Abstract
Background and Purpose
This review will focus on the emerging principles of neural repair after stroke, and on the overlap between cellular mechanisms of neural repair in stroke and clinical principles of recovery and rehabilitation.
Summary of Review
Stroke induces axonal sprouting and neurogenesis. Axonal sprouting occurs in tissue adjacent to the stroke and its connected cortical areas, and from sites that are contralateral to the infarct. Neurogenesis produces newly born immature neurons in peri-infarct striatum and cortex. Stimulation of both axonal sprouting and neurogenesis is associated with improved recovery in animal models of stroke. A unique cellular environment in the post-stroke brain supports neural repair: an association of angiogenic and remodeling blood vessels with newly born immature neurons in a neurovasclar niche. Controversies in the field of neural repair after stroke persist, and relate to the locations of axonal sprouting in animal models of stroke and how these correlate to patterns of human remapping and recovery, and to the different models of stroke used in studies of neurogenesis.
Conclusions
On a cellular level, the phenomenology of neural repair after stroke has been defined and unique regenerative environments in the post-stroke brain identified. As the field moves toward specific studies of causal mechanisms in post-stroke repair, it will need to maintain a perspective of the animal models suited to the study of neural repair after stroke as they relate to the patterns of recovery in humans in this disease.
Keywords: axonal sprouting, regeneration, neurogenesis, neurorehabilitation, angiogenesis
Neural Repair: the Target
Stroke is the leading cause of adult disability. An estimated 55% of the total yearly costs of stroke occur in the chronic setting1, which means that the long-term management of disabled stroke patients costs upwards of $34.5 billion per year2. From 1994 to 2004 the death rate from stroke declined 20.4 percent2. However, stroke incidence is expected to increase to an estimated 1.14 million per year in 20253. This translates to a disease with an ever-larger number of disabled survivors. These statistics have led to a research focus on mechanisms of neural repair as they relate to the patterns of human recovery after stroke.
The patterns of recovery in the human brain after stroke have been determined with functional imaging and transcranial magnetic (TMS) and direct current (TDC) stimulation. In the early stages after stroke, brain activation in sensorimotor tasks of the affected limb occurs in a wideranging network of cortex in primary motor, premotor and supplementary motor areas in both hemispheres. In patients that experience a good recover over time, a more focused set of cortical areas are involved in sensorimotor tasks, and these often relate to peri-infarct and connected cortical areas4–8. Patients with poorer recovery often retain a more diffuse, or contralesional, activation of cortical areas to sensorimotor tasks4. Similarly, de-activation with TMS or TDC of peri-lesional areas disrupts recovered functions after stroke9,10. De-activation of contralesional hemispheric areas may also disrupt motor performance in recovered stroke patients11, but these are often patients with larger stroke and/or poorer overall recovery12,13. Areas of cortical remapping in the stroke hemisphere undergo an expansion in cortical thickness14 that is reminiscent of the dendritic sprouting and increase in cortical volume in areas that mediate recovery of function in ischemic lesions in animals15,16. There is variability in this pattern of diffuse to focused, and bilateral to ipsilesional activation with stroke recovery in humans. At least some of this variability relates to lesion location and size, with larger lesions producing functional recruitment of contralesional cortical areas into a recovery network12,13,17,18. This review will focus on one major set of findings in these human studies or recovery after stroke: imaging, stimulation and recent structural studies indicate that a major pattern of successful recovery after stroke in humans establishes a target for the study of cellular and molecular mechanisms of neural repair in the peri-infarct and connected cortical areas ipsilateral to the stroke. Within these regions of peri-infarct and connected cortical areas two main cellular processes of neural repair have been extensively described, post-stroke axonal sprouting and post-stroke neurogenesis. This review will focus on the cellular and molecular events in peri-infarct and connected cortical areas that are associated with neurological recovery after stroke.
Post-Stroke Axonal Sprouting
Axonal sprouting occurs after injury in the peripheral nervous system, and with specific therapies, in the spinal cord and optic nerve19–22. In these sites, sprouting neurons activate specific molecular elements of a growth program to elaborate a growth cone, extend an axon and form new synapses. Nervous system injury also induces glial and meningeal growth-inhibitory proteins that block axonal sprouting23.
The unequivocal demonstration of post-stroke axonal sprouting has required direct axonal quantification. This is because the proteins associated with the growth cone, such as GAP43, which have been traditionally used to “map” sprouting axons are in fact not neuron- or sprouting-specific: GAP43 is found in astrocytes, oligodendrocytes and is induced in neurons with LTP24–28. With this requirement for a direct demonstration of post-stroke axonal sprouting, axonal sprouting after stroke has been shown in peri-infarct cortex, in the cervical spinal cord and brainstem, and between parietal and frontal lobes.
Long distance axonal sprouting after stroke has been shown in the brainstem and cervical spinal cord and in cortico-striatal projections. Axons sprout from the intact projections of the sensorimotor cortex contralateral to the stroke, into the de-afferented regions of cervical sprinal cord and midbrain that previously received a projection from the now infarcted sensorimotor cortex29,29. This sprouting can be unequivocally demonstrated as it develops a novel contralateral projection. A similar process occurs in the motor cortex contralateral to ischemic sensorimotor cortical lesions, where projections from the contralateral cortex sprout into the region of striatum on the other side of the brain that normally received input from the now infarcted sensorimotor cortex30.
In these cases of long-distance axonal sprouting in the rat, the sprouting axons appear to arise from intact, contralateral projections that were not injured by the stroke. It is not clear at present if this sprouting involves in situ axonal branch formation and growth, but it would be unlikely for axonal projections to grow de novo across the long distances from cortex to cervical spinal cord or brainstem after stroke. Axonal branch formation involves specific molecular events and these appear to be at least partially distinct from those that regulate axonal growth cone behavior31. For example, the guidance cues netrin-1 and semaphorin 3a (sema3a) and the growth factor fibroblast growth factor 2 (FGF2) can directly regulate the formation of axon branches independently of an effect on the growth cone32. The molecular control of cytoskeletal dynamics also involves different stathmin family proteins in growth cone vs. axon branching33. Thus, if post-stroke axonal sprouting from cortex contralateral to stroke into brainstem and cervical spinal cord is mediated by axonal branch formation, it may differ molecularly from that seen in axonal sprouting in peri-infarct cortex (see below). In terms of functional assessment, pharmacological stimulation of axonal sprouting from cortex contralateral to stroke into cervical spinal cord and brainstem is correlated with functional recovery28,34, suggesting that this axonal sprouting from intact, contralateral projections may mediate recovery in rats. However, generalizing this process from the rat to the human is problematic (see last section).
Stroke also induces axonal sprouting in the cortex adjacent to or connected with the stroke site. Small strokes in the rodent somatosensory cortex induce axonal sprouting in the cortex within 1–4 millimeters from the infarct. This sprouting causes a topographic re-mapping of the normal somatosensory connections35. In the more complex brain of the New World Monkey, stroke in the motor cortex induces axonal sprouting from premotor cortex in the frontal lobe into the somatosensory cortex of the parietal lobe. This axonal sprouting develops a novel projection from premotor cortex to somatosensory area 1/236. There is precedence for such a long distance axonal sprouting in the primate brain, in that de-afferentation of somatosensory cortex also leads to long-distance axonal sprouting in cortex37. In these examples of axonal sprouting in regions adjacent or connected to stroke, the axonal sprouting occurs in the same areas that are associated with functional recovery in humans. However, there has been no direct demonstration that axonal sprouting in peri-infarct or connected cortical areas promotes functional recovery.
