Abstract
Objective
To examine abnormal patterns of frontal cortical-subcortical activity in response to emotional stimuli in euthymic individuals with bipolar disorder type I in order to identify trait-like, pathophysiologic mechanisms of the disorder. We examined potential confounding effects of total psychotropic medication load and illness variables upon neural abnormalities.
Method
We analyzed neural activity in 19 euthymic bipolar and 24 healthy individuals to mild and intense happy, fearful and neutral faces.
Results
Relative to healthy individuals, bipolar subjects had significantly increased left striatal activity in response to mild happy faces (p < 0.05, corrected), decreased right dorsolateral prefrontal cortical (DLPFC) activity in response to neutral, mild and intense happy faces, and decreased left DLPFC activity in response to neutral, mild and intense fearful faces (p < 0.05, corrected). Bipolar and healthy individuals did not differ in amygdala activity in response to either emotion. In bipolar individuals, there was no significant association between medication load and abnormal activity in these regions, but a negative relationship between age of illness onset and amygdala activity in response to mild fearful faces (p = 0.007). Relative to those without comorbidities, bipolar individuals with comorbidities showed a trend increase in left striatal activity in response to mild happy faces.
Conclusions
Abnormally increased striatal activity in response to potentially rewarding stimuli and decreased DLPFC activity in response to other emotionally salient stimuli may underlie mood instabilities in euthymic bipolar individuals, and are more apparent in those with comorbid diagnoses. No relationship between medication load and abnormal neural activity in bipolar individuals suggests that our findings may reflect pathophysiologic mechanisms of the illness rather than medication confounds. Future studies should examine whether this pattern of abnormal neural activity could distinguish bipolar from unipolar depression.
Keywords: bipolar disorder, emotion processing, functional MRI
Bipolar disorder is one of the most debilitating illnesses worldwide (1). Bipolar disorder type I, in particular, is characterized by abnormalities in psychosocial and cognitive function as well as emotion and mood regulation that can persist outside of episodes of mania and depression, during remission (2-6), and likely reflect pathophysiologic mechanisms of the illness (7) that are not mood state dependant. The research agenda for DSM-V emphasizes a need to translate basic and clinical neuroscience findings into a new classification system for all psychiatric disorders based upon pathophysiologic and etiological processes (8, 9). Examining neural system abnormalities in euthymic individuals with bipolar disorder type I during paradigms specifically designed to measure emotion processing is therefore a first stage toward identifying biomarkers of bipolar disorder that reflect pathophysiologic neural mechanisms of the disorder (10). These, in turn, can then be included in future diagnostic classification systems for psychiatric disorders.
Most functional neuroimaging studies of neural systems implicated in emotion processing in bipolar disorder have not recruited exclusively euthymic individuals (11-18) and have thus been unable to investigate abnormal activity in neural regions involved in mood regulation that likely reflect persistent pathophysiologic mechanisms of bipolar disorder. Recent studies that have examined euthymic bipolar individuals provided conflicting reports of decreased (19) and increased (20-22) subcortical limbic activity in response to emotional stimuli. Differences in the nature of these emotion-processing paradigms and the stimuli employed in these studies may have contributed to their discrepant findings. Functional neuroimaging studies employing simple, well-validated facial expression paradigms in euthymic bipolar individuals would help clarify the functional abnormalities in sub-cortical limbic and prefrontal cortical regions implicated in emotion processing that likely reflect pathophysiologic processes of the illness.
Facial expressions are well-validated, socially salient emotional stimuli, and have been employed in numerous neuroimaging studies examining emotion processing in healthy and psychiatric populations (23), including studies of individuals with bipolar disorder. Aberrant labeling of facial expressions have, for instance, been reported in bipolar individuals when stable (18), as well as during mania (24, 25). One study (26) specifically demonstrated impaired labeling of facial expressions of disgust in euthymic bipolar individuals. Neuroimaging studies examining neural activity in response to facial expressions in remitted, or euthymic, individuals with bipolar disorder have reported significantly increased activity in subcortical limbic regions, including amygdala, striatum and parahippocampal gyrus, implicated in normal emotion and reward processing (27, 28) in response to happy (14, 16) and fearful faces (16, 18, 21). Additionally, decreased activity in dorsal prefrontal cortical regions implicated in voluntary inhibition of emotional behavior (29) has been shown in remitted bipolar individuals in response to emotional faces (16, 18). Together, these findings support theories proposing abnormally increased activity in subcortical limbic emotion-processing regions (e.g., amygdala and striatum) and decreased activity in dorsal and lateral prefrontal cortical emotion-regulation regions as a neural model representing emotion and mood dysregulation in bipolar disorder (7, 23).
Surprisingly few studies in bipolar disorder have examined associations between psychotropic medication and neural activity. Recent studies acknowledged that medication may have been a potential confound but did not examine this further (19), or they subdivided individuals into those taking versus those not taking different psychotropic medications (14, 16, 22). These latter studies reported either no significant associations between psychotropic medication and abnormal neural activity (22) or significantly decreased amygdala activity in medicated versus unmedicated subgroups in response to emotional stimuli (14). Similarly, studies examining neural activity during non-emotion-processing, cognitive control paradigms reported either no significant associations between psychotropic medication and magnitude of prefrontal cortical activity (30-32) or significantly increased dorsolateral prefrontal cortex (DLPFC) activity in medicated versus unmedicated bipolar subgroups (33). Other studies examined correlations between chlorpromazine dose equivalents and neural activity in bipolar individuals taking antipsychotic medication (18, 34). These studies reported significant negative correlations between chlorpromazine dose equivalent and amygdala activity in response to emotional stimuli in bipolar males (18) and significant positive correlations between antipsychotic dose and dorsal prefrontal cortical activity during selective attention tasks in bipolar adults (34).
Together, these findings indicate that medicated more than unmedicated bipolar individuals show decreased subcortical limbic activity during emotion processing, and increased dorsal prefrontal cortical activity during cognitive control paradigms, i.e., levels of activity in these regions resembling those of healthy individuals. These reports suggest that functional neural abnormalities observed in bipolar individuals likely reflect pathophysiologic processes that may be ameliorated by, rather than abnormalities that are secondary to, psychotropic medication. We have recently proposed the examination of impact of total psychotropic medication load, reflecting both dose and variety of different medications taken, on neural activity in bipolar disorder (35). No studies have yet examined this in bipolar disorder. This, therefore, remains a major limitation of current neuroimaging studies of the disorder.
