Abstract
Background and Purpose
To examine the neurophysiological basis for the pronounced differences in hyperactivity and impulsiveness that distinguish the Predominantly Inattentive type of Attention-Deficit/Hyperactivity Disorder (ADHD-PI) from the combined type of the disorder (ADHD-C).
Methods
Event-related brain responses to a go/no-go test of inhibitory control were measured with functional magnetic resonance imaging (fMRI) in 11 children with ADHD-C and nine children with ADHD-PI, aged 7 to 13 years, who were matched for age, sex, and intelligence.
Results
There were no significant group differences in task performance. Children with ADHD-C and ADHD-PI activated overlapping regions of right inferior frontal gyrus, right superior temporal lobe, and left inferior parietal lobe during inhibitory control. However, the magnitude of the activation in the temporal and parietal regions, as well as in the bilateral middle frontal gyrus, was greater in children with ADHD-PI than those with ADHD-C. Conversely, children with ADHD-C activated bilateral medial occipital lobe to a greater extent than children with ADHD-PI.
Conclusions
The results provide preliminary evidence that phenotypic differences between the ADHD-C and ADHD-PI subtypes are associated with differential activation of regions that have previously been implicated in the pathophysiology of ADHD and are thought to mediate executive and attentional processes.
Introduction
The predominantly inattentive type of attention-deficit/hyperactivity disorder (ADHD-PI) is characterized by difficulties with attention without the pronounced hyperactivity-impulsivity that is diagnostic for the more widely recognized combined type of ADHD (ADHD-C) 1. Attention problems also differ qualitatively between the subtypes, with more sluggish cognitive tempo in the ADHD-PI type 2, 3. However, linking these differences in behavioral phenotype with neuropsychological mechanisms has presented a challenge. Some studies have shown slower processing speed in the ADHD-PI subtype 4–6, consistent with reports of sluggish cognitive tempo. The literature is inconsistent with respect to differences in inhibitory control 6, with some showing longer stop signal reaction time (SSRT) 5, 7 or increased continuous performance test (CPT) commission errors 8 in children with ADHD-C, and others failing to find subtype differences on one or more of these measures 4, 9. Given the inconsistencies in results of neurocognitive studies of the ADHD-PI and ADHD-C subtypes, the use of functional magnetic resonance imaging (fMRI) may help to resolve continuing controversy concerning whether these subtypes are variants of the same condition or completely different disorders 10, 11. Beyond the heuristic value of this research, these results have implications for the selection of subjects for functional neuroimaging studies that seek to isolate specific cognitive processes involved in the etiology of ADHD.
Studies using fMRI to date have depicted a complicated picture of functional brain anomalies related to executive and inhibitory processes in ADHD. For example, of five studies that have used go/no-go paradigms to test inhibitory control, three reported increased activation of ventral prefrontal brain regions in children with ADHD 12, 13 and adolescents diagnosed with ADHD during childhood 14, a fourth study found decreased activation of ventral prefrontal cortex in children with ADHD 15, and the fifth reported no prefrontal cortical abnormalities in adolescents with ADHD-C 16. Similarly divergent results were reported regarding activation of the striatum and anterior cingulate gyrus during the go/no-go task 12–16. These inconsistencies have frustrated efforts to pinpoint the precise frontostriatal impairments in the pathophysiology of ADHD. Several factors are likely to contribute to these inconsistencies, including the heterogeneous nature of the ADHD samples that have been studied. The samples in most fMRI studies have combined individuals with ADHD-C and ADHD-PI 12, 13, 15, 17, 18 14, 19 despite evidence that the subtypes may imperfectly represent independent forms of ADHD with genetic influences specific to each 20. Recent studies have attempted to address this heterogeneity by restricting the sample to youth with ADHD-C only 16, 21, 22. However, no studies have directly compared the ADHD-C and ADHD-PI subtypes to disentangle subtype differences in the neural mechanisms of inhibitory control.
The present study used fMRI with a go/no-go task to examine event-related brain activation during inhibitory control in children with ADHD-C and ADHD-PI. The go/no-go task used in this study has been previously characterized in healthy children and adults 23, 24 and has been used to test children with ADHD 13. Based on the phenotypic differences between groups in impulsivity/hyperactivity, as well as the fMRI findings available at the time i.e., 12, 18, we predicted that children with ADHD-C would have worse inhibitory control and activate ventral prefrontal cortex and striatum less than children with ADHD-PI. In addition, given hypothesized deficits in alerting and orienting functions mediated by posterior parietal cortex in the ADHD-PI group 6, we were particularly interested in comparisons between subtypes in activation of this region. Further analyses examined subtype similarities in activation.
Method
Participants
Twenty children (14 boys, 6 girls) were recruited from a larger pool of 7 to 13 year-old participants in a study of the neurocognitive functioning of ADHD. The children all met DSM-IV diagnostic criteria for either the ADHD-C or ADHD-PI subtype as assessed by clinical interview and confirmed by parental responses to the NIMH Diagnostic Interview Schedule for Children-Version IV (NIMH-DISC) 25. In addition, all children met symptom severity thresholds of at least 1.5 standard deviations (SD) ≥ age and gender means on both Conners’ Parent Rating Scale-Revised (CPRS-R) 26 and Conners’ Teachers Rating Scale-Revised (CTRS-R) 27 according to diagnostic subtype: 1) DSM-IV Inattentive Symptoms scale score ≥ 65 for both subtypes; and 2) DSM-IV Hyperactive-Impulsive Symptoms scale score ≥ 65 for the ADHD-C subtype or < 65 for the ADHD-PI subtype. Finally, general cognitive ability was assessed with the Wechsler Intelligence Scale for Children-Third Edition (WISC-III). Children with a chronic medical or neurological condition, diagnosis of pervasive developmental disorder, psychosis, or Tourette’s Syndrome, WISC-III Full Scale Intelligence Quotient (FSIQ) < 80, or who were currently receiving psychotropic medication (other than stimulants) were excluded from participation in the parent study. The final sample consisted of 11 children with ADHD-C and 9 children with ADHD-PI.
