Abstract
Caspase-8 has a well defined canonical role as an apical protease of the extrinsic apoptosis pathway. Evidence is growing, however, that the protein has numerous other non-apoptotic functions. We have previously demonstrated that caspase-8 is required for efficient adhesion-induced activation of the Erk 1/2 pathway. We now show that caspase-8 is also necessary for the efficient activation of downstream events associated with epidermal growth factor (EGF) signaling. This promotion of EGF-induced Erk 1/2 activation is independent of caspase-8’s proteolytic activity and can be re-capitulated using only the protein’s pro-domains. In addition, we identify specific residues within the caspase-8 “RXDLL motif” that are essential for Erk pathway activation. Furthermore, these residues are also involved in forming a complex with the tyrosine kinase Src. Caspase-8 null cells and cells re-constituted with caspase-8 harboring point mutations of these critical amino acids also show defective EGF-induced migration as compared to cells re-constituted with the wild-type protein. In sum, we provide the first evidence for caspase-8 as an essential component of growth factor signaling and suggest this may be due to its association with Src. As the EGF/Src pathway activity has been demonstrated to promote oncogenic events, our findings that caspase-8 is necessary for these activities may help explain why it is rarely deleted or silenced in tumors.
Keywords: Caspase-8, EGF, Erk, Src, PDGF, neuroblastoma
INTRODUCTION
Caspase-8 is a well characterized protease of the “extrinsic” apoptotic pathway known to be important in death receptor-mediated killing. Recruitment of caspase-8 to activated death receptors results in its dimerization, activation, subsequent auto-processing and initiation of the “effector” caspase activity associated with classical apoptosis (1–4). This death receptor mediated apoptosis has been the focus of various attempts to induce cell death in tumors. Indeed, Tumor necrosis factor- Related Apoptosis- Inducing Ligand (TRAIL) has been shown to induce apoptosis in a variety of tumor, but not normal, cells (5, 6). Whilst resistance to death receptor ligands currently limits their efficacy, deletion or silencing of essential proteins of the cascade, such as caspase-8, occur only extremely infrequently in cancers (7). Caspase-8 has in fact been shown to have increased expression in lung cancers (8) supporting the hypothesis that the protein may be involved in other non-apoptotic but potentially pro-tumorigenic events.
Mounting evidence to support alternative non-apoptotic functions for caspase-8 has emerged in recent years (reviewed in (9–11)). Several reports have shown a role for caspase-8 in hematopoetic cell proliferation and “maturation” (e.g. (12–14)), whilst other laboratories have shown caspase-8 to be essential for activation of NF-kB (15–17). Further data describing a role for caspase-8 in adhesion and cell motility have recently accumulated. Involvement of caspase-8 in cell motility has been described by several independent laboratories (18–21). We recently demonstrated an essential role for caspase-8 in promoting cell adhesion-induced activation of the Erk 1/2 pathway via an association with Src (7). Intriguingly, this activation is independent of the catalytic activity of caspase-8 and can be re-capitulated in caspase-8 null cells using only the N-terminal “Death Effector Domains” (DEDs). Indeed, the DEDs alone are capable of forming a protein complex with Src and act indistinguishably from full-length caspase-8 in these biochemical and physiological analyses.
Here we show that caspase-8 is also critical for EGF-induced activation of the Erk pathway. Again the DEDs alone of caspase-8 are sufficient for this activation and we identify residues within the so called “RXDLL motif” that are essential for the promulgation of EGF signaling. We show that caspase-8 is required for EGF-induced cell migration and that point mutants of the RXDLL motif show impaired motility similar to that of caspase-8 null cells. In sum, we provide the first evidence that caspase-8 is an essential component of growth factor signaling pathways and that its effects in this regard may be due to the DEDs ability to associate with a protein complex containing Src. That caspase-8 is involved not only in adhesion- but also in growth factor-induced signaling may help explain why it is so seldom silenced or deleted in tumors. In addition, these findings suggest that potentially driving caspase-8 from non-apoptotic to more canonical pro-apoptotic signaling may be an important therapeutic intervention point in cancers.
MATERIALS AND METHODS
Reagents
Unless otherwise specified, all reagents were from Sigma-Aldrich, St. Louis, MO. AG1478, PP1 and SU6656 were from Calbiochem, La Jolla, CA. Matrigel is from BD Biosciences, La Jolla, CA. Zeocin and primocin are from InvivoGen (San Diego, CA). EGF was always used at a concentration of 100ng/mL and PDGF was used at 50ng/mL.
Cell culture, DNA transfections and stable cell line generation
293T, and HeLa cells were cultured in standard DME medium supplemented with 10% FBS and penicillin/ streptomycin/ L-Glutamine (Omega Scientific Inc, Tarzana, CA). MDA-MB-231 cells were cultured in F12:DMEM (1:1) with 10% FBS and penicillin/ streptomycin/ L-Glutamine whilst SKOV3 cells were grown in McCoy’s 5A medium with 10% FBS, non-essential amino acids (Hyclone, Logan, UT) and penicillin/ streptomycin/ L-Glutamine. SH-SY5Y cells were cultured in alpha-MEM plus 10% FBS and penicillin/ streptomycin/ L-Glutamine. Caspase-8 deficient NB7 cells were a kind gift from Dr. Jill Lahti (St. Jude Children's Research Hospital, Memphis, TN) and were maintained in RPMI 1640 supplemented with 10% FBS, penicillin/ streptomycin/ L-Glutamine and 100 µg/mL Primocin (InvivoGen, San Diego, CA). Plasmids encoding shRNAmirs were obtained from OpenBiosystems (Huntsville, AL). Caspase-8 cDNA and a c-Src expression plasmid were kind gifts from Drs. Guy Salvesen and Sara Courtneidge, respectively (both at Burnham Institute for Medical Research, La Jolla, CA). Plasmid DNA was transfected using Fugene 6 (Roche Applied Science, Indianapolis, IN) as per the manufacturers’ instructions and point mutants were generated using a QuikChange XL Mutagenesis kit (Stratagene, Cedar Creek, TX). For stable cell line production, the cDNAs of interest were cloned into MSCV-IRES-zeo plasmids as standard. The subsequent DNA integrity was confirmed by sequencing and transfected into PheNX-A packaging cells as above. Viral supernatants were removed (at 48 and 72 h), the debris pelleted by centrifugation and polybrene added to a final concentration of 8 µg/mL before being added to the cells to be infected. Cells were cultured in viral supernatants as such for 48 h before selection with 10 µg/mL zeocin for 14 days. Alternatively, cells transfected with shRNAmir-encoding DNA were selected 48 h later in 1 µg/mL puromycin for approximately 10 days until antibiotic resistance had been established. Cell lysates were then assayed for protein expression by immunoblotting.