Additionally, few studies have examined associations between illness duration variables, comorbid psychiatric diagnoses and abnormal neural activity in individuals with bipolar disorder. One study reported a significant negative correlation between age of illness onset and activity within DLPFC during facial expression labeling in remitted individuals (18).
Our first aim was to examine functional abnormalities in subcortical limbic regions and DLPFC implicated in emotional processing and emotion regulation, respectively (23), in euthymic individuals with bipolar type I in response to well-validated, socially salient emotional stimuli: happy and fearful faces, previously employed in studies in bipolar disorder (15-17). In addition to faces displaying intense emotional expressions, we included faces displaying milder-intensity expressions. These are thought to be ecologically more valid (21, 36) and we have previously shown increased subcortical limbic activity in response to these milder emotional stimuli, in particular mild happy facial expressions, in bipolar disorder (16). We therefore hypothesized greater subcortical limbic (including striatum and amygdala), and decreased dorsal prefrontal cortical activity in response to both mild and intense happy and fearful facial expressions in euthymic bipolar relative to healthy individuals. Our second exploratory aim was to examine associations between total psychotropic medication load, illness duration variables and activity in neural regions showing abnormal activity in response to these facial expressions in euthymic bipolar relative to healthy individuals.
Methods
Participants
A total of 22 bipolar individuals who had not participated in any of our previous neuroimaging studies were identified using DSM-IV criteria with the Structured Clinical Interview for DSM-IV (SCID-I) (37). All bipolar individuals were recruited from the Western Psychiatric Institute and Clinic, Mood Disorders Treatment and Research Program, University of Pittsburgh, Pittsburgh, PA, USA (47% female). Three of these were excluded from analyses due to excessive movement (>5 mm) during or inability to complete scanning procedures, allowing data of 19 bipolar individuals to be analyzed. Euthymic status was defined, a priori, as having been in remission for at least two months as assessed by SCID and clinical interview. All but one bipolar individual (who scored 11) scored <7 on the Hamilton Rating Scale for Depression 25-item version (HRSD-25) (38). This individual was included in the analyses because clinical evaluation deemed eligibility for inclusion into the study on grounds other than the rating on the HRSD-25, i.e., SCID interview. All bipolar individuals scored <10 on the Young Mania Rating Scale (39). For means, standard deviations, mean illness duration and age of illness onset, see Table 1. Eleven of these bipolar individuals had other (multiple) comorbid diagnoses, such as eating (binge eating) disorder (n = 3), substance use disorder (n = 5), specific (n = 2) or social (n = 1) phobia, panic disorder (n = 2), generalized anxiety disorder (n = 1), anxiety disorder not otherwise specified (NOS) (n = 1) and obsessive compulsive disorder (n = 1). Three bipolar individuals were symptomatic for comorbidities of specific phobia (n = 1), social phobia (n = 1), and anxiety disorder NOS (n = 1) in the past month prior to their assessment. All but one of the bipolar individuals were taking medication; one was taking mood-stabilizer monotherapy (lithium), five were taking antipsychotic monotherapy, and 12 were taking multiple medications for at least one month prior to the study. For details of medication combinations, see Table 2. Additionally recruited by advertisement were 24 healthy individuals gender ratio matched with bipolar individuals (54% female) without current and lifetime personal (SCID-I criteria) or family history of psychiatric disorder. There were no significant between-group differences in age or verbal IQ as estimated by National Adult Reading Test (NART) (40). For means, standard deviations and range see Table 1.
Table 1.
Demographic information and bipolar individual characteristics
| Bipolar individuals (n = 19) | Healthy individuals (n = 24) | |
|---|---|---|
| Handedness | Right | Right |
| Gender | ||
| Female | 9 | 13 |
| Male | 10 | 11 |
| Age, mean (SD) | 32.47 (8.8) range: 29; 23−52 | 27.78 (8.7) range: 34; 18−52 |
| NART, mean (SD) | 114.9 (6.1) range: 21.68; 100−121 | 117.4 (5.4) range: 24.03; 101−125 |
| Illness duration, mean (SD) | 10.6 (6.61) range: 25; 1−26 | n/a |
| Age of illness onset, mean (SD) | 22.47 (8.01) range: 20; 12−32 | n/a |
| HRSD-25, mean (SD) | 1.94 (2.59) range: 11; 0−11 | n/a |
| YMRS, mean (SD) | 1.37 (2.67) range: 9; 0−9 | n/a |
NART = National Adult Reading Test; HRSD-25 = Hamilton Rating Scale for Depression 25-item version; YMRS = Young Mania Rating Scale.
Table 2.
Medication information for bipolar individuals
| Medication | Mood stabilizers | Antipsychotics | Antidepressants | Anxiolytics |
|---|---|---|---|---|
| aLithium; n = 6 | bRisperidone; n = 3 | cBupropion; n = 1 | dLorazepam; n = 4 | |
| eValproate semisodium; n = 1 | fAripiprazole; n = 7 | gSertraline; n = 2 | ||
| hLamotrigine; n = 1 | iQuetiapine; n = 2 | jMirtazapine; n = 1 | ||
| kCarbamazepine; n = 2 | lOlanzapine; n = 1 | mTrazodone; n = 1 | ||
| nOxycarbamazepine; n = 1 | oClozapine; n = 1 | pVenlafaxine; n = 2 | ||
| qGabapentin; n = 1 | rCitalopram; n = 1 | |||
| sValproic acid; n = 1 | tFluoxetine; n = 1 | |||
| Monotherapy | n = 1 (a) | n = 5 (f, l,o) | n = 0 | n=0 |
| Combination treatments | |
|---|---|
| Mood stabilizers and antipsychotics | n = 2 [(a, h, e) (f, b)] |
| Mood stabilizers, antipsychotics, antidepressant, and lorazepam | n = 1 [(a, q) (f, b) (r) (d)] |
| Mood stabilizers and antidepressants | n = 4 [(a, h, n, s) (p, m, t)] |
| Mood stabilizer, antidepressants, and lorazepam | n = 2 [(k) (c, g) (d)] |
| Antipsychotic and antidepressants | n = 2 [(i) (g, j)] |
| Antipsychotic and lorazepam | n = 1 [(b) (d)] |
Superscripts denote the types of medication taken in either monotherapies or combination treatment.
