Abstract
To study mechanisms governing fetoplacental vascular function, we have established a primary ovine fetoplacental artery endothelial (OFPAE) cell line. These OFPAE cells produce nitric oxide (NO), proliferate, and migrate in response to fibroblast growth factor 2 (FGF2) and vascular endothelial growth factor (VEGF). To overcome the senescence crisis that this primary OFPAE cell line will eventually enter, we attempted to establish a functional OFPAE cell line with a prolonged life span by transfecting cells with plasmids containing a neomycin resistance gene and a simian virus 40 gene (SV40) expressing large T (T) and small t (t) antigens. The OFPAE cells at passage 8 were transfected. After neomycin selection, the surviving OFPAE (designated SV40 OFPAE) cells were expanded up to passage 80. Up to passage 30, these SV40 OFPAE cells maintained a morphology similar to untransfected OFPAE cells. Expression of T and t antigens in SV40 OFPAE cells was confirmed by immunocytochemistry. These SV40 OFPAE cells exhibited positive uptake of acetylated low-density lipoprotein (Ac-LDL) and positive staining for NO synthase 3 (NOS3) and formed capillary-like tube structures on Matrigel. Up to passages 20–23, these SV40 OFPAE cells proliferated (P < 0.05) and produced (P < 0.05) NO in response to both FGF2 and VEGF. Moreover, this cell proliferation stimulated by FGF2 and VEGF was dose-dependently inhibited (P < 0.05) by PD98059 (a selective mitogen-activated protein kinase 1 and 2 [MAP2K1/2, also termed MEK1/2] inhibitor) or by LY294002 (a selective phosphoinositide 3-kinase [PI3K] inhibitor). These data indicate that SV40 OFPAE cells, at least at passage 23, retain endothelial phenotypes and functions similar to their parental, untransfected OFPAE cells. Thus, a functional OFPAE cell line with an extended life span has been successfully established, potentially providing a valuable cell model for studying fetoplacental endothelial function.
Keywords: cell proliferation, growth factors, kinases, mitogen-activated protein kinase 3/1, nitric oxide, placenta, placental endothelial cells, signal transduction, simian virus 40, v-akt murine thymoma viral oncogen homolog 1
INTRODUCTION
Angiogenesis and vasodilatation are essential processes that allow dramatic increases in placental blood flows, which are directly correlated with fetal growth, fetal survival, and neonatal birth weight [1, 2]. Placental angiogenesis and vasodilatation are believed to be actively regulated by fibroblast growth factor 2 (FGF2) and vascular endothelial growth factor (VEGF), two potent angiogenic factors [1–4]. This is supported by the observation that the expression of placental FGF2 and VEGF increases during normal pregnancy, along with an elevation in placental vascular density [1–4]. Moreover, alternations in the expression of these angiogenic factors are associated with changes in placental vascular growth and development in complicated pregnancies, such as preeclampsia [3, 4]. Similar to these angiogenic factors, the placental production of nitric oxide (NO), a vasodilator, is also elevated during normal pregnancies and is associated with increases in the expression of nitric oxide synthase 3 (NOS3) [4, 5]. The inhibition of NOS activity causes increases in fetoplacental vascular resistance, leading to the reduction of fetoplacental blood flows [4, 5]. These data indicate that NO plays an active role in regulating placental vascular tone and blood flows during pregnancy [4, 5]. Given that the endothelium is a fundamental element of angiogenesis and a major source of NO production responsible for vasodilatation, it is obviously an essential component in the regulation of placental blood flows [4, 5].
To study the cellular and molecular mechanisms that regulate fetoplacental angiogenesis and vasodilatation, we have established a primary ovine fetoplacental artery endothelial (OFPAE) cell line [4]. These OFPAE cells maintain many classical endothelial phenotypes in vitro. For example, they express NOS3 and receptors for FGF2 and VEGF [6]. More importantly, these OFPAE cells proliferate and produce NO in response to the stimulation of FGF2 and VEGF [6] (unpublished results). Thus, this cell line provides us with a valuable in vitro cell model for studying fetoplacental endothelial function.
Like other normal human somatic cells, however, this primary OFPAE cell line will eventually enter senescence, which limits its availability for future studies. One efficient approach to overcome this senescence crisis is to introduce the simian virus 40 (SV40) gene, bearing T and t antigens, into the primary cells [7–11]. The SV40 gene has been widely used to immortalize or greatly extend the life span of many endothelial cell lines with different origins, including those from human cerebromicrovessels [12], the umbilical veins (HUVE) [13, 14], and the placental microvasculature [15]. The SV40 exerts its effects primarily by binding and inactivating retinoblastoma (Rb) and p53 tumor suppressor proteins as well as inhibiting protein phosphatase 2 (PPP2, also known as PP2A), thus releasing cell division from any further restrictions [7–11].
One of the well-documented characteristics of endothelial cells is their responsiveness to FGF2 and VEGF. Both FGF2 and VEGF play important roles in regulating angiogenesis [16] and the vasomotor tone via controlling the production of vasodilators (i.e., NO) [6, 17–22]. The cellular responses to FGF2 and VEGF are mediated by activating their specific receptor-tyrosine kinases, which, in turn, phosphorylate and activate a cascade of downstream protein kinases. These protein kinases include mitogen-activated protein kinase 1 and 2 (MAP2K1/2) and phosphoinositide 3-kinase (PI3K), which are the primary kinases for activating mitogen-activated protein kinase 3/1 (MAPK3/1, also known as ERK1/2) and v-akt murine thymoma viral oncogen homolog 1 (AKT1) [16], respectively. In OFPAE cells, the inhibition of MAP2K1/2 and PI3K activation greatly attenuates FGF2- and VEGF-induced cell proliferation and migration (unpublished results). Both MAPK3/1 and AKT1 have also been implicated in the alteration of NOS3 activity via phosphorylating NOS3 [22]. In OFPAE cells, both increased NOS3 protein levels (facilitated by FGF2) [6] and increased acute NO production (facilitated by VEGF) (unpublished results) are mediated via activation of the MAP2K1/2/MAPK3/1 cascade.
In the present study, we have attempted to establish a functional OFPAE cell line with a prolonged life span using a pRNS-1 plasmid that contains a modified SV40 genome [23]. We determined 1) whether SV40-transfected OPFAE cells had a prolonged life span while maintaining the same characteristics of endothelial cells as their parental, untransfected OFPAE cells; 2) whether these SV40 OFPAE cells proliferated and produced NO in response to FGF2 and VEGF; and 3) whether FGF2- and VEGF-stimulated SV40 OFPAE cell proliferation was mediated partly via the MAP2K/1/2/MAPK3/1 pathway, the PI3K/AKT1 pathway, or both.
MATERIALS AND METHODS
OFPAE Cell Preparation and SV40 Transfection
OFPAE cells were established in our laboratory [6, 24]. Protocols for endothelial isolation and experimental procedures were approved by the Research Animal Care Committees of both the Medical School and the College of Agriculture and Life Sciences and by the Institutional Review Board, University of Wisconsin at Madison.