Growth Promotion
Axonal sprouting in stroke, in neural development and in the regenerating peripheral nervous system occurs through the elaboration of a molecular growth program in temporal phases after stroke22. Within days after injury, abnormal patterns of neuronal activity are generated in injured neurons or brain regions that will undergo sprouting30,38 . Within the first three days after ischemic cortical lesions, post-lesion neuronal activity takes the form of rhythmic neuronal discharges that develop in peri-infarct and then contralateral cortex and are tightly linked to axonal sprouting in this model30 . At this early phase after stroke, molecular events associated with axonal sprouting are initiated. This molecular growth program includes molecules that may form a link between membrane signaling events, intracellular signaling cascades, transcriptional activation and cytoskeletal reorganization. Gene expression profiles of peri-infarct cortex within the first day after stroke demonstrate induction of neuronal growth factors (IGF-1) and transcription factors, such as members of the ANIA class and ARC, that are associated with neuronal plasticity39,40 . Within the specific region of post-stroke axonal sprouting in peri-infarct cortex over the one-month period of axonal sprouting, specific patterns of growth-promoting genes are present41 . The AP-1 transcription factor c-jun, the lipid raft growth cone proteins CAP32 and GAP43, and SPRR1 are activated within the first week after stroke, with CA23, GAP43 and c-jun mRNA induction increased for one-month after stroke41 . At later time points, between one and three weeks after stroke, genes involved in axonal outgrowth or cytoskeletal reorganization, such as L1-NCAM, p21/Waf1, MARKS and Tα1 tubulin are induced. At 28 days after stroke in the rat, new patterns of cortical connections can be detected. During this later stage, genes involved in microtubule reorganization are still being activated, such as the stathmin family members, SCLIP and SCG1041.
This pattern of growth-promoting gene activation differs from that seen in peripheral nerve sprouting. Genes associated with cytoskeletal reorganization, including the cytoskeletal reorganizing proteins SCLIP and SCG10 are induced late in peri-infarct cortex after stroke and RB3 and stathmin gene expression decline in the region of axonal sprouting in peri-infarct cortex after stroke41. However, RB3 is upregulated in peripheral nerve regeneration39,40 and SCG10 and SCLIP are induced throughout the sprouting response in peripheral nerve41. Similarly, gene expression for p21 and SPRR1are induced throughout the duration of the peripheral nerve sprouting response43 unlike their transient induction in the region of post-stroke axonal sprouting in peri-infarct cortex41. These differences between PNS and CNS post-injury axonal sprouting may reflect true differences in the molecular response of these two systems, and may account for the limited ability of the CNS to sprout. However, the current gene expression profiling of axonal sprouting after stroke has involved whole tissue analysis—isolation of the region of axonal sprouting and its constituent glia, blood vessels, inflammatory cells, many types of neurons and relatively small number of sprouting neurons. A definitive study of the molecular growth program of the sprouting neurons after stroke will require selective isolation of this cell population.
Growth Inhibition
There are three main classes of axonal growth-inhibitory molecules23,45 Myelin-associated proteins include NogoA, myelin-associated glycoprotein (MAG) and oligodendrocyte myelin associated glycoprotein (OMgp). Extracellular matrix proteins include the chondroitin and heparin sulfate proteoglycans and tenascin. These organize with other extracellular proteins in a complex three dimensional matrix that my inhibit axonal growth, mediate axon fasciculation or even promote growth through the binding of growth factors (such as FGF246). The developmentally associated axonal guidance molecules are loosely grouped in a category based on their initial description in neurodevelopment. These include members of ephrin A and B and EphA/B tyrosine kinase signaling systems and sema 3a/neuropilin 1. Stroke alters the expression of all three classes of growth-inhibitory molecules both locally near the infarct and in distant areas in a temporal and spatial pattern that places several of these molecules in a direct position to block post-stroke axonal sprouting.
Stroke massively induces the expression of chondroitin sulfate proteoglycans and other growth inhibitory molecules in the immediate vicinity of the infarct, within the glial scar. This is a region in which axonal growth promoting proteins are also induced, such as GAP43, CAP23 and SPRR141,47,48. Thus glial scar can be identified as an area of massive simultaneous upregulation of both growth inhibitory and growth promoting genes41. However, this region of glial scar is not the major area of post-stroke axonal sprouting. As demonstrated with tract tracing experiments, this region includes more distant areas of peri-infarct cortex, away from the glial scar32. In this region, only a small subset of growth-inhibitory molecules are induced by stroke during the initial periods of axonal sprouting: neurocan, NG2, EphB1, ephrin A5 and MAG41,49. These molecules are induced directly in regions in which growth cone proteins, such as GAP43, CAP23, MARCKS and SPRR1 are upregulated, and in a pattern of overlap with these molecules in peri-infarct cortex41. EphrinA5 and MAG are of further interest in the biology of post-stroke axonal sprouting because they are not only induced by stroke in the region of axonal sprouting in peri-infarct cortex in young adult animals, but also are induced to even higher levels by stroke in this region in the aged brain50. Because stroke largely occurs in aged individuals, EphrinA5 an MAG may provide targets to manipulate axonal sprouting in a clinically important manner.
Stroke causes a reduction in specific chondroitin sulfate proteoglycans in the region of post-stroke axonal sprouting. The chondroitin sulfate proteoglycans aggrecan, versican and phosphacan are organized as peri-neuronal nets around both inhibitory and pyramidal neurons in cortex51. After stroke, the mRNA for these genes is at steady state until a late induction38, but immunohistochemical staining for these proteins is lost within the first week41,52. This early loss of protein levels suggests that stroke causes enzymatic cleavage of chondroitin sulfate proteoglycans in perineuronal nets in peri-infarct cortex. Peri-neuronal nets modulate cortical plasticity, as they appear in cortex during the closure of the critical period, the time period in the developing brain in which environmental alterations can produce large scale changes in physiology and structure in neurons53. Enzymatic removal of peri-neuronal nets prolongs the critical period, and behavioral maneuvers that prolong the critical period delay deposition of chondroitin sulfate proteoglycans into peri-neuronal nets53. Thus, stroke may promote axonal sprouting in a region of peri-infarct cortex through enzymatic removal of chondroitin sulfate proteoglycans in manner analogous to the initial period of connection formation in the developing cortex.