Exclusion criteria included borderline personality disorder (SCID-II criteria), history of head injury or neurological disease, non-right handedness (Annett criteria) (41) and failure to meet magnetic resonance imaging (MRI) screening criteria (pregnancy, metallic fragments, cardiac pacemaker, or claustrophobia). Additionally, patients reporting drug and alcohol dependence and abuse within the past three months (except episodic abuse related to mood episodes) were excluded. After complete description of the study to participants, written informed consent was obtained. The University of Pittsburgh Institutional Review Board approved this study.
Paradigm
All participants completed two six-minute, event-related neuroimaging paradigms that are reliably associated with subcortical limbic activity in healthy individuals (42), and that have been employed in several previous neuroimaging studies of mood-disordered populations (16, 43, 44). In one experiment, participants viewed 20 neutral, 20 mild and 20 intense happy facial expressions, and in the other experiment, they viewed 20 neutral, 20 mild and 20 intense fearful facial expressions. Both paradigms included neutral faces and a baseline fixation cross; hence, three different types of facial stimuli were included in each of the two experiments (16). All facial stimuli were taken from a standardized series (45). Ten different models posed each neutral and emotional expression, so that in each experiment, each of the 30 individual stimuli was presented twice. Mild emotional facial expressions were included as these are more representative of the non-intense facial expressions observed in everyday life. Furthermore, in a previous study of remitted bipolar individuals, we demonstrated increased amygdala and striatal activity in bipolar relative to healthy individuals that was particularly apparent in response to these mild emotional facial expressions (16). Participants judged the gender of each face, a task of implicit emotion processing reliably associated with activity in subcortical limbic regions in healthy individuals (14, 42, 46). To determine emotion-labeling accuracy, 15 of the bipolar and 21 of the healthy individuals also performed an emotion-labeling task prior to scanning. Here, participants viewed 45 faces depicting sadness, anger, fear, happiness, disgust or neutral expressions (47), and chose corresponding emotion labels from a list of six options. Discrepant numbers of participants taking part in neuroimaging tasks and facial expression labeling tasks outside of the scanner were due to problems in accommodating all scanning and offline procedures in a small number of bipolar and healthy individuals.
Data acquisition
Neuroimaging data were collected using a 3.0 Tesla Siemens Allegra MRI scanner at the University of Pittsburgh/Carnegie Mellon University Brain Imaging Research Center. Structural 3D Sagittal MPRAGE images were acquired [echo time (TE): 2.48 ms, repetition time (TR): 1630 ms, flip angle 8 , field of view (FOV): 200 mm, slice thickness: 1 mm, matrix: 256 × 256, 192 continuous slices]. Mean blood-oxygenation-level-dependent (BOLD) images were acquired with a gradient echo planar imaging (EPI) sequence: 33 axial slices (3 mm thick, 0 mm gap; TR/TE = 2000/25 ms, FOV = 24 cm, matrix = 64 × 64).
Imaging analyses
Data were preprocessed and analysed using statistical parametric mapping software (SPM5; http://www.fil.ion.ucl.ac.uk/spm). Data for each participant were first corrected for differences in acquisition time between slices, realigned using the first slice as a reference, and unwarped to correct for static inhomogeneity of the magnetic field and movement by inhomogeneity interactions. They were then coregistered with the subject's anatomical image, segmented, normalized to standard Montreal Neurological Institute (MNI) template, resampled to 3 × 3 × 3mm3 voxels, and spatially smoothed with a Gaussian kernel of 6 mm full-width at half-maximum (FWHM).
A first-level, fixed-effect model was constructed with three emotion intensities (neutral, mild, intense) in both experiments (fearful, happy) entered as separate conditions in an event-related design with fixation cross as baseline in the design matrix. Movement parameters from the realignment stage were entered as covariates of no interest to control for participant movement. Trials were modeled using the Canonical Haemodynamic Response Function. The three intensities were then entered as separate t-contrasts into second-level analyses.
A second-level, random-effects group analysis was conducted on the t-contrasts generated in the previous single-subject analyses in a 2 (group) by 3 (intensity) repeated-measures ANOVA for each experiment to examine the main effect of group and group-by-intensity interaction. Significant activation clusters were verified using small volume correction. BOLD signal in any a-priori regions (i.e., subcortical limbic regions, including amygdala and striatum, and DLPFC) identified in these whole-brain analyses was extracted using MarsBar 0.40 toolbox (48). If the above a-priori regions were not detected at whole-brain level analyses, these were subsequently defined using the Wake Forest University (WFU) PickAtlas (49).We further examined any findings regarding main effect of group and group-by-intensity interaction in any of the above a-priori neural regions of interest emerging from whole-brain analyses in post hoc independent t-tests, using extracted values of BOLD signal change in these regions and SPSS (SPSS 15.0 for Windows, Rel. 15.0.0. 06 September 2006; Chicago, IL, USA: SPSS, Inc.). We used an adjusted statistical threshold of p = 0.05/(number of comparisons plus number of regions examined) to allow for multiple between-group comparisons in these post hoc analyses.
Examination of gray matter volume in a-priori regions
Potential contributions of gray matter volume in a-priori regions (i.e., subcortical limbic regions, including amygdala and striatum, and DLPFC) were assessed using SPM5. Regions for which no between-group differences of functional magnetic resonance imaging (fMRI) data were reported (bilateral amygdalae) were defined anatomically using the WFU PickAtlas (49). Using SPM5, previously converted files were first segmented into gray and white matter and normalized to the standard international consortium for brain mapping template using a unified model (50). Voxel values were modulated by the Jacobian determinants derived from the spatial normalization: when brain structures showed volume reduction after spatial normalization, the total volume generated took into account regional decreases proportional to the degree of total volume. Voxel resolution after normalization was 2 × 2 × 2 mm. Images were smoothed using a 12 mm Gaussian kernel. In a-priori regions, proportional gray matter volumes were extracted and the mean values of each region exported to SPSS for group comparison.