OFPAE cells at passage 8 were used for SV40 transfection. Cells were seeded in six-well plates and cultured in Dulbecco modified Eagle Medium (DMEM) that consisted of 5% fetal bovine serum (FBS), 5% calf serum (CS), and 1% penicillin/streptomycin (P/S) (all from Gibco, Grand Island, NY). When cells reached 70% confluence, SV40 transfection was conducted as described [15]. Briefly, after rinsing with media (OPTI-MEM1; Gibco), cells were incubated with a freshly prepared transfection mixture that consisted of 300 µl of OPTI-MEM1, 15 ng of pRNS-1 plasmids with an origin-defective SV40 genome expressing T and t antigens and resistance to neomycin (kindly provided by Dr. Harvey L. Ozer, New Jersey Medical School, Newark, NJ) [23], and 45 µl of FuGENE6 reagent (Roche, Nutley, NJ). Prior to transfection, the presence of T and t antigen and neomycine resistance sequences in pRNS-1 plasmids was confirmed with restriction enzyme digestions and PCR reactions. After 24 h of culture, cells were cultured with DMEM that consisted of 5% FBS, 5% CS, and 1% P/S, supplemented with neomycin (1 mg/ml; Invitrogen, Carlsbad, CA) to select SV40 positively transfected cells. After 12 days of culture with a daily medium change, the surviving cells (indicative of positive SV40 transfection) were designated passage 1 SV40 OFPAE cells. These SV40 OFPAE cells were expanded for 10 passages with a continuous neomycin selection. After passage 10, cells were reselected at every other five passages by culturing cells with the medium that contained neomycin.
Immunocytochemistry
To confirm successful transfection, expression of T and t antigens in SV40 OFPAE cells was determined by immunocytochemistry as described [6, 24]. Subconfluent SV40 OFPAE cells at passage 4 grown in eight-well chamber slides (Nunc, Naperville, IL) were fixed in 4% formaldehyde (pH 7.4; Electron Microscopy Sciences, Hatfield, PA). Immunolocalization was accomplished with a specific mouse monoclonal antibody (4 µg/ml, SV40 Pab108; BD Biosciences, Franklin Lakes, NJ) against T and t antigens and indirect immunoperoxidase detection via the avidin-biotin-peroxidase complex method (Vector Laboratories, Burlingame, CA) with 3-amino-9-ethyl carbazole (AEC) substrate (Vector Laboratories) as a chromogen. After immunostaining, cells were counterstained with hematoxylin (InnoGenex, San Ramon, CA). Preimmune mouse immunoglobulin G (IgG) at the same concentration as the primary antibody was used as a control. Untransfected OFPAE cells and SV40-transfected human placental microvascular endothelial cells (SV40 HPME; kindly provided by Dr. Peter Friedl, Institut für Biochemie, Technische Universität Darmstadt, Darmstadt, Germany) [15] were also run in parallel as negative and positive staining controls, respectively. These SV40 HPME cells with an extensive life span (at least up to 80 passages) were transfected with the same pRNS-1 plasmids as those used in the present study [15].
Characterization of SV40 OFPAE Cells
Endothelial phenotypes of these SV40 OFPAE cells were verified for 1) expression of NOS3 with a mouse monoclonal antibody (4 µg/ml; BD Biosciences) by immunochemistry as described above, 2) uptake of acetylated low-density lipoprotein (Ac-LDL), and 3) capillary-like tube formation assays (see below). After passage 20, SV40 OFPAE cells were cultured with molecular cell developmental biology medium (MCDB131) supplemented with 10% FBS, 10% CS, 10 mM glutamine, and 1% P/S. Cellular responses (i.e., kinase activation, proliferation, and NO production) to FGF2 and VEGF were also assessed.
Uptake of DiI Ac-LDL
Uptake of Ac-LDL was carried out as described [4]. SV40 OFPAE cells at passage 8 were cultured in media in the absence or presence of DiI Ac-LDL (10 µg/ml; Biomedical Technologies, Stoughton, MA) for 4 h at 37°C. After rinsing with serum-free medium, cells were fixed and mounted in a mounting reagent containing 4′,6′-diamidino-2-phenylindole (DAPI; Vector Laboratories). Untransfected OFPAE and SV40 HPME cells were run in parallel as controls.
Capillary-Like Tube Formation
SV40 OFPAE cells at passage 23 (80 000 cells/well) were seeded onto a 24-well culture plate coated with 300µl of Growth Factor Reduced Matrigel (~0.5-mm thickness; BD Biosciences) and incubated in MCDB131 with serum. After 26 h of culture, the capillary-like tube formation was examined under a microscope. Untransfected OFPAE and SV40 HPME cells were run in parallel as controls.
Cell Proliferation Assays
To determine whether SV40 OFPAE cells responded to stimulation by growth factors, cell proliferation assays were performed as described [6, 24]. Cells at passages 20 and 21 were seeded in 96-well plates (2000 cells/well) in MCDB131 media with serum and cultured for 16 h. After serum starvation for 48 h, cells were treated with bovine FGF2 (R&D Systems, Minneapolis, MN) or human recombinant VEGF165 (VEGF; PeproTech, Rocky Hill, NJ) at 0 (controls), 0.01, 0.1, 1, 10, or 100 ng/ml. After an additional 48 h of culture, the number of cells was counted as described previously [6, 24]. Wells with known cell numbers (0, 2500, 5000, 10 000, 20 000, or 40 000 cells/well; six wells/cell density) were evaluated in a similar fashion to establish standard curves. After determination of an optimal dose, additional cells were treated with FGF2 or VEGF in the absence or presence of PD98059 (CalBiochem, San Diego, CA), a selective MAP2K1/2 inhibitor, or LY294002 (CalBiochem), a PI3K inhibitor, in serum-free MCDB131 (six wells per concentration) to examine the effects of MAP2K1/2/MAPK3/1 and PI3K/AKT1 on FGF2- and VEGF-induced SV40 OFPAE cell proliferation.
NO Production Assay
The NO generated by SV40 OFPAE cells was determined by a real-time NO detection method with a fluorescence probe, 4-amino-5-methylamino-2′,7′-difluorescein diacetate (DAF-FM-DA; Molecular Probes, Eugene, OR) as described [24]. In this assay, DAF-FM-DA is deacetylated inside the cells and converted to DAF-FM. In the presence of NO, DAF-FM reacts with NO and forms fluorescent products, which can be detected. Briefly, cells at passages 20–23 were grown on 96-well, clear-bottom plates in MCDB131 with 5% FBS, 5% CS, 10 mM glutamine, and 1% P/S. When a confluence of 70%–80% had been reached, cells were washed twice and incubated in freshly prepared modified Krebs-Ringer phosphate buffer (mKRPH, pH 7.4). After 1 h of equilibration in mKRPH, DAF-FM-DA (final concentration at 5 µM) was added, followed by 1 h of incubation. After rinsing twice, cells were treated with FGF2 or VEGF at 1, 10, or 100 ng/ml in the presence of 10 µM l-arginine. Fluorescent signals were detected every 5 min for up to 2 h with a Synergy microplate fluorescence reader (Bio-TEK Instrument, Winooski, VT) having an excitation/emission wavelength of 485/528 nm. All data reported were corrected by subtracting the relative fluorescent unit obtained from the cells treated with DAF-FM-DA only.