Participants were scanned on average 2.1 ± 1.3 years following their initial evaluation for the neurocognitive study. The children remained symptomatic as demonstrated by mean ± SD scores on the DSM-IV Inattentive and Hyperactive-Impulsive Symptoms scales of the CPRS-R of 75.4 ± 11.0 and 77.9 ± 9.5 for children with ADHD-C and 70.0 ± 13.2 and 58.5 ± 11.7 for children with ADHD-PI. The ADHD-C and ADHD-PI groups did not differ in mean ± SD elapsed time since evaluation (2.4 ± 1.5 years versus 1.6 ± 1.0 years), age (11.2 ± 1.9 years versus 10.7 ± 1.3 years), FSIQ (108.0 ± 13.8 versus 107.9 ± 10.0), gender (64% male versus 78% male) or handedness (64% right-handed vs. 78% right-handed) (all p > .10). One child with ADHD-C and two children with ADHD-PI had specific learning disabilities. SES, as indexed by mother’s education, was equivalent between groups in that 57% of the ADHD-C and 50% of the ADHD-PI had completed a four-year college degree. None of the families in either group were receiving public assistance.
ADHD-CB and ADHD-PI groups were nearly equal with respect to the percentages who were stimulant-naïve (18% vs. 22%, respectively) or who had been treated with stimulants for more than one month (73% vs. 67%, respectively. At the time of the study, five (44%) children with ADHD-C and six (67%) children with ADHD-PI were receiving stimulant treatment and all were withdrawn from medication for at least 24 hours prior to the scan. The study was approved by the Institutional Review Board committee of the Mount Sinai School of Medicine. Written informed consent was obtained from the parents of participants and signed assent from the children. Participants and parents were compensated for their participation.
Go/No-Go Paradigm
Durston et al 13, 23, 24 designed the go/no-go task used in this study to measure the ability to inhibit responses to rare non-targets (No-go trials) in the context of frequent targets (Go trials). The task consisted of five 238 second blocks that began with a 10-second period of fixation. Each block contained 57 trials, with 43 (75%) Go trials and 14 (25%) No-go trials, yielding a total of 70 No-go trials across the task. The Lilo and Stitch characters from the “Lilo and Stitch” movie (© Disney Enterprises, Inc.) served as the stimuli for Go trials, the stimulus for No-go trials was the Cobra Bubble character from the same movie, and fixation was depicted by a small image of Earth. Stimuli were presented for 500 msec with an interstimulus interval of 3500 msec. Trial order was pseudorandomized so that the occurrence of No-go trials was jittered from four to 20 seconds (i.e., preceded by one to five Go trials). Participants were reminded at the beginning of each block to respond as quickly as possible while trying not to make mistakes.
The go/no-go task was compiled and run using E-Prime™ software (Psychology Software Tools, Inc., Pittsburgh, PA; 28). Stimuli were projected via an SVGA projector system onto a rear-projection screen mounted at the head of the magnet bore. Participants viewed the stimuli through a mirror on the head coil positioned above their eyes and responded with the right hand using the BrainLogics fiber optic button system (Psychology Software Tools, Inc., Pittsburgh, PA). Responses were recorded on a personal computer and provided measures of reaction time and accuracy.
MRI Image Acquisition
All structural and functional scans were acquired on the same 3.0 Tesla Siemens Allegra (Siemens Medical Systems, Erlangen, Germany) head-dedicated MRI scanner equipped with a high-performance head gradient system that was designed especially for functional brain imaging. Firm foam padding was used to restrict head motion. Following sagittal localization and shimming, a high-resolution T2-weighted anatomical volume of the whole brain was acquired with a turbo spin-echo (TSE) pulse sequence (28 axial slices, repetition time [TR] = 5380 msec, echo time [TE] = 99 msec, flip angle = 170°, field of view [FOV] = 210 mm, matrix = 512 × 336, voxel size = 0.41 × 0.41 × 4 mm). Functional T2*-weighted images depicting the blood oxygenation level-dependent (BOLD) signal were acquired at the same 28 slice locations using gradient-echo echo-planar images (EPI) with a TR of 2000 msec, TE of 40 msec, flip angle of 90°, FOV of 210 mm, and a matrix of 64 × 64. Each functional image comprised a full brain volume of 28 axial slices (thickness = 3 mm, skip = 1 mm, in-plane resolution = 3.28 × 3.28 mm). All images were acquired with slices positioned parallel to the anterior commissure–posterior commissure line. The participants each completed 5 runs of 238 seconds, resulting in 120 time points per participant
Statistical Analyses
Group differences in the percent of commission errors on No-go trials of the go/no-go task, as well as the percent of omission errors and mean reaction time on Go trials, were analyzed with Student’s t-tests. Pearson’s product-moment correlations between percent change in MRI signal intensity and behavioral measures were calculated for regions that demonstrated significant activation. The two-tailed probability level was set at p < .05 for statistical significance.