Getting in the Mood for Growth
Neuronal connections form during development when immature neurons that are in a growth state project through a series of inhibitory and facilitating guidance cues to a target. Axonal sprouting in the developing brain thus has two sides to the process: a growth program that is active in the embryonic/early postnatal sprouting neurons54, and a favorable environment or set of cues through which to grow. As neurons mature this growth state is lost, and in parallel myelin and chondroitin sulfate proteoglycans are deposited and form a more inhibitory growth environment23,45. If the process of initial axonal sprouting in the developing brain is a guide for axonal sprouting during neural regeneration, to stimulate axonal sprouting after stroke or other CNS injuries in the adult will take both blockade of axonal growth inhibitors, and stimulation of neurons into a growth state. The idea that both stimulation of a growth program, and blockade of growth inhibitors, is necessary for CNS regeneration is supported by recent in vivo experiments. MAG or NogoA knockout animals do not show enhanced neuronal sprouting in spinal cord injury55,56. Blockade of either NogoA signaling or of RhoA, a common intracellular effector for MAG, NogoA, chondroitin sulfate proteoglycans and ephrins, alone in optic nerve injury does not promote robust regeneration across the lesion site. However, blockade of NogoA or RhoA signaling and induction of a neuronal growth state do promote substantial axonal sprouting across the lesion site20,21. Similarly, in peripheral nerve sprouting into the spinal cord, degradation of chondroitin sulfate proteoglycans and stimulation of a neuronal growth state are both required for robust regeneration57. Activation of a neuronal growth state after stroke may be achieved with pharmacological treatments, such as inosine or amphetamine28,34. However, it is possible that a neuronal growth state may be activated through a behavioral paradigm, such as a neurorehabilitation protocol. Exposure to an enriched environment or over-use of the affect limb after stroke promotes neuronal sprouting and synaptogenesis in cortex16. Motor learning is associated with cortical re-mapping and increases in synaptic number in corresponding motor cortex56. In humans, overuse of the affected limb after stroke promotes cortical remapping within the peri-infarct and connected cortical areas60,61. Thus, it is possible that neurons in peri-infarct and connected cortical areas after stroke may be activated for neuronal growth—dendritic and/or axonal sprouting—through behavioral measures to overuse the affected region of cortex, and that this can be coupled with blockade of the key growth-inhibitory molecule(s) to promote functionally important axonal sprouting after stroke.
Post-Stroke Neurogenesis
Stroke induces cell proliferation within the subventricular zone, migration of newly born immature neurons into peri-infarct tissues and long-term survival and maturation into a small number of cells with a mature neuronal phenotype and ultrastructural evidence for synapses59–63. Post-stroke neurogenesis appears to divert migratory immature neurons from their normal path in the rostral migratory stream (RMS)66. The phenomenon of post-stroke neurogenesis has been the subject of several excellent reviews67,68. Recent research has begun to describe the cellular environments that may lead to post-stroke neurogenesis and immature neuron migration. In the ischemic striatum, immature neurons, identified through their staining for the microtubule-associated protein doublecortin, are found in association with astrocytes. Activated astrocytes in the ischemic striatum secrete stromal-derived factor-1 (SDF-1) and this induces immature neuron migration into this area69. This cellular relationship for migrating immature neurons in stroke has similarities to the normal process of migration for these cells in the RMS, where they migrate within an astrocyte boundary from the SVZ to the olfactory bulb. Within the migratory path of the RMS, specific metalloproteinases are involved in the astrocytic boundaries of the pathway70. Interestingly, in post-stroke neuroblast migration, the immature neurons themselves elaborate a metalloproteinase that may facilitate movement through the brain parenchyma71.
Post-stroke neurogenesis also occurs in close association with the vasculature. Newly born immature neurons can be found associated with blood vessels after stroke65,66,72. Xenotransplants of stem/progenitor cells also home to the ischemic tissue and associate with blood vessels after stroke73,74. In peri-infarct cortex, newly born neurons migrate into the region near the stroke site and form a tight physical association with blood vessels in the first week after stroke in a neurovascular niche in peri-infarct cortex. This vascular/neuroblast association occurs with blood vessels that are actively remodeling after stroke, and undergoing angiogenesis. Pharmacological blockade of angiogenesis after stroke significantly reduces the number of immature neurons that are present in peri-infarct cortex, by almost 90%66. Thus angiogenesis is causally linked to neurogenesis after stroke. This finding of a neurovascular niche for neurogenesis after stroke is supported by the many growth factors or pharmacological agents that appear capable of inducing both of these processes together, such as VEGF, erythropoietin, FGF2, statins and phosphodieseterase type 5 inhibitors75–78. These data linking angiogenic blood vessels with newly born immature neurons in peri-infarct cortex appear at odds with the reported association of immature neurons and astrocytes in the ischemic striatum69. As reviewed below, the differences in association of newly born immature neurons in stroke predominantly with astrocytes in ischemic striatum and with angiogenic blood vessels in peri-infarct cortex likely relates to important, and often overlooked, differences in the stroke models used in neural repair studies.
Cellular Environments for Neural Repair after Stroke
Angiogenesis, neurogenesis and axonal sprouting occur in common areas of peri-infarct tissue after stroke and may form a unique regenerative triad that supports neural repair in this disease. Studies in stroke have defined specific receptor-ligand signaling systems that link angiogenesis and neurogenesis. As noted above, blocking angiogenesis severely reduces post-stroke neurogenesis. These angiogenic blood vessels in peri-infarct cortex secrete SDF-1 and angiopoietin-1 in the first week after stroke. Administration of SDF-1 or ang-1 stimulates neuroblast migration into peri-infarct cortex, and blockade of their receptors, CXCR4 and Tie2, blocks or disperses the migration of immature neurons after stroke66. This work identifies two intercellular signaling systems that mediate post-stroke neurogenesis within a neurovascular niche in peri-infarct cortex. Erythropoietin (EPO) and VEGF are also in a position to mediate a neurovascular coupling of angiogenic blood vessels and migrating neuroblasts. EPO is induced in blood vessels and astrocytes in peri-infarct tissue after stroke79. This endogenous increase in post-stroke EPO production promotes post-stroke neurogenesis76. Pharmacological doses of EPO also promote angiogenesis and neurogenesis after stroke75. VEGF is induced in peri-infarct tissue after stroke and may be secreted by angiogenic blood vessels. VEGF receptor blockade downregulates post-stroke neurogenesis and exogenous VEGF promotes post-stroke neurogenesis78. VEGF is also produced by neurons and astrocytes in peri-infarct cortex75,76 and is strongly bound to the extracellular matrix, so the exact cellular communication pattern within the VEGF system in stroke remains to be determined.
Angiogenesis, neurogenesis and axonal sprouting are more broadly linked in neurodevelopment. This linkage has been particularly supported for members of the ephrin B/EphB signaling system. Members of the ephrin B tyrosine kinase signaling system serve as important axonal guidance cues in many areas of the nervous system80. Ephrin B molecules also play key roles in vascular sprouting, vasculogenesis and angiogenesis. Ephrin B2 guides vascular sprouting, in parallel to its mechanism for axonal sprouting81–83. Ephrin B2 is upregulated during angiogenesis and guides mural cell recruitment to angiogenic blood vessels84. Ephrin B class members also mediate neuroblast migration from the normal SVZ and control an aspect of neural crest progenitor migration85,86. Thus ephrin B/EphB proteins on immature neurons or neural progenitors and vascular endothelium and are known to mediate sprouting, cell migration and recruitment. EphB receptors are upregulated in peri-infarct cortex in the region of axonal sprouting, angiogenesis and neurogenesis during the time course for these processes41. A similar overlap between angiogenesis, neurogenesis and axonal sprouting, and an induction in expression level peri-infarct cortex in stroke, is present for the ephrin A system41,87,88 and sema 3a/neuropilin 1/VEGF41,83,89. In the neuropilin 1 system, sema 3a or VEGF competitively bind the neuropilin 1 receptor83, to mediate their distinct cellular effects, such as axonal repulsion or neurite outgrowth and neurogenesis. Because astrocytes, meningeal fibroblasts and blood vessels will be sources for sema 3a and/or VEGF, this suggests that neuropilin 1 signaling in the neurovascular niche after stroke will be a dynamic representation of local ligand concentration as the processes of angiogenesis and astrocytosis develop along their different time courses. A common theme within ephrin and semaphorin systems is that these operate within growing axons, blood vessels and immature neurons, are induced within the region of the neurovascular niche in peri-infarct cortex and may provide a molecular network for coordinated regulation of these three processes as the brain repairs after ischemic injury.
