Abstract
Background
Mild cognitive impairment (MCI) is a transitional state between normal ageing and dementia, at least for some patients. Behavioral symptoms in MCI are associated with a higher risk of dementia, but their association with dementia risk in patients without MCI is unknown. Mild Behavioral Impairment (MBI) refers to a late life syndrome with prominent psychiatric and related behavioral symptoms in the absence of prominent cognitive symptoms, which may also be a dementia prodrome.
Objective
To compare MCI and MBI patients and to estimate the risk of dementia development in these two groups.
Method
A consecutive series of 358 patients (239 with MCI; and 119 with MBI) presenting to an outpatient general hospital specialty clinic were followed for up to 5 years until conversion to dementia or censoring.
Results
34% of MCI patients and over 70% of patients with MBI developed dementia (Logrank p=0.011). MBI patients without cognitive symptoms were more likely to develop dementia (Logrank p<0.001). MBI patients were more likely to develop dementia due to frontotemporal degeneration (FTD) as opposed to Alzheimer’s dementia (AD).
Conclusion
MBI appears to be a transitional state between normal ageing and dementia. MBI (specifically those without cognitive symptoms) may confer a higher risk for dementia than MCI and is likely an FTD prodrome in many cases. These findings have implications for the early detection, prevention, and treatment of patients with dementia in late life, by focusing on the emergence of new behavioral symptoms.
Introduction
Dementia is a major public health problem because of its growing prevalence, and economic impact.1-3 It is a chronic condition with global consequences that seriously impacts patients, their families, and society.4 An understanding of the prodromal states or early clinical presentations of dementia is a significant priority since it would aide early detection, facilitate early treatment, and could lead to effective prevention. Mild cognitive impairment (MCI) is a cognitive disturbance of older persons, more severe than what would be expected for age and education, but not of sufficient severity for a diagnosis of dementia.5 Several operational definitions for MCI have been proposed 5-6 and at least two subtypes have been described7, amnestic thought to be mainly the prodrome of Alzheimer dementia (AD), and non-amnestic thought to be mainly a prodrome of other dementias such as frontotemporal (FTD), vascular (VaD) or Lewy Body dementia (LBD).
In the last several years, there has been growing awareness of the importance of neuropsychiatric symptoms (NPS) in dementia, given their near universal occurrence over the course of dementia, associated caregiver burden, and association with early institutionalization 8-12. Whereas dementia is still defined as a cognitive disorder, neuropsychiatric symptoms are now regarded as an intrinsic aspect of dementia and the underlying causes usually a neurodegenerative processes.
Although neuropsychiatric symptoms are common in dementia 8-12 they have received less attention in the prodromal states to dementia. In a population-based study the most common NPS in MCI were apathy, depression, agitation, delusions, hallucinations, and sleep impairment13. In MCI patients, the occurrence of NPS was associated with a higher risk of dementia onset. For example depression in MCI has been reported to double the risk of dementia14. Furthermore cognitively normal elderly individuals who develop depression are at increased risk of subsequent MCI15. However, not all prodromal states involve prominent cognitive impairment. Many patients develop NPS as the first indicator of impending dementia. This is most common in patients with FTD, but is also the case in patients with AD. For example we reported that in 50% of a series of dementia patients who consulted our service NPS were the first indication of change, before the occurrence of cognitive symptoms. Of the latter patients, 36% had FTD, 28% had AD; 18% had VaD and 18% had other types of dementia16. As a result we proposed the syndrome of “Mild Behavioral Impairment” (MBI) consisting of: (1) persistent behavioral changes and mild psychiatric symptoms, especially disinhibition; (2) no serious cognitive complaints; (3) normal activities of daily living; and (4) absence of dementia16-18. MBI has been hypothesized to confer increased risk for dementia development, especially of FTD, whether or not significant cognitive symptoms are present. Here we report a validation of the MBI construct in a longitudinal study. Our aims were to compare MBI with MCI patients, and to examine the risk of dementia development, especially FTD, in MBI patients compared to MCI patients.
Methods
Design and setting
This was a prospective cohort study of outpatients with MBI and MCI. The study was performed under the oversight of an institutional review board. Each participant or a legal representative provided informed consent for participation.
Participants
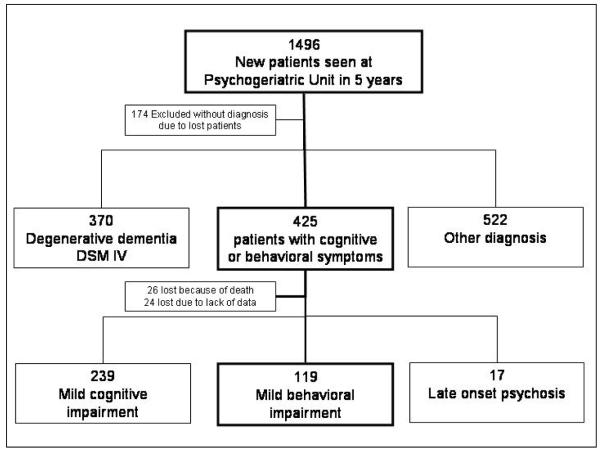
Between January 2001 and January 2006, a consecutive series of 1496 new elderly (≥65) outpatients was evaluated at the Psychogeriatric Unit of CEMIC University in Buenos Aires, Argentina. Patients were referred from two sources: 1133 from the CEMIC Department of Internal Medicine, and 363 from community general practitioners. After a complete neuropsychiatric assessment, 425 presented with cognitive and or behavioral symptoms; of these 119 were found to have MBI and 239 MCI. Another 17 presented with late onset primary psychotic disorders.
