Abstract
Methyl-piperidino-pyrazole (MPP), an estrogen receptor α (ERα)-selective antagonist we developed, has a basic side chain (BSC) attached to an ERα-selective agonist ligand, methyl pyrazole triol (MPT) through an ether linkage. To remove the possibility that metabolic cleavage of the BSC in MPP would regenerate MPT, we have replaced the N-piperidinylethoxy moiety with an N-piperidinylpropyl group, giving MPrP. This new analog retains the high ERα-selective binding affinity and antagonist potency of MPP.
Keywords: pyrazoles, SERMs, binding affinity, antagonist, estrogen receptor
Estrogens can have remarkable tissue-selective effects, and this has led to the development of compounds termed selective estrogen receptor modulators (SERMs), which function as estrogen agonists in some tissues (bone, brain and the cardiovascular system) but as antagonists in others (uterus and breast).1,2 Estrogen receptors (ERs) can bind a variety of steroidal and non-steroidal ligands, and the search for better SERMs has driven efforts to increase the chemical diversity of these compounds, especially the non-steroidal ones. In fact, subtle changes in ligand structure can have a dramatic impact on receptor conformation and the resulting biological activities.3-7 A prominent feature of SERMs is a basic side chain (BSC), typically an aminoethyl group, appended to a core non-steroidal ER ligand by a phenyl ether linkage. The precise structure and orientation of the BSC can modulate SERM activity.8,9
Estrogen action is mediated through two ER subtypes, ERα and ERβ, which have distinct target tissue distributions and functional activities.10-14 Classical SERMs (e.g., tamoxifen and raloxifene), however, have essentially no selectivity for either ER subtype. Compounds capable of stimulating ERα very selectively have been developed,15-17 and we found that members of the triarylpyrazole class, such as propyl-pyrazole-triol (PPT), have ca. 1000-fold higher affinity and agonist potency on ERα than on ERβ.16,17 By attaching a BSC onto members of the pyrazole triol family of non-steroidal ER ligands, we obtained antagonist compounds that retained this affinity and potency preference for ERα.18 Of the seven BSC-pyrazole combinations that we investigated, the most selective was a methyl-piperidino-pyrazole, which we termed MPP (Scheme 1). In binding and transcription activation assays, MPP is very ERα selective, with its antagonistic activity on ERα being complete at concentrations at which it has neither agonist nor antagonist activity on ERβ.18
Scheme 1.
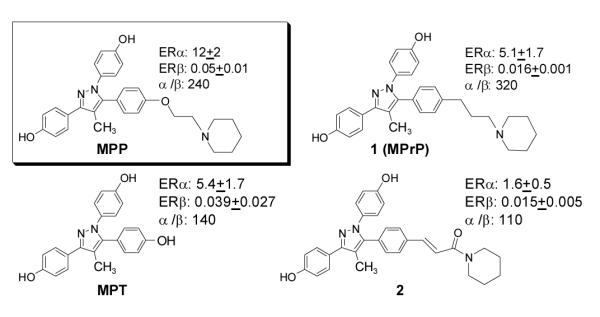
Structures and ERα and ERβ relative binding affinity (RBA, estradiol = 100%) values of MPP, its precursor MPT, and analogs 1 (MPrP) and compound 2. RBA values are the average of duplicate or triplicate determinations ± SD.
While MPP appeared to be a complete ERα antagonist in cell-based assay systems and has been widely used by others to evaluate the role of ERα in various estrogen-responsive systems, it resembles a SERM. Therefore, it was not surprising that we (unpublished) and others19 found MPP to have some agonist activity in certain animal models of estrogenic activity.
Structurally, MPP is based on and prepared from a pyrazole triol, methyl-pyrazole-triol (MPT; Scheme 1), which is an ERα agonist, though of modest potency.16 Thus, in principle, metabolic cleavage of the BSC might reveal latent agonist activity in an MPT metabolite. To investigate this possibility, we synthesized two MPP analogs in which the side chain was modified so as to preclude an ether metabolic cleavage that could convert MPP to MPT. The best of these analogs, MPrP, maintains excellent selectivity for ERα in terms of binding affinity and antagonist potency in transcription activation assays.