Atque inter silvas Academi quaerere verum: And Seek for Truth in the Garden of Academus
With the phenomena of axonal sprouting and neurogenesis now described, the field of neural repair after stroke appears poised to move into a productive series of studies on causal molecular mechanisms. However, at least three lingering problems remain in the studies of neural repair that may impact how these mechanisms are translated into the clinic. First, the exact sites of post-stroke neurogenesis remain controversial. Many investigators have not been able to identify the long-distance migration of immature neurons from the SVZ into peri-infarct cortex. Long distance migration in post-stroke neurogenesis will be a key process if this is to have relevance to the human. Several studies document robust local migration of immature neurons from the SVZ into the immediately adjacent ischemic striatum, but not beyond this site63,64,69,90. Similarly, one study found that astrocytes in the ischemic striatum produce SDF-1 and that this is a tropic signal for migration of immature neurons69, whereas in peri-infarct cortex blood vessels are a source for SDF-191 and for its migratory signal to immature neuroblasts69. Second, many studies have documented axonal sprouting from cortex contralateral to the stroke site in rats, and established that this contralateral sprouting into denervated brainstem and cervical spinal cord mediates a degree of functional recovery28,29,92. However, human studies suggest that cortex contralateral to stroke does not mediate direct control of the affected limb during recovery and that activation of contralateral cortex after stroke negatively correlates with recovery4–7,8,9 or occurs primarily after larger strokes11,12. Third, as evidence mounts for a cellular link between angiogenesis, neurogenesis and axonal sprouting in peri-infarct tissue, stroke studies employ a variety of models with differing amounts of reperfusion and penumbral area, and in some cases utilize animal models in which there are genetic mutations associated with the vasculature that limit angiogenesis and angiogenic growth factors and affect neurogenesis, such as the spontaneously hypertensive rat93–95.
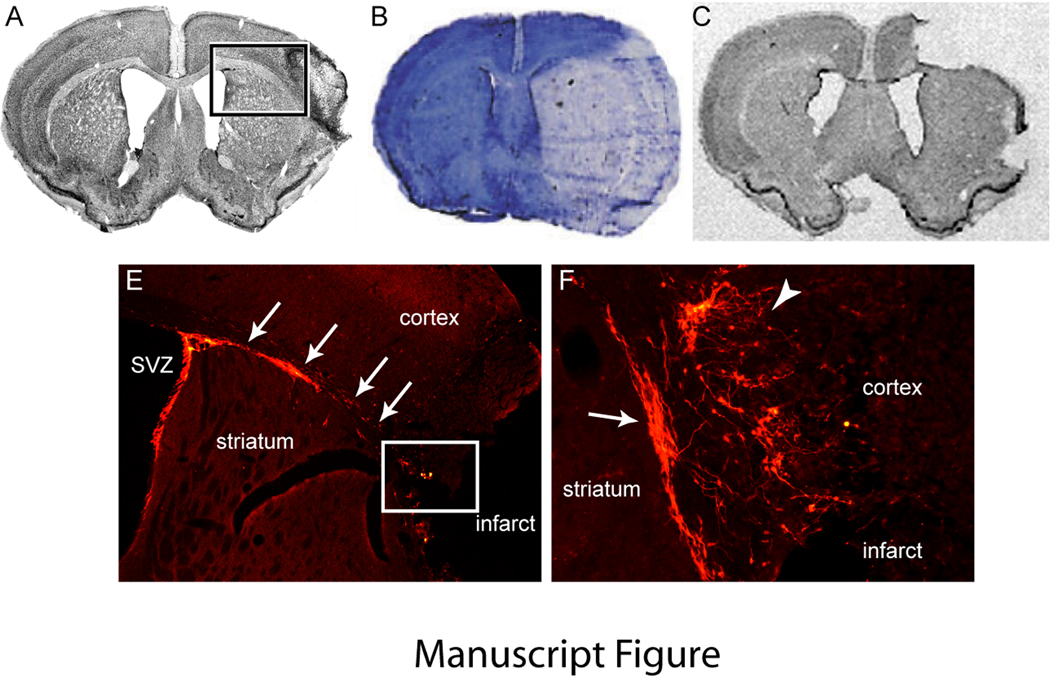
The type of stroke model used in a particular study will dramatically affect the type of neural repair observed in that study. The middle cerebral artery occlusion model with permanent, or one to two hours of occlusion, produces infarcts in a substantial portion of the cerebral hemisphere, from 21–45%, and usually spares a small region of medial striatum adjacent to the SVZ (Figure)96. While rodents survive this type of stroke, this amount of ischemic damage in humans leads to death or a devastated neurological outcome96. Similarly, the three vessel occlusion model with distal middle cerebral artery occlusion, also damages much of the cortex in the affected hemisphere (Figure)96. Because in these two models there is not much peri-infarct and connected cortex remaining after stroke, it is likely that axonal sprouting and neural recovery will need to occur in contralateral cortex. This has been reported in humans, where patients with the largest infarcts maintain contralateral activation of cortex as compared to the transfer of cortical function to peri-infarct and connected areas that occurs in smaller infarcts4. In terms of neurogenesis in the middle cerebral artery occlusion models, it is likely that there is no migration of immature neurons to peri-infarct cortex because this region is either dying or dead (Figure) or that the migratory route is encased in gliotic scar, in which secreted proteins are inhibitory to the migration of immature neurons97. Further, the region of long-term tissue survival and immature neuron migration in middle cerebral artery occlusion models contains a significant astrocytic response that in many cases directly abuts the SVZ98–101. In stroke models with restricted cortical infarcts, there is a large distance between the infarct and the SVZ, with several millimeters of normal white matter and striatum between the two96. Thus immature neurons that migrate out of the SVZ in the middle cerebral artery occlusion models migrate though a very different environment than in stroke models in which the stroke site is well removed from the SVZ, and this may account for the different cell signaling patterns between the two models. The middle cerebral artery and other large stroke models provide consistent measures of cell death for neuroprotection studies. However, this consistency comes in the setting of damage to many of the regions associated with recovery. Thus for neural repair stroke studies, rather than consistency of cell death, there should be a focus on the damage in the regions that survive. To paraphrase Horace, as one seeks for truths in the field of neural repair, it is important to remember the garden in which one is seeking.
Figure. Stroke Model Determines Degree of Surviving Tissue and Pattern of Post-Stroke Neurogenesis.
A. Focal cortical stroke produced by permanent occlusion of a distal branch of the MCA and brief bilateral common carotid occlusion29. Box shows the region seen in panel D. B. Large hemispheric stroke produced by 90 minutes of MCA suture occlusion129. C. Large cortical infarct produced by permanent distal MCA and ipsilateral CCA occlusion and transient contralateral CCA occlusion130. D. Doublecortin positive immature neurons (orange) migrate from the SVZ along the white matter dorsal to the striatum to the peri-infarct cortex (arrows) at 7 days after stroke. Box shows the region that is enlarged in panel E. E. Doublecortin positive cells migrate just ventral to the infarct (arrow) and extend into peri-infarct cortex. Within peri-infarct cortex, immature neurons extend local processes (arrowhead). A comparison of the large infarcts in panels B and D indicate that the region of migration and neurogenesis in peri-infarct cortex would not be present in these stroke models as this tissue is dead. Panel B is reprinted with permission from Brain, Oxford University Press. Panel C is reprinted with permission from Journal of Cerebral Blood Flow & Metabolism, Nature Publishing Group.