Western Blot Analysis for MAPK3/1 and AKT1
Western blot analysis was performed as described previously [6, 24]. After 48 h of serum deprivation, SV40 OFPAE cells grown in 60-mm culture dishes were treated with FGF2 and VEGF for 0, 5, 10, 15, 30, or 60 min. The cells were washed twice with cold PBS, harvested, and lysed by sonification in a lysis buffer (4 mM sodium pyrophosphate, 50 mM Hepes [pH 7.5], 100 mM NaCl, 10 mM EDTA, 10 mM sodium fluoride, 2 mM sodium orthovanadate [Na3VO4], 1 mM phenylmethyl sulfonyl fluoride, 1% Triton X-100, leupeptin [5 µg/ml], and aprotinin [5 µg/ml]). The cell lysates were centrifuged at 12 000 × g for 10 min at 4°C, and the protein concentrations of the supernatant were detected with a bicinchoninic acid protein assay (Pierce, Rockford, IL). Proteins (15 µg/sample) were separated on 12% SDS-PAGE gels, electroblotted onto Immobilon-P membranes (Millipore, Bedford, MA), and immunoblotted with a rabbit antibody against phosphor-specific or total (1:2000) MAPK3/1 or phosphor-specific or total AKT1 (Ser493) (1:500 or 1:1000, respectively) (all antibodies were purchased from Cell Signaling Technology, Beverly, MA). These proteins were detected with enhanced chemiluminescence (ECL) or ECL plus detection systems (Amersham Bioscience, Piscataway, NJ).
Statistical Procedures
For growth factor dose responses and NO production, data were analyzed by a one-way ANOVA (SigmaStat; Jandel Scientific, San Rafael, CA). When an F test was significant, data were compared with their respective control by the Bonferroni multiple comparison test or the Student t-test. Data are reported as means ± SEM.
RESULTS
Characterization of SV40 OFPAE Cells
After 12 days of SV40 transfection and continuous neomycin selection, surviving SV40 OFPAE cells were expanded up to passage 80. Up to passage 30, these cells had a similar morphology (i.e., a cobblestone appearance at confluence) but exhibited less contact inhibition than did the untransfected OFPAE cells. After passage 30, these cells progressively formed a heterogeneous morphology (i.e., spindle shaped with the appearance of large granules inside cytoplasm).
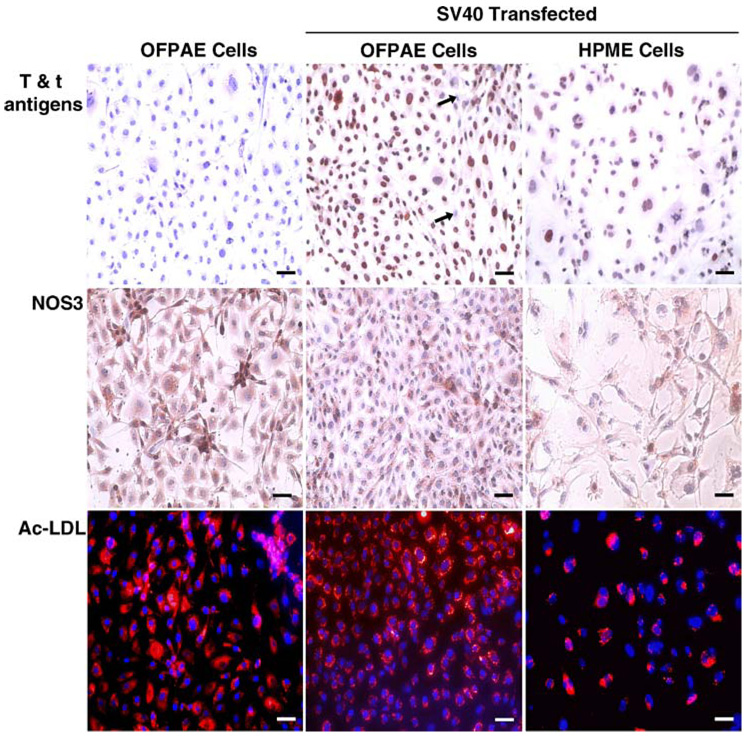
To confirm transfection, the expression of T and t antigens in SV40 OFPAE cells was examined by immunocytochemistry. We observed that T and t antigens were present in the majority of SV40 OFPAE and SV40 HPME cells, localized predominantly in the nucleus (Fig. 1). No staining was observed in untransfected OFPAE cells (Fig. 1) or in mouse IgG controls for untransfected OFPAE, SV40 OFPAE, and SV40 HPME cells (data not shown). These observations indicate a successful SV40 transfection and the establishment of an OFPAE cell line with a prolonged life span.
FIG. 1.
Characterization of SV40 OFPAE cells. Upper and middle panels: immunolocalization of T and t antigens and NOS3, respectively. A mouse monoclonal antibody against T and t antigens or NOS3 was used with AEC as a chromogen. After immunostaining, cells were counterstained with hematoxylin. Brownish staining indicates positive staining for T and t antigens or NOS3. Blue nuclear staining is due to hematoxylin counterstaining. Arrows: negative cells. No positive staining was observed in IgG controls (data not shown). Bottom panels: DiI-Ac-LDL uptake. Cells were incubated with DiI-Ac-LDL for 4 h at 37°C, fixed, and mounted. Red fluorescence indicates accumulation of DiI-Ac-LDL. Blue fluorescence is due to DAPI counterstaining. Untransfected OFPAE and SV40 HPME cells were run in parallel, serving as controls. Bar = 50 µm.
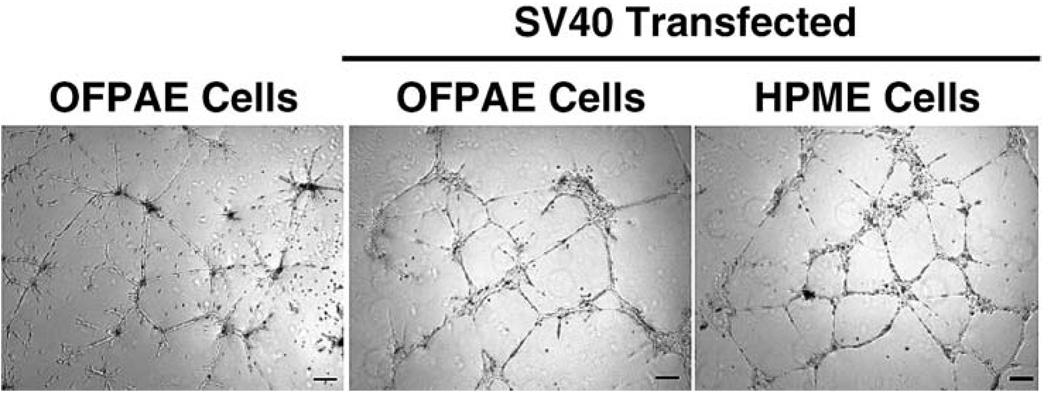
Similar to the untransfected OFPAE and SV40 HPME cells, these SV40 OFPAE cells exhibited positive NOS3 staining and uptake of Ac-LDL (Fig. 1). These SV40 OFPAE cells grown on Matrigel also formed capillary-like tube structures when cultured in MCDB131 with serum (Fig. 2), indicating that SV40 OFPAE cells maintained the same endothelial phenotypes as untransfected OFPAE cells [4].
FIG. 2.