Image preprocessing and analyses were conducted using statistical parametric mapping (SPM2) (www.fil.ion.ucl.ac.uk/spm/spm2.html). The first five volumes of each functional time series were discarded and the functional scans were then realigned to the remaining first volume, coregistered with the high-resolution TSE image, spatially normalized to a Montreal Neurological Institute (MNI) T2-weighted template image, and spatially smoothed. General linear modeling (GLM) was then conducted for the functional scans from each participant by modeling the observed event-related BOLD signals and regressors to identify the relationship between the experimental parameters and the hemodynamic response. Event-related analyses were performed by convolving the default SPM basis function with a train of delta functions that represent the individual trial events to create regressors, the linear combination of which was used to model the hemodynamic response to four conditions: correct and incorrect NOGO and GO trials. The six parameters generated during motion correction were entered as covariates in the GLM. The four conditions were then estimated for each participant and the effects of inhibitory control were tested by applying appropriate linear contrasts to the parameter estimates for the correct No-go minus correct Go contrast, resulting in a contrast image for each participant.
The contrast images of all participants were entered into second-level group analyses conducted with random-effects statistical models that accounted for intrasubject variability. The first analysis examined regions in which there was evidence of a conjunction between inhibitory control-related activations in the ADHD-C and ADHD-PI groups. We employed here the method described by Friston et al. 29–31 that tests the Minimum T Statistic against the Global Null Hypothesis with a global false-positive rate of .01 for the height threshold. This is one of two accepted methods for conducting conjunction analyses, yielding a more inclusive interpretation of common activations.
The a priori hypothesis that children with ADHD-CB would activate ventral prefrontal cortex and striatum less than children with ADHD-PI was then tested with direct between-group contrasts. The resultant voxel-wise statistical maps were then thresholded for significance using a cluster-size algorithm that protects against an inflation of the false-positive rate. Results are reported at an uncorrected height (intensity) threshold of p < 0.05 and an extent threshold of k = 100 voxels, corresponding to a whole brain false-positive rate of approximately 0.05, according to a Monte Carlo simulation of the current brain volume 32. This more liberal alpha-level was selected in order to minimize Type II error, given that this first fMRI comparison of subtypes was intended to be exploratory and hypothesis-generating rather than definitive. Simultaneously, however, we employed a relatively conservative extent threshold of 100 voxels, rather than 50, in order to provide additional protection against findings of spurious activations. Coordinates of activation were converted to the Talairach and Tournoux 33 coordinate system using a nonlinear transformation (http://www.mrc-cbu.cam.ac.uk/Imaging/mnispace.html).
Results
Behavioral Data
There were no significant differences between children with ADHD-C and those with ADHD-PI in percent commission errors on No-go trials [t18 = .48, p = .64] or mean reaction time [t18 = .90, p = .38] and percent of omission errors on Go trials [t18 = .21, p = .84]. Both groups of children made a large number of commission errors; percent commission errors on No-go trials was 41.2 ± 16.6% for children with ADHD-C and 37.9 ± 13.1% for children with ADHD-PI. Mean reaction time and percent omission errors on Go trials were 460 ± 55 msec and 6.4% ± 7.4% for the ADHD-C group and 488 ± 84 msec and 5.7% ± 5.7% for the ADHD-PI group. Mean translational movement during the echoplanar time series was 1.74 ± .1.19 mm for children with ADHD-C and 1.71 ± .85 mm for children with ADHD-PI [t18 = .06, p = .95]. Mean rotational displacement was less than .10 for both groups [t18 = .95, p = .36].
fMRI Data
Conjunction Analysis
Brain regions that were conjointly activated during inhibitory control (i.e., correct No-go event minus correct Go event) by children with ADHD-C and ADHD-PI are shown in Figure 1 and listed in Table 1. Regions with overlapping activation included: Brodmann’s Area (BA 47) in the ventrolateral convexity of the right inferior frontal gyrus; the right superior temporal gyrus (BA 22); and the left inferior parietal lobule (BA 40). There were no significant correlations between the magnitude of these activations and the percent of commission errors on No-go trials (all p > .10).
Figure 1.
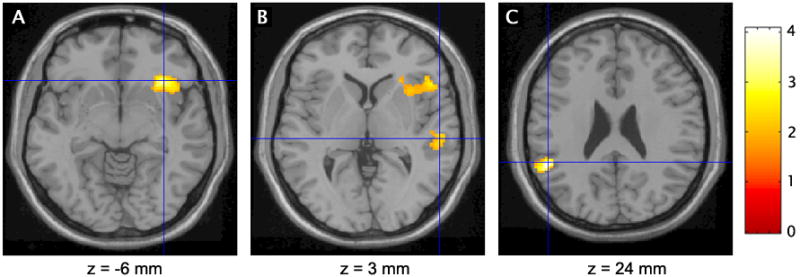
Children with the combined and predominantly inattentive types of attention-deficit/hyperactivity disorder conjointly activated the right inferior frontal gyrus (A), right superior temporal gyrus (B), and left inferior parietal lobule (C) during inhibitory control (t > 1.96, p < .05). Crosshairs indicate cluster maxima. Values below the images refer to the Talairach and Tournoux (1988) coordinates for the sections.
TABLE 1.