Diagnostic criteria
MBI was defined as a behavioral disturbance not meeting DSM-IV19 or NINCS-ADRDA criteria for dementia, psychosis or another major psychiatric condition, and also not meeting criteria for MCI of any type. It was operationalized using the following criteria for inclusion in the study: a) the presence of a major change in patient behavior; b) this change occurring later in life (>60) that is persistent (>6 months); c) no complaint of cognitive impairment by patient/informant d) normal occupational and social functioning, e) normal activities of daily living, f) absence of dementia. Loss of independence criteria was crucial for ruling out dementia at baseline and follow up and it was operationalized as follows: cognitive deficits caused significant impairment in normal occupational and/or normal social functioning and/or normal activities of daily living.
For the purpose of this study, a patient with cognitive complaints and psychiatric symptoms (plus remaining criteria) was considered to have MCI. A patient with major persistent change in behavior but no cognitive complaints from either the patient or the caregiver was considered to have MBI regardless of whether cognitive impairment was subsequently found on testing or not.
MBI was considered absent if the patient a) had another concomitant neurological or psychiatric disorder that could better explain disturbances (i.e. epilepsy, major stroke, tumors, schizophrenia etc) b) behavioral disturbances of acute onset, c) alcohol or substance abuse.
Examples of major persistent changes in patient behavior that might have led to a diagnosis of MBI are as follows: agitation, anxiety symptoms, apathy, aspontaneity, delusion symptoms, depressive symptoms, disinhibition, emotional lability, euphoria, impulsivity, indifference, irritability, lack of empathy, loss of insight, loss of personal hygiene, loss of social tact, oral/dietary changes, perseverant behavior, sleep disorders.
Mild cognitive impairment20 was defined as cognitive decline not meeting DSM-IV criteria for dementia. It was operationalized using results of neuropsychological testing in 2 groups as follows, with both groups considered as one in this study; (1) MCI amnestic-type if they met the following criteria: memory complaint, normal activities of daily living, normal general cognitive function, abnormal memory function for age (defined as a score on a standardized test that was 1.5 SD below the mean compared with individuals of the same age and level of education); (2) Non amnestic MCI if they met the following criteria: cognitive complaint, normal activities of daily living, abnormal cognitive function for age (defined as a deterioration in at least 1 cognitive domain not including memory, or 1 abnormal test 1.5 SD below the mean adjusted for age and education in at least 2 other domains).
Follow up and outcome assessment
Patients were assessed at baseline and every 4 months or when necessary using a comprehensive approach, for up to 5 years. The median follow up for MBI was 30 months and for MCI 24. Loss to follow-up was <12%: 26 patients died, and 24 were lost to follow up for other reasons (Figure 1).
Figure 1.
Recruitment flow
Data collected at baseline included socio-demographic and clinical variables such as age at assessment, years of education, gender, marital and retirement state, socio-economic level and number of times patients were seen by psychiatrists during the study. Physical examination and laboratory analysis were performed as clinically appropriate for each patient. Neuroimaging examinations using brain CT, and as appropriate MRI or SPECT were assessed. At each visit, neurological examination findings, such as primitive reflexes, were assessed, as were medical history, blood pressure, medications, physical function, social support. The following cognitive assessment battery was also administered by blinded raters to group using validated translated versions: Signoret memory tests21, Wechsler Adult Scale — revised22, Boston naming Test in Buenos Aires23, Mini Mental24, Trail Making Test25. At each examination, we used the Neuropsychiatric Inventory (NPI)26 to assess the occurrence and severity of NPS. NPI has wide acceptance as a measure of NPS associated with cognitive disorders. It is a fully structured interview, which obtains its information from an informant knowledgeable about the participant. It focuses on observable symptoms and behaviors. Depressive symptoms were assessed using the Beck Inventory 27. Several of the authors (FT, RFA) reviewed the data from each visit to determine at each time point whether a given patient had converted to dementia using the baseline DSM-IV or NINCS-ADRDA criterias19,28-30.
Statistical analysis
Initially analyses were performed to compare the MCI, and MBI patients at baseline. Categorical variables were expressed as percentages; for continuous variables, mean and standard deviations were estimated, while for non-normally distributed variables, medians and percentiles were considered. When frequency differences were compared by diagnosis, univariate analyses were carried out using chi-square tests. Student t-tests were used to compare groups on continuous variables, while the nonparametric Wilcoxon ranksum test was applied to compare groups on non-normally distributed variables. Survival analyses were then conducted to compare groups on time to onset of dementia. The main outcome was diagnosis of dementia. The time to dementia was considered the outcome of interest. The follow-up period was from the initial observation to dementia conversion or to the end of the study. Cox proportional hazards models were also estimated to test multivariate associations between multiple explanatory variables and dementia conversion in patients with MBI compared to MCI. Effects are shown as hazard ratios (HR), with 95% confidence intervals (95%CI). For all analyses, the STATA 8.0 statistical software package was used.
