α-Hydroxy carbonyl compounds represent a structural type of both synthetic and biological importance. We previously noted that the enol allyl carbonates of α-siloxycarbonyl compounds underwent smooth palladium catalyzed decarboxylative asymmetric allylic alkylation (AAA)1 to allylated α-siloxyaldehydes using a Pd complex bearing the Lanth ligand regardless of the regioisomeric nature of the starting material (ie. I or II in eq. 1).2 The regioselectivity may be interpreted as a faster equilibration between the Pd enolate A and B compared to the rate of alkylation which occurs faster via B (ie. k > k2 > k1). However, if A and B exist as either tight ion-pairs or covalently bonded enolates as we proposed before,1c the Pd catalyst should be involved in both R migration and enolate alkylation steps. Thus, by tuning the ligand and the potential migrating group, we envisioned that we could change the reaction pattern in favor of the formation of α-hydroxyketones III, which have attracted much attention due to their versatile roles in organic synthesis.1k, 3 Herein, we report our success in the highly regio- and enantio-selective synthesis of α-acyloxyketones by such an approach.
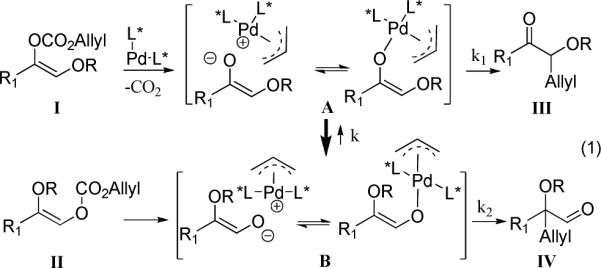
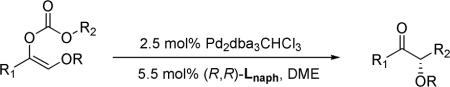
Initially we investigated the role of ligands by using carbonate 1a (R = tert-butyldimethylsilyl, TBS) as the substrate, which as we reported previously, in the presence of Lanth decarboxylatively alkylated to the corresponding siloxyaldehyde 4a with high regioselectivity (Table 1, entry 1). PHOX ligands, which, similar to Lanth, have also been successfully used to catalyze the decarboxylative AAA of enol allyl carbonates, favored the formation of the aldehyde product in a ratio of 4.2 to1; however, the ee of 3a was much lower (17%, entry 4). In contrast to these results, varying our ligands to Lstd and Lstlb, slightly favored the formation of the ketone product (entry 2 and 3). The best selectivity (3a/4a = 17/1) was achieved by using Lnaph (entry 5). Replacement of OTBS with OAc almost completely suppressed the formation of the aldehyde product (entry 6). Changing solvent from dioxane to 1,2-dimethoxyethane (DME), kept the excellent regioselectivity but also improved the ee of 3b to 90% (entry 7).4 The ligand-dependence of the product distribution of 1b was similar to that of 1a, although in all cases the ketone product was the major one (entry 8−11). Besides acetoxy other ester groups were also investigated and 3d with R = pivaloyl (Piv) had the highest ee value (94%, entry 13). Starting from 2 (R = TBS or Piv), which, after decarboxylation initially generated the more stable Pd enolate B, only the aldehyde product was exclusively generated in the presence of Lnaph (entry 14 and 15).This suggests that the equilibrium between A and B is slower than the alkylation steps (k < k1, k2), in stark contrast to the reaction catalyzed by Lanth. The same reaction catalyzed by PHOX ligand (entry 16), however, gave a similar amount of aldehyde 4a (3a/4a = 1/7.7) as in the reaction of entry 4 (3a/4a = 1/4.2), implying a faster equilibrium and comparably slower alkylation (k > k1 ≈ k2).
Table 1.