Notably, other SERMs having aminoethoxy BSCs could, in principle, also be metabolized to more agonistic phenols,20 and while this possibility has been considered as a mechanism for tamoxifen resistance in breast cancer,21,22 we have found only one case in which the propyl for ethoxy group substitution in a SERM has been made.23 This compound, however, was characterized only as a potent antifertility agent, similar in activity to the SERM nafoxidine.23
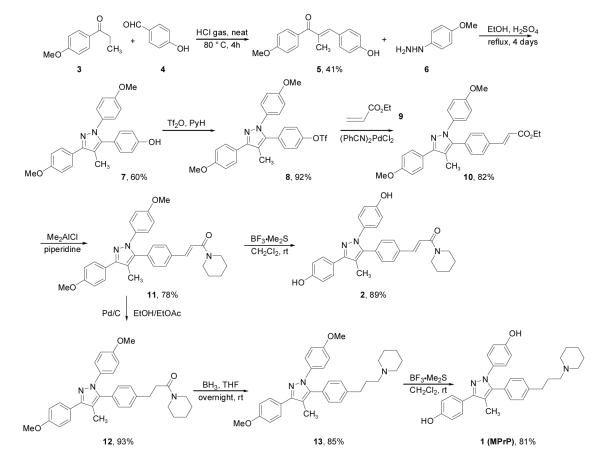
To prepare the BSC pyrazole 1 (MPrP) and its analog 2, we first performed an aldol condensation of 1-(4-methoxy-phenyl)-propan-1-one 3 and p-hydroxybenzaldehyde 4, according to a modified literature procedure (Scheme 2).24 The highly crystalline enone 5 underwent reaction with 4-methoxylphenylhydrazine 6 under vigorous conditions to give pyrazole 7 from which the triflate 8 was obtained. Heck-type coupling with ethyl acrylate and a catalytic amount of (PhCN)2PdCl2 in toluene afforded the desired pyrazole ester 10 in 82% yield. Piperidinolysis with stoichiometric dimethyl-aluminum chloride in CH2Cl2 then gives compound 11 in good yield. Hydrogenation gave the saturated amide 12, which was reduced by borane to the corresponding amine 13. Methyl ether cleavage with BF3·Me2S gave from 13 the desired product 1 (MPrP) in 81% yield and from 11, the unsaturated amide 2.
Scheme 2.
Synthesis of MPP analogs 1 (MPrP) and 2.
The ERα and ERβ binding affinities, determined by a competitive radiometric binding assay,25,26 shown in Scheme 1, are expressed as relative binding affinity (RBA) values (estradiol = 100%). The nature of the BSC affects binding affinity, and compound 1 (MPrP) has an ERα binding affinity (5.1%) slightly lower than that of MPP (12%), but because its ERβ binding is further reduced from that of MPP, MPrP has a somewhat increased ERα binding selectivity (320-fold). The ERα selectivity of MPrP is also about 2.3-fold greater than that of the triol agonist MPT, which was the parent of MPP. The binding of the unsaturated amide (2) is markedly lower.
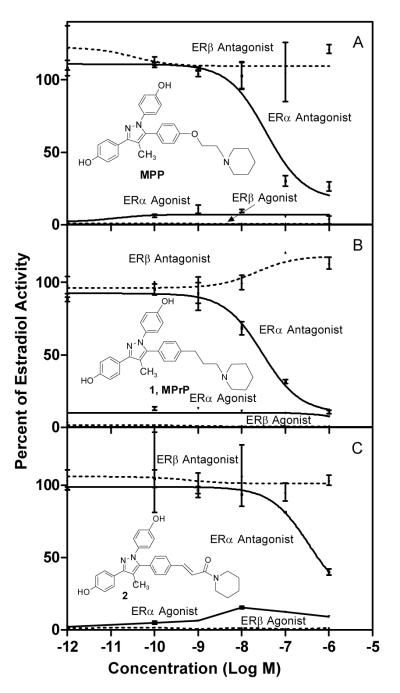
The ERα and ERβ transcriptional activity of MPP and compounds 1 (MPrP) and 2 was determined by estrogen-responsive reporter gene cotransfection assays in human endometrial cancer cells (HEC-1; Figure 1; IC50 values are given in the legend).27 All three compounds are ERα antagonists with no significant agonist or antagonist activity on ERβ. The potency of analog 1 (MPrP) appears to be somewhat higher than that of MPP itself, and it also lacks the residual, low ERα partial agonist activity of MPP. The potency of amide (2) is somewhat lower.