Regions Conjointly Activated During Inhibitory Control By Children with the Combined and Predominantly Inattentive Types of Attention-Deficit/Hyperactivity Disorder
| Coordinates | Cluster Size | ||||||
|---|---|---|---|---|---|---|---|
| Brain Region | BA | Side | x | y | z | t | |
| Inferior frontal gyrus | 47 | R | 34 | 23 | −6 | 3.34 | 938 |
| Superior temporal gyrus | 22 | R | 55 | −25 | 3 | 2.33 | 143 |
| Inferior parietal lobule | 40 | L | −51 | −43 | 24 | 4.07 | 281 |
Note: BA refers to Brodmann’s area; L and R refer to the left and right cerebral hemispheres; x, y, and z refer to the Talairach and Tournoux33 coordinates of the maximally activated voxel; t refers to the t score of the of maximally activated voxel (p < .05).
Between-Group Analyses
Significant differences in activation between children with ADHD-C and those with ADHD-PI revealed by direct voxel-by-voxel between-group comparisons are depicted in Figure 2 and detailed in Table 2. Children with ADHD-PI had markedly greater activation of bilateral middle frontal gyrus (BA 9/10) and inferior parietal lobule (BA 40) during the inhibition of prepotent responses than children with ADHD-C. In addition, the magnitude of activation of the right superior temporal gyrus (BA 22) region identified in the conjunction analysis was greater in the ADHD-PI than the ADHD-C group. Conversely, children with ADHD-C had greater activation of a large bilateral region of the cuneus (BA 19) during inhibitory control than children with ADHD-PI (see Figure 2 and Table 2). There were no significant correlations between activation and the percent of commission errors on No-go trials (all p > .10).
Figure 2.
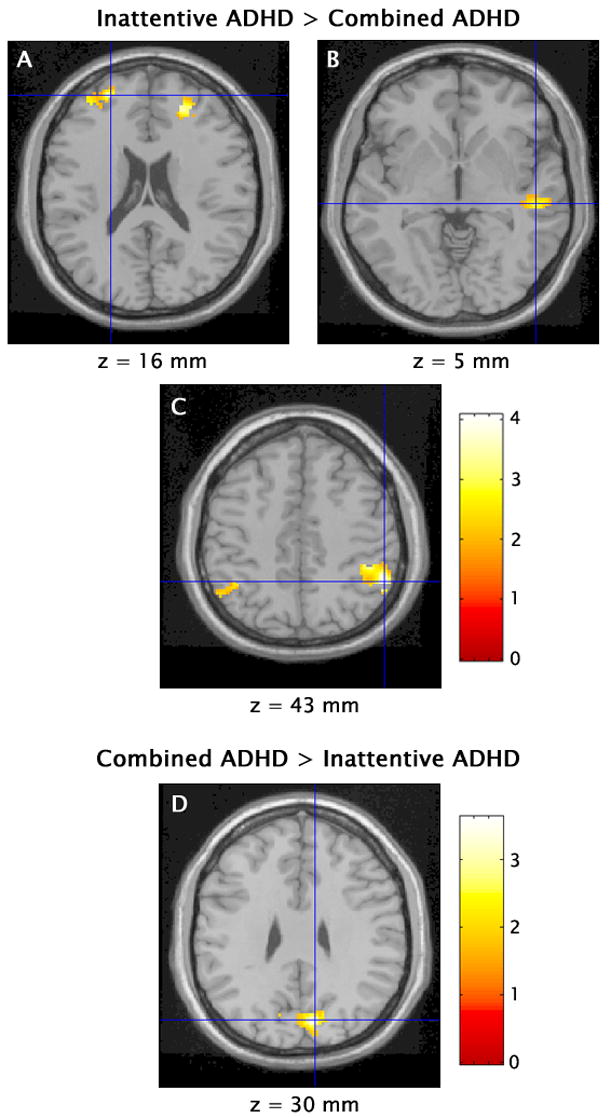
Children with the combined and predominantly inattentive types of attention-deficit/hyperactivity disorder had significant differences in activation during inhibitory control in bilateral middle frontal gyrus (A), right superior temporal gyrus (B), bilateral inferior parietal lobule (C), and bilateral cuneus (D) (t > 1.96, p < .05). Crosshairs indicate cluster maxima. Values below the images refer to the Talairach and Tournoux (1988) coordinates for the sections.
TABLE 2.
Regions of Significant Differences in Activation During Inhibitory Control n Children with the Combined Type Versus the Predominantly Inattentive Type of Attention-Deficit/Hyperactivity Disorder
| Combined ADHD > Inattentive ADHD | Inattentive ADHD > Combined ADHD | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coordinates | Coordinates | ||||||||||||||
| Brain Region | BA | Side | x | y | z | t | Cluster size | Brain Region | BA | Side | x | y | z | t | Cluster size |
| Cuneus | 19 | R | 10 | −74 | 30 | 3.63 | 563 | Middle frontal gyrus | 9 | R | 22 | 44 | 16 | 3.50 | 287 |
| 19 | L | −10 | −71 | 24 | 2.82 | 10 | L | −24 | 51 | 16 | 4.46 | 115 | |||
| Superior temporal gyrus | 22 | R | 50 | −25 | 5 | 2.95 | 111 | ||||||||
| Inferior parietal lobule | 40 | R | 53 | −46 | 43 | 4.21 | 695 | ||||||||
| 40 | L | −40 | −48 | 41 | 2.83 | 167 | |||||||||
Note: BA refers to Brodmann’s area; L and R refer to the left and right cerebral hemispheres; x, y, and z refer to the Talairach and Tournoux 33 coordinates of the maximally activated voxel; t refers to the t score of the of maximally activated voxel (p < .05).
