Abstract
We present a case of globosus amorphus delivered from a goat and subjected to radiography and histological examination. Radiography revealed a lack of development of any organ system; histological sections showed evidence of lymphoid aggregations, mononuclear infiltrations, blood capillaries, and dense fibroblasts.
Résumé
Un rare cas de globosus amorphus chez une chèvre. Nous présentons un cas de globosus amorphus mis-bas d’une chèvre et soumis à un examen radiographique et histologique. La radiographie a révélé une absence de développement de tous les systèmes d’organes; des sections histologiques ont montré des signes d’agrégations lymphoïdes, d’infiltrations mononucléaires, de capillaires et de fibroblastes denses.
(Traduit par Isabelle Vallières)
Fifty-one of 251 does presented to the Obstetrics Unit of Madras Veterinary College teaching hospital during 2006–2007 were diagnosed as dystocia due to maternal or fetal cause. This paper describes the gross morphology, radiography, and histological observations on a rare case of caprine fetal globosus amorphous encountered. Globosus amorphus is an asymmetrical spherical mass covered in skin and without a functional heart that is attached to the placenta of a normal twin (1,2). Its incidence is rare in domestic animals.
Case description
A 2-year-old Kani-adu goat was presented in lateral recumbency, with a history of inappetance, full-term pregnancy, and labor pain for 10 h. On clinical examination the goat was dull, with slightly congested mucous membranes and with a rectal temperature of 40.3°C (reference range: 38.6°C to 39.8°C). The vulval lips were edematous and there was a clear vaginal discharge. Fetal viability was checked by ultrasonography.
On vaginal examination, the cervix was fully dilated and the amniotic sac was intact. On manual rupture of the amniotic sac, the fetus was found to be in an anterior–longitudinal (P1), dorso-sacral (P2) position with bilateral shoulder flexion (P3). The case was tentatively diagnosed as dystocia due to postural abnormality. The condition was corrected to deliver 2 fully developed dead fetuses, followed by a normal live fetus that was delivered by gentle traction. On careful exploration of the uterus, a roughly spherical mass covered with a thick membrane was felt deep in the uterine horn. The mass was carefully removed through the birth canal, and was diagnosed as an anomalous fetus.
The dam was stabilized with 500 mL of multiple electrolyte solution (Parenteral Drugs, Mumbai, India) administered intravenously. Oxytocin (Pitocin, Parke Devis, Mumbai, India), 15 IU, was injected intramuscularly to hasten uterine involution and expulsion of placental debris. Intamox (amoxicillin and cloxacillin; Neovet Intas Pharmaceuticals, Ahmedabad, India), 500 mg, was administered intravenously.
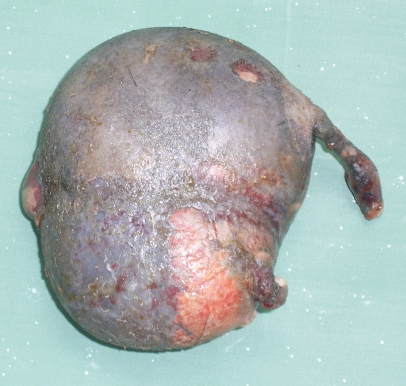
The anomalous fetus was covered with pigmented skin with a few hairs (Figure 1). It was slightly flattened and roughly spherical, measured 9.3 × 6.1 × 3.4 cm, and weighed 786 g. One pole of the anomalous fetus had a soft tissue protuberance and the other had 2 unequal and undifferentiated limbs. The cranial and caudal ends could not be identified and no oral or anal openings were discernible.
Figure 1.
Gross morphology of amorphus globosus with pigmented skin and a few hairs.
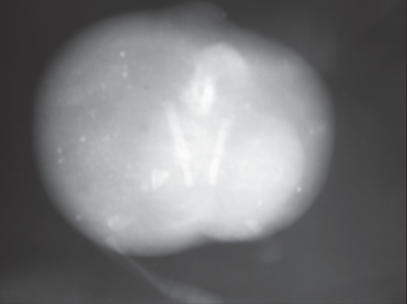
The radiographic image showed an irregular round soft tissue mass with soft tissue protrusion on 1 side and a partly developed appendicular structure with undifferentiated bone and a rudimentary appendicular protrusion on the other side (pole, Figure 2). The tissue mass was divided into 2 zones by a radio-dense soft tissue layer near the periphery. Two unequal linear radio-opaque structures in the center were made of bone that resembles the pelvic girdle. An irregular oval mass between the linear bony structure and the soft tissue protrusion resembled undifferentiated fused spines. Radiologically there was no recognizable organ system.
Figure 2.
Radiograph of anomalous fetus showing irregular soft round tissue mass with soft tissue protrusion on one side.
A medial linear incision was made to identify the development of various anatomical structures. Undifferentiated muscle and prominent blood vessels were observed, but there was no recognizable organ system. The bony mass could not be differentiated as forelimb or hind limb. Achordia was evident.