Preparation of protein extracts and immunoblot analysis
Cells were treated as described for experimental conditions and protein extracts generated exactly as in Finlay and Vuori (7). Protein extracts (20 µg) were resolved on SDS-polyacrylamide gels and electrophoretically transferred to PVDF membranes (Immobilon-P, Millipore) by standard methodology. Membranes were blocked for 1 h in TBSTw (20 mM Tris-HCl, pH 7.6, 150 mM NaCl, 0.05% Tween-20) containing 5% non-fat dried milk (or 5% BSA for anti-phosphotyrosine studies) and were incubated rocking overnight at 4°C with the appropriate primary antibody: anti-Erk (# 9102, 1:2000), anti-phospho-Erk (# 9101, 1:2000), anti-Akt (# 9272, 1:2000), anti-phospho-Akt-S473 (# 9271, 1:2000), anti-phospho-Src-Y416 (#2101, 1:2000), anti-EGFR (C74B9 #2646, 1:2000) (all from Cell Signaling Technology Inc., Beverly, MA); anti-Src (mAb 327, 1:1000) (from Calbiochem, La Jolla, CA); anti-HA (3F10, 1:5000) (Roche Applied Science, Indianapolis, IN); anti-c-Src (EC10, 1:2000) (Upstate, Charlottesville, VA) or anti-Caspase-8 (C15, 1:500) (kind gift from Dr. Marcus Peter, University of Chicago, Chicago, IL). After incubation for 1 h with anti-rabbit IgG (111-035-003), anti-mouse IgG (115-035-003) or anti-rat IgG (712-035-150) secondary antibodies conjugated to horseradish peroxidase (Jackson ImmunoResearch Laboratories Inc.), bands were visualized using enhanced chemiluminescence (SuperSignal® West Pico Chemiluminescent substrate, #34080, Pierce, Rockford, IL). All analyses were performed at least three times.
Cell migration assays
Cell migration was assayed essentially as described (21). Briefly, wells of a 24-well dish were coated with 10 µg/mL fibronectin at 37°C overnight. Cells of interest were seeded (~200k/well) in the absence of serum and allowed to adhere for 2 h at 37°C in a 5% CO2 humidified incubator. The confluent cell monolayers were then wounded using a conditioned pipette tip as standard and the cells treated with serum-free media supplemented with 100ng/mL EGF in the presence of 1µg/mL aphidicolin. The cells were allowed to migrate and the distances measured at 24 and 48 h. All experiments were carried out in triplicate at least twice.
RESULTS AND DISCUSSION
Caspase-8 is essential for EGF-induced activation of the Erk 1/2 pathway
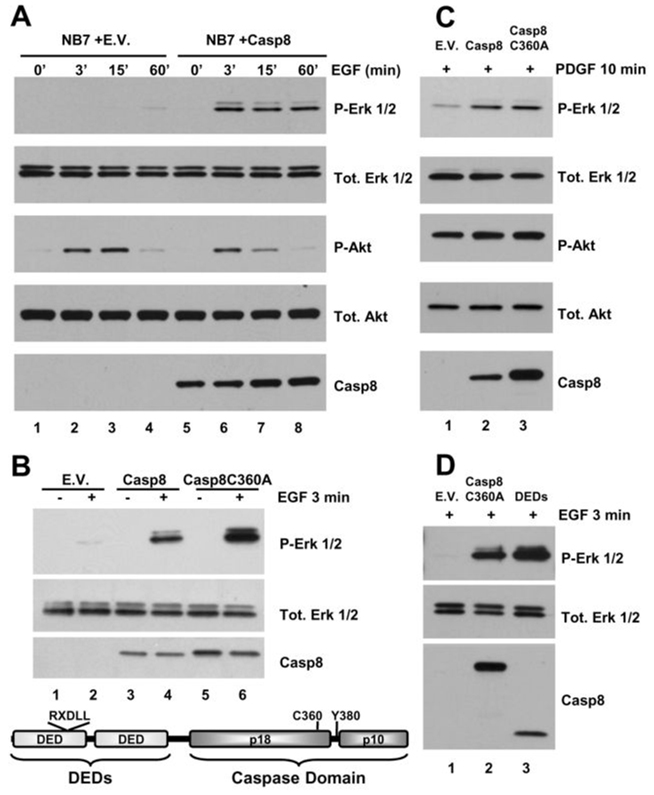
We have previously demonstrated a role for caspase-8 in promoting cell adhesion and downstream activation of the Erk 1/2 pathway (7), and others have shown a role for caspase-8 in adhesion and motility ((19–21) and reviewed in (22)). Surprisingly, whilst investigating EGF-induced motility, we discovered that caspase-8 also plays a critical role in growth factor signaling (Fig. 1). NB7 neuroblastoma cells deficient in caspase-8 ((7, 23), Fig. 1A, lanes 1–4) show defective EGF-induced activation of the Erk 1/2 pathway as compared to cells re-constituted with wild-type caspase-8 (Fig. 1A, lanes 5–8) over the course of one hour at all EGF concentrations tested (not shown). This is not a cell specific effect as SH-SY5Y neuroblastoma cells with no detectable caspase-8 expression also show impaired activation of the Erk pathway in response to EGF as compared to cells re-constituted with the protein (Supp. Fig. S1). We note that EGF also induces phosphorylation of Akt at serine 473 but that this activation is independent of the presence or absence of caspase-8, suggesting that the PI3-kinase pathway is not affected. The activation of the Erk pathway in response to EGF (100 ng/mL, 3 min) is not dependent on the proteolytic activity of caspase-8 as expression of a catalytically inactive point mutant of caspase-8 (C360A, (24)) promotes activity comparable to that of the wild-type protein (Fig. 1B). A schematic diagram of caspase-8 indicating domains and residues of interest is also shown. We note that targeted depletion of caspase-8 (>90%) using shRNAmirs is insufficient to inhibit EGF-induced Erk signaling, implying a signaling role that can be effected by relatively low levels of the protein (Supp Fig. S2). As we have previously shown that adhesion-induced activation of the Erk pathway is also dependent on caspase-8, these findings suggest the protein may have a more generalized, non-apoptotic role as a critical factor in several signaling events. To investigate if other growth factors were dependent on caspase-8 for efficient Erk pathway signaling, caspase-8 deficient NB7 cells (NB7, Fig. 1C, lane 1) or NB7 cells reconstituted with wild-type caspase-8 (NB7+Casp8, lane 2) or a catalytically inactive point mutant of caspase-8 (NB7+Casp8C360A, lane 3) were treated with 50ng/ml PDGF for 10 min and analyzed by immunoblotting. Again, the caspase-8 null NB7 cells showed impaired activation of the Erk- , but not Akt-pathway as compared to cells expressing wild-type or a proteolytically inactive form of caspase-8. Caspase-8 was also found to be essential for TNFα-induced activation of the Erk pathway in these cells (data not shown).