Exploratory Analyses
Exploratory analyses capitalized on the large number of commission errors on NOGO trials in the present study to test the neural consequences of error trials. Error trials were modeled individually in 18 of the 19 children with the incorrect NOGO events minus correct GO events contrast using the methods described above; one child with PI did not make enough commission errors to model incorrect NOGO events. Conjunction analysis of the error trials contrasts revealed several regions that were activated following commission errors by both the CB and PI groups (Table 3). Both groups of children produced robust activation of bilateral inferior frontal gyrus (BA 45/47) that extended into the insula, as well as of regions in the left anterior (BA 24/32) and middle cingulate gyrus (BA 24). Direct between-group comparisons revealed no clusters of significant differences in activation between the CB and PI groups.
Table 3.
Regions of Significant Activation During Error Trials (incorrect NOGO trials minus correct GO trials) in Children with Both the Combined and Predominantly Inattentive Types of ADHD
| Coordinates
|
Cluster Size
|
||||||
|---|---|---|---|---|---|---|---|
| Brain Region | BA | Side | x | y | z | t | |
| Inferior frontal gyrus/insula | 45/47 | R | 55 | 16 | 1 | 3.54 | 1,884 |
| L | −38 | 20 | 3 | 3.99 | 628 | ||
| Anterior cingulate gyrus | 24/32 | L | −4 | 24 | 23 | 3.01 | 947 |
| Middle cingulate gyrus | 24 | L | −6 | −16 | 36 | 2.93 | 293 |
Note: x, y, z refer to Talairach and Tournoux 33 of maximally activated voxel (p < .01 uncorrected). Cluster size refers to the number of voxels in region of activation.
Discussion
The present results provide the first evidence of both of common and distinct neural mechanisms during the successful inhibition of prepotent responses in school-aged children with ADHD-C and ADHD-PI. Specifically, both groups of children activated the right ventrolateral prefrontal cortex, right superior temporal lobe, and left inferior parietal lobe during successful inhibition, but the magnitude of the activation in the latter two regions, as well as bilaterally in ventral-most prefrontal cortex, was greater in children with ADHD-PI than those with ADHD-C. In contrast, children with ADHD-C activated a bilateral region of superior occipital lobe to a greater extent during inhibitory control than children with ADHD-PI. The equally poor performances on the go/no-go task by the two groups of children suggest that these differences in brain activation reflect differences in the magnitude to which these regions were engaged under conditions that elicited comparable performance 34 and are not simply artifacts of impaired performance 35. Under these circumstances, greater activation by one group suggests less efficient processing by the brain regions in question.
Similar activation of ventrolateral prefrontal, dorsal superior temporal, and inferior parietal regions during inhibitory control by children with ADHD-C and ADHD-PI is both consistent with findings of largely similar executive function deficits in the subtypes 6, 36 and noteworthy given previous findings of abnormal activation of these regions during go/no-go tasks in youth with ADHD 13–15. The dorsal superior temporal and inferior parietal regions together form the temporo-parietal junction (TPJ), a cortical association area specialized for the detection of behaviorally relevant signals 37. The TPJ provides afferent input to the ventrolateral prefrontal cortex 38, 39, which in turn, is unique among frontal regions in that it contains populations of neurons that code for sensory cues that signal the suppression rather than execution of behavior 40. The data suggest that children with both subtypes recruited a similar neural network, and presumably cognitive processes, to successfully inhibit responses.
The finding of significantly greater activation of the parietal and temporal regions by children with ADHD-PI than those with ADHD-C, in the context of equivalent task performance, suggests that the ADHD-PI group recruits alerting and/or orienting processes less efficiently than does the ADHD-C group. The use of a control group as well as a task such as the Attentional Network Task 37, 41, which more specifically assesses orienting and alerting functions than does the Go/No-go task used in this study, will be necessary to definitively determine whether the ADHD-PI group is more or less effective in the recruitment of these processes than children without ADHD and children with ADHD-C. In this context, it may be noteworthy that performance differences in spatial orienting have not been found in several other studies that have compared the subtypes 6, 42–44. Differences in the activation of bilateral cuneus between children with ADHD-C and ADHD-PI point to other potential differences in attentional processes between children with ADHD-C and those with ADHD-PI. The cuneus is also a part of the dorsal visual system 45 and has afferent connections with the inferior parietal cortex among other regions 46. In addition to its role in visual processing, this medial parietal region has been implicated in uncued spatial and non-spatial shifts in attention 47, 48. The divergent recruitment of inferior and medial parietal regions by children with ADHD-PI and ADHD-C during inhibitory control suggests subtle subtype differences in attentional and/or sensory processing that may relate to phenotypic differences between the ADHD-C and ADHD-PI subtypes. This result is intriguing given that most investigations have focused on the motor output stage of information processing in ADHD e.g 49, 50.
Children with ADHD-C and ADHD-PI also differed in bilateral activation of the BA 10 region in the rostral-most portion of the prefrontal cortex. This region receives mnemonic input from retrosplenial cortex and polysensory input from superior temporal regions, and is ideally positioned to exert high-order executive control over cognition through reciprocal connections with adjacent prefrontal regions 51. However, the exact executive process mediated by this prefrontal region is still debated 52, 53. The present results suggest that recruitment of higher-order executive processes during inhibitory control may account for some of the phenotypic differences between children with ADHD-C and ADHD-PI and could explain the discrepant findings of abnormal BA 10 activation during the go/no-go task that has been found in ADHD samples composed of both the ADHD-C and ADHD-PI subtypes 13–15, but not in a sample limited to the ADHD-C subtype 16.