Examination of potential confounding effects of medication load and illness variables
We have developed a strategy for measuring total medication load in bipolar individuals by coding the dose of each antidepressant, mood stabilizer, antipsychotic and anxiolytic medication as absent = 0, low = 1, or high = 2. For antidepressants and mood stabilizers, we converted each medication into low- or high-dose groupings using a previously employed approach (35, 51, 52). Individuals on Levels 1 and 2 of these criteria were coded as low dose, those with Levels 3 and 4 as high dose. We added a no-dose subtype for those not taking these medications. We converted antipsychotic doses into chlorpromazine dose equivalents, and coded as 0, 1, or 2, for no medication, chlorpromazine equivalents dose equal or below, or above the mean effective daily dose (ED50) of chlorpromazine as defined by Davis and Chen (53). Lorazepam dose was similarly coded as, 0, 1 or 2, with reference to the midpoint of the Physician's Desk Reference-recommended daily dose range. We generated a composite measure of total medication load, reflecting dose and variety of different medications taken, by summing all individual medication codes for each medication category for each individual bipolar participant. We used Spearman rank correlational analyses to examine associations between total medication load and magnitude of BOLD signal change in a-priori neural regions showing significant group-by-condition interactions and/or a significant main effect of group.
To examine potential effects of illness duration, age of illness onset and current (subthreshold) depression severity, we conducted post hoc correlational analyses between these variables and BOLD signal change in a-priori neural regions (i.e., subcortical limbic regions, including amygdala and striatum, and DLPFC). Since 11 out of 19 patients had multiple comorbid diagnoses, and seven of these had comorbid anxiety disorders, we additionally compared BOLD signal change in our a-priori neural regions of interest in bipolar individuals with comorbid diagnoses versus those without any such diagnoses.
For the above analyses, we used an adjusted statistical threshold of p = 0.05/(number of comparisons plus number of regions examined) to allow for multiple between-group comparisons.
Results
Neuroimaging data analyses
To test our hypotheses, we examined the main effect of group and interaction between group and intensity upon neural activity during both experiments. To examine further any significant main effects or group-by-intensity interactions, specific between-group contrasts for each emotion intensity were assessed.
Happy
Main effects and interactions
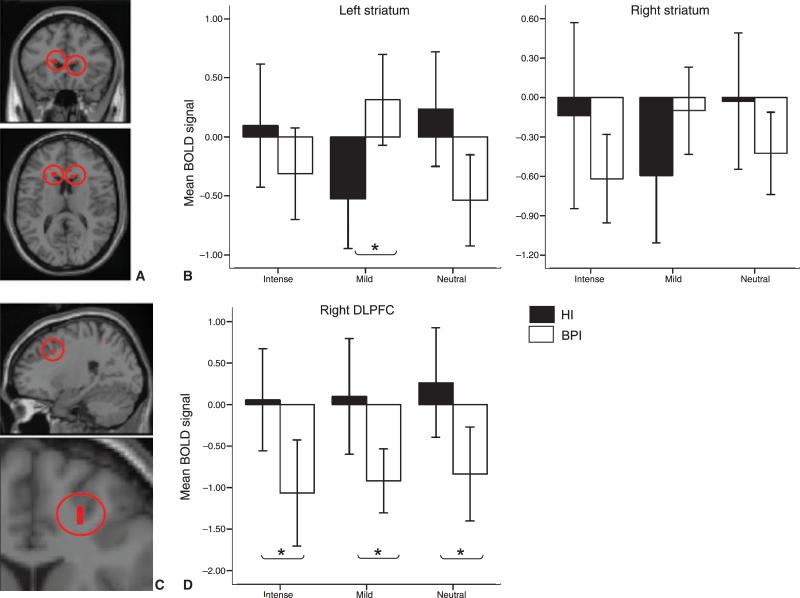
Whole-brain ANOVA revealed a significant group-by-intensity interaction for several clusters including those in a-priori regions: bilateral striatum [putamen/head-of-caudate-nucleus: left: F(6,123) = 10.39, p (corrected) = 0.008, Cohen's d = 0.98; right: F(6,123) = 9.20, p (corrected) = 0.018, Cohen's d = 0.95]. There was also a main effect of group (bipolar versus healthy individuals) for several clusters including right DLPFC [F(6,123) = 15.27, p (corrected) = 0.01, Cohen's d = 1.25 (Table 3)]. Bipolar relative to healthy individuals had significantly higher left striatal (putamen/caudate nucleus) activity in response to mild happy faces [F(1, 41) = 8.562, p (corrected) = 0.006, Cohen's d = 0.6 (Fig. 1 A, B)], and decreased activity in right DLPFC to all three intensities [neutral: t(1,41) = 3.32, p = 0.036, Cohen's d = 0.6; mild: t(1,41) = 3.41, p = 0.028, Cohen's d = 0.62; intense: t(1,41) = 3.49, p = 0.023, Cohen's d = 0.63 (Fig. 1 C, D)]. Activity within the right striatum (caudate nucleus) was decreased in response to all intensities relative to baseline in both groups. There were nonsignificant trends for greater decreases in activity in this region in response to intense happy and neutral faces in bipolar relative to healthy individuals [intense happy faces: mean for bipolar individuals: −0.94; mean for healthy individuals: −0.15, F(1,41) = 3.02, p = 0.09; neutral faces: mean for bipolar individuals: −0.69; mean for healthy individuals: −0.16; p > 0.1], but greater decreases in activity in response to mild happy faces in healthy relative to bipolar individuals [mild happy faces: mean for bipolar individuals: −0.11; mean for healthy individuals: −0.75, F(1,41) = 2.97, p = 0.093].
Table 3.