Results
Comparison of the MBI and MCI groups at baseline
Table 1 shows demographic characteristics of the study groups. There were no differences between the MBI and MCI groups on demographic characteristics. The MBI participants were followed up longer by 6 months at the median, but had a similar median number of follow up visits.
Table 1.
Demographic characteristics at baseline
| MCI (n=239) |
MBI (n=119) |
Comparison | |
|---|---|---|---|
| Demographics | |||
| Age at evaluation, mean (SD) | 72.3 ± 7.8 | 72.91 (8.9) | t = 0.626 p = 0.531 (***) |
| Sex, male (n, %) | 98 (41) | 56 (47.05) | χ2(1) = 1.188 p = 0.276 (**) |
| Married (n, %) | 159 (67) | 84 (70.58) | χ2(1) = 0.600 p = 0.438 (**) |
| Education, median | 12 | 12 | z = 1.187 p = 0.235 (*) |
| Retired (n, %) | 182 (76) | 109 (92) | χ2(1) = 2.350 p = 0.125 (**) |
| Follow-up in study | |||
| Median (months) | 24.15 | 30.00 | z = -4.213 p< 0.001 (*) |
| 10the percentile | 9.00 | 12.00 | |
| 90th percentile | 51.89 | 60.00 | |
| Median number of visits | 7 | 8 | z = -2.723 p = 0.006 (*) |
| 10the percentile | 2 | 4 | |
| 90th percentile | 21 | 21 |
Wilcoxon ranksum test
Pearson χ2
t test
Table 2 compares the groups on baseline clinical and laboratory characteristics. The MCI group was more likely to have dyslipidemia31,32, or hypothyroidism33 found on questioning of past medical history but not in the lab screening for altered thyroid gland by the determination of serum thyroid-stimulating hormone (TSH) levels. The MCI group was also more likely to be on antidepressants and benzodiazepines (substance-induced persisting amnestic disorder patients were already excluded at baseline) which might explain their relatively low anxiety and depression scores on the NPI (below). In contrast, the MBI group were more likely to have neurological signs and primitive reflexes34,35. Altered hematocrit and hypercholesterolemia were the laboratory values with significant differences between groups.
Table 2.
Baseline clinical and labs characteristics
| MCI (n=239) |
MBI (n=119) |
Comparison | |
|---|---|---|---|
| n (%) | n (%) | ||
| Medical history | Pearson χ2 | ||
| Arterial Hypertension | 79 (33.1) | 45 (37.8) | χ2(1) = 0.795 p = 0.372 |
| Diabetes | 19 (7.9) | 7 (5.9) | χ2(1) = 0.504 p = 0.478 |
| Dyslipidemia | 72 (30.1) | 13 (10.9) | χ2(1) = 16.177 p < 0.001 |
| Mild hypothyroidism | 35 (14.6) | 8 (6.7) | χ2(1) = 4.717 p = 0.030 |
| Psychiatric medications | Pearson χ2 | ||
| Anti depressives | 99 (41.4) | 21 (17.6) | χ2(1) = 20.152 p < 0.001 |
| Antipsychotic | 33 (13.8) | 26 (21.8) | χ2(1) = 3.732 p = 0.053 |
| Benzodiazepines | 85 (35.6) | 23 (19.3) | χ2(1) = 9.942 p = 0.002 |
| Family history of dementia | 40 (16.7) | 26 (21.8) | χ2(1) = 1.380 p = 0.240 |
| Pyramidal signs on exam | 28 (11.7) | 26 (21.8) | χ2(1) = 6.368 p = 0.012 |
| Extrapyramidal signs on exam | 22 (9.2) | 40 (33.6) | χ2(1) = 33.054 p < 0.001 |
| Primitive reflexes | 38 (15.9) | 59 (49.6) | χ2(1) = 45.621 p < 0.001 |
| Altered hematocrit | 2 (0.8) | 5 (4.2) | Fisher’s exact = 0.043 |
| Hypercholesterolemia | 23 (9.6) | 4 (3.4) | Fisher’s exact = 0.035 |
Table 3 compares the groups on baseline cognitive complaints and test results. By definition, all MCI patients had memory complaints. In the MBI group, we found that 49.6% of patients had cognitive symptoms discovered by raters, even though these symptoms were not a complaint for either the patient or the family. MCI patients had lower scores on memory tests while MBI patients had lower scores on tests of executive function and IQ domains.
Table 3.