Capillary-like tube formation in SV40 OFPAE cells. Cells were seeded (80 000 cells/well) in 24-well culture plates coated with Matrigel and cultured in MCDB131 with serum. After 26 h of culture, capillary-like tube formation was examined. Untransfected OFPAE and SV40 HPME cells were run in parallel, serving as controls. Bar = 200 µm.
Effects of FGF2 and VEGF on SV40 OFPAE Cell Proliferation
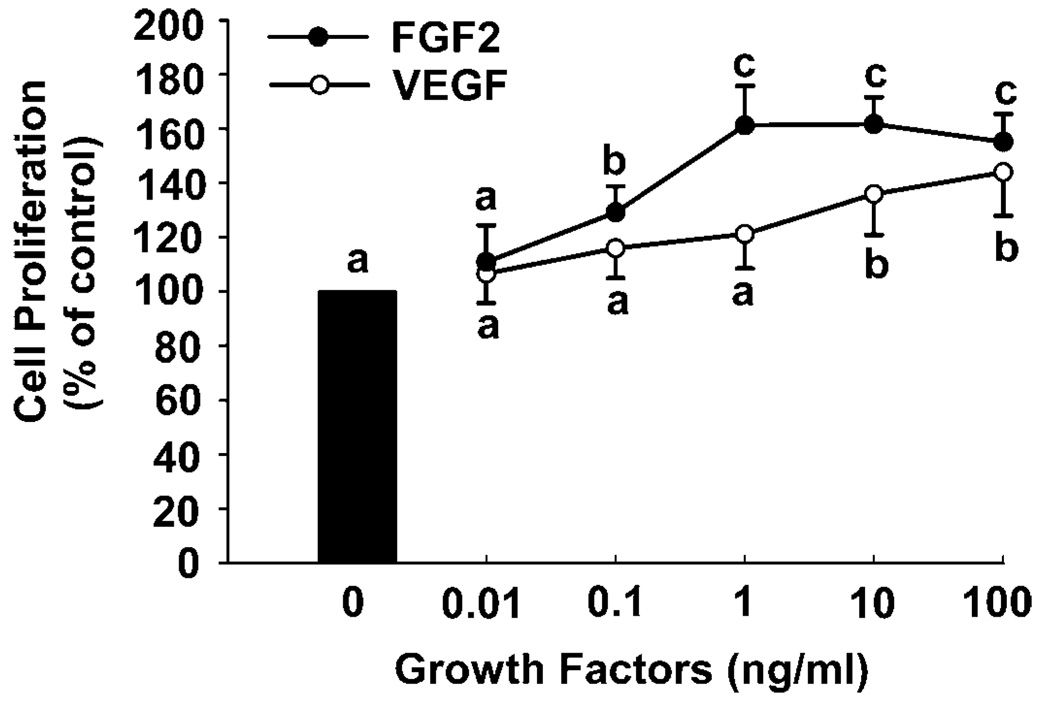
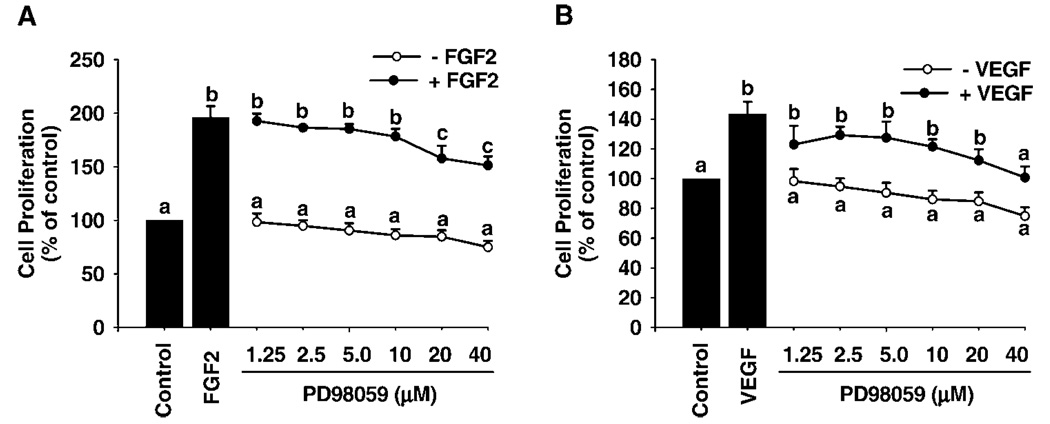
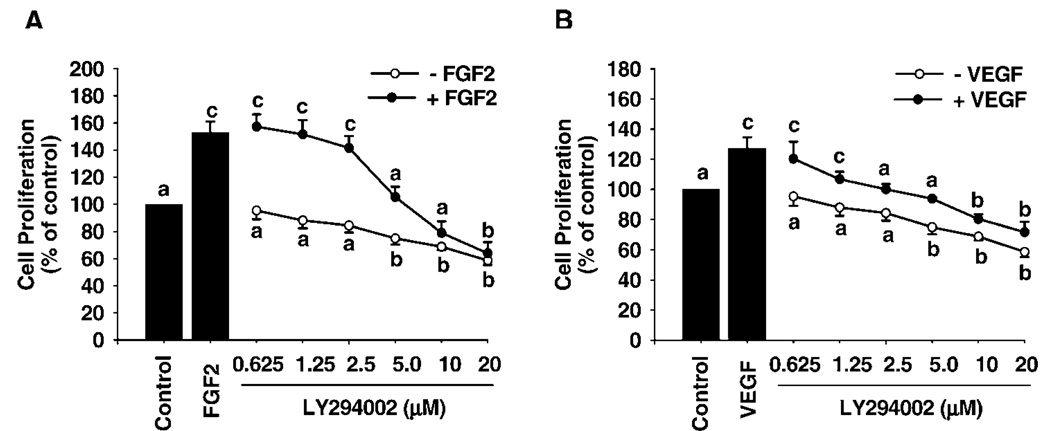
Both FGF2 and VEGF dose-dependently stimulated SV40 OFPAE cell proliferation (Fig. 3). FGF2 stimulated cell proliferation with a 30% increase (P < 0.05) over the control at 0.1 ng/ml, reached its maximum effect (~60%) at 1 and 10 ng/ml, and then declined slightly (~50%) at 100 ng/ml (Fig. 3). For VEGF, the threshold stimulatory effect (P < 0.05) occurred at 10 ng/ml (~30%) and increased slightly up to 100 ng/ml (~40%; Fig. 3). Moreover, the FGF2- and VEGF-stimulated SV40 OFPAE cell proliferation was dose-dependently inhibited (P < 0.05) by both PD98059 and LY294002 treatments (Fig. 4 and Fig. 5). PD98059 at 20 and 40 µM inhibited (P < 0.05) FGF2- and VEGF-stimulated SV40 OFPAE cell proliferation respectively (Fig. 4), while the inhibitory (P < 0.05) effects of LY294002 were observed at 5 and 2.5 µm for the FGF2- and VEGF-stimulated cell proliferations, respectively (Fig. 5).
FIG. 3.
Effects of FGF2 and VEGF on proliferation of SV40 OFPAE cells. Cells were seeded in 96-well plates (2000 cells/well). After serum starvation, cells were treated with FGF2 or VEGF. Cells were counted after 48 h of treatment. Data for each point were averaged from four and three individual experiments for FGF2 and VEGF, respectively, and expressed as means ± SEM percentage of the control. Within each growth factor treatment, means with different letters differ significantly (P < 0.05) from the control.