Group by emotion intensity interaction and main effect of group results for bipolar individuals and healthy individuals for both the happy and fearful face experiments
| Talairach coordinates |
|||||||
|---|---|---|---|---|---|---|---|
| Region | Side | x | y | z | F | z | p (FWE corrected) |
| Happy condition | |||||||
| Group-by-condition interaction | |||||||
| Striatum/head of caudate nucleus | R | 10 | 20 | 11 | 11.02 | 3.95 | 0.005 |
| Striatum/head of caudate nucleus | L | −17 | 20 | 14 | 10.39 | 3.82 | 0.008 |
| Angular gyrus, BA39 | L | −46 | −56 | 36 | 7.69 | 3.19 | 0.05 |
| Main effect of group | |||||||
| Dorsolateral prefrontal cortex; BA46 | R | 20 | 14 | 35 | 15.79 | 3.67 | 0.011 |
| Inferior occipital gyrus; BA17 | R | 18 | −91 | −10 | 14.52 | 3.52 | 0.015 |
| Middle temporal gyrus; BA42/22 | R | 49 | −53 | 2 | 13.63 | 3.40 | 0.026 |
| Precuneus; BA19 | R | 20 | −56 | 42 | 12.50 | 3.25 | 0.04 |
| Lingual gyrus; BA17 | L | −14 | −96 | −11 | 11.88 | 3.16 | 0.045 |
| Fearful condition | |||||||
| Main effect of group | |||||||
| Angular gyrus; BA39 | R | 39 | −62 | 31 | 13.78 | 3.43 | 0.023 |
| Inferior parietal cortex; BA39 | R | 36 | −64 | 39 | 11.94 | 3.17 | 0.049 |
| Precuneus; BA39 | R | 36 | −64 | 36 | 11.80 | 3.15 | 0.05 |
| Dorsolateral prefrontal cortex; BA8 | L | −30 | 5 | 42 | 11.79 | 3.15 | 0.05 |
FWE = family-wise error; R = right; L = left.
Fig. 1.
(A) Loci of the significant group-by-condition interaction [p (corrected) = 0.05] for the happy-face paradigm. (B) Activity is shown for bilateral striatum (head of caudate/putamen; left: [−17, 20, 14]; right: [15, 11, 16]). (C) Specific between-group contrasts revealed that bipolar individuals (BPI) relative to healthy individuals (HI) showed increased activity in left striatum to mild happy faces (*p < 0.05). (D) A significant main effect of group is shown for the right dorsolateral prefrontal cortex (DLPFC) [20, 14, 35], with BPI showing significantly decreased activity in response to happy as well as neutral faces (*p < 0.05). BOLD = blood-oxygenation-level-dependent.
Fearful
Main effects and interactions
Whole-brain ANOVA revealed a significant main effect of group for a cluster within left DLPFC [F(6,12) = 11.79, p (corrected) = 0.05, Cohen's d = 1.08] but no significant group-by-intensity interaction. Bipolar relative to healthy individuals showed significant decreases in activity in response to all three intensities [neutral: F(1,41) = 11.49, p = 0.002, Cohen's d = 1.06; mild: F(1,41) = 5.76, p = 0.02, Cohen's d = 0.75; intense: t(1,41) = 8.96, p = 0.005, Cohen's d = 0.94].
Amygdala
As we had specific hypotheses regarding amygdala activity, we used the WFU PickAtlas amygdale template to define bilateral amygdalae regions of interest from which we extracted mean BOLD signal to examine between-group differences in amygdala activity. There was no significant main effect of group and no significant group-by-intensity interaction upon amygdala activity in response to either emotion at any intensity.
Regional gray matter volume
There were no between-group differences in gray matter volume in a-priori regions emerging from analyses of between-group differences (left striatum and bilateral DLPFC) or in bilateral amygdalae.
Medication
There were no significant correlations between medication load and magnitude of activity within any of the five a-priori regions (left striatum and bilateral DLPFC emerging from whole-brain analyses and template-derived bilateral amygdalae), using a stringent statistical threshold of p = 0.001 to control for multiple testing resulting from analyses in these five regions for two emotion categories (happy, fearful) and three intensities (neutral, mild, intense emotion), or even using a more lenient threshold of p = 0.05.
Illness variables
There was a near-significant negative correlation between right amygdala mean BOLD signal in response to mild fearful faces and age of illness onset (r = −0.596, p = 0.007), using the stringent statistical threshold of p = 0.001. Neither illness duration nor depression severity showed significant correlations with BOLD signal within any of the five a-priori regions (left striatum and bilateral DLPFC emerging from whole-brain analyses and template-derived bilateral amygdalae) (all p > 0.05). Controlling for multiple comparisons using a stringent statistical threshold of p = 0.001, there was a trend only when comparing between-group differences in bipolar individuals with any comorbid Axis I diagnosis versus those without such comorbid diagnoses in mean BOLD signal within left striatum in response to mild happy faces (t = 2.71, p = 0.04). Bipolar individuals with comorbid diagnoses had increased BOLD signal within this region (mean: 0.83) relative to bipolar individuals without such comorbid diagnoses (mean: −0.12). Examining differences between bipolar individuals with comorbid anxiety disorders only versus those without such comorbid diagnoses yielded no significant differences within any of the five a-priori regions (left striatum and bilateral DLPFC emerging from whole-brain analyses and template-derived bilateral amygdalae).
Relationships between medication load and illness variables
There were no significant correlations between medication load and age of illness onset, illness duration, or depression severity using either a statistical threshold of p = 0.01 to allow for multiple comparisons or at a more lenient threshold of p = 0.05. Additionally, medication load did not differentiate between those patients with a diagnosis of any comorbid condition versus those without, nor between those with a diagnosis of comorbid anxiety disorder versus those without (p > 0.05).
Behavioral findings
Using Mann Whitney U-test for analyses, there were no between-group differences in facial emotion-labeling accuracy for bipolar and healthy individuals (U = 144.5, p > 0.05) for the task performed outside the scanner; nor were there between-group differences in gender-labeling accuracy for the task performed during the neuroimaging experiments (U = 147, p > 0.05).
Discussion
We wished to examine functional abnormalities in neural regions underlying emotion regulation in bipolar individuals to better understand pathophysiologic mechanisms of this disorder. Data support our hypotheses of abnormal patterns of subcortical limbic and dorsal prefrontal cortical activity in response to emotional faces in bipolar individuals. We show increased activity in bipolar versus healthy individuals to mild happy faces in a region of striatum centered on left putamen/head of caudate nucleus, and decreased DLPFC activity in response to the majority of faces in the happy and fearful face experiments. These findings are consistent with neural models of bipolar disorder which postulate increased activity within subcortical limbic emotion-processing regions, together with decreased activity in dorsal prefrontal cortical emotion regulation regions, as the neural basis for emotion and mood dysregulation in the disorder (7, 54).