Cognitive characteristics at baseline
| MCI (n=239) |
MBI (n=119) |
Comparison | |
|---|---|---|---|
| Cognitive symptoms n, (%) | 239 (100) | 59 (49.6) | χ2(1) = 141.878 p < 0.001 (**) |
| Mini Mental State Exam score, mean (SD) | 27.4 (1.8) | 26.1 (2.1) | t = 4.420 p < 0.001 (***) |
| Signoret Memory Test | |||
| Paragraph recall, median | 5 | 7 | z = -7.064 p < 0.001 (*) |
| Paragraph delay recall, median | 4 | 5 | z = -3.781 p < 0.001 (*) |
| List of words, median | 7 | 8 | z = -5.099 p < 0.001 (*) |
| Retention, median | 4 | 6 | z = -5.305 p < 0.001 (*) |
| Recall with clues, median | 7 | 8 | z = -1.607 p = 0.108 (*) |
| Recognition, median | 11 | 10 | z = 2.790 p = 0.005 (*) |
| Boston Naming Test, mean (SD) | 49.6 (0.43) | 47.9 (0.64) | t = 2.2663 p = 0.024 (***) |
| Semantic fluency, median | 15 | 14 | z = 2.169 p = 0.030 (*) |
| Fonologic fluency, median | 12 | 11 | z = 0.433 p = 0.665 (*) |
| Digit Span, median | 8 | 6 | z = 6.760 p < 0.001 (*) |
| Trail making B, mean (SD) | 125.7 (8.37) | 158.9 (8.50) | t = -2.616 P = 0.009 (***) |
| Wechsler Abbreviated Scale of Intelligence | |||
| Vocabulary, median | 55 | 50 | z = -0.359 p = 0.719 (*) |
| Similarities, median | 48 | 37 | z = -0.359 p = 0.719 (*) |
| Block Design, median | 42 | 32 | z = -4.707 p < 0.001 (*) |
| Matrix Reasoning, median | 40 | 36.5 | z = -3.856 p < 0.001 (*) |
| Verbal IQ, mean (SD) | 107.8 (1.88) | 96.7 (1.03) | t = 4.3335 p < 0.001 (***) |
| Performance Scale IQ, mean (SD) | 95.5 (1.04) | 88.9 (0.91) | t = 4.3467 p < 0.001 (***) |
| Global IQ, mean (SD) | 101.1 (0.96) | 92.3 (0.85) | t = 6.2819 p < 0.001 (***) |
Wilcoxon ranksum test
Pearson χ2
t test
Table 4 compares the groups on NPS. Because of the inclusion criteria, all MBI patients had persistent changes in behavior. While 85 (35.5%) MCI patients had behavioral disturbances reported by relatives on the NPI (χ2(1) =134.562 p< 0.001), a smaller group of 23 (9.6%) showed a persistent major change in patient behavior occurring later in life (χ2(1) = 271.1424 p < 0.001). The two groups differed on a number of NPS. Depression and anxiety scores were lower in the MCI group possibly related to their more frequent use of antidepressants. Although change in behavior was an inclusion criterion for MBI, no patients had a psychotic specific disorder.
Table 4.
Neuropsychiatric characteristics at baseline
| MCI (n=239) n (%) |
MBI (n=119) n (%) |
Comparison | |
|---|---|---|---|
| Neuropsychiatric symptoms as assessed on the NPI |
85 (35.5) | 119 (100) | χ2(1) = 134.562 p < 0.001 (**) |
| Delusions | 22 (9.2) | 67 (56) | χ2(1) = 94.3368 p < 0.001 (**) |
| Hallucinations | 9 (3.8) | 22 (18.49) | χ2(1) = 21.7688 p < 0.001 (**) |
| Agitation | 22 (9.2) | 31 (26.1 ) | χ2(1) = 17.8737 p < 0.001 (**) |
| Depression | 40 (16.7) | 57 (47.9) | χ2(1) = 39.0560 p < 0.001 (**) |
| Anxiety | 40 (16.7) | 54 (45.4) | χ2(1) = 33.6585 p < 0.001 (**) |
| Euphoria / Negation | 6 (2.5) | 9 (7.6) | χ2(1) = 5.0520 p < 0.001 (**) |
| Apathy / Indifference | 28 (11.7) | 35 (29.4) | χ2(1) = 17.1565 p < 0.001 (**) |
| Disinhibition | 26 (10.9) | 58 (48.7) | χ2(1) = 63.4130 p < 0.001 (**) |
| Irritability | 41 (17.2) | 55 (46.2) | χ2(1) = 34.1945 p < 0.001 (**) |
| Aberrant motor behavior | 10 (4.2) | 26 (21.8) | χ2(1) = 27.4081 p < 0.001 (**) |
| Sleep | 23 (9.6) | 60 (50.4) | χ2(1) = 74.2451 p < 0.001 (**) |
| Appetite and Eating Disorders | 19 (7.9) | 32 (26.9) | χ2(1) = 23.3305 p < 0.001 (**) |
| Beck Depression Inventory (median) | 9 | 12 | z = -4.583 p < 0.001 (*) |
| Complaint of a major change in patient behavior occurring later in life and persistent |
23 (9.6) | 119 (100) | χ2(1) = 271.1424 p < 0.001 (**) |
Wilcoxon ranksum test
Pearson χ2
Table 5 compares the groups on neuroimaging characteristics. Most MBI patients had brain imaging carried out for clinical reasons. While there were no major differences in CT or MRI findings, the MBI group was more likely to show decreased perfusion in frontal or temporal lobes on SPECT.