Selected Optimization Studiesa
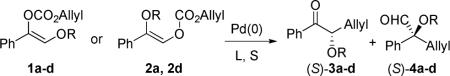 | ||||||
|---|---|---|---|---|---|---|
| entry | substrate (R) | ligand | solvent | yieldb | 3/4c | ee of 3d |
| 1 | 1a (TBS) | Lanth | dioxane | 95% | 1/33 | - |
| 2 | 1a | Lstd | dioxane | 93% | 2.7/1 | 77% |
| 3 | 1a | Lstlb | dioxane | 93% | 2.8/1 | 91% |
| 4 | 1a | PHOX | dioxane | 77% | 1/4.2 | −17% |
| 5 | 1a | Lnaph | dioxane | 91% | 17/1 | 85% |
| 6 | 1b (Ac) | Lnaph | dioxane | 99% | 98/2 | 82% |
| 7 | 1b | Lnaph | DME | 99% | 98/2 | 90% |
| 8 | 1b | Lanth | DME | 27% | 3/2 | 25% |
| 9 | 1b | Lstd | DME | 93% | 25/1 | 79% |
| 10 | 1b | Lstlb | DME | 53% | 7/1 | 83% |
| 11 | 1b | PHOX | DME | 46% | 11/1 | −11% |
| 12 | 1c (Bz) | Lnaph | DME | 95% | 98/2 | 74% |
| 13 | 1d (Piv) | Lnaph | DME | 99% | 98/2 | 94% |
| 14 | 2a (TBS) | Lnaph | dioxane | 99% | 2/98 | - |
| 15 | 2d (Piv) | Lnaph | DME | 79% | 2/98 | - |
| 16 | 2a (TBS) | PHOX | dioxane | 88% | 1/7.7 | −18% |
Unless otherwise indicated, all reactions were performed on a 0.2 mmol scale at 0.1 M concentration at 23 °C for 16 h, using 2.5 mol% Pd2(dba)3CHCl3 and 5.5 mol% ligand.
The yields were combined isolated yields of 3 and 4.
The molar ratios of 3 and 4 were determined by 1H-NMR of the crude products.
The ee values were determined by HPLC on a chiral stationary phase.
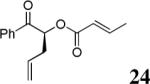
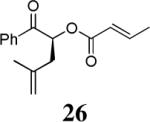
The scope of the reaction has been investigated and the results are summarized in Table 2. Besides the aromatic ketones (entry 1−5), enones such as 12b and 12d (entry 6 and 7), as well as aliphatic ketones such as 14 and 16 (entry 8 and 9) can be obtained in good yields and high ee's. In general, pivolate protected α-hydroxyketones have moderately higher ee's than the corresponding acetate protected ones; however, acetate is easier to be removed without loss of the enantioselectivity of the α-hydroxyketone. Substrates with a substituted allylic moiety also reacted with full conversions, in some cases at slightly warmer temperature (40 °C) (entry 10−12). The dr's of the corresponding products are over 95/5, and the ee values of the major diastereomers are higher than that of 3b. These high dr's are reflected in the excellent ee's of 29 and 30 by the hydrogenations of 20 and 22 respectively (eq. 2). More interestingly, OR can be a functionalized group as in 24, 26, and 28 (entry 13−15). Such functionality can be useful in further structural elaboration as illustrated by the treatment of 24 with Grubbs II catalyst to afford lactone 31 without any erosion of enantioselectivity (eq. 3).
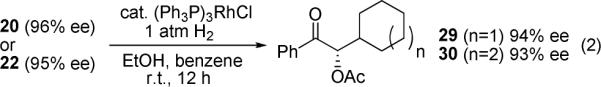
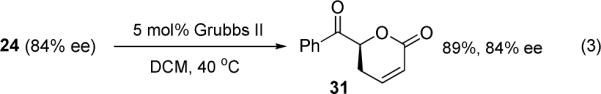
Table 2.