Conclusions
Stroke induces not only a region of cell death and scar formation, but regions of neural repair and reorganization. In peri-infarct cortex, this reorganization and repair are seen in the formation of new patterns of connections and in the generation of new neurons. Axonal sprouting and neurogenesis occur in the same cellular environment as angiogenesis after stroke. Angiogenesis, neurogenesis and axonal sprouting share many molecular signaling systems in development, and it appears that stroke induces a microenvironment in per-infarct brain regions in which these three processes share molecular signals during tissue regeneration. Future studies will better define the molecules that control tissue regeneration in peri-infarct cortex and striatum so as to generate pharmacological targets for neural repair after stroke. Current studies have shown an association of axonal sprouting and neurogenesis with neurological recovery. Future studies will also need to determine if these two processes have a causal role in recovery after stroke.
Acknowledgments and Funding
This work is supported by NS053957, NS45729 and the Miriam and Sheldon Adelson Program in Neural Repair and Rehabilitation.
References
- 1.Taylor TN, Davis PH, Torner JC, Holmes J, Meyer JW, Jacobson MF. Lifetime cost of stroke in the United States. Stroke. 1996;27:1459–1466. doi: 10.1161/01.str.27.9.1459. [DOI] [PubMed] [Google Scholar]
- 2.American Heart Association Heart. Disease and Stroke Statistics—2007. Circulation. 2007;115:e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 3.Broderick JP, William M. Feinberg Lecture: stroke therapy in the year 2025: burden, breakthroughs, and barriers to progress. Stroke. 2004;35:205–211. doi: 10.1161/01.STR.0000106160.34316.19. [DOI] [PubMed] [Google Scholar]
- 4.Ward NS, Frackowiak RS. (2006) The functional anatomy of cerebral reorganisation after focal brain injury. J Physiol Paris. 2006;99:425–436. doi: 10.1016/j.jphysparis.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Traversa R, Cicinelli P, Bassi A, et al. Mapping of motor cortical reorganization after stroke. A brain stimulation study with focal magnetic pulses. Stroke. 1997;28:110–117. doi: 10.1161/01.str.28.1.110. [DOI] [PubMed] [Google Scholar]
- 6.Calautti C, Leroy F, Guincestre J, et al. Sequential activation brain mapping after subcortical stroke: changes in hemispheric balance and recovery. Neuroreport. 2001;12:3883–3886. doi: 10.1097/00001756-200112210-00005. [DOI] [PubMed] [Google Scholar]
- 7.Ward NS, Brown MM, Thompson AJ, Frackowiak RS. (2003) Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain. 2003;126:2476–2496. doi: 10.1093/brain/awg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tombari D, Loubinoux I, Pariente J, et al. A longitudinal fMRI study: in recovering and then in clinically stable sub-cortical stroke patients. Neuroimage. 2004;23:827–839. doi: 10.1016/j.neuroimage.2004.07.058. [DOI] [PubMed] [Google Scholar]
- 9.Fridman EA, Hanakawa T, Chung M, et al. Reorganization of the human ipsilesional premotor cortex after stroke. Brain. 2004;127:747–758. doi: 10.1093/brain/awh082. [DOI] [PubMed] [Google Scholar]
- 10.Talelli P, Rothwell J. (2006) Does brain stimulation after stroke have a future? Curr Opin Neurol. 2006;19:543–550. doi: 10.1097/WCO.0b013e32801080d1. [DOI] [PubMed] [Google Scholar]
- 11.Lotze M, Markert J, Sauseng P, Hoppe J, Plewnia C, Gerloff C. The role of multiple contralesional motor areas for complex hand movements after internal capsular lesion. J Neurosci. 2006;26:6096–6102. doi: 10.1523/JNEUROSCI.4564-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johansen-Berg H, Rushworth MF, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci U S A. 2002;99:14518–14523. doi: 10.1073/pnas.222536799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winhuisen L, Thiel A, Schumacher B, Kessler J, Rudolf J, Haupt WF, Heiss WD. Role of the contralateral inferior frontal gyrus in recovery of language function in poststroke aphasia: a combined repetitive transcranial magnetic stimulation and positron emission tomography study. Stroke. 2005;36:1759–1763. doi: 10.1161/01.STR.0000174487.81126.ef. [DOI] [PubMed] [Google Scholar]
- 14.Schaechter JD, Moore CI, Connell BD, Rosen BR, Dijkhuizen RM. (2006) Structural and functional plasticity in the somatosensory cortex of chronic stroke patients. Brain. 2006;129:2722–2733. doi: 10.1093/brain/awl214. [DOI] [PubMed] [Google Scholar]
- 15.Jones TA, Schallert T. Overgrowth and pruning of dendrites in adult rats recovering from neocortical damage. Brain Res. 1992;581:156–160. doi: 10.1016/0006-8993(92)90356-e. [DOI] [PubMed] [Google Scholar]
- 16.Biernaskie J, Corbett D. Enriched rehabilitative training promotes improved forelimb motor function and enhanced dendritic growth after focal ischemic injury. J Neurosci. 2001;21:5272–5280. doi: 10.1523/JNEUROSCI.21-14-05272.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerloff C, Bushara K, Sailer A, Wassermann EM, Chen R, Matsuoka T, Waldvogel D, Wittenberg GF, Ishii K, Cohen LG, Hallett M. Multimodal imaging of brain reorganization in motor areas of the contralesional hemisphere of well recovered patients after capsular stroke. Brain. 2006;129:791–808. doi: 10.1093/brain/awh713. [DOI] [PubMed] [Google Scholar]
- 18.Hamzei F, Liepert J, Dettmers C, Weiller C, Rijntjes M. Two different reorganization patterns after rehabilitative therapy: an exploratory study with fMRI and TMS. Neuroimage. 2006;31:710–720. doi: 10.1016/j.neuroimage.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 19.Ramon-Cueto A, Cordero MI, Santos-Benito FF, Avila J. Functional recovery of paraplegic rats and motor axon regeneration in their spinal cords by olfactory ensheathing glia. Neuron. 2000 Feb;25(2):425–435. doi: 10.1016/s0896-6273(00)80905-8. [DOI] [PubMed] [Google Scholar]
- 20.Fischer D, He Z, Benowitz LI. Counteracting the Nogo receptor enhances optic nerve regeneration if retinal ganglion cells are in an active growth state. J Neurosci. 2004;24:1646–1651. doi: 10.1523/JNEUROSCI.5119-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fischer D, Petkova V, Thanos S, Benowitz LI. Switching mature retinal ganglion cells to a robust growth state in vivo: gene expression and synergy with RhoA inactivation. J Neurosci. 2004;24:8726–8740. doi: 10.1523/JNEUROSCI.2774-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raivich G, Makwana M. The making of successful axonal regeneration: genes, molecules and signal transduction pathways. Brain Res Rev. 2007;53:287–311. doi: 10.1016/j.brainresrev.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Liu BP, Cafferty WB, Budel SO, Strittmatter SM. Extracellular regulators of axonal growth in the adult central nervous system. Philos Trans R Soc Lond B Biol Sci. 2006 Sep 29;361(1473):1593–1610. doi: 10.1098/rstb.2006.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sensenbrenner M, Lucas M, Deloulme JC. Expression of two neuronal markers, growth-associated protein 43 and neuron-specific enolase, in rat glial cells. J Mol Med. 1997;75:653–663. doi: 10.1007/s001090050149. [DOI] [PubMed] [Google Scholar]