FIG. 4.
Effects of PD98059 on FGF2- and VEGF-stimulated SV40 OFPAE cell proliferation. Cells were treated with FGF2 and VEGF in the absence or presence of PD98059. Cells were counted after 48 h of treatment. Data for each point were averaged from four experiments and expressed as means ± SEM (percentage of the control). Within each growth factor treatment, means with different letters differ significantly (P < 0.05) from the control.
FIG. 5.
Effects of LY294002 on FGF2- and VEGF-stimulated SV40 OFPAE cell proliferation. Cells were treated with FGF2 and VEGF in the absence or presence of LY294002. Cells were counted after 48 h of treatment. Data for each point were averaged from four experiments and expressed as means ± SEM (percentage of the control). Within each growth factor treatment, means with different letters differ significantly (P < 0.05) from the control.
Phosphorylation of MAPK3/1 and AKT1 Induced by FGF2 and VEGF
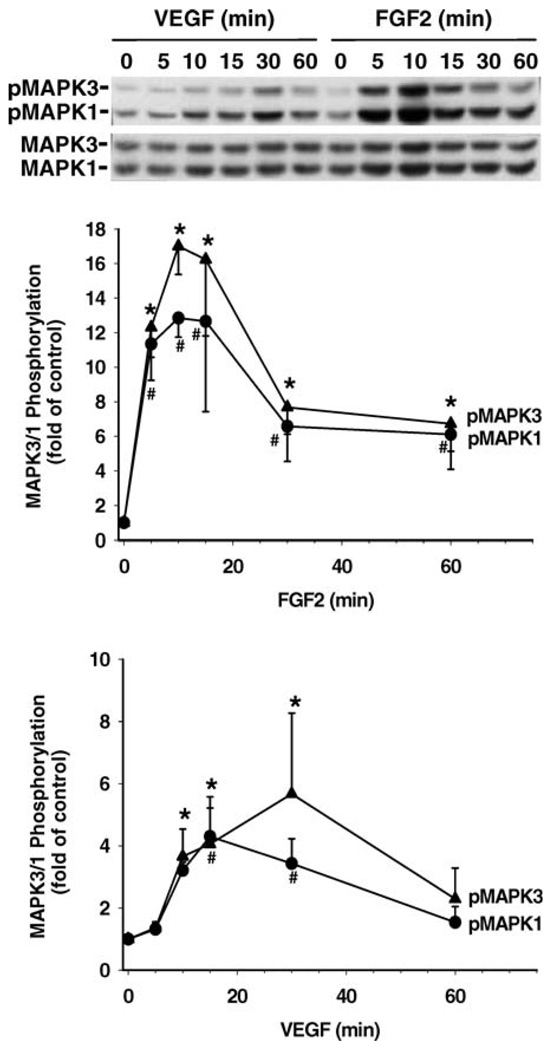
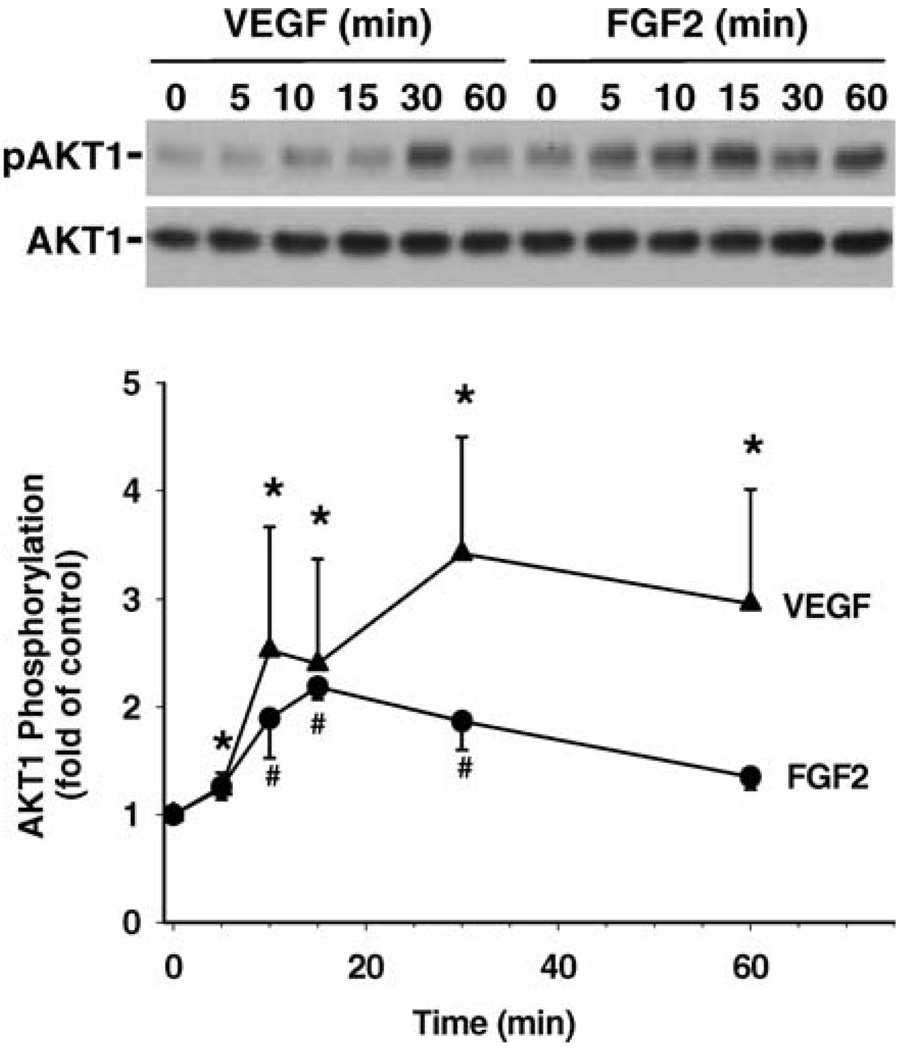
Phosphorylation of MAPK3/1 and AKT1 by FGF2 and VEGF was determined in SV40 OFPAE cells by Western blot analysis (Fig. 6 and Fig. 7). Both FGF2 and VEGF time-dependently elevated (P < 0.05) phosphorylation of MAPK3/1 and AKT1 but did not alter protein levels of total MAPK3/1 and AKT1 (Fig. 6 and Fig. 7). For MAPK3/1, FGF2 stimulatory effects (~11- and 10-fold above the control for MAPK3 and MAPK1, respectively; P < 0.05) were first detected at 5 min of treatment and reached their maximal elevations at 10 and 15 min. This FGF2-induced MAPK3/1 phosphorylation declined but still maintained a relatively high level (~7- to 8-fold above the control) at 30 and 60 min (Fig. 6). VEGF also elevated (P < 0.05) the phosphorylation levels of MAPK3/1 by approximately 3-fold at 10 min (Fig. 6). This VEGF-elevated MAPK3/1 phosphorylation was maintained for up to 30 min and then returned to the basal level at 60 min (Fig. 6). For AKT1, FGF2-induced (P < 0.05) phosphorylation of AKT1 was observed at 10–30 min and was elevated ~2-fold above the control and then returned to the basal levels at 60 min. VEGF-increased (P < 0.05) AKT1 phosphorylation was first detected at 5 min, reached its maximal elevation of ~4-fold at 30 min, and then was maintained at a relatively high level (~3-fold) at 60 min (Fig. 7).