Table 5.
Neuroimaging comparison at baseline
| MCI (n=239) |
MBI (n=119) |
Comparison | |
|---|---|---|---|
| n (%) | N (%) | ||
| CT or MRI | |||
| No image | 87 (36.4) | 5 (4.2) | Pearson χ2(7) = 44.147 p < 0.001 |
| Normal | 30 (12.6) | 18 (15.1) | |
| 1 mild generalized atrophy | 45 (18.8) | 22 (18.5) | |
| 2 leucoareosis | 34 (14.2) | 10 (8.4) | |
| 3 mild focal atrophy | 21 (8.8) | 22 (18.5) | |
| 1+2 | 11 (4.6) | 4 (3.4) | |
| 1+3 | 2 (0.8) | 2 (1.7) | |
| 2+3 | 9 (3.8) | 0 (0.0%) | |
| SPECT | |||
| No image | 178 (74.5) | 6 (5) | Pearson χ2(4) = 141.870 p < 0.001 |
| Normal | 22 (9.2) | 7 (5.9) | |
| Decreased perfusion in parietal or temporal parietal lobes |
18 (7.5) | 15 (12.6) | |
| Decreased perfusion in several areas |
1 (0.4) | 4 (3.4) | |
| Decreased perfusion in frontal or frontal temporal lobes |
20 (8.4) | 51 (42.9) | |
Conversion to dementia
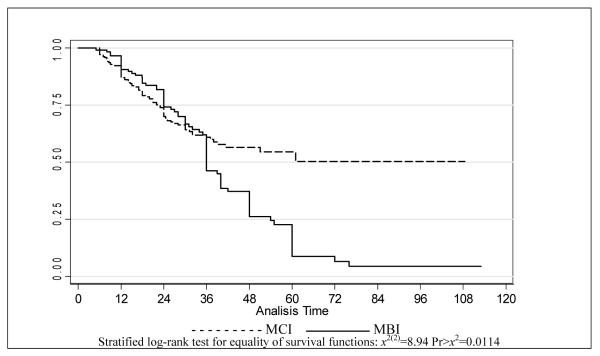
Figure 2 compares time to dementia onset between the two groups on Kaplan-Meier plots, adjusted for age. Dementia onset was faster in MBI patients (log-rank test for equality of survivor functions: P=0.0114). Cox proportional hazard model estimation revealed that the risk of onset was 43% higher in MBI than in MCI (HR=1.43, 95% CI 1.01- 2.03). After adjusting for age at diagnosis this hazard ratio was essentially unchanged (HR=1.48, 95% CI 1.04-2.11).
Figure 2.
Survival estimates adjusted for age, by inicial diagnosis
MBI participants with and without cognitive symptoms, and MCI participants with and without psychiatric symptoms
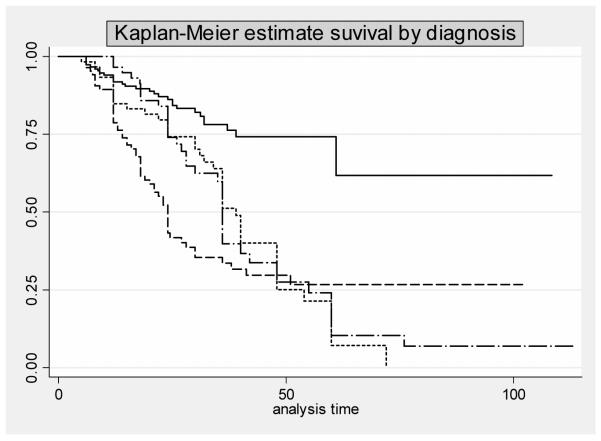
As shown in table 3, when the clinician examiners evaluated specifically for cognitive symptoms in MBI patients 49.6% reported such symptoms. Also, as shown in table 4 when they investigated specifically for psychiatric symptoms in MCI patients 35.5% reported such symptoms. Consequently an analysis was performed to compare rates of conversion to dementia in the following groups: 1) MCI without neuropsychiatric symptoms, 2) MCI with neuropsychiatric symptoms, 3) MBI with cognitive symptoms 4) MBI without cognitive symptoms. MCI patients with psychiatric symptoms differed from MCI patients with no psychiatric symptoms as they had 4- fold increased risk of conversion to dementia (HR 4.01, 95% Conf. Interval 2.5-6.3). Their risk was similar to that of MBI with cognitive symptoms (χ2(1) =2.46 p= 0.116). We also found, as shown in Figure 3, that MCI without NPS, and MBI without cognitive symptoms were quite different in the time to dementia onset, which was much faster for MBI without cognitive symptoms than for MCI without NPS (log-rank test χ2(3) = 42.87 p<0.001). Table 6 displays data regarding dementia conversion rates and the type of dementia to which patients converted. While MCI patients without NPS patients converted mainly to AD, and MBI without cognitive symptoms mainly converted to FTD, both MCI with NPS and MBI with cognitive symptoms showed similar conversion risks. Overall, there were 68 incident cases of FTD compared to 92 of AD and the higher risk of conversion for all types of dementia was seen in the MBI no cognitive group.