Figure 1.
Transcription activation through ERα (solid lines) and ERβ (dotted lines) of compounds MPP, 1 (MPrP), and 2. HEC-1 cells were transfected with expression plasmids for ERα or ERβ and the estrogen responsive gene 2xERE-pS2-Luc and were incubated with the indicated ligand for 24 h. Antagonist assays were done in the presence of 1 nM estradiol (E2). Values are the mean (± SD) of two or more experiments, expressed as a percent of the activity of ERα and ERβ with 1 nM E2, which is set at 100%. IC50 values from the antagonist profiles: MPP, ERα 80 nM, compound 1 (MPrP), ERα 20 nM, and compound 2, ERα 1 μM.
In this study, we have developed a synthetic strategy to generate 1 (MPrP), a novel analog of our ERα-selective antagonist MPP, in which the 2-(N-piperidino)ethoxy moiety has been replaced by the 3-(N-piperidino)propyl moiety, removing a potential metabolic liability that might engender agonist activity. This new analog retains the high affinity and antagonist potency selectivity for ERα of the parent ligand and should be a useful probe for the biological activity of ERα.
Acknowledgments
Supported by grants from the NIH (PHS 5R37 DK15556 to JAK and 5R01 CA11819 to BSK). Funding for NMR and MS instrumentation is from the Keck Foundation, NIH and NSF. We are grateful to Dr. Sung Hoon Kim for helpful comments.
References
- 1.Park WC, Jordan VC. Trends Mol. Med. 2002;8:82. doi: 10.1016/s1471-4914(02)02282-7. [DOI] [PubMed] [Google Scholar]
- 2.Sato M, Grese TA, Dodge JA, Bryant HU, Turner CH. J. Med. Chem. 1999;42:1. doi: 10.1021/jm980344o. [DOI] [PubMed] [Google Scholar]
- 3.Zhou HB, Nettles KW, Bruning JB, Kim Y, Joachimiak A, Sharma S, Carlson KE, Stossi F, Katzenellenbogen BS, Greene GL, Katzenellenbogen JA. Chem Biol. 2007;14:659–669. doi: 10.1016/j.chembiol.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Fink BE, Mortensen DS, Stauffer SR, Aron ZD, Katzenellenbogen JA. Chem. Biol. 1999;6:205. doi: 10.1016/S1074-5521(99)80037-4. [DOI] [PubMed] [Google Scholar]
- 5.Barlaam B, Bernstein P, Dantzman C, Warwick P. PCT Int. Appl. Astrazeneca AB; Swed.: 2002. p. 71. Wo. [Google Scholar]
- 6.Teo CC, Kon OL, Sim KY, Ng SC. J. Med. Chem. 1992;35:1330. doi: 10.1021/jm00086a002. [DOI] [PubMed] [Google Scholar]
- 7.Palkowitz AD, Glasebrook AL, Thrasher KJ, Hauser KL, Short LL, Phillips DL, Muehl BS, Sato M, Shetler PK, Cullinan GJ, Pell TR, Bryant HU. J. Med. Chem. 1997;40:1407. doi: 10.1021/jm970167b. [DOI] [PubMed] [Google Scholar]
- 8.Zhou HB, Sheng S, Compton DR, Kim Y, Joachimiak A, Sharma S, Carlson KE, Katzenellenbogen BS, Nettles KW, Greene GL, Katzenellenbogen JA. J. Med. Chem. 2007;50:399. doi: 10.1021/jm061035y. [DOI] [PubMed] [Google Scholar]
- 9.Wallace OB, Bryant HU, Shetler PK, Adrian MD, Geiser AG. Bioorg. Med. Chem. Lett. 2004;14:5103–5106. doi: 10.1016/j.bmcl.2004.07.072. [DOI] [PubMed] [Google Scholar]