Figure 1. Caspase-8 is essential for EGF-induced activation of the Erk 1/2 pathway.
A) Immunoblot analysis of a time course of EGF (100 ng/mL)-induced Erk and Akt pathway activation in NB7 cells lacking caspase-8 (lanes 1–4) or same re-constituted with wild type protein (lanes 5–8). B) Immunoblot analysis of EGF (100 ng/mL, 3 min)-induced Erk pathway activation in NB7 cells lacking caspase-8 and reconstituted with empty vector (E.V. lanes 1 and 2), or with wild type protein (Casp8, lanes 3 and 4), or an inactivating point mutant of caspase-8 (Casp8C360A, lanes 5 and 6). Schematic depiction of caspase-8 showing domains and residues of interest in this study (below). C) Immunoblot analysis of PDGF (50 ng/mL, 10 min)-induced Erk and Akt pathway activation in NB7 cells lacking caspase-8 re-constituted with empty vector, wild type protein or an inactivating point mutant of caspase-8 (lanes 1–3, respectively). D) Immunoblot analysis of EGF-induced Erk pathway activation in NB7 cells lacking caspase-8 and re-constituted with empty vector, Casp8C360A or with the form of caspase-8 containing DEDs alone (lanes 1–3, respectively).
Our prior studies suggested that caspase-8 effected adhesion-mediated activation of the Erk pathway by facilitating Src family kinase activity via association with its Death Effector Domain (DED) N-terminal pro-domain. In these studies, caspase-8 was further shown to maintain Src in a detergent soluble fraction, presumably to facilitate appropriate cellular localization (7). Here, NB7 cells re-constituted with only the DEDs of caspase-8 demonstrate EGF-induced Erk pathway activation at least comparable to that of cells expressing the Caspase-8C360A protein (Fig. 1D, lanes 3 and 2, respectively). Thus, these findings suggest that the DEDs of caspase-8 play an essential role in facilitating both EGF- (Fig. 1) and adhesion-induced (7) Erk pathway activation.
Caspase-8’s “RXDLL motif” is critical for EGF-induced Erk pathway activation
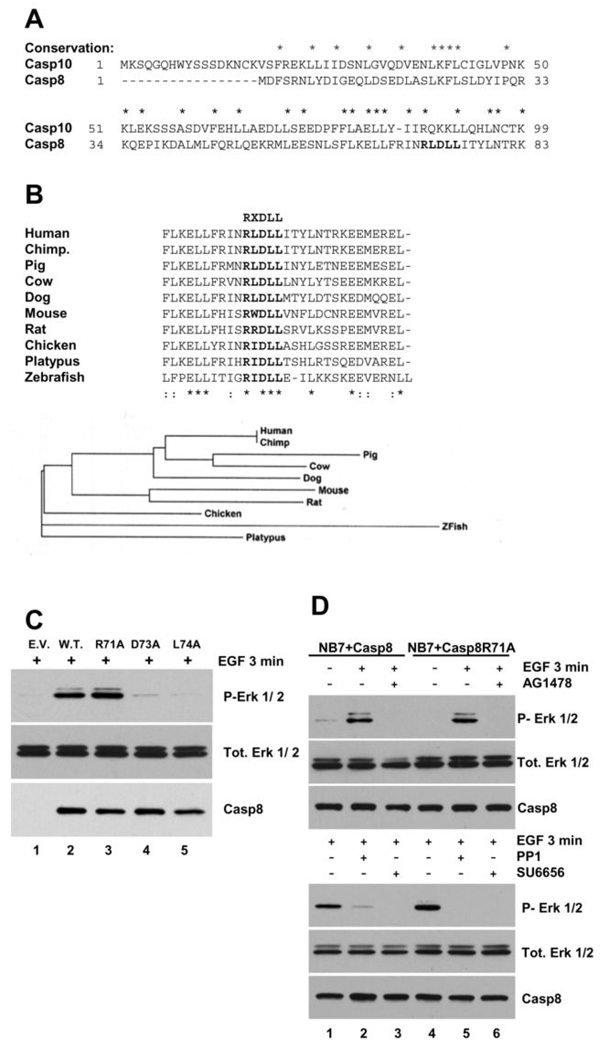
As the DEDs of caspase-8 are sufficient to promote both the adhesion- and EGF-induced activation of the Erk 1/2 pathway, it was of interest to identify specific residues that may be involved. Although NB7 cells do not express caspase-8 they do express its related homologues, FADD, c-FLIP and caspase-10 (data not shown). Presence of caspase-10 in humans appears to result from a recent evolutionary duplication of caspase-8 due to its close homology (Fig 2A) and absence from some other mammalian species such as mouse (25). Whilst caspase-10 has been shown to be interchangeable with caspase-8 in some apoptotic death receptor events (26), the impaired EGF signaling in caspase-8 null cells occurs in the presence of caspase-10 suggesting a divergence of function between the two proteins. Further to these findings Muppidi and colleagues identify specific residues within the adaptor protein FADD that are conserved in many DED containing proteins and are essential for its self association (27). Interestingly, this “conserved RXDLL motif” is not conserved in caspase-10 (Fig. 2A).