FIG. 6.
Phosphorylation of MAPK3/1 induced by FGF2 and VEGF in SV40 OFPAE cells. Cells were treated with 10 ng/ml of FGF2 or VEGF for up to 60 min. Proteins (15 µg/lane) were separated, and MAPK3/1 was determined. A representative blot is shown. Data were averaged from five independent experiments and expressed as means ± SEM (fold of control). For each MAPK isoform, means with asterisks or number symbols differ significantly (P < 0.05) from the control. pMAPK3/1, Phosphorylated MAPK3/1; MAPK3/1, total MAPK3/1.
FIG. 7.
Phosphorylation of AKT1 induced by FGF2 and VEGF in SV40 OFPAE cells. Cells were treated with 10 ng/ml of FGF2 or VEGF for up to 60 min. Proteins were separated, and AKT1 was determined. A representative blot is shown. Data were averaged from four independent experiments and expressed as means ± SEM (fold of control). Within each growth factor treatment group, means with asterisks or number symbols differ significantly (P < 0.05) from the control. pAKT1, Phosphorylated AKT1; AKT1, total AKT1.
Acute NO Production by SV40 OFPAE Cells
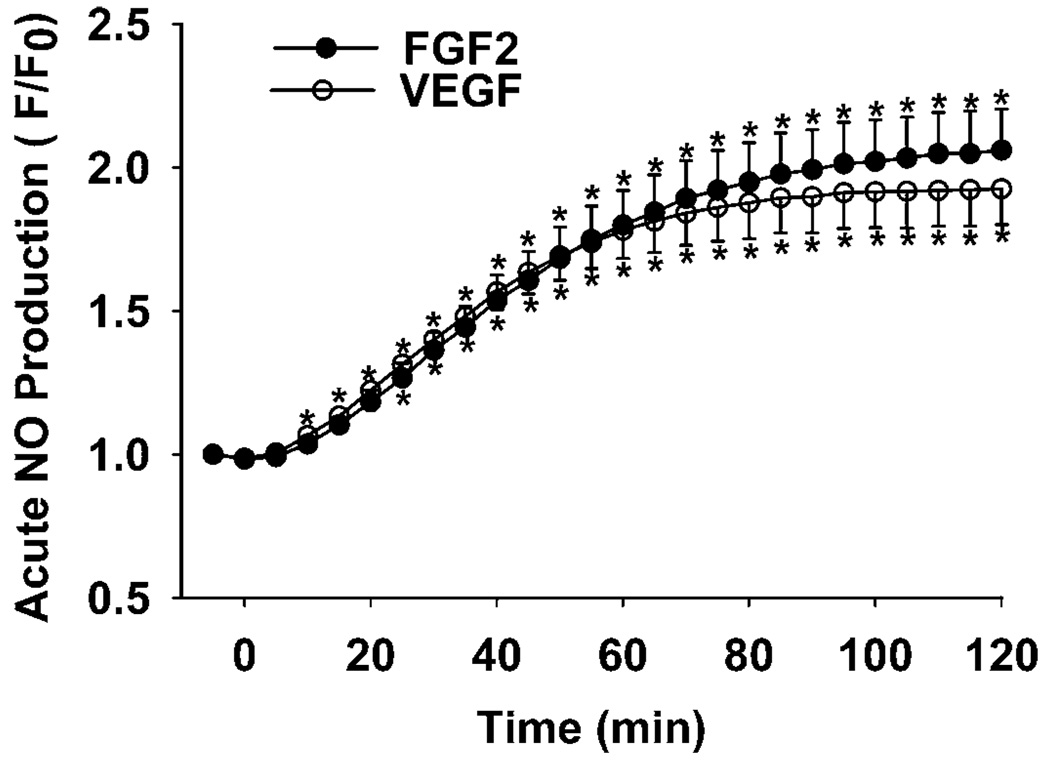
Both FGF2 and VEGF stimulated (P < 0.05) NO production in SV40 OFPAE cells in a time-dependent manner (Fig. 8). The stimulatory effect was first observed after 5 min of treatment, reached a plateau at 30 min, and then was maintained at this level for up to 2 h (Fig. 8).
FIG. 8.
Effects of FGF2 and VEGF on NO production by SV40 OFPAE cells. Cells were incubated with 10 ng/ml of FGF2 or VEGF, and NO production was determined every 5 min for up to 2 h. Data were averaged from three independent experiments and expressed as means ± SEM (ratio of F:F0). Means with asterisks differ (P < 0.05) from the control.
DISCUSSION
A better understanding of the cellular and molecular mechanisms regulating fetoplacental angiogenesis and vasodilatation is important, since such mechanisms are directly relevant to fetal development and survivability [1–4]. These mechanisms may ultimately provide an important tool to manipulate fetoplacental blood flows, thus yielding more optimal birth weights and healthier offspring. One approach to the study of these mechanisms is to use in vitro primary cell line models. Use of these primary cell lines in studies, however, is restricted because of their limited proliferative capacity and functional variation. In the present study, we have demonstrated the successful establishment of a functional SV40 OFPAE cell line with an extended life span (up to passage 80). Similar to SV40-transfected endothelial cells previously described [12–15], these SV40 OFPAE cells exhibit many endothelial phenotypes (i.e., positive uptake of Ac-LDL, expression of NOS3, and formation of capillary-like tube structures—three widely used indicators of endothelial cells). More importantly, these SV40 OFPAE cells, even after extensive expansion in vitro, undergo proliferation and produce NO in response to FGF2 and VEGF. Thus, this SV40-transfected cell line retains many of the key characteristics of its parental, untransfected cells [4] and provides a valuable cellular model for further studying the mechanism that governs fetoplacental angiogenesis and vasodilatation.
Transfection with the SV40 gene has been used to immortalize or extend the life span of endothelial cells from different origins, including human cerebromicrovessels [12], the HUVE [13, 14], and the placental microvasculature [15]. SV40-transfected cells are characterized by their immortalization or a greatly extended life span [12–15]. Compared to their parental, untransfected cells, SV40 OFPAE cells have acquired an extended life span. This prolonged life span of SV40 OFPAE cells may be caused largely by the combined actions of SV40 T and t antigens: the T antigen abrogates the function of Rb and p53, while the t antigen suppresses PPP2 activity [8–11]. To date, it is unclear whether they have attained immortalization. However, we have observed that these SV40 OFPAE cells maintain relatively high proliferative capacity even after several days of serum deprivation, suggesting that they have been transformed [10].