Figure 3.
| MCI without NPS | — | MBI with cognitive impairment | -.-.- |
| MCI with NPS | ---- | MBI without cognitive impairment | ......... |
Table 6.
Comparison of groups on dementia conversion
| MCI without NPS |
MCI with NPS | MBI with cognitive symptoms |
MBI without cognitive symptoms |
||
|---|---|---|---|---|---|
| (n=154) | (n = 85) | (n = 59) | (n = 60) | Comparison | |
| Patients converted (n, %) | 29 (18.8) | 54 (63.5) | 41 (69.4) | 44 (73.3) | Log-rank test for equality of survivor functions χ2(3) = 42.87 p<0.001 |
| Outcome | |||||
| Frontotemporal, n (%) | 0 (0) | 15 (17.6) | 12 (20.3) | 41 (68.3) | Pearson χ2(6) = 142.38 p < 0.001 |
| Alzheimer’s, n (%) | 28 (18.2) | 37 (43.5) | 25 (42.4) | 2 (3.3) | |
| Lewy Bodies, n (%) | 1 (0.6) | 2 (2.4) | 4 ( 6.8) | 1 (1.6) |
Discussion
We examined conversion to dementia in 358 patients over a 5-year period, 239 with MCI and 119 with MBI. We were specifically interested in the construct of MBI, which, as with MCI, has been proposed to represent a transitional state between normal ageing and dementia. An obstacle to research progress in this area has been the lack of agreement on what constitutes behavioral impairment. In this study MBI was defined using criteria first proposed in 2003. NPS were consistently and robustly associated with faster time to dementia conversion across groups. Although this has previously been reported, since the MBI group without cognitive complaints converted to dementia faster than the MCI group without psychiatric complaints, this study emphasizes the importance of NPS even in the absence of cognitive symptoms. Rates of dementia conversion in the MBI group with cognitive complaints were comparable to those in the MCI group with neuropsychiatric symptoms suggesting that these two groups could probably be considered a single one. Finally, the presence of MBI was associated with clinical and neuroimaging evidence of abnormalities in the frontal regions of the brain, and with a greater risk of conversion to FTD than to AD. Hence, MBI, specifically in the absence of cognitive symptoms, probably represents an FTD prodrome, at least in about half of cases.
Confidence in these findings is supported by several factors. First, the clinical definition of MBI was based on a standardized clinical examination, supplemented by the administration of widely used neuropsychiatric scales by experienced physicians. Second, the effect of NPS on conversion to dementia was observed with both dementia types (DAT and FTD) reducing the likelihood that diagnostic imprecision affected results. Third, the availability of 119 subjects with MBI with high follow up participation over five years improved power to estimate associations between behavior, cognitive tests, clinical findings, brain imaging findings and dementia incidence.
There are several limitations to consider. One is the relatively short median follow-up of 30 months. Another is that the results were based on a selected group of patients referred for consultation to a psychogeriatric service. The fact that participants were referred by general practitioners is a strength that indicates the growing importance these practitioners are giving to NPS in late life. Nevertheless, they might have been biased to prescribe fewer psychiatric medications to MBI patients with apathy/indifference, which might in part explain the heterogeneous distribution of medications. While we can not be entirely sure that MCI patients, who were more likely to be taking antidepressants and benzodiazepines, did not have MBI at some point previous this study was started, we certainly looked for that possibility by searching records and questioning patients and family about a change in behavior.
Another limitation is that lack of insight might have affected the reporting of both cognitive and behavioral complaints, which affected whether a diagnosis of MCI or MBI was made. Many patients lack insight into their own cognitive changes, and often times family members, and patients, do not recognize behavioral changes. Nevertheless, the presence of specific complaints in either the cognitive or behavioral area provides validity as being of great clinical significance: this typically is the reason for which care is sought. The point is further reinforced by the analyses showing strong associations between MBI without cognitive complaints and dementia conversion. In light of this, we believe that the groups of MBI — MCI defined in this way are different enough to think of them as two separate groups for the purposes of this report. We are aware that there is overlap between the MCI with-NPS and MBI with-cognitive impairment groups. Therefore, the “no cognitive complaints criterion” of the MBI diagnosis should be improved in future investigations to better differentiate these groups.
One more limitation is the lack of investigation of conversion from MBI to MCI. We know that the mean (SD) MMSE of the MBI group without cognitive symptoms was 26.9 (1.42), while the mean for the MBI group with cognitive symptoms was 25.7 (1.67) t=3.761 p< 0.001. We are conscious that many patients might have converted from MBI to MCI, but we did not investigate this possibility in the study. This would seem to be an important aspect of future research. While some of these limitations may affect the external validity (generalizability) of the study they should not affect its internal validity. Nevertheless, it is important to replicate this study in other settings.