Reaction Scope.a
 | ||||
|---|---|---|---|---|
| entry | substrate | product | yield | ee |
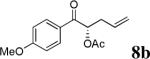
| 1 |  |
 |
98% | 89% |
| 2 | 5d (R3 = Piv) | 6d | 99% | 91% |
| 3b | 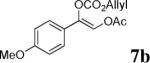 |
 |
84% | 87% |
| 4 | 7d (R3 = Piv) | 8d | 96% | 95% |
| 5 | 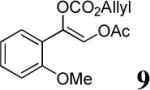 |
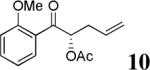 |
83% | 92% |
| 6 | 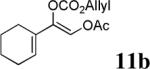 |
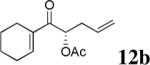 |
96% | 89% |
| 7 | 11d (R3 = Piv) | 12d | 97% | 90% |
| 8 | 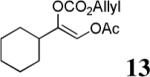 |
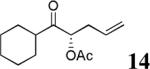 |
61% | 83% |
| 9 | 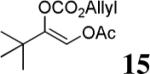 |
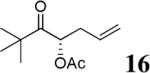 |
65% | 82% |
| 10c | 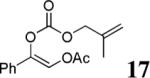 |
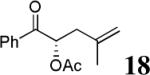 |
96% | 94% |
| 11 | 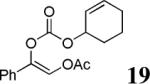 |
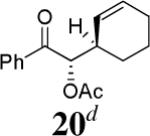 |
97% | 96% |
| 12c | 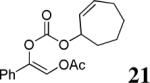 |
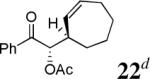 d d
|
99% | 95% |
| 13 | 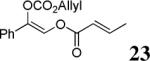 |
 |
92% | 84% |
| 14 | 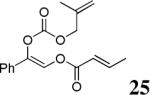 |
 |
93% | 94% |
| 15 | 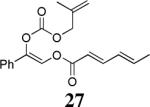 |
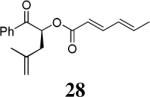 |
88% | 93% |
All reactions were performed on a 0.2 mmol scale at 0.1 M in DME at 23 °C for 16 h, using 2.5 mol% Pd2(dba)3CHCl3 and 5.5 mol% Lnaph; the yields were isolated yields and ee values were determined by chiral HPLC.
Reaction was performed at 4 °C.
Reaction was performed at 40 °C.
Greater than 95/5 dr.
In summary, the palladium catalyzed decarboxylative AAA of 1,2-enediol carbonates can be precisely controlled by the selection of the ligand to generate either regio-isomer. Interestingly, although acyl migration in sodium enolates is fast even at −78 °C,5 such equilibration is much slower with these Pd enolates and shows a ligand dependence. In the case of using Lnaph as ligand it is slower than the alkylation, so that no migration is observed even above room temperature. This supports the concept that the decarboxylative AAA of ketones reacts through a tight ion pair or covalently bonded Pd enolate intermediates.
Supplementary Material
Acknowledgement
We thank the National Science Foundation and the National Institutes of Health, General Medical Sciences Grant GM13598, for their generous support of our programs. J. Xu has been supported by Abbott Laboratories Fellowships. We thank Chirotech (now Dow) for their generous gifts of ligands and Johnson Matthey for gifts of palladium salts.
Footnotes
Supporting Information Available: Experimental procedures and characterization data for all new compounds (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Behenna DC, Stoltz BM. J. Am. Chem. Soc. 2004;126:15044. doi: 10.1021/ja044812x.;Trost BM, Xu J. J. Am. Chem. Soc. 2005;127:2846. doi: 10.1021/ja043472c.;Trost BM, Xu J. J. Am. Chem. Soc. 2005;127:17180. doi: 10.1021/ja055968f.; For reviews on decarboxylative AAA of simple ketones, see: You S-L, Dai L-X. Angew. Chem. Int. Ed. 2006;45:5246. doi: 10.1002/anie.200601889.;Braun M, Meier T. Angew. Chem., Int. Ed. 2006;45:6952. doi: 10.1002/anie.200602169.;Braun M, Meier T. Syn. Lett. 2006;5:661.;Stoltz BM, Mohr JT. Chem. Asian J. 2007;2:1476. doi: 10.1002/asia.200700183.. For more examples in metal catalyzed AAA of simple ketones, see: Trost BM, Schroeder GM. J. Am. Chem. Soc. 1999;121:6759.;Trost BM, Schroeder GM. Chem. Eur. J. 2005;11:174.;Braun M, Laicher F, Meier T. Angew. Chem., Int. Ed. 2000;39:3494. doi: 10.1002/1521-3773(20001002)39:19<3494::aid-anie3494>3.0.co;2-4.;Yan XX, Liang CG, Zhang Y, Hong W, Cao BX, Dai LX, Hou XL. Angew. Chem., Int. Ed. 2005;44:6544. doi: 10.1002/anie.200502020.;Graening T, Hartwig JF. J. Am. Chem. Soc. 2005;127:17192. doi: 10.1021/ja0566275.;Bélanger É, Cantin K, Messe O, Tremblay M, Paquin J-F. J. Am. Chem. Soc. 2007;129:1034. doi: 10.1021/ja067501q.;Zheng W-H, Zheng B-H;, Yan Z, Hou X-L. J. Am. Chem. Soc. 2007;129:7718. doi: 10.1021/ja071098l.;Doyle AG;, Jacobsen EN. Angew. Chem., Int. Ed. 2007;46:3701. doi: 10.1002/anie.200604901.;He H, Zheng X-J, Li Y, Dai L-X, You S-L. Org. Lett. 2007;9:4339. doi: 10.1021/ol7019394.;Braun M, Meier T, Laicher F, Meletis P, Fidan M. Adv. Synth. Catal. 2008;350:303.