SV40-transfected cells are also characterized by their dramatically increased growth rate and resistance to apoptosis [12–15]. The patterns of proliferative responses observed for SV40 OFPAE cells at passages 20 and 21 to FGF2 and VEGF in the present study are similar to those reported for untransfected OFPAE cells [6], although a direct quantitative comparison is improper, because those untransfected OFPAE cells are used at much earlier passages (passages 8–10) and are maintained and treated under different media (i.e., DMEM for untransfected OFPAE cells and MCDB131 for SV40 OFPAE cells). On the other hand, we have also noted that the growth rate of SV40 OFPAE cells is almost doubled when they are cultured in media that contain serum, compared with untransfected OFPAE cells. Moreover, these SV40 OFPAE cells have much higher basal levels of MAPK3/1 and AKT1 phosphorylation (indicative of the activation of these kinases), along with a relatively high proliferative capacity under serum-free conditions. Thus, SV40 OFPAE cells must be deprived of serum for at least 48 h (vs. 24 h for untransfected OFPAE cells [6]) to lower the basal levels enough to detect their responses (proliferation and kinase phosphorylation) to FGF2 and VEGF. These hyperactive basal levels are not surprising, since the expression of SV40 T and t antigens abrogates the negative control of the cell cycle processes and elevates the phosphorylation of MAPK3/1 and AKT1 [8–11].
The MAP2K1/2/MAPK3/1 and PI3K/AKT1 signaling pathways are critical for mediating FGF2- and VEGF-activated cell growth and function [4, 6, 16]. Our current findings that the FGF2- and VEGF-stimulated SV40 OFPAE cell proliferation is mediated at least via these two pathways are similar to those observed in their parental untransfected cells (unpublished results), indicating that even after SV40 transfection, these two signaling pathways still play a role in regulating cell function. Intriguingly, we observed that instead of returning to the basal levels after a rapid activation in untransfected OFPAE cells [6], the FGF2-(but not the VEGF-) induced MAPK3/1 and the VEGF- (but not the FGF2-) induced AKT1 phosphorylation in SV40 OFPAE cells were maintained at relatively higher levels even after 30 min of stimulation (Fig. 6 and Fig. 7). These data suggest that a differential regulation exists between FGF2- and VEGF-induced MAPK3/1 and AKT1 activation in SV40 OFPAE cells. Similar phenomena have been reported in other immortalized cells. For example, in t-antigen-expressing endogenous telomerase immortalized human mammary epithelial cells, peak levels of AKT1 phosphorylation at 10 min of endothelial growth factor stimulation were maintained for up to 8 h, whereas MAPK3/1 phosphorylation was decreased [25]. The exact mechanism governing this differential regulation remains to be elucidated. It is, however, postulated that this is primarily because the t antigen inhibits PPP2 activity, differentially perturbing the signaling transduction pathways [25]. This concept is also supported by our recent findings that after the suppression of PPP2 expression with specific PPP2 small interfering RNA, OFPAE cells exhibited a similar differential regulation of MAPK3/1 and AKT1 in response to treatment with FGF2 and VEGF (unpublished results). Additionally, since a lower dose of PD98059 is sufficient to inhibit FGF2-induced cell proliferation than that used for the action of the VEGF and vice versa for LY294002 in the action of the VEGF (Fig. 4 and Fig. 5), these data suggest the predominance of each pathway mediating FGF2- and VEGF-induced SV40 OFPAE cell proliferation: the MAP2K1/2/MAPK3/1 pathway is predominant in the FGF2- induced cell proliferation as opposed to the PI3K/AKT1 pathway in the action of the VEGF.
It is known that SV40 transfection can alter some characteristics of cells. For example, it has been reported that the secretion of protein activator inhibitors by SV40-transfected HUVE cells is significantly decreased, along with a loss of the ability to produce prostacyclin [14]. Expression of the von Willebrand factor is variable among human glomerular endothelial cell lines after SV40 transfection [26]. SV40 OFPAE cells after passage 30 also show a more heterogeneous morphology and a less strict contact inhibition, as observed in SV40 HPME cells [15]. This phenomenon may be caused by a disruption of the expression of adhesion proteins (i.e., integrins α2), which function as receptors of collagen, laminin, and fibronectin on endothelial cells, as suggested by Kirchhofer et al. [27].
Unlike their untransfected cells in which only VEGF, not FGF2, induces an acute (up to 2 h after stimulation) production of NO (unpublished results), FGF2 stimulates an acute production of NO in SV40 OFPAE cells (Fig. 8). This phenomenon seems in contrast with the previous report by Pace et al. [28]. This study demonstrated that the mechanisms controlling acetylcholine- and estradiol-stimulated NO production are conserved in an ovine fetal pulmonary artery endothelial cell line immortalized by the E6/E7 region of human papillomavirus type 16, which also acts on Rb and p53. Our present data, however, suggest that SV40 transfection alters the sensitivity of NOS3 activation in OFPAE cells in response to FGF2, presumably by altering NOS3 phosphorylation. It is known that the t antigen exerts its transformation action by inhibiting PPP2 activity [8–11] and that PPP2 plays a key role in mediating NO production [29, 30] by regulating the PI3K/AKT1 pathway [31, 32]. Thus, it is possible that the expression of the t antigen in OFPAE cells suppresses PPP2 activity, which, in turn, modifies the activation of the signaling pathways (i.e., prolonging MAPK3/1 activation, as shown in Fig. 6) and NOS3 phosphorylation levels, thus leading to the increased acute NO production induced by FGF2.
ACKNOWLEDGMENTS
The authors appreciate Dr. Y. P. Liu and K. M. Grindle, University of Wisconsin-Madison, for technical support for the SV40 transfection assays.
Footnotes
Supported in part by the National Institutes of Health grants HL64703 and HD38843S2 to J.Z.