We conclude that MBI, specifically in the absence of cognitive symptoms, as with MCI, is a transitional state between normal aging and dementia, at least for some patients. MBI confers a higher risk of dementia conversion than MCI, with or without NPS, especially of FTD. These findings emphasize the importance of the emergence of NPS in later life as worrisome. Early detection of dementia and targeting of therapies, possibly even prevention will likely be served well by a better understanding of these observations. Further, it is possible that targeted treatment for MBI using available psychopharmaca might delay conversion to dementia.
Acknowledgments
Funding/Support: The authors thank the René Baron Foundation, from the CEMIC School of Medicine, for providing research facilities.
Dr. Taragano was supported by Lina Esevich grant #310618 to the CEMIC University Hospital Dementia Research Unit.
Dr. Lyketsos’ effort was supported by National Institute on Aging grant P50AG005146 to the Johns Hopkins Alzheimer’s Disease Research Center.
Footnotes
Conflict of interest statement: None--
Authors’ affiliations CEMIC University Institute, Buenos Aires, Argentina (Taragano, Allegri, Krupitzki, Serrano, Sarasola, Lon) Johns Hopkins Bayview and Johns Hopkins University, Baltimore, Maryland, USA (Lyketsos)
Ethics committee approval: The study was performed accordantly to institutional review board regulations of the CEMIC University and each participant gave oral informed consent.
REFERENCES
- 1.Allegri RF, Butman J, Arizaga RL, Machnicki G, Serrano C, Taragano FE, Sarasola D, Lon L. Economic impact of dementia in developing countries: an evaluation of costs of Alzheimer-type dementia in Argentina. Int Psychogeriatr. 2006 Jul 27;:1–14. doi: 10.1017/S1041610206003784. [DOI] [PubMed] [Google Scholar]
- 2.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88(9):1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Helmer C, Pasquier F, Dartigues JF. Epidemiology of Alzheimer disease and related disorders. Med Sci (Paris) 2006 Mar;22(3):288–96. doi: 10.1051/medsci/2006223288. [DOI] [PubMed] [Google Scholar]
- 4.Rabins PV, Lyketsos CG, Steele C. Practical Dementia Care. Oxford University Press; New York, NY: 1999. [Google Scholar]
- 5.Gauthier S, Reisberg B, Zaudig M, Petersen RC, Ritchie K, Broich K, Belleville S, Brodaty H, Bennett D, Chertkow H, Cummings JL, de Leon M, Feldman H, Ganguli M, Hampel H, Scheltens P, Tierney MC, Whitehouse P, Winblad B. International Psychogeriatric Association Expert Conference on Mild cognitive impairment. Lancet. 2006 Apr 15;367(9518):1262–70. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- 6.Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, Berg L. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001 Mar;58(3):397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 7.Petersen RC. Mild cognitive impairment: transition from aging to Alzheimer’s disease. In: Iqbal K, Sisodia SS, Winblad B, editors. Alzheimer’s disease: advances in etiology, pathogenesis and therapeutics. John Wiley & Sons; West Sussex, England: 2001. pp. 141–151. [Google Scholar]
- 8.Steinberg M, Shao H, Zandi P, Lyketsos CG, Welsh-Bohmer KA, Norton MC, Breitner JC, Steffens DC, Tschanz JT, Cache County Investigators Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: the Cache County Study. Int J Geriatr Psychiatry. 2007 Jul 3; [Google Scholar]
- 9.Finkel SI, e Silva J Costa, Cohen G, Miller S, Sartorius N. Behavioral and psychological signs and symptoms of dementia. Int Psychogeriatr. 1996;8(Suppl 3):497–500. doi: 10.1017/s1041610297003943. [DOI] [PubMed] [Google Scholar]
- 10.Jeste DV, Finkel SI. Psychosis and Alzheimer’s disease. Am J Geriatr Psychiatry. 2000 Winter;8(1):29–34. doi: 10.1097/00019442-200002000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Pollero A, Allegri RF, Taragano FE. Neuropsychiatric symptoms in patients with Alzheimer disease. Vertex. 2004 Mar-May;15(55):5–9. [PubMed] [Google Scholar]
- 12.Lyketsos CG, Rabins PV, Breitner JCS. An evidence-based proposal for the classification of neuropsychiatric disturbance in Alzheimer’s disease. Int J Geriatr Psychiatry. 2001;16(11):1037–1042. doi: 10.1002/gps.440. [DOI] [PubMed] [Google Scholar]
- 13.Lyketsos CG, Lopez O, Jones B, et al. Prevalence of Neuropsychiatric Symptoms in Dementia and Mild Cognitive Impairment. JAMA. 2002 Sep 25;288(12):1475–1483. doi: 10.1001/jama.288.12.1475. [DOI] [PubMed] [Google Scholar]
- 14.Modrego PJ, Ferrández J. Depression in patients with mild cognitive impairment increases the risk of developing dementia of Alzheimer type: a prospective cohort study. Arch Neurol. 2004 Aug;61(8):1290–3. doi: 10.1001/archneur.61.8.1290. [DOI] [PubMed] [Google Scholar]