These findings must be considered in the context of several important methodological limitations, including the small sample size, and the lack of a control group against which to compare neural function in children with ADHD-C and ADHD-PI, and the analytic approach. It is certainly possible that greater statistical power in the present study would have revealed significant group similarities and distinctions in activation of other regions in the brain (e.g., striatum). For example, the absence of striatal activation during inhibitory control in either group of children with ADHD was unexpected and could reflect the relatively small sample size. However, this finding is not without precedent. Previous studies that used the same go/no-go task have found striatal activation in healthy adults but not healthy children 24 and in healthy children but not children with ADHD 13. Regrettably, the significance of the lack of striatal activation in this study cannot be determined without a healthy control group against which to compare the children with ADHD. Striatal activation during inhibition may not differ between children with ADHD-C and ADHD-PI (hence the negative finding here), but may very well differ between the two groups of children with ADHD and healthy controls. It is also important that future studies compare groups which are either homogeneous or rigorously matched with respect to such variables as gender, presence of learning disabilities, and history of stimulant treatment which may affect brain activation. An additional consideration relates to the approach used in conducting the conjunction analysis. There is currently disagreement in the field regarding which of the two approaches is superior, and for which types of analyses. This study followed the method recommended by Friston et al. 29–31 that tests the conjunction effect against the Global Null Hypothesis that none of the comparisons are significant and yields a significant effect for the conjunction if any of the comparisons are significant. An alternative method, advocated by Nichols 54 tests the conjunction effect against the Conjunction Null Hypothesis that at least one comparison is non-significant, thus yielding a significant result for the conjunction only if all of the comparisons are significant. The Friston et al. method yields a more inclusive interpretation of common activations, while the method of Nichols et al. yields a more restricted view. We acknowledge that using the alternative approach might have yielded a somewhat different pattern of results.
Finally, a methodological limitation of the current study concerns the use of a 40 ms echo time, which is fairly long for the 3T. Current usage is 27 ms. Although some effects in orbital and ventral prefrontal cortex were nonetheless visualized, as reported, it may be that we have underestimated the activation in these regions.
In sum, the present study provides preliminary evidence of both similarities and distinctions in the neural generators of inhibitory control in children with the ADHD-C and ADHD-PI subtypes. The overlapping regions of ventrolateral prefrontal, inferior parietal, and superior temporal activation in children with ADHD-C and ADHD-PI suggests some convergence in the cognitive strategies used to guide the inhibitory control of behavior. In contrast, differential activation of ventral-most prefrontal and medial parietal regions that have previously been implicated in the pathophysiology of ADHD and are thought to mediate executive and attentional processes may account for some of the phenotypic differences between the ADHD-C and ADHD-PI subtypes. The implications of these findings for the classification of the ADHD subtypes and the search for causal factors of the disorder awaits further investigation with larger samples that include a healthy comparison group.
Acknowledgments
This work was supported by National Institute of Mental Health grants R03 HD37803 (MVS), K01 MH070892 (KPS), and R21 MH066360 (JHN), and an Elaine Schlosser Lewis Award for Research in ADHD from the American Academy of Child and Adolescent Psychiatry (MVS, JHN). We wish to express our sincere thanks to B.J. Casey for consultation to the study and A.F.T. Arnsten for comments on the manuscript.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, D.C: Author; 1994. [Google Scholar]
- 2.Hartman CA, Willcutt EG, Rhee SH, Pennington BF. The relation between sluggish cognitive tempo and DSM-IV ADHD. Journal of Abnormal Child Psychology. 2004;32:491–503. doi: 10.1023/b:jacp.0000037779.85211.29. [DOI] [PubMed] [Google Scholar]
- 3.McBurnett K, Pfiffner LJ. Symptom properties as a function of ADHD type: An argument for continued study of sluggish cognitive tempo. Journal of Abnormal Child Psychology. 2001;29:207–213. doi: 10.1023/a:1010377530749. [DOI] [PubMed] [Google Scholar]
- 4.Chhabildas N, Pennington B, Willcutt EG. A comparison of the neuropsychological profiles of the DSM-IV subtypes of ADHD. Journal of Abnormal Child Psychology. 2001;29:529–540. doi: 10.1023/a:1012281226028. [DOI] [PubMed] [Google Scholar]