Figure 2. Caspase-8’s “RXDLL motif” is critical for EGF-induced Erk pathway activation.
A) Alignment of the N-terminal protein sequences of caspase-8 and caspase-10. B) Multi-species alignment of caspase-8 protein sequences of a 30 aa region containing the “RXDLL motif” (top). Cladogram of the alignment presented above (bottom). C) Immunoblot analysis of EGF-induced Erk pathway activation in NB7 cells lacking caspase-8 re-constituted with empty vector (E.V.), caspase-8 (W.T.) or with R71A, D73A or L74A point mutants of caspase-8 (lanes 1–5, respectively). D) Immunoblot analysis of EGF-induced Erk pathway activation in NB7 cells re-constituted with wild-type Caspase-8 (Casp8; lanes 1–3) or with R71A point mutant of caspase-8 (Casp8R71A; lanes 4–6) in the presence and absence of AG1478 (10 µM, top panels) or PP1 (1 µM) or SU6656 (10 µM) (bottom panels).
We noticed that the RXDLL motif of caspase-8 is widely conserved across many divergent species from human to platypus or zebrafish (Fig. 2B), suggesting an important role. To investigate the role of the RXDLL motif we generated NB7 cells re-constituted with caspase-8 wild-type or point mutants of this region and tested their respective ability to transduce EGF signaling. Whilst the mutation of Arginine 71 to Alanine (R71A) showed Erk pathway activation comparable to wild-type protein, the D73A and L74A mutants showed extremely impaired signaling similar to the caspase-8 null cells (Fig. 2C). We used further pharmacological analysis of the responsive cell lines (NB7+Casp8 and NB7+Casp8R71A) to investigate canonical signaling. EGF-induced activation of the Erk pathway was blocked by the EGF receptor inhibitor AG1478 in both cell lines (Fig. 2D, upper panels). In addition, EGF activation of the Erk pathway was also impaired by Src kinase inhibitors PP1 or SU6656 (Fig. 2D, lower panels).
The caspase-8 “RXDLL motif” is essential both for association with Src and for EGF-induced cell motility
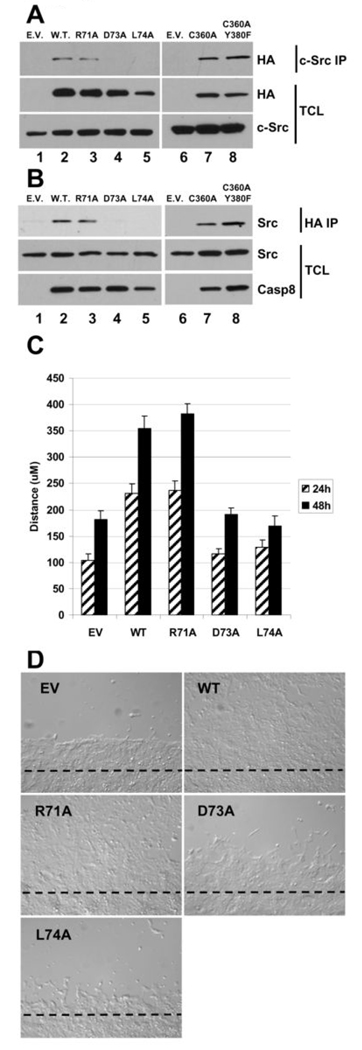
The observation that Src family members may be involved in caspase-8-mediated signaling events (Fig. 2D) is consistent with our previous findings that caspase-8 can associate with a protein complex containing Src (7). We previously showed that endogenous caspase-8 and Src are associated in U87 glioblastoma cells and that the DEDs alone of caspase-8 were sufficient yet critical for this protein complex formation. Furthermore, others have demonstrated that Src can phosphorylate caspase-8 (on Y380), suggesting that at least a temporal association indeed takes place (28). Using transient expression studies we show that c-Src co-immunoprecipitates with wild-type and the R71A mutant of caspase-8. Consistent with the impaired Erk signaling, however, both the D73A and L74A mutants fail to associate with c-Src under the same experimental conditions (Fig. 3A, left panels). We also confirm that the catalytic activity of caspase-8 is not required for this association as the C360A mutant is also co-immunoprecipitated with c-Src. Interestingly, we show that this association is not affected by a dual C360A/Y380F mutation of caspase-8 (Fig. 3A, right panels). These co-immunoprecipitations were also confirmed in the reverse direction. We show that the wild-type caspase-8 or the R71A mutant co-immunoprecipitate with endogenous Src in NB7 cells whilst the D73A and L74A point mutants are defective in this respect (Fig. 3B, left panels). Consistent with our previous finding (Fig. 3A), non-proteolytic (C360A) and the C360A/Y380F dual mutant of caspase-8 also co-precipitate with endogenous Src (Fig. 3B, right panels).
Figure 3. The caspase-8 “RXDLL motif” is essential both for association with Src and for EGF-induced cell motility.
