editors. Pathogenic Mycobacteria in Water. A Guide to Public Health Consequences Monitoring and Management. TJ International Ltd; Padstow, Cornwall, UK: 2004. [Google Scholar]
- 16.Prammananan T, Phunpruch S, Tingtoy N, Srimuang S, Chaiprasert A. Distribution of hsp65 PCR-restriction enzyme analysis patterns among Mycobacterium avium complex isolates in Thailand. J Clin Microbiol. 2006;44:3819–3821. doi: 10.1128/JCM.02495-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oliveira R, Sircili M, Oliveira E, Balian S, Ferreira J, Leão S. Identification of Mycobacterium avium genotypes with distinctive traits by combination of IS1245-based restriction fragment length polymorphism and restriction analysis of hsp65. J Clin Microbiol. 2003;41:44–49. doi: 10.1128/JCM.41.1.44-49.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ritacco V, Kremer K, van der Laan T, Pijnenburg EM, de Haas PEW, van Soolingen D. Use of IS901 and IS1245 in RFLP typing of Mycobacterium avium complex: Relatedness among serovar reference strains, human and animal isolates. Int J Tuberc Lung Dis. 1998;2:242–251. [PubMed] [Google Scholar]
- 19.Gerlach H. Bacteria. In: Ritchie W, Harrison GJ, Harrison LR, editors. Avian Medicine: Principles and Application Lake Worth. Florida: Wingers Publ; 1994. pp. 971–975. [Google Scholar]
- 20.Kearns KS, Loudis B. Avian Mycobacteriosis. Recent Advances in Avian Infectious Diseases. [Last accessed June 10, 2009]; [updated 2003 March 10; cited 2006 Dec 12] Available from http://www.ivis.org/advances/Kearns/kearns3/chapter_frm.asp?LA=1.
- 21.Kent TP, Kubica GP. Atlanta Centers for Disease Control; 1985. Public health mycobacteriology, a guide for the level III laboratory. [Google Scholar]
- 22.Leão S, Briones MR, Sircili MP, Baleen SC, Mores N, Ferreira-Neto JS. Identification of two novel Mycobacterium avium allelic variants in pig and human isolates from Brazil by PCR-restriction enzyme analysis. J Clin Microbiol. 1999;37:2592–2597. doi: 10.1128/jcm.37.8.2592-2597.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Telenti A, Marchesi F, Balz M, Bally F, Böttger EC, Bodmer T. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J Clin Microbiol. 1993;31:175–178. doi: 10.1128/jcm.31.2.175-178.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cardoso-Leao S, Bernardelli A, Cataldi A, et al. Multicenter evaluation of mycobacteria identification by PCR restriction enzyme analysis in laboratories from Latin America and the Caribbean. J Microbiol Methods. 2005;61:193–199. doi: 10.1016/j.mimet.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 25.Turenne CY, Wallace R, Jr, Behr MA. Mycobacterium avium in the postgenomic era. Clin Microbiol Rev. 2007;20:205–229. doi: 10.1128/CMR.00036-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mijs W, de Haas P, Rossau R, et al. Molecular evidence to support a proposal to reserve the designation Mycobacterium avium subsp. avium for bird-type isolates and ‘M. avium subsp. hominissuis’ for the human/porcine type of M. avium. Int J Syst Evol Microbiol. 2002;52:1505–1518. doi: 10.1099/00207713-52-5-1505. [DOI] [PubMed] [Google Scholar]
- 27.Bieta F, Boschirolib ML, Thorelb MF, Guilloteaua LA. Zoonotic aspects of Mycobacterium bovis and Mycobacterium avium-intracellulare complex (MAC) Vet Res. 2005;36:411–436. doi: 10.1051/vetres:2005001. [DOI] [PubMed] [Google Scholar]
- 28.Samuelson J. Enfermedades infecciosas. In: Cotran RS, Robbins SL, Kumar V, editors. Patología Estructural y Funcional. 6a ed. Madrid-Espana : McGraw-Hill Interamericana; 2000. pp. 349–424. [Google Scholar]
- 29.Murcia MI, Cardoso-Leao S, Rittaco V, et al. Distribución de patrones PRA en aislamientos clínicos del complejo Mycobacterium avium procedentes de España y Suramérica. Biomédica. 2004;24:60–64. [PubMed] [Google Scholar]