- 25.Yamada K, Goto S, Oyama T, Inoue N, Nagahiro S, Ushio Y. In vivo induction of the growth associated protein GAP43/B-50 in rat astrocytes following transient middle cerebral artery occlusion. Acta Neuropathol (Berl) 1994;88:553–557. doi: 10.1007/BF00296492. [DOI] [PubMed] [Google Scholar]
- 26.Namgung U, Matsuyama S, Routtenberg A. Long-term potentiation activates the GAP-43 promoter: selective participation of hippocampal mossy cells. Proc Natl Acad Sci U S A. 1997;94:11675–11680. doi: 10.1073/pnas.94.21.11675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fox MA, Afshari FS, Alexander JK, Colello RJ, Fuss B. Growth conelike sensorimotor structures are characteristic features of postmigratory, premyelinating oligodendrocytes. Glia. 2006;53:563–566. doi: 10.1002/glia.20293. [DOI] [PubMed] [Google Scholar]
- 28.Chen P, Goldberg DE, Kolb B, Lanser M, Benowitz LI. Inosine induces axonal rewiring and improves behavioral outcome after stroke. Proc Natl Acad Sci U S A. 2002;99:9031–9036. doi: 10.1073/pnas.132076299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papadopoulos CM, Tsai SY, Alsbiei T, O'Brien TE, Schwab ME, Kartje GL. Functional recovery and neuroanatomical plasticity following middle cerebral artery occlusion and IN-1 antibody treatment in the adult rat. Ann Neurol. 2002;51:433–441. doi: 10.1002/ana.10144. [DOI] [PubMed] [Google Scholar]
- 30.Carmichael ST, Chesselet M-F. Synchronous neuronal activity is a signal for axonal sprouting after cortical lesions in the adult. J. Neurosci. 2002;22:6062–6070. doi: 10.1523/JNEUROSCI.22-14-06062.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kornack DR, Giger RJ. Probing microtubule +TIPs: regulation of axon branching. Curr Opin Neurobiol. 2005;15:58–566. doi: 10.1016/j.conb.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 32.Dent EW, Barnes AM, Tang F, Kalil K. Netrin-1 and semaphorin 3A promote or inhibit cortical axon branching, respectively, by reorganization of the cytoskeleton. J Neurosci. 2004;24:3002–3012. doi: 10.1523/JNEUROSCI.4963-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poulain FE, Sobel A. The "SCG10-LIke Protein" SCLIP is a novel regulator of axonal branching in hippocampal neurons, unlike SCG10. Mol Cell Neurosci. 2007;34:137–146. doi: 10.1016/j.mcn.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 34.Ramic M, Emerick AJ, Bollnow MR, O'Brien TE, Tsai SY, Kartje GL. Axonal plasticity is associated with motor recovery following amphetamine treatment combined with rehabilitation after brain injury in the adult rat. Brain Res. 2006;1111:176–186. doi: 10.1016/j.brainres.2006.06.063. [DOI] [PubMed] [Google Scholar]
- 35.Carmichael ST, Wei L, Rovainen CM, Woolsey TA. New patterns of intra-cortical connections after focal stroke. Neurobiol Dis. 2001;8:910–922. doi: 10.1006/nbdi.2001.0425. [DOI] [PubMed] [Google Scholar]
- 36.Dancause N, Barbay S, Frost SB, Plautz EJ, Chen D, Zoubina EV, Stowe AM, Nudo RJ. Extensive cortical rewiring after brain injury. J Neurosci. 2005;25:10167–10179. doi: 10.1523/JNEUROSCI.3256-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Florence SL, Taub HB, Kaas JH. Large-scale sprouting of cortical connections after peripheral injury in adult macaque monkeys. Science. 1998;282:1117–1121. doi: 10.1126/science.282.5391.1117. [DOI] [PubMed] [Google Scholar]
- 38.Cummins TR, Waxman SG. (1997) Downregulation of tetrodotoxin-resistant sodium currents and upregulation of a rapidly repriming tetrodotoxin-sensitive sodium current in small spinal sensory neurons after nerve injury. J Neurosci. 1997;17:3503–3514. doi: 10.1523/JNEUROSCI.17-10-03503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raghavendra Rao VL, Bowen KK, Dhodda VK, Song G, Franklin JL, Gavva NR, Dempsey RJ. Gene expression analysis of spontaneously hypertensive rat cerebral cortex following transient focal cerebral ischemia. J Neurochem. 2002;83:1072–1086. doi: 10.1046/j.1471-4159.2002.01208.x. [DOI] [PubMed] [Google Scholar]
- 40.Lu A, Tang Y, Ran R, Clark JF, Aronow BJ, Sharp FR. Genomics of the periinfarction cortex after focal cerebral ischemia. J Cereb Blood Flow Metab. 2003;23:786–810. doi: 10.1097/01.WCB.0000062340.80057.06. [DOI] [PubMed] [Google Scholar]
- 41.Carmichael ST, Archibeque I, Luke L, Nolan T, Momiy J, Li S. Growth-Associated Gene Expression after Stroke: Evidence for a growth-promoting region in peri-infarct cortex. Exptl Neurol. 2005;193:291–311. doi: 10.1016/j.expneurol.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 42.Bonilla IE, Tanabe K, Strittmatter SM. Small proline-rich repeat protein 1A is expressed by axotomized neurons and promotes axonal outgrowth. J Neurosci. 2002;22:1303–1315. doi: 10.1523/JNEUROSCI.22-04-01303.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Costigan M, Befort K, Karchewski L, Griffin RS, D'Urso D, Allchorne A, Sitarski J, Mannion JW, Pratt RE, Woolf CJ. Replicate high-density rat genome oligonucleotide microarrays reveal hundreds of regulated genes in the dorsal root ganglion after peripheral nerve injury. BMC Neurosci. 2002;3:16. doi: 10.1186/1471-2202-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iwata T, Namikawa K, Honma M, Mori N, Yachiku S, Kiyama H. Increased expression of mRNAs for microtubule disassembly molecules during nerve regeneration. Brain Res Mol Brain Res. 2002;102:105–159. doi: 10.1016/s0169-328x(02)00187-0. [DOI] [PubMed] [Google Scholar]
- 45.Sandvig A, Berry M, Barrett LB, Butt A, Logan A. (2004) Myelin-, reactive glia-, and scar-derived CNS axon growth inhibitors: expression, receptor signaling, and correlation with axon regeneration. Glia. 2004;46:225–251. doi: 10.1002/glia.10315. [DOI] [PubMed] [Google Scholar]
- 46.Milev P, Monnerie H, Popp S, Margolis RK, Margolis RU. The core protein of the chondroitin sulfate proteoglycan phosphacan is a high-affinity ligand of fibroblast growth factor-2 and potentiates its mitogenic activity. J Biol Chem. 1998;273 doi: 10.1074/jbc.273.34.21439. 21439-214. [DOI] [PubMed] [Google Scholar]
- 47.Stroemer RP, Kent TA, Hulsebosch CE. Neocortical neural sprouting, synaptogenesis, and behavioral recovery after neocortical infarction in rats. Stroke. 1995;26:2135–2144. doi: 10.1161/01.str.26.11.2135. [DOI] [PubMed] [Google Scholar]