Increased left striatal activity in response to mild happy faces in bipolar versus healthy individuals supports our earlier finding of increased left putamen activity in response to mild happy faces in bipolar versus healthy individuals (16), and is consistent with the left hemisphere's role in positive emotion perception (55). Striatal regions are implicated in the perception of potentially rewarding stimuli, e.g., food (56), happy faces (44), or during reward anticipation (57). Increased left striatal activity in response to mild happy faces in euthymic bipolar individuals suggests increased reward perception in these stimuli, even in the absence of subthreshold manic symptoms. These findings contrast with those in medicated and unmedicated unipolar depressed individuals, who show decreased striatal activity in response to positive stimuli (44). Increased striatal activity in response to mild happy faces may, therefore, represent an important pathophysiologic process specific to bipolar disorder. Future studies should compare bipolar and unipolar individuals on positive and negative emotion-processing tasks.
The pattern of decreased left and right DLPFC activity in response to mild happy and mild fearful faces, respectively, in bipolar relative to healthy individuals is consistent with findings of reduced DLPFC activity in bipolar females (18), and decreased activity in dorsal prefrontal cortical regions implicated in emotion regulation in bipolar disorder (7, 17). The abnormal left- versus right-sided DLPFC activity in response to mild happy and fearful faces, respectively, in bipolar individuals supports theories emphasizing lateralization of positive and negative emotion processing (55). Interestingly, both increased striatal activity and reduced DLPFC activity in bipolar individuals were demonstrated predominantly in response to mild-intensity emotions. Mild facial expressions may be more representative of expressions observed in everyday life than the intense facial expressions in the standardized series employed here. Abnormal striatal and DLPFC activity in bipolar individuals in response to mild-intensity expressions may therefore underlie the dysfunctional social interactions in bipolar individuals (2-4), even when euthymic. This finding is consistent with data from our previous study in remitted bipolar individuals (16).
Contrary to our hypotheses, bipolar relative to healthy individuals did not demonstrate increased amygdala activity in response to any faces. Previous studies in bipolar individuals report increased amygdala activity in response to negative (11, 16, 18) and positive (14) emotional faces. Unlike these studies, we examined euthymic individuals only. The absence of amygdala activity may, therefore, reflect the euthymic status of our recruited bipolar individuals.
Included in the second, exploratory aim of our study was examination of the potential contribution of psychotropic medication to abnormal activity in neural regions implicated in emotion processing in bipolar disorder. Most neuroimaging studies of bipolar disorder have recruited medicated individuals, as these individuals are more representative of the bipolar population, and it is often clinically unfeasible and ethically problematic to withdraw such individuals from medication. We employed a new strategy (35, 51) based on previous methods (52, 53) to compute a measure of total medication load that included dosage and number of different medications taken. This novel approach for the study of potential medication effects in neuroimaging studies of bipolar populations has several advantages over the study of medicated versus unmedicated individuals. First, bipolar individuals (particularly those with type I) who tolerate being medication free are unlikely to be matched for illness severity with those who require medication. Direct comparison of medicated and unmedicated individuals with bipolar disorder may be confounded, therefore, by between-group differences in illness severity. Second, medicated individuals with bipolar disorder type I are more representative of the bipolar population at large compared with bipolar individuals who can tolerate being medication free (see 35). Third, compared with previously employed approaches of examining individuals taking, versus those not taking, each class of psychotropic medication (e.g., 16, 22), the calculation of total medication load has the additional advantage of avoiding multiple comparisons of medication subgroups comprising smaller numbers of individuals. Finally, total medication load also reflects both the dose and variety of different medications taken by bipolar individuals who have been medicated in real-world contexts, and are typically treated with a number of different medications and medication combinations. Our findings indicate no significant association between this measure and magnitude of activity in any neural regions in which significantly abnormal activity was observed in bipolar individuals. Our findings are consistent with previous studies showing no significant effect of psychotropic medication upon neural activity in bipolar individuals (14, 22, 32). Other studies have shown predominantly ameliorative, rather than confounding, effects of psychotropic medication upon neural activity in bipolar individuals (16, 30, 33, 34) or in male patients with bipolar disorder (18). Our findings, therefore, suggest that it is possible to identify neuroimaging measures that may reflect pathophysiologic processes of bipolar disorder that persist during euthymic phases of illness, rather than measures that are confounds of psychotropic medication.
There was a trend toward a negative association between age of illness onset and amygdala activity in response to mild fearful faces in bipolar individuals. This relationship may have obscured any between-group difference in amygdala activity in response to fearful faces that has been demonstrated in previous studies (11, 14, 16), as some of our recruited bipolar individuals had an older age of illness onset. The relationship between age of illness onset and amygdala activity did not relate to illness duration per se, as there was no significant difference in this latter measure between bipolar individuals with earlier versus later age of illness onset. There was also a trend for bipolar individuals with comorbid Axis I diagnoses to have higher left striatal activity in response to mild happy faces. This suggests that the presence of comorbid psychiatric diagnoses might have contributed to the pattern of elevated striatal activity observed in the bipolar individuals in this study. Bipolar disorder often presents with comorbid Axis I conditions, and it is known that these negatively impact the severity of the course of illness. The presence, therefore, of comorbid diagnoses that affect general functioning and well-being should be examined further by studying the extent to which the spectra of comorbid conditions in bipolar disorder impact patterns of abnormal subcortical limbic and DLPFC activity in response to emotional stimuli in individuals with the disorder.
There was no significant between-group difference in facial emotion labeling performed in a separate task outside the scanner or in gender labeling performed during the scan. Most findings indicate subtle abnormalities in emotion labeling only (e.g., 24-26), and no gender labeling diffculties (16), in bipolar individuals. Our findings suggest that abnormal neural activity in response to facial emotion in bipolar individuals may reflect abnormal implicit processing of these emotional stimuli rather than an inability to subsequently label facial emotion or gender explicitly. Additionally, there were no significant between-group differences in gray matter volume in left striatum, bilateral amygdalae or DLPFC, indicating that between-group differences in neural activity were not secondary to abnormal regional gray matter volume in bipolar individuals.
Limitations
Although this study represents one of the larger functional neuroimaging investigations of euthymic bipolar individuals to date, the number of patients included in this study is relatively modest. Our findings would need to be replicated in a larger sample of euthymic bipolar individuals. Nearly all bipolar individuals in our study were taking psychotropic medication. Our findings suggest no significant impact of medication load upon neural activity in bipolar individuals in the present study. Future studies should, however, examine this further, both by inclusion of our approach of using an index of psychotropic medication load (35) and by including unmedicated bipolar individuals (as in, e.g., 58). In our study, a number of patients had multiple comorbid Axis I diagnoses, as is generally observed in bipolar individuals. Further examination of the relationship between the spectra of comorbid conditions and patterns of abnormal subcortical limbic and DLPFC activity is needed in bipolar individuals.