A) Anti-HA immunoblot of anti-c-Src immunoprecipitates from 293T cells transfected with c-Src and either empty vector (E.V. lane 1) or expression vectors for caspase-8-HA (W.T. lane 2), caspase-8R71A-HA (lane 3), caspase-8D73A-HA (lane 4) or caspase-8L74A-HA (lane 5). Anti-HA and anti-c-Src immunoblots of the total cell lysates (TCL) used for immunoprecipitations are shown in the lower panels. B) Anti-endogenous Src immunoblot of anti-HA immunoprecipitates from NB7 cells stably infected with either empty vector (E.V. lane 1) or expression vectors for caspase-8-HA (W.T. lane 2), caspase-8R71A-HA (lane 3), caspase-8D73A-HA (lane 4) or caspase-8L74A-HA (lane 5). Anti-caspase-8 and anti-c-Src immunoblots of the total cell lysates (TCL) used for immunoprecipitations are shown in the lower panels. C) Migration assay of the cells used in Fig. 2C and Fig 3B above was carried out as described. Monolayers were wounded, the cells treated with serum-free media supplemented with 100 ng/mL EGF and allowed to migrate for 24 and 48 h. D) Representative images (48 h) of the cell migration assay described in Fig. 3C.
To investigate if this ability to associate with Src and promote Erk pathway signaling is involved in EGF-induced cell motility we employed migration assays as described by Barbero et al. (21). Caspase-8 null NB7 cells and NB7 cells re-constituted with the wild-type protein or with the R71A, D73A or L74A mutants (Fig. 2) were analyzed for cell motility as described. NB7 cells re-constituted with wild-type or R71A caspase-8 demonstrated enhanced EGF-induced cell motility compared to caspase-8 null NB7 cells or cells expressing the D73A or L74A mutants (Fig. 3C). Representative images of the cell migration in wound healing assays used to generate quantitative data are depicted in Figure 3D. Furthermore, the caspase-8 null NB7 cells seeded in matrigel failed to migrate and/ or invade the matrix remaining instead as individual cells. Cells re-expressing caspase-8 however showed enhanced invasive properties by migrating through the matrigel and demonstrating an elongated phenotype (Supp. Fig. S3).
Caspase-8 appears to modulate EGF signaling via Src
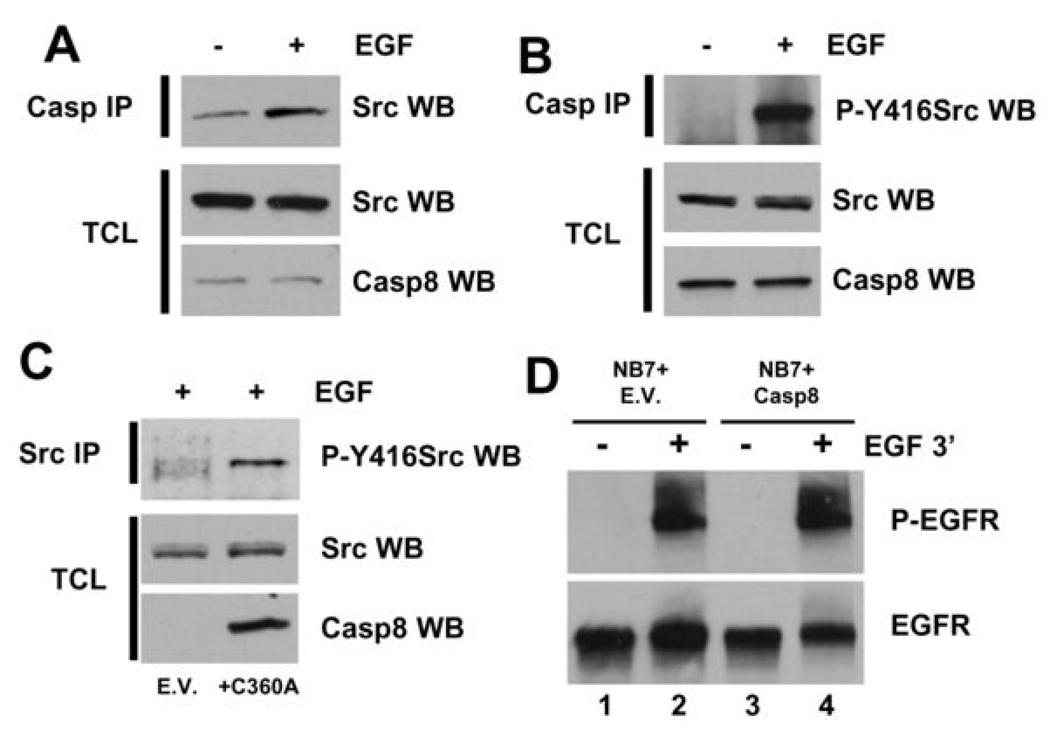
As caspase-8 associates with a protein complex containing Src it was of interest to further elucidate mechanistically if it could modulate Src signaling. We demonstrate that EGF promotes the association of caspase-8 and Src (Fig. 4A) and furthermore that the Src coupled with caspase-8 in response to EGF is catalytically active (Fig. 4B). More striking evidence is provided by our observation that cells lacking caspase-8 show impaired EGF-induced Src activation as compared to cells re-constituted with caspase-8C360A (Fig. 4C). Also we present data showing that the presence or absence of caspase-8 has no effect on the phosphorylation of the EGFR in response to EGF (Fig. 4D). Taken together, these data suggest that caspase-8 modulates the observed effects in growth factor signaling not at the level of the growth factor receptor itself but via Src activation. Presently, it remains to be determined if caspase-8 can modulate EGF signaling in a Src-independent manner, or whether the role of caspase-8 in EGF-induced ERK activation is limited to cells in which EGF-induced ERK activation is Src-dependent.
Figure 4. Caspase-8 appears to modulate EGF signaling via Src.