- 48.Li Y, Jiang N, Powers C, Chopp M. Neuronal damage and plasticity identified by microtubule-associated protein 2, growth-associated protein 43, and cyclin D1 immunoreactivity after focal cerebral ischemia in rats. Stroke. 1998;29:1972–1980. doi: 10.1161/01.str.29.9.1972. [DOI] [PubMed] [Google Scholar]
- 49.Deguchi K, Takaishi M, Hayashi T, Oohira A, Nagotani S, Li F, Jin G, Nagano I, Shoji M, Miyazaki M, Abe K, Huh NH. Expression of neurocan after transient middle cerebral artery occlusion in adult rat brain. Brain Res. 2005;1037:194–199. doi: 10.1016/j.brainres.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 50.Li S, Carmichael ST. Growth-associated gene and protein expression in the region of axonal sprouting in the aged brain after stroke. Neurobiol Dis. 2006;23:362–373. doi: 10.1016/j.nbd.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 51.Celio MR, Spreafico R, De Biasi S, Vitellaro-Zuccarello L. Perineuronal nets: past and present. Trends Neurosci. 1998;21:510–515. doi: 10.1016/s0166-2236(98)01298-3. [DOI] [PubMed] [Google Scholar]
- 52.Hobohm C, Gunther A, Grosche J, Rossner S, Schneider D, Bruckner G. Decomposition and long-lasting downregulation of extracellular matrix in perineuronal nets induced by focal cerebral ischemia in rats. J Neurosci Res. 2005;80:539–548. doi: 10.1002/jnr.20459. [DOI] [PubMed] [Google Scholar]
- 53.Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- 54.Cai D, Qiu J, Cao Z, McAtee M, Bregman BS, Filbin MT. Neuronal cyclic AMP controls the developmental loss in ability of axons to regenerate. J Neurosci. 2001;21:4731–4739. doi: 10.1523/JNEUROSCI.21-13-04731.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bartsch U, Bandtlow CE, Schnell L, Bartsch S, Spillmann AA, Rubin BP, Hillenbrand R, Montag D, Schwab ME, Schachner M. Lack of evidence that myelin-associated glycoprotein is a major inhibitor of axonal regeneration in the CNS. Neuron. 1995;15:1375–1381. doi: 10.1016/0896-6273(95)90015-2. [DOI] [PubMed] [Google Scholar]
- 56.Steward O, Zheng B, Banos K, Yee KM, et al. "Axon Regeneration in Young Adult Mice Lacking Nogo-A/B." Neuron 38, 187–199. Neuron. 2007;54:191–195. doi: 10.1016/j.neuron.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 57.Steinmetz MP, Horn KP, Tom VJ, Miller JH, Busch SA, Nair D, Silver DJ, Silver J. Chronic enhancement of the intrinsic growth capacity of sensory neurons combined with the degradation of inhibitory proteoglycans allows functional regeneration of sensory axons through the dorsal root entry zone in the mammalian spinal cord. J Neurosci. 2005;25:8066–8076. doi: 10.1523/JNEUROSCI.2111-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- 59.Kleim JA, Barbay S, Cooper NR, Hogg TM, Reidel CN, Remple MS, Nudo RJ. Motor learning-dependent synaptogenesis is localized to functionally reorganized motor cortex. Neurobiol Learn Mem. 2002;77:63–77. doi: 10.1006/nlme.2000.4004. [DOI] [PubMed] [Google Scholar]
- 60.Wittenberg GF, Chen R, Ishii K, Bushara KO, Eckloff S, Croarkin E, Taub E, Gerber LH, Hallett M, Cohen LG. Constraint-induced therapy in stroke: magnetic-stimulation motor maps and cerebral activation. Neurorehabil Neural Repair. 2003;17:48–57. doi: 10.1177/0888439002250456. [DOI] [PubMed] [Google Scholar]
- 61.Schaechter JD, Kraft E, Hilliard TS, Dijkhuizen RM, Benner T, Finklestein SP, Rosen BR, Cramer SC. Motor recovery and cortical reorganization after constraint-induced movement therapy in stroke patients: a preliminary study. Neurorehabil Neural Repair. 2002;16:326–338. doi: 10.1177/154596830201600403. [DOI] [PubMed] [Google Scholar]
- 62.Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci U S A. 2001;98:4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 64.Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52:802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- 65.Yamashita T, Ninomiya M, Hernandez Acosta P, Garcia-Verdugo JM, Sunabori T, Sakaguchi M, Adachi K, Kojima T, Hirota Y, Kawase T, Araki N, Abe K, Okano H, Sawamoto K. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J Neurosci. 2006;26:6627–6636. doi: 10.1523/JNEUROSCI.0149-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–130016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lichtenwalner RJ, Parent JM. Adult neurogenesis and the ischemic forebrain. J Cereb Blood Flow Metab. 2006;26:1–20. doi: 10.1038/sj.jcbfm.9600170. [DOI] [PubMed] [Google Scholar]
- 68.Zhang RL, Zhang ZG, Chopp M. Neurogenesis in the adult ischemic brain: generation, migration, survival, and restorative therapy. Neuroscientist. 2005;11:408–416. doi: 10.1177/1073858405278865. [DOI] [PubMed] [Google Scholar]
- 69.Thored P, Arvidsson A, Cacci E, Ahlenius H, Kallur T, Darsalia V, Ekdahl CT, Kokaia Z, Lindvall O. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24:739–747. doi: 10.1634/stemcells.2005-0281. [DOI] [PubMed] [Google Scholar]
- 70.Yang P, Baker KA, Hagg T. A disintegrin and metalloprotease 21 (ADAM21) is associated with neurogenesis and axonal growth in developing and adult rodent CNS. J Comp Neurol. 2005;490:163–179. doi: 10.1002/cne.20659. [DOI] [PubMed] [Google Scholar]
- 71.Lee SR, Kim HY, Rogowska J, Zhao BQ, Bhide P, Parent JM, Lo EH. Involvement of matrix metalloproteinase in neuroblast cell migration from the subventricular zone after stroke. J Neurosci. 2006;26:3491–3495. doi: 10.1523/JNEUROSCI.4085-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang L, Zhang ZG, Zhang RL, Gregg SR, Hozeska-Solgot A, LeTourneau Y, Wang Y, Chopp M. Matrix metalloproteinase 2 (MMP2) and MMP9 secreted by erythropoietin-activated endothelial cells promote neural progenitor cell migration. J Neurosci. 2006;26:5996–6003. doi: 10.1523/JNEUROSCI.5380-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, Yoshikawa H, Tsukamoto Y, Iso H, Fujimori Y, Stern DM, Naritomi H, Matsuyama T. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114:330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, Walsh CA, Snyder EY, Khoury SJ. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A. 2004;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004 Jul;35(7):1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- 76.Tsai PT, Ohab JJ, Kertesz N, Groszer M, Matter C, Gao J, Liu X, Wu H, Carmichael ST. A critical role of erythropoietin receptor in neurogenesis and post-stroke recovery. J Neurosci. 2006;26:1269–1274. doi: 10.1523/JNEUROSCI.4480-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li L, Jiang Q, Zhang L, Ding G, Gang Zhang Z, Li Q, Ewing JR, Lu M, Panda S, Ledbetter KA, Whitton PA, Chopp M. Angiogenesis and improved cerebral blood flow in the ischemic boundary area detected by MRI after administration of sildenafil to rats with embolic stroke. Brain Res. 2007;1132:185–192. doi: 10.1016/j.brainres.2006.10.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Greenberg DA, Jin K. Growth factors and stroke. NeuroRx. 2006;3:458–465. doi: 10.1016/j.nurx.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang ZG, Zhang L, Tsang W, Soltanian-Zadeh H, Morris D, Zhang R, Goussev A, Powers C, Yeich T, Chopp M. Correlation of VEGF and angiopoietin expression with disruption of blood-brain barrier and angiogenesis after focal cerebral ischemia. J Cereb Blood Flow Metab. 2002;22:379–392. doi: 10.1097/00004647-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 80.McLaughlin T, O'Leary DD. Molecular gradients and development of retinotopic maps. Annu Rev Neurosci. 2005;28:327–355. doi: 10.1146/annurev.neuro.28.061604.135714. [DOI] [PubMed] [Google Scholar]