Conclusions
Our findings are the first to demonstrate increased striatal activity in euthymic bipolar individuals in response to mildly rewarding stimuli. This, in combination with decreased DLPFC activity in bipolar individuals in response to the majority of facial expressions, may underlie the mood instability observed even in euthymic individuals, and may represent an important pathophysiologic neural mechanism of bipolar disorder. Furthermore, we report no significant association between a novel composite measure of total psychotropic medication load and magnitude of activity in the above neural regions in bipolar individuals. Future studies should examine whether this pattern of abnormal neural activity can be used as a criterion to distinguish bipolar from unipolar depression.
Acknowledgements
All work was carried out within the Department of Psychiatry, University of Pittsburgh; neuroimaging data were collected at the Brain Imaging Research Center, University of Pittsburgh and Carnegie Mellon University. We thank Dr. K. J. Jung, S. Kurdilla, and D. Vizslay for their help acquiring neuroimaging data. The research in this study was supported in part by R01 MH076971-01A2 (MLP) and by a NARSAD Independent Investigator Award (MLP). MLP is the NARSAD Nellie Blumenthal Investigator.
Footnotes
The authors of this paper do not have any commercial associations that might pose a conflict of interest in connection with this manuscript.
References
- 1.Murray CJ, Lopez AD. Evidence-based health policy – lessons from the Global Burden of Disease Study. Science. 1996;274:740–743. doi: 10.1126/science.274.5288.740. [DOI] [PubMed] [Google Scholar]
- 2.Blairy S, Linotte S, Souery D, et al. Social adjustment and self-esteem of bipolar patients: a multicenter study. J Affect Disord. 2004;79:97–103. doi: 10.1016/S0165-0327(02)00347-6. [DOI] [PubMed] [Google Scholar]
- 3.Romans SE, McPherson HM. The social networks of bipolar affective disorder patients. J Affect Disord. 1992;25:221–228. doi: 10.1016/0165-0327(92)90079-l. [DOI] [PubMed] [Google Scholar]
- 4.Goodwin FK, Jamison KR. Manic-Depressive Illness: Bipolar Disorder and Recurrent Depression. 2nd edn. Oxford University Press; New York: 2007. [Google Scholar]
- 5.Malhi GS, Ivanovski B, Hadzi-Pavlovic D, Mitchell PB, Vieta E, Sachdev P. Neuropsychological deficits and functional impairment in bipolar depression, hypomania and euthymia. Bipolar Disord. 2007;9:114–125. doi: 10.1111/j.1399-5618.2007.00324.x. [DOI] [PubMed] [Google Scholar]
- 6.Krueger S, Seminowicz D, Goldapple K, Kennedy SH, Mayberg HS. State and trait influences on mood regulation in bipolar disorder: blood flow differences with an acute mood challenge. Biol Psychiatry. 2003;54:1274–1283. doi: 10.1016/s0006-3223(03)00691-7. [DOI] [PubMed] [Google Scholar]
- 7.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol Psychiatry. 2003;54:512–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 8.Charney DS, Nemeroff CB, Lewis L, et al. National Depressive and Manic-Depressive Association consensus statement on the use of placebo in clinical trials of mood disorders. Arch Gen Psychiatry. 2002;59:262–270. doi: 10.1001/archpsyc.59.3.262. [DOI] [PubMed] [Google Scholar]
- 9.Phillips ML, Frank E. Redefining bipolar disorder: toward DSM-V. Am J Psychiatry. 2006;163:1135–1136. doi: 10.1176/ajp.2006.163.7.1135. [DOI] [PubMed] [Google Scholar]
- 10.Kupfer DJ. The increasing medical burden in bipolar disorder. JAMA. 2005;293:2528–2530. doi: 10.1001/jama.293.20.2528. [DOI] [PubMed] [Google Scholar]
- 11.Altshuler L, Bookheimer S, Proenza MA, et al. Increased amygdala activation during mania: A Functional Magnetic Resonance Imaging Study. Am J Psychiatry. 2005;162:1211–1213. doi: 10.1176/appi.ajp.162.6.1211. [DOI] [PubMed] [Google Scholar]
- 12.Blumberg HP, Stern E, Ricketts S, et al. Rostral and orbital prefrontal cortex dysfunction in the manic state of bipolar disorder. Am J Psychiatry. 1999;156:1986–1988. doi: 10.1176/ajp.156.12.1986. [DOI] [PubMed] [Google Scholar]
- 13.Blumberg HP, Stern E, Martinez D, et al. Increased anterior cingulate and caudate activity in bipolar mania. Biol Psychiatry. 2000;48:1045–1052. doi: 10.1016/s0006-3223(00)00962-8. [DOI] [PubMed] [Google Scholar]
- 14.Blumberg HP, Donegan NH, Sanislow CA, et al. Preliminary evidence for medication effects on functional abnormalities in the amygdala and anterior cingulate in bipolar disorder. Psychopharmacology. 2005;183:308–313. doi: 10.1007/s00213-005-0156-7. [DOI] [PubMed] [Google Scholar]
- 15.Chen CH, Lennox B, Jacobs R, et al. Explicit and implicit facial affect recognition in manic and depressed states of bipolar disorder: A Functional Magnetic Resonance Imaging Study. Biol Psychiatry. 2006;59:31–39. doi: 10.1016/j.biopsych.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Lawrence NS, Williams AM, Surguladze S, et al. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguished patients with bipolar disorder and major depression. Biol Psychiatry. 2004;55:578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 17.Lennox BR, Jacob R, Calder AJ, Lupson V, Bullmore ET. Behavioural and neurocognitive responses to sad facial affect are attenuated in patients with mania. Psychol Med. 2004;34:795–802. doi: 10.1017/s0033291704002557. [DOI] [PubMed] [Google Scholar]
- 18.Yurgelun-Todd DA, Gruber SA, Kanayama G, Killgore WDS, Baird AA, Young AD. fMRI during affect discrimination in bipolar affective disorder. Bipolar Disord. 2000;2:237–248. doi: 10.1034/j.1399-5618.2000.20304.x. [DOI] [PubMed] [Google Scholar]