- 5.Nigg JT, Blaskey LG, Huang-Pollock CL, Rappley MD. Neuropsychological executive functions and DSM-IV subtypes. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:59–66. doi: 10.1097/00004583-200201000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Solanto MV, Gilbert SN, Raj A, et al. Neurocognitive functioning in AD/HD, Predominantly Inattentive Subtype. Journal of Abnormal Child Psychology. 2007;35:729–744. doi: 10.1007/s10802-007-9123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geurts HM, Verte S, Oosterlaan J, Roeyers H, Sergeant JA. ADHD subtypes: do they differ in their executive functioning profile? Archives of Clinical Neuropsychology. 2004;20:457–477. doi: 10.1016/j.acn.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Hinshaw SP, Carte ET, Sami N, Treuting JJ, Zupan BA. Preadolescent girls with attention-deficit/hyperactivity disorder: II. Neuropsychological performance in relation to subtypes and individual classification. Journal of Consulting and Clinical Psychology. 2002;70:1099–1111. doi: 10.1037//0022-006x.70.5.1099. [DOI] [PubMed] [Google Scholar]
- 9.Bauermeister JJ, Matos M, Reina G, et al. Comparison of the DSM-IV combined and inattentive types of ADHD in a school-based sample of Latino/Hispanic children. Journal of Child Psychology and Psychiatry. 2005;46:166–179. doi: 10.1111/j.1469-7610.2004.00343.x. [DOI] [PubMed] [Google Scholar]
- 10.Lahey BB. Should the combined and predominantly inattentive types of ADHD be considered distinct and unrelated disorders? Not now, at least. Clinical Psychology: Science and Practice. 2001;8:494–497. [Google Scholar]
- 11.Milich R, Balentine A, Lynam D. ADHD Combined Type and ADHD Predominantly Inattentive Type are distinct and unrelated disorders. Clinical Psychology: Science & Practice. 2001;8:463–488. [Google Scholar]
- 12.Vaidya CJ, Austin G, Kirkorian G, et al. Selective effects of methylphenidate in attention deficit hyperactivity disorder: A functional magnetic resonance study. Proceedings of the National Academy of Science. 1998;95:14494–14495. doi: 10.1073/pnas.95.24.14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durston S, Tottenham NT, Thomas KM, et al. Differential patterns of striatal activation in young children with and without ADHD. Biological Psychiatry. 2003;15:871–878. doi: 10.1016/s0006-3223(02)01904-2. [DOI] [PubMed] [Google Scholar]
- 14.Schulz KP, Fan J, Tang CY, et al. Response inhibition in adolescents diagnosed with attention deficit/hyperactivity disorder during childhood: an event-related fMRI study. American Journal of Psychiatry. 2004;161:1650–1657. doi: 10.1176/appi.ajp.161.9.1650. [DOI] [PubMed] [Google Scholar]
- 15.Booth JR, Burman DD, Meyer JR, et al. Larger deficits in brain networks for response inhibition than for visual selective attention in attention deficit hyperactivity disorder (ADHD) Journal of Child Psychology and Psychiatry. 2005;46:94–111. doi: 10.1111/j.1469-7610.2004.00337.x. [DOI] [PubMed] [Google Scholar]
- 16.Tamm L, Menon V, Ringel J, Reiss AL. Event-related fMRI evidence of frontotemporal involvement in aberrant response inhibition and task switching in Attention-Deficit/Hyperactivity Disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43:1430–1440. doi: 10.1097/01.chi.0000140452.51205.8d. [DOI] [PubMed] [Google Scholar]
- 17.Bush G, Frazier JA, Rauch SL, et al. Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the counting Stroop. Biological Psychiatry. 1999;45:1542–1552. doi: 10.1016/s0006-3223(99)00083-9. [DOI] [PubMed] [Google Scholar]
- 18.Rubia K, Overmeyer S, Taylor E, et al. Hypofrontality in Attention Deficit Hyperactivity Disorder during higher-order motor control: A study with functional MRI. American Journal of Psychiatry. 1999;156:891–896. doi: 10.1176/ajp.156.6.891. [DOI] [PubMed] [Google Scholar]
- 19.Schulz KP, Tang CY, Fan J, et al. Differential prefrontal cortex activation during inhibitory control in adolescents with and without childhood attention-deficit/hyperactivity disorder. Neuropsychology. 2005;19:390–402. doi: 10.1037/0894-4105.19.3.390. [DOI] [PubMed] [Google Scholar]
- 20.Rasmussen ER, Neuman RJ, Heath AC, Levy F, Hay DA, Todd RD. Familial clustering of latent class and DSM-IV defined attention-deficit/hyperactivity disorder (ADHD) subtypes. Journal of Child Psychology and Psychiatry. 2004;45:589–598. doi: 10.1111/j.1469-7610.2004.00248.x. [DOI] [PubMed] [Google Scholar]
- 21.Rubia K, Smith AB, Brammer MJ, Toone B, Taylor E. Abnormal brain activation during inhibition and error detection in medication-naive adolescents with ADHD. American Journal of Psychiatry. 2005;162:1067–1075. doi: 10.1176/appi.ajp.162.6.1067. [DOI] [PubMed] [Google Scholar]
- 22.Vaidya CJ, Bunge SA, Dudukovic NM, Zalecki CA, Elliott GR, Gabrielli JDE. Altered neural substrates of cognitive control in childhood ADHD: evidence from functional magnetic resonance imaging. AmericanJournal of Psychiatry. 2005;162:1605–1613. doi: 10.1176/appi.ajp.162.9.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Durston S, Thomas KM, Worden MS, Yang Y, Casey BJ. The effect of preceding context on inhibition: an event-related fMRI study. Neuroimage. 2002;16:449–453. doi: 10.1006/nimg.2002.1074. [DOI] [PubMed] [Google Scholar]
- 24.Durston S, Thomas KM, Yang Y, Ulug AM, Zimmerman R, Casey BJ. A neural basis for development of inhibitory control. Developmental Science. 2002;5:9–16. [Google Scholar]
- 25.Shaffer D, Fisher P, Dulcan M, et al. The NIMH Diagnostic Interview Schedule for Children Version 2.3 (DISC-2.3): Description, acceptability, prevalence rates, and performance in the MECA study. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:865–877. doi: 10.1097/00004583-199607000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Conners CK, Sitarenios G, Parker JDA, Epstein JN. The revised Conners’ Parent Rating Scale (CPRS-R): Factor structure, reliability, and criterion validity. Journal of Abnormal Child Psychology. 1998;25:257–268. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- 27.Conners CK, Sitarenios G, Parker J, Epstein JN. Revision and restandardization of the Conners’ Teacher Rating Scale (CTRS-R): Factor structure, reliability, and criterion validity. Journal of Abnormal Child Psychology. 1998;26:279–291. doi: 10.1023/a:1022606501530. [DOI] [PubMed] [Google Scholar]