REFERENCES
- 1.Reynolds LP, Redmer DA. Utero-placental vascular development and placental function. J Anim Sci. 1995;73:1839–1851. doi: 10.2527/1995.7361839x. [DOI] [PubMed] [Google Scholar]
- 2.Magness RR, Zheng J. Maternal cardiovascular alterations during pregnancy. In: Gluckman PD, Heymann MA, editors. Pediatric and Perinatal Perspectives: The Scientific Basic. London: Edward Arnold Publishers; 1996. pp. 762–772. [Google Scholar]
- 3.Mayhew TM, Charnock-Jones DS, Kaufmann P. Aspects of human fetoplacental vasculogenesis and angiogenesis. III. Changes in complicated pregnancies. Placenta. 2004;25:127–139. doi: 10.1016/j.placenta.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 4.Zheng J, Bird IM, Chen DB, Magness RR. Angiotensin II regulation of ovine fetoplacental artery endothelial functions: interactions with nitric oxide. J Physiol. 2005;565:59–69. doi: 10.1113/jphysiol.2004.082420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sladek SM, Magness RR, Conrad KP. Nitric oxide and pregnancy. Am J Physiol. 1997;272:R441–R463. doi: 10.1152/ajpregu.1997.272.2.R441. [DOI] [PubMed] [Google Scholar]
- 6.Zheng J, Bird IM, Melsaether AN, Magness RR. Activation of the mitogen-activated protein kinase cascade is necessary but not sufficient for basic fibroblast growth factor- and epidermal growth factor-stimulated expression of endothelial nitric oxide synthase in ovine fetoplacental artery endothelial cells. Endocrinology. 1999;140:1399–1407. doi: 10.1210/endo.140.3.6542. [DOI] [PubMed] [Google Scholar]
- 7.Neufeld DS, Ripley S, Henderson A, Ozer HL. Immortalization of human fibroblasts transformed by origin-defective simian virus 40. Mol Cell Biol. 1987;7:2794–2802. doi: 10.1128/mcb.7.8.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gazdar AF, Butel JS, Carbone M. SV40 and human tumours: myth, association or causality? Nat Rev Cancer. 2002;2:957–964. doi: 10.1038/nrc947. [DOI] [PubMed] [Google Scholar]
- 9.Comerford SA, Clouthier DE, Hinnant EA, Hammer RE. Induction of hepatocyte proliferation and death by modulation of T-antigen expression. Oncogene. 2003;22:2515–2530. doi: 10.1038/sj.onc.1206259. [DOI] [PubMed] [Google Scholar]
- 10.Ahuja D, Saenz-Robles MT, Pipas JM. SV40 large T antigen targets multiple cellular pathways to elicit cellular transformation. Oncogene. 2005;24:7729–7745. doi: 10.1038/sj.onc.1209046. [DOI] [PubMed] [Google Scholar]
- 11.Arroyo JD, Hahn WC. Involvement of PP2A in viral and cellular transformation. Oncogene. 2005;24:7746–7755. doi: 10.1038/sj.onc.1209038. [DOI] [PubMed] [Google Scholar]
- 12.Muruganandam A, Herx LM, Monette R, Durkin JP, Stanimirovic DB. Development of immortalized human cerebromicrovascular endothelial cell line as an in vitro model of the human blood-brain barrier. FASEB J. 1997;11:1187–1197. doi: 10.1096/fasebj.11.13.9367354. [DOI] [PubMed] [Google Scholar]
- 13.Hohenwarter O, Waltenberger A, Strutzenberger K, Katinger H. Human endothelial cell lines established by mutated forms of the simian virus 40 large T oncogene. J Biotechnol. 1997;54:131–137. doi: 10.1016/s0168-1656(97)00044-8. [DOI] [PubMed] [Google Scholar]
- 14.Fitzgerald U, Hettle S, Macdonald C, Mclean JS. Umbilical cord endothelial cells expressing large T antigen: comparison with primary cultures and effect of cell age. In Vitro Cell Dev Biol Anim. 2000;36:222–227. doi: 10.1290/1071-2690(2000)036<0222:UCECEL>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 15.Schutz M, Teifel M, Friedl P. Establishment of a human placental endothelial cell line with extended life span after transfection with SV40 T-antigens. Eur J Cell Biol. 1997;74:315–320. [PubMed] [Google Scholar]
- 16.Cross MJ, Claesson-Welsh L. FGF and VEGF function in angiogenesis: signaling pathways, biological responses and therapeutic inhibition. Trends Pharmacol Sci. 2001;22:201–207. doi: 10.1016/s0165-6147(00)01676-x. [DOI] [PubMed] [Google Scholar]
- 17.Cuevas P, Carceller F, Ortega S, Zazo M, Nieto I, Gimenez-Gallego G. Hypotensive activity of fibroblast growth factor. Science. 1991;254:1208–1210. doi: 10.1126/science.1957172. [DOI] [PubMed] [Google Scholar]
- 18.Ku DD, Zaleski JK, Liu S, Brock TA. Vascular endothelial growth factor induces EDRF-dependent relaxation in coronary arteries. Am J Physiol. 1993;265:H586–H592. doi: 10.1152/ajpheart.1993.265.2.H586. [DOI] [PubMed] [Google Scholar]
- 19.Kadota O, Ohta S, Kumon Y, Sakaki S, Matsuda S, Sakanaka M. Role of basic fibroblast growth factor in the regulation of rat basilar artery tone in vivo. Neurosci Lett. 1995;199:99–102. doi: 10.1016/0304-3940(95)12040-b. [DOI] [PubMed] [Google Scholar]
- 20.Ni Y, May V, Braas K, Osol G. Pregnancy augments utero placental vascular endothelial growth factor (VEGF) gene expression and vasodilators in rat uterine circulation. Am J Physiol. 1997;273:H938–H944. doi: 10.1152/ajpheart.1997.273.2.H938. [DOI] [PubMed] [Google Scholar]
- 21.Kostyk SK, Kourembanas S, Wheeler EL, Medeiros D, McQuillan LP, D’Amore PA, Braunhut SJ. Basic fibroblast growth factor increases nitric synthase production in bovine endothelial cells. Am J Physiol. 1995;269:H1583–H1589. doi: 10.1152/ajpheart.1995.269.5.H1583. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Zheng J, Bird IM, Magness RR. Effects of pulsatile shear stress on nitric oxide production and endothelial cell nitric oxide synthase expression by ovine fetoplacental artery endothelial cells. Biol Reprod. 2003;69:1053–1059. doi: 10.1095/biolreprod.102.013474. [DOI] [PubMed] [Google Scholar]
- 23.Litzkas P, Jha KK, Ozer HL. Efficient transfer of cloned DNA into human diploid cells: protoplast fusion in suspension. Mol Cell Biol. 1984;4:2549–2552. doi: 10.1128/mcb.4.11.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng J, Wen Y, Chen DB, Bird IM, Magness RR. Angiotensin II elevates nitric oxide synthase 3 expression and nitric oxide production via a mitogen-activated protein kinase cascade in ovine fetoplacental artery endothelial cells. Biol Reprod. 2005;72:1421–1428. doi: 10.1095/biolreprod.104.039172. [DOI] [PubMed] [Google Scholar]
- 25.Zhao JJ, Gjoerup OV, Subramanian RR, Cheng Y, Chen W, Roberts TM, Hahn WC. Human mammary epithelial cell transformation through the activation of phosphatidylinositol 3-kinase. Cancer Cell. 2003;3:483–495. doi: 10.1016/s1535-6108(03)00088-6. [DOI] [PubMed] [Google Scholar]
- 26.Harada T, Batsford S, Morioka T, Yao J, Arakawa M, Gejyo F, Oite T. Establishment of immortalized human glomerular endothelial cell lines and their application. Nephron Exp Nephrol. 2005;99:e38–e45. doi: 10.1159/000083096. [DOI] [PubMed] [Google Scholar]
- 27.Kirchhofer D, Languino LR, Ruoslahti E, Pierschbacher MD. Alpha 2 beta 1 integrins from different cell types show different binding specificities. J Biol Chem. 1990;265:615–618. [PubMed] [Google Scholar]
- 28.Pace MC, Chambliss KL, German Z, Yuhanna IS, Mendelsohn ME, Shaul PW. Establishment of an immortalized fetal intrapulmonary artery endothelial cell line. Am J Physiol Lung Cell Mol Physiol. 1999;277:L106–L112. doi: 10.1152/ajplung.1999.277.1.L106. [DOI] [PubMed] [Google Scholar]
- 29.Greif DM, Kou R, Michel T. Site-specific dephosphorylation of endothelial nitric oxide synthase by protein phosphatase 2A: evidence for crosstalk between phosphorylation sites. Biochemistry. 2002;41:15845–15853. doi: 10.1021/bi026732g. [DOI] [PubMed] [Google Scholar]
- 30.Michell BJ, Chen ZP, Tiganis T, Stapleton D, Katsis F, Power DA, Sim AT, Kemp BE. Coordinated control of endothelial nitric-oxide synthase phosphorylation by protein kinase C and the cAMP-dependent protein kinase. J Biol Chem. 2001;276:17625–17628. doi: 10.1074/jbc.C100122200. [DOI] [PubMed] [Google Scholar]
- 31.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]