- 2.Trost BM, Xu J, Markus R. J. Am. Chem. Soc. 2007;129:282. doi: 10.1021/ja067342a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Protocols for the asymmetric synthesis of α-hydoxyketones include: oxidation of enolates, enol ethers or enol esters: Davis FA, Chen BC. In: Houben-Weyl: Methods of Organic Chemistry. Helmchen G, Hoffmann RW, Mulzer J, Schaumann E, editors. E 21. Georg Thieme Verlag; Stuttgart: 1995. p. 4497.;Davis FA, Chen BC. Chem. Rev. 1992;92:919., and references therein Hashiyama T, Morikawa K, Sharpless KB. J. Org. Chem. 1992;57:5067.;Morikawa K, Park J, Andersson PG, Hashiyama T, Sharpless KB. J. Am. Chem. Soc. 1993;115:8463.;Zhu Y, Tu Y, Yu H, Shi Y. Tetrahedron Lett. 1998;39:7819.; aminoxylation of ketones: Momiyama N, Yamamoto H. J. Am. Chem. Soc. 2003;125:6038. doi: 10.1021/ja0298702.;Bøgevig A, Sundén H, Córdova A. Angew. Chem., Int. Ed. 2004;43:1109. doi: 10.1002/anie.200353018.;Hayashi Y, Yamaguchi J, Sumiya T, Shoji M. Angew. Chem., Int. Ed. 2004;43:1112. doi: 10.1002/anie.200353085.; benzoin-type reactions: Enders D, Kallfass U. Angew. Chem., Int. Ed. 2002;41:1743. doi: 10.1002/1521-3773(20020517)41:10<1743::aid-anie1743>3.0.co;2-q.;Linghu X, Potnick JR, Johnson JS. J. Am. Chem. Soc. 2004;126:3070–3071. doi: 10.1021/ja0496468.; reduction of 1,2-diketones: Koike T, Murata K, Ikariya T. Org. Lett. 2000;2:3833. doi: 10.1021/ol0002572.; oxidation of 1,2-diols: Adam W, Fell RT, Saha-Moller CR, Zhao C-G. Tetrahedron: Asymmetry. 1998;9:397.; the rearrangement of α-tertiary hydroxyaldehydes: Ooi T, Saito A, Maruoka K. J. Am. Chem. Soc. 2003;125:3220. doi: 10.1021/ja0292851.;Ooi T, Ohmatsu K, Maruoka K. J. Am. Chem. Soc. 2007;129:2410. doi: 10.1021/ja063051q.; and alkylation: Andrus MB, Hicken EJ, Stephens JC, Bedke DK. J. Org. Chem. 2005;70:9470. doi: 10.1021/jo051568z.
- 4.The absolute configuration was assigned by the NMR study of the corresponding O-methylmandelate ester. Trost BM, Belletire JL, Godleski S, McDougal PG, Balkovec JM, Baldwin JJ, Christy ME, Ponticello GS, Varga SL, Springer JP. J. Org. Chem. 1986;51:2370.
- 5.Observed in the preparation of substrates. See Supporting Information for details.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