- 28.Schneider W, Eschman A, Zuccolotto A. E-Prime Reference Guide. Pittsburgh, PA: Psychology Software Tools, Inc; 2002. [Google Scholar]
- 29.Price CJ, Friston KJ. Cognitive conjunction: a new approach to brain activation experiments. NeuroImage. 1997;5:261–270. doi: 10.1006/nimg.1997.0269. [DOI] [PubMed] [Google Scholar]
- 30.Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: characterizing differential differential responses. NeuroImage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- 31.Friston KJ, Holmes AP, Price CJ, Buchel C, Worsley KJ. Multi-subject fMRI studies of conjunction analyses. NeuroImage. 1999;10:385–396. doi: 10.1006/nimg.1999.0484. [DOI] [PubMed] [Google Scholar]
- 32.Slotnick SD, Moo LR, Segal JB, Hart JJ. Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Brain Research Cognitive Brain Research. 2003;17:75–82. doi: 10.1016/s0926-6410(03)00082-x. [DOI] [PubMed] [Google Scholar]
- 33.Talairach J, Tournoux M. Co-planar stereotaxic atlas of the human brain. New York: Thieme Medical; 1988. [Google Scholar]
- 34.Callicott JH, Weinberger DR. Neuropsychiatric dynamics: The study of mental illness using functional magnetic resonance imaging. European Journal of Radiology. 2000;30:95–104. doi: 10.1016/s0720-048x(99)00048-0. [DOI] [PubMed] [Google Scholar]
- 35.Casey BJ. Neuroscience: Windows into the human brain. Science. 2002;296:1408–1409. doi: 10.1126/science.1072684. [DOI] [PubMed] [Google Scholar]
- 36.Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biological Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 37.Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. Neuroimage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 38.Petrides M, Pandya DN. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. European Journal of Neuroscience. 2002:16. doi: 10.1046/j.1460-9568.2001.02090.x. [DOI] [PubMed] [Google Scholar]
- 39.Romanski LM, Bates JF, Goldman-Rakic PS. Auditory belt and parabelt projections to the prefrontal cortex in the rhesus monkey. Journal of Comparative Neurology. 1999;403:141–157. doi: 10.1002/(sici)1096-9861(19990111)403:2<141::aid-cne1>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 40.Sakagami M, Tsutsui KI, Lauwereyns J, Koizumi M, Kobayashi S, Hikosaka O. A code for behavioral inhibition on the basis of color, but not motion, in ventrolateral prefrontal cortex of macaque monkey. Journal of Neuroscience. 2001;21:4801–4808. doi: 10.1523/JNEUROSCI.21-13-04801.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. Journal of Cognitive Neuroscience. 2002;14:340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- 42.Huang-Pollock CL, Nigg JT, Halperin JM. Single dissociation findings of ADHD deficits in vigilance but not anterior or posterior attention systems. Neuropsychology. 2006;20:420–429. doi: 10.1037/0894-4105.20.4.420. [DOI] [PubMed] [Google Scholar]
- 43.Oberlin BG, Alford JL, Marrocco PT. Normal attention orienting but abnormal stimulus alerting and conflict effect in combined subtype of ADHD. Behavioural Brain Research. 2005;165:1–11. doi: 10.1016/j.bbr.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 44.Booth JE, Carlson CL, Tucker DM. Performance on a neurocognitive measure of alerting differentiates ADHD combined and inattentive subtypes: A preliminary report. Archives of Clinical Neuropsychology. 2007:3. doi: 10.1016/j.acn.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 45.Ungerleider LG, Mishkin M. Two visual systems. In: Ingle DJ, Goodale MA, Mansfield RJW, editors. Analysis of Visual Behavior. Cambridge, M.A: MIT Press; 1982. pp. 549–586. [Google Scholar]
- 46.Divac I, Lavail JH, Rakic P, Winston KR. Heterogeneous afferents to the inferior parietal lobule of the rhesus monkey revealed by the retrograde transport method. Brain Research. 1977;123:197–207. doi: 10.1016/0006-8993(77)90474-7. [DOI] [PubMed] [Google Scholar]
- 47.Corbetta M, Miezin FM, Shulman GL, Petersen SE. A PET study of visuospatial attention. Journal of Neuroscience. 1993;13:1202–1226. doi: 10.1523/JNEUROSCI.13-03-01202.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Le TH, Pardo JV, Hu X. 4 T-fMRI study of nonspatial shifting of selective attention: cerebellar and parietal contributions. Journal of Neurophysiology. 1998;79:1535–1548. doi: 10.1152/jn.1998.79.3.1535. [DOI] [PubMed] [Google Scholar]
- 49.Castellanos FX. Neural substrates of attention-deficit hyperactivity disorder. Advances in Neurobiology. 2001;85:197–206. [PubMed] [Google Scholar]
- 50.Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- 51.Petrides M, Pandya DN. The frontal cortex. In: Paxinos G, Mai JK, editors. The Frontal Cortex. 2. San Diego: Elsevier Academic Press; 2004. pp. 950–972. [Google Scholar]
- 52.Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J. The role of the anterior prefrontal cortex in human cognition. Nature. 1999;399:148–151. doi: 10.1038/20178. [DOI] [PubMed] [Google Scholar]
- 53.Szameitat AJ, Schubert T, Muller K, Von Cramon DY. Localization of executive functions in dual-task performance with fMRI. Journal of Cognitive Neuroscience. 2002;14:1184–1199. doi: 10.1162/089892902760807195. [DOI] [PubMed] [Google Scholar]
- 54.Nichols T, Brett M, Andersson J, Wager T, Poline J. Valid conjunction inference with the minimum statistic. NeuroImage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]