- 15.Geda YE, Knopman DS, Mrazek DA, Jicha GA, Smith GE, Negash S, Boeve BF, Ivnik RJ, Petersen RC, Pankratz VS, Rocca WA. Depression, apolipoprotein E genotype, and the incidence of mild cognitive impairment: a prospective cohort study. Arch Neurol. 2006 Mar;63(3):435–40. doi: 10.1001/archneur.63.3.435. [DOI] [PubMed] [Google Scholar]
- 16.Taragano F, Allegri RF. Mild Behavioral Impairment: The Early Diagnosis Int Psychogeriatr. 2003;Vol. 15(Supplement 2):386. http://www.ipaonline.net/pdfs/29766_IPA_7x10.pdf (page 12)
- 17.Lyketsos CG. Neuropsychiatric symptoms of dementia: Nature and treatment. Plenary Lecture, 9th International Conference on Alzheimer’s Disease and Related Disorders; Philadelphia Convention Center, Philadelphia, Pennsylvania. July 20th, 2004. [Google Scholar]
- 18.Scholzel-Dorenbos CJ. Mild Behavioral Impairment: a prodromal stage of frontotemporal lobar degeneration. J Am Geriatr Soc. 2006 Jan;54(1):180–1. doi: 10.1111/j.1532-5415.2005.00575_11.x. [DOI] [PubMed] [Google Scholar]
- 19.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 20.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999 Mar;56(3):303–8. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 21.Signoret JL, Whiteley A. Memory battery scale. Intern. Neuropsych Soc Bull. 1979:2–26. [Google Scholar]
- 22.Wechsler Adult Scale — reduced. WASI The Psychological Corporation; USA: 1999. [Google Scholar]
- 23.Allegri RF, Mangone CA, Rymberg S, Fernandez A, Taragano FE. Spanish version of the Boston naming Test in Buenos Aires. The Clinical Neuropsychologist. 1997;11(4):416–420. [Google Scholar]
- 24.Folstein MF, Folstein SE, McHugh PR. “Mini mental state” a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 25.Reitan RM. Validity of the Trail Making Test as an indication of organic brain damage. Percept Mot Skills. 1958;8:271. [Google Scholar]
- 26.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994 Dec;44(12):2308–14. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 27.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961 Jun;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 28.Mc Khann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 29.Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546–54. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 30.McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with lewy bodies: report of the consorttium on DLB international workshop. Neurolgy. 1996;47:1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 31.Skoog I. Vascular aspect in Alzheimer disease. J Neural Transm Suppl. 2000;59:37–43. doi: 10.1007/978-3-7091-6781-6_6. [DOI] [PubMed] [Google Scholar]
- 32.Kivipelto M, Helkala EL, Hanninen T, Laakso MP, Hallikainen M, Alhainen K, Soininen H, Tuomilehto J, Nissinen A. Midlife vascular risk factors and late-life mild cognitive impairment: A population-based study. Neurology. 2001 Jun 26;56(12):1683–9. doi: 10.1212/wnl.56.12.1683. [DOI] [PubMed] [Google Scholar]
- 33.Luboshitzky R, Oberman AS, Kaufman N, Reichman N, Flatau E. Prevalence of cognitive dysfunction and hypothyroidism in an elderly community population. Isr J Med Sci. 1996 Jan;32(1):60–5. [PubMed] [Google Scholar]
- 34.Van Boxtel MP, Bosma H, Jolles J, Vreeling FW. Prevalence of primitive reflexes and the relationship with cognitive change in healthy adults: a report from the Maastricht Aging Study. J Neurol. 2006 Jul;253(7):935–41. doi: 10.1007/s00415-006-0138-7. Epub 2006 Mar 6. [DOI] [PubMed] [Google Scholar]
- 35.Borroni B, Broli M, Costanzi C, Gipponi S, Gilberti N, Agosti C, Padovani A. Primitive reflex evaluation in the clinical assessment of extrapyramidal syndromes. Eur J Neurol. 2006 Sep;13(9):1026–8. doi: 10.1111/j.1468-1331.2006.01404.x. [DOI] [PubMed] [Google Scholar]
- 36.Forsell Y, Palmer K, Fratiglioni L. Psychiatric symptoms/syndromes in elderly persons with mild cognitive impairment. Data from a cross-sectional study. Acta Neurol Scand. 2003;179:25–8. doi: 10.1034/j.1600-0404.107.s179.4.x. [DOI] [PubMed] [Google Scholar]
- 37.Baquero M, Blasco R, Campos-Garcia A, Garces M, Fages EM, Andreu-Catala M. Descriptive study of behavioural disorders in mild cognitive impairment. Rev Neurol. 2004;38:323–6. [PubMed] [Google Scholar]
- 38.Hwang TJ, Masterman DL, Ortiz F, Fairbanks LA, Cummings JL. Mild cognitive impairment is associated with characteristic neuropsychiatric symptoms. Alzheimer Dis Assoc Disord. 2004;18:17–21. doi: 10.1097/00002093-200401000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Feldman H, Scheltens P, Scarpini E, Hermann N, Mesenbrink P, Mancione L, et al. Behavioral symptoms in mild cognitive impairment. Neurolog. 2004;62:1199–1201. doi: 10.1212/01.wnl.0000118301.92105.ee. [DOI] [PubMed] [Google Scholar]