- 10.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Proc. Natl. Acad. Sci. U. S. A. 1996;93:5925. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mosselman S, Polman J, Dijkema R. FEBS Lett. 1996;392:49. doi: 10.1016/0014-5793(96)00782-x. [DOI] [PubMed] [Google Scholar]
- 12.Pettersson K, Gustafsson JA. Annu. Rev. Physiol. 2001;63:165. doi: 10.1146/annurev.physiol.63.1.165. [DOI] [PubMed] [Google Scholar]
- 13.Dechering K, Boersma C, Mosselman S. Curr. Med. Chem. 2000;7:561. doi: 10.2174/0929867003375010. [DOI] [PubMed] [Google Scholar]
- 14.Katzenellenbogen BS, Montano MM, Ediger TR, Sun J, Ekena K, Lazennec G, Martini PG, McInerney EM, Delage-Mourroux R, Weis K, Katzenellenbogen JA. Recent Prog. Horm. Res. 2000;55:163–193. discussion 194. [PubMed] [Google Scholar]
- 15.Larrea F, Garcia-Becerra R, Lemus AE, Garcia GA, Perez-Palacios G, Jackson KJ, Coleman KM, Dace R, Smith CL, Cooney AJ. Endocrinology. 2001;142:3791. doi: 10.1210/endo.142.9.8401. [DOI] [PubMed] [Google Scholar]
- 16.Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. J. Med. Chem. 2000;43:4934. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- 17.Kraichely DM, Sun J, Katzenellenbogen JA, Katzenellenbogen BS. Endocrinology. 2000;141:3534. doi: 10.1210/endo.141.10.7698. [DOI] [PubMed] [Google Scholar]
- 18.Sun J, Huang YR, Harrington WR, Sheng S, Katzenellenbogen JA, Katzenellenbogen BS. Endocrinology. 2002;143:941. doi: 10.1210/endo.143.3.8704. [DOI] [PubMed] [Google Scholar]
- 19.Davis AM, Ellersieck MR, Grimm KM, Rosenfeld CS. Mol. Reprod. Dev. 2006;73:1034. doi: 10.1002/mrd.20520. [DOI] [PubMed] [Google Scholar]
- 20.Murphy CS, Langan-Fahey SM, McCague R, Jordan VC. Mol. Pharmacol. 1990;38:737. [PubMed] [Google Scholar]
- 21.Osborne CK, Jarman M, McCague R, Coronado EB, Hilsenbeck SG, Wakeling AE. Cancer Chemother. Pharmacol. 1994;34:89. doi: 10.1007/BF00685924. [DOI] [PubMed] [Google Scholar]
- 22.Wolf DM, Langan-Fahey SM, Parker CJ, McCague R, Jordan VC. J. Natl. Cancer Inst. 1993;85:806. doi: 10.1093/jnci/85.10.806. [DOI] [PubMed] [Google Scholar]
- 23.Lednicer D, Lyster SC, Duncan GW. J. Med. Chem. 1967;10:78. doi: 10.1021/jm00313a016. [DOI] [PubMed] [Google Scholar]
- 24.Huang YR, Katzenellenbogen JA. Org. Lett. 2000;2:2833. doi: 10.1021/ol0062650. [DOI] [PubMed] [Google Scholar]
- 25.Carlson KE, Choi I, Gee A, Katzenellenbogen BS, Katzenellenbogen JA. Biochemistry. 1997;36:14897. doi: 10.1021/bi971746l. [DOI] [PubMed] [Google Scholar]
- 26.Katzenellenbogen JA, Johnson HJ, Jr., Myers HN. Biochemistry. 1973;12:4085–4092. doi: 10.1021/bi00745a010. [DOI] [PubMed] [Google Scholar]
- 27.Sun J, Meyers MJ, Fink BE, Rajendran R, Katzenellenbogen JA, Katzenellenbogen BS. Endocrinology. 1999;140:800. doi: 10.1210/endo.140.2.6480. [DOI] [PubMed] [Google Scholar]