A) Anti-Src immunoblot of anti-HA immunoprecipitates from NB7+Casp8C360A-HA cells serum-starved for 24 h and then treated with either vehicle or EGF (100 ng/mL) for 3 min. Anti-Src and anti-Caspase-8 immunoblots of the total cell lysates (TCL) used for immunoprecipitations are shown in the lower panels. B) Anti-P-Y416Src immunoblot of anti-HA immunoprecipitates from NB7+Casp8C360A-HA cells serum-starved for 24 h and then treated with either vehicle or EGF (100 ng/mL) for 3 min. Anti-Src and anti-Caspase-8 immunoblots of the total cell lysates (TCL) used for immunoprecipitations are shown in the lower panels. C) Anti-P-Y416Src immunoblot of anti-Src immunoprecipitates from NB7+E.V. or NB7+Casp8C360A-HA cells serum-starved for 24 h and then treated with EGF (100 ng/mL) for 3 min. Anti-Src and anti-Caspase-8 immunoblots of the total cell lysates (TCL) used for immunoprecipitations are shown in the lower panels. D) Immunoblot analysis of vehicle (lanes 1 and 3) or EGF (100 ng/mL, 3 min)-induced EGFR activation in NB7 cells lacking caspase-8 (lanes 1 and 2) or same re-constituted with wild type protein (lanes 3 and 4) is shown.
Caspase-8 tyrosine 380 may be involved in negative regulation of EGF signaling
Recent studies of caspase-8’s role in cell adhesion and motility, and in particular a potential involvement of phosphorylation at Y380, have yielded some seemingly contradictory results. Caspase-8 has been shown to be phosphorylated by Src on this residue in response to EGF or adhesion to fibronectin (28). Whilst we have observed no differences in the capability of the caspase-8C360A/Y380F dual mutant to affect cell adhesion, other groups have demonstrated a role for the Y380-site in cell motility studies that utilized the caspase-8Y380F mutant. To assess the potential involvement of caspase-8 Y380 in EGF-induced signaling we first analyzed Erk and Akt pathway activities in cells lacking caspase-8 and in cells that had been re-constituted with either the N-terminal DEDs alone (this region lacks the Y380 site), or the C-terminal “Caspase Domain” alone (this region contains the Y380 site) (Fig. 5A, lanes 1–3 respectively). As noted earlier, the DEDs alone are sufficient to permit activation of the Erk pathway in response to EGF to a similar extent as the wild-type protein (Fig 1D and Fig. 5A, lane 2). Cells expressing the “Caspase Domain” only-mutant in turn show impaired Erk activation that is comparable to that observed in the caspase-8 null cells (Fig. 5A, lane 3). Thus, these results suggest that the C-terminus of caspase-8 (which contains the Y380 site) does not directly contribute to the Erk pathway activation by EGF, and that the DED domains of caspase-8 are both necessary and sufficient to mediate Erk activation. Of note, we see no impairment of activation of the Akt pathway in the absence or presence of caspase-8 (Fig. 1A) or in cells expressing either the DEDs alone- or “Caspase Domain” alone-forms of caspase-8 (Fig. 5A).
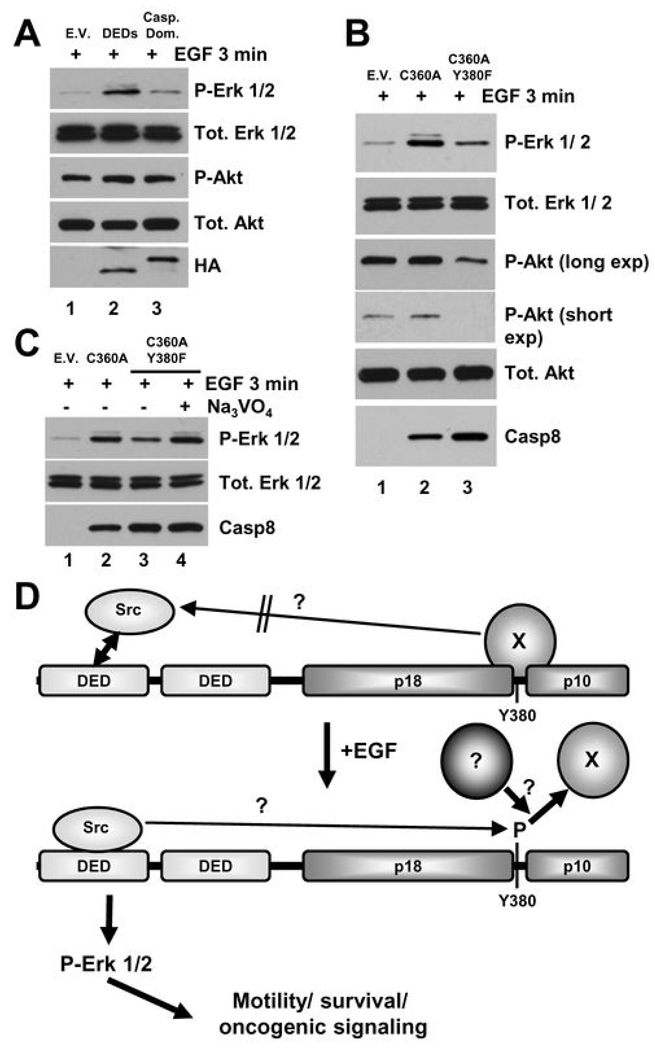
Figure 5. Caspase-8 tyrosine 380 may be involved in negative regulation of EGF signaling.
A) Immunoblot analysis of EGF-induced Erk and Akt pathway activation in NB7 cells lacking caspase-8 re-constituted with empty vector (E.V.), the DEDs alone (DEDs) or the “caspase domain” of caspase-8 (Casp. Dom.) (lanes 1–3, respectively). B) Immunoblot analysis of EGF-induced Erk and Akt pathway activation in NB7 cells lacking caspase-8 re-constituted with empty vector (E.V.), the catalytically inactive point mutant (C360A) or the C360A/Y380F dual mutant of caspase-8 (C360A/Y380F) (lanes 1–3, respectively). C) Immunoblot analysis of EGF-induced Erk pathway activation in NB7 cells lacking caspase-8 re-constituted with empty vector, the catalytically inactive point mutant (C360A) or the C360A/Y380F dual mutant of caspase-8 (as in Fig 5B) with or without sodium orthovanadate (200 µM) as described (lanes 1–4, respectively). D) Simplified schematic depiction of the proposed model of the critical role for caspase-8 in EGF induction of Erk pathway signaling events.