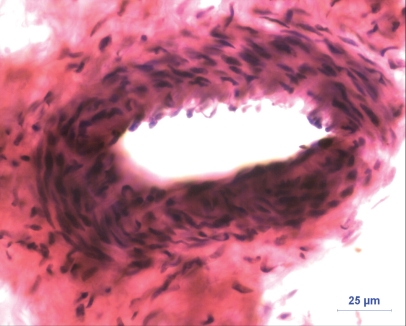
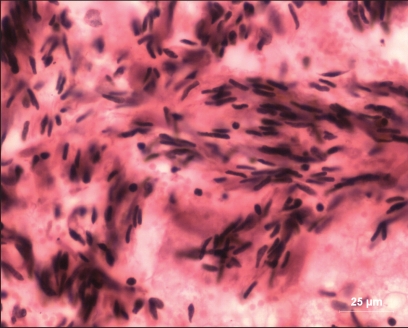
Tissues from the anamolous fetus were fixed in neutral-buffered formalin and processed for histology. Sections of 4–5 μm thickness were cut and stained with hematoxylin and eosin (3). There were numerous lymphoid aggregations and blood-filled capillaries. Capillary endothelium showed oval to elongated nuclei. Some areas showed epithelial type cells with eosinophilic granular cytoplasm. Arteries were present (Figure 3). Some areas showed scattered mononuclear cell infiltration, whereas other areas revealed dense fibroblasts (Figure 4) arranged in various directions.
Figure 3.
Histological section of amorphus globosus showing prominent artery (hematoxylin and eosin; bar = 25 μm).
Figure 4.
Histological section showing mononuclear infiltration with dense fibroblasts (hematoxylin and eosin; bar = 25 μm).
Discussion
A few cases of amorphus fetus have been reported in cattle (2,4–6) and in a mare (7), but globosus amorphus in a goat is very rare. The strange entity is said to be attached with fetal membranes of the normal fetus (8). Complete absence of gross tissue organization and umbilical cord, but with microscopic development of the body axis has been reported in humans (9). In another case, the acardiac twin was described as being the result of abnormal placental vascular anastomoses termed as twin reversed arterial perfusion (TRAP) theory (10).
Radiographical findings revealed only a small piece of bone with no characteristic features suggestive of any organized skeletal development. These findings are consistent with previous reports in cattle (5,6). Presence of vasculature with no heart or pump-like structures in the present case was similar to observations in a previous report (6). Histological findings revealed that the amorphus fetus consisted of several tissues showing various degrees of development. Microscopically, the subcutaneous tissue consisted of connective tissue and muscle, with mononuclear infiltration and lymphoid structures as reported by others (2,5).
The incidence of globosus amorphus in farm animals is greater than generally believed. In 2 separate reports in cattle (2,5) the amorphous fetus had the same chromosomal sex as the co-twin. However, another report (6) suggested that the globosus amorphus could develop from dizygotic twins. Unfortunately, a karyotypic study could not be carried out in the present case due to poor cooperation from the owner. Hence, it was not possible to determine whether this fetus amorphous was of monozygotic or dizygotic origin.
Acknowledgments
The authors thank Dr. D. Kathiresan, Director of clinics, TANUVAS, Dr. S. Prathaban, Professor and Head, Department of Clinics, Madras Veterinary College (MVC), Chennai for the facilities that were provided for the study. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office ( hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Roberts SJ. Veterinary Obstetrics and Genital Diseases. 3rd ed. Woodstock, Vermont: Roberts, SJ; 1986. Gestation period — Embryology, fetal membrane and placenta — Teratology; pp. 80–81. [Google Scholar]
- 2.Hishinuma M, Takahashi Y, Kanagawa H. Histological and cytological observation on a bovine acardius amorphus. Jpn J Vet Sci. 1987;49:195–197. doi: 10.1292/jvms1939.49.195. [DOI] [PubMed] [Google Scholar]
- 3.Bancroft JD, Gamble M. A Textbook on Theory and Practice of Histological Technique. 6th ed. New York: Churchill Livingston; 2007. [Google Scholar]
- 4.Czarnecki CM. Bovine holocardius amorphus monster. Can Vet J. 1976;17:109–110. [PMC free article] [PubMed] [Google Scholar]
- 5.Kamimura S, Enomoto S, Goto K, Hamana K. A globosus amorphous from an in vitro fertilized embryo transferred to a Japanese black cow. Theriogenol. 1993;40:853–858. doi: 10.1016/0093-691x(93)90220-y. [DOI] [PubMed] [Google Scholar]
- 6.Hishinuma M, Hoshi N, Takahashi Y, Kanagawa H. Vasculature and chromosomal composition in bovine acardius amorphus. Jpn J Vet Sci. 1988;50:1139–1141. doi: 10.1292/jvms1939.50.1139. [DOI] [PubMed] [Google Scholar]
- 7.Crossman PJ, Dicken PS. Amorphus globosus in the mare. Vet Rec. 1974;95:22. doi: 10.1136/vr.95.1.22-b. [DOI] [PubMed] [Google Scholar]
- 8.Arthur GH. Wright’s Veterinary Obstetrics. 3rd ed. London: Balliere Tindall; 1964. Anomalies of development of the conceptus; pp. 92–95. [Google Scholar]
- 9.Hanley LC, Boyd TK, Hecht JL. Acardiac twin presenting as fetus amorphous with an attenuated umbilical cord. Pediatr Dev Pathol. 2007;10:487–490. doi: 10.2350/07-02-0222.1. [DOI] [PubMed] [Google Scholar]
- 10.Kariappa TM, Chidananda HT, Mamatha R. Acardiac twin: An unusual case report. Indian J Pathol Microbiol. 2007;50:801–803. [PubMed] [Google Scholar]