- 81.Adams RH, Wilkinson GA, Weiss C, Diella F, Gale NW, Deutsch U, Risau W, Klein R. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 1999;13:295–306. doi: 10.1101/gad.13.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oike Y, Ito Y, Hamada K, Zhang XQ, Miyata K, Arai F, Inada T, Araki K, Nakagata N, Takeya M, Kisanuki YY, Yanagisawa M, Gale NW, Suda T. Regulation of vasculogenesis and angiogenesis by EphB/ephrin-B2 signaling between endothelial cells and surrounding mesenchymal cells. Blood. 2002;100:1326–1333. [PubMed] [Google Scholar]
- 83.Suchting S, Bicknell R, Eichmann A. Neuronal clues to vascular guidance. Exp Cell Res. 2006;312:668–675. doi: 10.1016/j.yexcr.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 84.Foo SS, Turner CJ, Adams S, Compagni A, Aubyn D, Kogata N, Lindblom P, Shani M, Zicha D, Adams RH. Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell. 2006;124:161–173. doi: 10.1016/j.cell.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 85.Klein R. Eph/ephrin signaling in morphogenesis, neural development and plasticity. Curr Opin Cell Biol. 2004;16:580–589. doi: 10.1016/j.ceb.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 86.Conover JC, Doetsch F, Garcia-Verdugo JM, Gale NW, Yancopoulos GD. Alvarez-Buylla A. Disruption of Eph/ephrin signaling affects migration and proliferation in the adult subventricular zone. Nat Neurosci. 2000;3:1091–1097. doi: 10.1038/80606. [DOI] [PubMed] [Google Scholar]
- 87.Bolz J, Uziel D, Muhlfriedel S, Gullmar A, Peuckert C, Zarbalis K, Wurst W, Torii M, Levitt P. Multiple roles of ephrins during the formation of thalamocortical projections: maps and more. J Neurobiol. 2004;59:82–94. doi: 10.1002/neu.10346. [DOI] [PubMed] [Google Scholar]
- 88.Holmberg J, Armulik A, Senti KA, Edoff K, Spalding K, Momma S, Cassidy R, Flanagan JG, Frisen J. Ephrin-A2 reverse signaling negatively regulates neural progenitor proliferation and neurogenesis. Genes Dev. 2005;19:462–471. doi: 10.1101/gad.326905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marin O, Yaron A, Bagri A, Tessier-Lavigne M, Rubenstein JL. Sorting of striatal and cortical interneurons regulated by semaphorin-neuropilin interactions. Science. 2001;293:872–875. doi: 10.1126/science.1061891. [DOI] [PubMed] [Google Scholar]
- 90.Komitova M, Mattsson B, Johansson BB, Eriksson PS. Enriched environment increases neural stem/progenitor cell proliferation and neurogenesis in the subventricular zone of stroke-lesioned adult rats. Stroke. 2005;36:1278–1282. doi: 10.1161/01.STR.0000166197.94147.59. [DOI] [PubMed] [Google Scholar]
- 91.Stumm RK, Rummel J, Junker V, Culmsee C, Pfeiffer M, Krieglstein J, Hollt V. Schulz S A dual role for the SDF-1/CXCR4 chemokine receptor system in adult brain: isoform-selective regulation of SDF-1 expression modulates CXCR4-dependent neuronal plasticity and cerebral leukocyte recruitment after focal ischemia. J Neurosci. 2002;22:5865–5878. doi: 10.1523/JNEUROSCI.22-14-05865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Seymour AB, Andrews EM, Tsai SY, Markus TM, Bollnow MR, Brenneman MM, O'Brien TE, Castro AJ, Schwab ME, Kartje GL. Delayed treatment with monoclonal antibody IN-1 1 week after stroke results in recovery of function and corticorubral plasticity in adult rats. J Cereb Blood Flow Metab. 2005;25:1366–1375. doi: 10.1038/sj.jcbfm.9600134. [DOI] [PubMed] [Google Scholar]
- 93.Jesmin S, Togashi H, Mowa CN, Ueno K, Yamaguchi T, Shibayama A, Miyauchi T, Sakuma I, Yoshioka M. Characterization of regional cerebral blood flow and expression of angiogenic growth factors in the frontal cortex of juvenile male SHRSP and SHR. Brain Res. 2004;1030:172–182. doi: 10.1016/j.brainres.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 94.Wang H, Olszewski B, Rosebury W, Wang D, Robertson A, Keiser JA. Impaired angiogenesis in SHR is associated with decreased KDR and MT1-MMP expression. Biochem Biophys Res Commun. 2004;315:363–368. doi: 10.1016/j.bbrc.2004.01.059. [DOI] [PubMed] [Google Scholar]
- 95.Kronenberg G, Lippoldt A, Kempermann G. Two genetic rat models of arterial hypertension show different mechanisms by which adult hippocampal neurogenesis is increased. Dev Neurosci. 2007;29:124–133. doi: 10.1159/000096217. [DOI] [PubMed] [Google Scholar]
- 96.Carmichael ST. Rodent models of focal stroke: size, mechanism, and purpose. NeuroRx. 2005:396–409. doi: 10.1602/neurorx.2.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kearns SM, Laywell ED, Kukekov VK, Steindler DA. Extracellular matrix effects on neurosphere cell motility. Exp Neurol. 2003;182:240–244. doi: 10.1016/s0014-4886(03)00124-9. [DOI] [PubMed] [Google Scholar]
- 98.Clark RK, Lee EV, Fish CJ, White RF, Price WJ, Jonak ZL, Feuerstein GZ, Barone FC. Development of tissue damage, inflammation and resolution following stroke: an immunohistochemical and quantitative planimetric study. Brain Res Bull. 1993;31:565–572. doi: 10.1016/0361-9230(93)90124-t. [DOI] [PubMed] [Google Scholar]
- 99.Mennel HD, El-Abhar H, Schilling M, Bausch J, Krieglstein J. Morphology of tissue damage caused by permanent occlusion of middle cerebral artery in mice. Exp Toxicol Pathol. 2000;52:395–404. doi: 10.1016/S0940-2993(00)80070-6. [DOI] [PubMed] [Google Scholar]
- 100.Li Y, Chen J, Zhang CL, Wang L, Lu D, Katakowski M, Gao Q, Shen LH, Zhang J, Lu M, Chopp M. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia. 2005;49:407–417. doi: 10.1002/glia.20126. [DOI] [PubMed] [Google Scholar]
- 101.Wegener S, Weber R, Ramos-Cabrer P, Uhlenkueken U, Sprenger C, Wiedermann D, Villringer A, Hoehn M. Temporal profile of T2-weighted MRI distinguishes between pannecrosis and selective neuronal death after transient focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 2006;26:38–47. doi: 10.1038/sj.jcbfm.9600166. [DOI] [PubMed] [Google Scholar]