As shown previously, the proteolytically inactive C360A mutant of caspase-8 promotes EGF-induced activation of the Erk pathway to the same extent as the wild-type protein (Fig. 1B and Fig. 5B). Unexpectedly, we found that the caspase-8C360A/Y380F dual mutant fails to allow efficient EGF-induced Erk pathway activation to take place. Taken in isolation, this result could imply a direct role for theY380 residue in caspase-8 dependent Erk signaling. However, this result is quite surprising in light of the fact that the “Caspase Domain” alone-form does not contribute to Erk activation, and that the DEDs alone-form fully restores Erk activation downstream of EGF (Fig. 5A). More intriguingly, we found that although wild-type caspase-8 expression, or expression of the “Caspase Domain” alone-form of caspase-8, has no effect on Akt pathway activation (see above), cells expressing the C360A/Y380F dual mutant show impaired Akt signaling (Fig. 5B, lane 3). In other words, expression of the caspase-8C360A/Y380F mutant appears to have a negative effect on both Erk and Akt signaling. Thus, we postulate that an “inhibitory signaling moiety” may be associated with the C360A/Y380F protein and, as a corollary, with the wild-type caspase-8 protein when not phosphorylated at Y380. Accordingly, we suggest that EGF facilitates phosphorylation of caspase-8 on Y380, resulting in a loss of inhibition of the Erk and Akt signaling pathways by the Y380 site. Upon caspase-8 phosphorylation on Y380, the DED-associated Src complex then additionally potentiates Erk pathway activity (but not of the Akt signaling pathway).
At present time, it remains to be determined how the unphosphorylated Y380-site (or the Y380F mutant site) could exert its negative effects. The phosphorylation status of this site does not appear to affect the association of the DED-domains with the Src protein complex (Fig. 3A and B). As the most attractive candidates for negative regulation of classical kinase cascades are phosphatases, we decided to ascertain if general phosphatase activity was involved in our model of negative signaling by the caspase-8 C360A/Y380F protein. To test this hypothesis we repeated the previous experiment but assayed Erk pathway activity in the cells expressing the C360A/Y380F dual mutant in the presence or absence of the phosphatase inhibitor sodium orthovanadate. We show that the ablation of phosphatase activity restores the Erk signaling of the C360A/Y380F dual mutant to levels comparable to, but not exceeding, those of the C360A mutant (Fig. 5C). We also demonstrate that this is a true caspase-8 response as treatment of NB7 cells lacking caspase-8 with sodium orthovanadate either alone or in combination with EGF has no effect on Erk pathway activation (Supp. Fig. S4). Thus, we provide preliminary evidence not only of an inhibitory moiety potentially associated with caspase-8 Y380 but also suggest that phosphatase activity may be involved. Future proteomic analyses of the components of the caspase-8 signaling complex should allow for identification of the phosphatase potentially involved, thus facilitating more robust confirmation. A very basic diagram displaying the proposed mechanism of our model system as discussed is shown in Fig. 5D. We note however that the system is likely to be more complicated and may vary based on temporal, dynamic and/ or contextual factors. Indeed others have demonstrated that phosphorylation of caspase-8 may allow binding of the p85 subunit of PI3-kinase, potentially displacing an inhibitory molecule or complex (20).
In sum, we provide further evidence to suggest that caspase-8 is involved in non-apoptotic and indeed potentially anti-apoptotic roles. We show that caspase-8 is essential for EGF (and PDGF)-induced-activation of the Erk, but not Akt pathway. We further show that the protein effects these actions via its DED pro-domain and identify residues within the “RXDLL motif” that are critical. We postulate that this is presumably via an ability to associate with a protein complex containing Src as point mutants unable to do so show impaired EGF-induced signaling and cell migration. We further show that cells lacking caspase-8 show impaired activation of Src in response to EGF. We also provide the first preliminary evidence that phosphorylation of caspase-8 at Y380 might relieve inhibitory signaling rather than positively potentiating the EGF response directly per se. As the EGF-Src axis has been strongly implicated in oncogenic progression (e.g. reviewed (29)) further research should confirm this unexpected layer of complexity and potentially identify novel therapeutic intervention points. Indeed EGFR and Src kinase activities have been the focus of the development of novel anti-tumor treatments (e.g. reviewed (30, 31)). Thus these findings and the recent observations from other laboratories (7, 19–21) may explain why caspase-8 is rarely absent in cancers.
Acknowledgments
This work was supported by grants from the National Institutes of Health (to KV).
REFERENCES
- 1.Muzio M, Chinnaiyan AM, Kischkel FC, et al. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death--inducing signaling complex. Cell. 1996;85:817. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 2.Srinivasula SM, Ahmad M, Fernandes-Alnemri T, et al. Molecular ordering of the Fas-apoptotic pathway: the Fas/APO-1 protease Mch5 is a CrmA-inhibitable protease that activates multiple Ced-3/ICE-like cysteine proteases. Proc Natl Acad Sci U S A. 1996;93:14486. doi: 10.1073/pnas.93.25.14486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medema JP, Scaffidi C, Kischkel FC, et al. FLICE is activated by association with the CD95 death-inducing signaling complex (DISC) EMBO J. 1997;16:2794. doi: 10.1093/emboj/16.10.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vincenz C, Dixit VM. Fas-associated death domain protein interleukin-1beta-converting enzyme 2 (FLICE2), an ICE/Ced-3 homologue, is proximally involved in CD95- and p55-mediated death signaling. J Biol Chem. 1997;272:6578. doi: 10.1074/jbc.272.10.6578. [DOI] [PubMed] [Google Scholar]
- 5.Wiley SR, Schooley K, Smolak PJ, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 6.Walczak H, Miller RE, Ariail K, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 7.Finlay D, Vuori K. Novel noncatalytic role for caspase-8 in promoting SRC-mediated adhesion and Erk signaling in neuroblastoma cells. Cancer Res. 2007;67:11704. doi: 10.1158/0008-5472.CAN-07-1906. [DOI] [PubMed] [Google Scholar]
- 8.Shen J, Behrens C, Wistuba II, et al. Identification and validation of differences in protein levels in normal, premalignant, and malignant lung cells and tissues using high-throughput Western Array and immunohistochemistry. Cancer Res. 2006;66:11194. doi: 10.1158/0008-5472.CAN-04-1444. [DOI] [PubMed] [Google Scholar]
- 9.Algeciras-Schimnich A, Barnhart BC, Peter ME. Apoptosis-independent functions of killer caspases. Curr Opin Cell Biol. 2002;14:721. doi: 10.1016/s0955-0674(02)00384-8. [DOI] [PubMed] [Google Scholar]
- 10.Schwerk C, Schulze-Osthoff K. Non-apoptotic functions of caspases in cellular proliferation and differentiation. Biochem Pharmacol. 2003;66:1453. doi: 10.1016/s0006-2952(03)00497-0. [DOI] [PubMed] [Google Scholar]
- 11.Park SM, Schickel R, Peter ME. Nonapoptotic functions of FADD-binding death receptors and their signaling molecules. Curr Opin Cell Biol. 2005;17:610. doi: 10.1016/j.ceb.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Olson NE, Graves JD, Shu GL, et al. Caspase activity is required for stimulated B lymphocytes to enter the cell cycle. J Immunol. 2003;170:6065. doi: 10.4049/jimmunol.170.12.6065. [DOI] [PubMed] [Google Scholar]
- 13.Salmena L, Lemmers B, Hakem A, et al. Essential role for caspase 8 in T-cell homeostasis and T-cell-mediated immunity. Genes Dev. 2003;17:883. doi: 10.1101/gad.1063703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Reilly LA, Divisekera U, Newton K, et al. Modifications and intracellular trafficking of FADD/MORT1 and caspase-8 after stimulation of T lymphocytes. Cell Death Differ. 2004;11:724. doi: 10.1038/sj.cdd.4401408. [DOI] [PubMed] [Google Scholar]
- 15.Chaudhary PM, Eby MT, Jasmin A, et al. Activation of the NF-kappaB pathway by caspase 8 and its homologs. Oncogene. 2000;19:4451. doi: 10.1038/sj.onc.1203812. [DOI] [PubMed] [Google Scholar]
- 16.Hu WH, Johnson H, Shu HB. Activation of NF-kappaB by FADD, Casper, and caspase-8. J Biol Chem. 2000;275:10838. doi: 10.1074/jbc.275.15.10838. [DOI] [PubMed] [Google Scholar]
- 17.Su H, Bidere N, Zheng L, et al. Requirement for caspase-8 in NF-kappaB activation by antigen receptor. Science. 2005;307:1465. doi: 10.1126/science.1104765. [DOI] [PubMed] [Google Scholar]
- 18.Barnhart BC, Legembre P, Pietras E, et al. CD95 ligand induces motility and invasiveness of apoptosis-resistant tumor cells. EMBO J. 2004;23:3175. doi: 10.1038/sj.emboj.7600325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helfer B, Boswell BC, Finlay D, et al. Caspase-8 promotes cell motility and calpain activity under nonapoptotic conditions. Cancer Res. 2006;66:4273. doi: 10.1158/0008-5472.CAN-05-4183. [DOI] [PubMed] [Google Scholar]
- 20.Senft J, Helfer B, Frisch SM. Caspase-8 interacts with the p85 subunit of phosphatidylinositol 3-kinase to regulate cell adhesion and motility. Cancer Res. 2007;67:11505. doi: 10.1158/0008-5472.CAN-07-5755. [DOI] [PubMed] [Google Scholar]
- 21.Barbero S, Barila D, Mielgo A, et al. Identification of a critical tyrosine residue in caspase 8 that promotes cell migration. J Biol Chem. 2008;283:13031. doi: 10.1074/jbc.M800549200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frisch SM. Caspase-8: fly or die. Cancer Res. 2008;68:4491. doi: 10.1158/0008-5472.CAN-08-0952. [DOI] [PubMed] [Google Scholar]
- 23.Teitz T, Wei T, Valentine MB, et al. Caspase 8 is deleted or silenced preferentially in childhood neuroblastomas with amplification of MYCN. Nat Med. 2000;6:529. doi: 10.1038/75007. [DOI] [PubMed] [Google Scholar]
- 24.Boldin MP, Goncharov TM, Goltsev YV, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell. 1996;85:803. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 25.Eckhart L, Ballaun C, Hermann M, et al. Identification of novel mammalian caspases reveals an important role of gene loss in shaping the human caspase repertoire. Mol Biol Evol. 2008;25:831. doi: 10.1093/molbev/msn012. [DOI] [PubMed] [Google Scholar]
- 26.Kischkel FC, Lawrence DA, Tinel A, et al. Death receptor recruitment of endogenous caspase-10 and apoptosis initiation in the absence of caspase-8. J Biol Chem. 2001;276:46639. doi: 10.1074/jbc.M105102200. [DOI] [PubMed] [Google Scholar]
- 27.Muppidi JR, Lobito AA, Ramaswamy M, et al. Homotypic FADD interactions through a conserved RXDLL motif are required for death receptor-induced apoptosis. Cell Death Differ. 2006;13:1641. doi: 10.1038/sj.cdd.4401855. [DOI] [PubMed] [Google Scholar]
- 28.Cursi S, Rufini A, Stagni V, et al. Src kinase phosphorylates Caspase-8 on Tyr380: a novel mechanism of apoptosis suppression. EMBO J. 2006;25:1895. doi: 10.1038/sj.emboj.7601085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishizawar R, Parsons SJ. c-Src and cooperating partners in human cancer. Cancer Cell. 2004;6:209. doi: 10.1016/j.ccr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Wakeling AE. Inhibitors of growth factor signalling. Endocr Relat Cancer. 2005;12 Suppl 1:S183. doi: 10.1677/erc.1.01014. [DOI] [PubMed] [Google Scholar]
- 31.Hatake K, Tokudome N, Ito Y. Next generation molecular targeted agents for breast cancer: focus on EGFR and VEGFR pathways. Breast Cancer. 2007;14:132. doi: 10.2325/jbcs.977. [DOI] [PubMed] [Google Scholar]