- 19.Malhi GS, Lagopoulos J, Owen AM, Ivanovski B, Shnier R, Sachdev P. Reduced activation to implicit affect induction in euthymic bipolar patients: an fMRI study. J Affect Disord. 2007;97:109–122. doi: 10.1016/j.jad.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Lagopoulos JM, G.S. A functional magnetic resonance imaging study of emotional Stroop in euthymic bipolar disorder. Neuroreport. 2007;8:1583–1587. doi: 10.1097/WNR.0b013e3282efa07a. [DOI] [PubMed] [Google Scholar]
- 21.Malhi GS, Lagopoulos J, Sachdev PS, Ivanovski B, Shnier R, Ketter TE. Is the lack of disgust something to fear? A functional magnetic resonance imaging facial emotion recognition study in euthymic bipolar disorder patients. Bipolar Disord. 2007;9:345–357. doi: 10.1111/j.1399-5618.2007.00485.x. [DOI] [PubMed] [Google Scholar]
- 22.Wessa M, Houenou J, Paillere-Martinot ML, et al. Frontostriatal overactivation in euthymic bipolar patients during an emotional go/nogo task. Am J Psychiatry. 2007;164:638–646. doi: 10.1176/ajp.2007.164.4.638. [DOI] [PubMed] [Google Scholar]
- 23.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 24.Getz GE, Shear PK, Strakowski SM. Facial affect recognition deficits in bipolar disorder. J Int Neuropsychol Soc. 2003;9:623–632. doi: 10.1017/S1355617703940021. [DOI] [PubMed] [Google Scholar]
- 25.Lembke A, Ketter TE. Impaired recognition of facial emotion in mania. Am J Psychiatry. 2002;159:302–304. doi: 10.1176/appi.ajp.159.2.302. [DOI] [PubMed] [Google Scholar]
- 26.Harmer CJ, Grayson L, Goodwin GM. Enhanced recognition of disgust in bipolar illness. Biol Psychiatry. 2002;51:298–304. doi: 10.1016/s0006-3223(01)01249-5. [DOI] [PubMed] [Google Scholar]
- 27.Elliot R, Ogilvie A, Rubinsztein JS, Calderone G, Dolan RJ, Sahakian BJ. Abnormal ventral frontal response during performance of an affective go/no-go task in patients with mania. Biol Psychiatry. 2004;55:1163–1170. doi: 10.1016/j.biopsych.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Elliot R, Newman JL, Longe OA, William Deaking JF. Instrumental responding for reward is associated with enhanced neuronal response in subcortical reward systems. NeuroImage. 2004;21:984–990. doi: 10.1016/j.neuroimage.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 30.Adler CM, Holland SK, Schmithorst V, Tuchfarber MJ, Strakowski SM. Changes in neuronal activation in patients with bipolar disorder during performance of a working memory task. Bipolar Disord. 2004;6:540–549. doi: 10.1111/j.1399-5618.2004.00117.x. [DOI] [PubMed] [Google Scholar]
- 31.Blumberg HP, Leung HC, Skudlarski P, et al. A functional magnetic resonance imaging study of bipolar disorder: state- and trait-related dysfunction in ventral prefrontal cortices. Arch Gen Psychiatry. 2003;60:601–609. doi: 10.1001/archpsyc.60.6.601. [DOI] [PubMed] [Google Scholar]
- 32.Kronhaus DM, Lawrence NS, Williams AM, et al. Stroop performance in bipolar disorder: further evidence for abnormalities in the ventral prefrontal cortex. Bipolar Disord. 2006;8:28–39. doi: 10.1111/j.1399-5618.2006.00282.x. [DOI] [PubMed] [Google Scholar]
- 33.Strakowski SM, Adler CM, Holland SK, Mills NP, DelBello MP, Eliassen JC. Abnormal FMRI brain activation in euthymic bipolar disorder patients during a counting Stroop interference task. Am J Psychiatry. 2005;162:1697–1705. doi: 10.1176/appi.ajp.162.9.1697. [DOI] [PubMed] [Google Scholar]
- 34.Gruber SA, Rogowska J, Yurgelun-Todd DA. Decreased activation of the anterior cingulate in bipolar patients: an fMRI study. J Affect Disord. 2004;82:191–201. doi: 10.1016/j.jad.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 35.Phillips ML, Travis MJ, Fagiolini A, Kupfer DJ. Medication effects in neuroimaging studies of bipolar disorder. Am J Psychiatry. 2008;165:313–320. doi: 10.1176/appi.ajp.2007.07071066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russel JA. Reading Emotions from and Into Faces: Resurrecting a Dimensional-Contextual Perspective. Cambridge University Press; New York, NY: 1997. [Google Scholar]
- 37.First MB, Gibbon ML, Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-IV (SCID-I): User's Guide and Interview, Research Version. Biometrics Research Department, New York Psychiatric Institute; New York, NY: 1995. [Google Scholar]
- 38.Williams JB. A Structured Interview Guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry. 1988;45:742–747. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]
- 39.Young RC, Biggs JT, Ziegler VE, Meyer DA. A Rating Scale for Mania: Reliability, Validity and Sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 40.Blair JR, Spreen O. Predicting premorbid IQ: a revision of the national adult reading test. Clin Neuropsychol. 1989;3:129–136. [Google Scholar]
- 41.Annett M. A classification of hand preference by association. Br J Psychol. 1970;61:303–321. doi: 10.1111/j.2044-8295.1970.tb01248.x. [DOI] [PubMed] [Google Scholar]
- 42.Surguladze SA, Brammer MJ, Young AW, et al. A preferential increase in the extrastriate response to signals of danger. Neuroimage. 2003;19:1317–1328. doi: 10.1016/s1053-8119(03)00085-5. [DOI] [PubMed] [Google Scholar]
- 43.Jollant F, Lawrence NS, Giampietro V, et al. Orbitofrontal cortex response to angry faces in men with histories of suicide attempts. Am J Psychiatry. 2008;165:740–748. doi: 10.1176/appi.ajp.2008.07081239. [DOI] [PubMed] [Google Scholar]
- 44.Surguladze S, Brammer MJ, Keedwell P, et al. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol Psychiatry. 2005;57:201–209. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 45.Young AW, Perrett D, Calder A. Facial Expressions of Emotions: Stimuli and Test (FEEST) Thames Valley Test Company; Thurstone: 2002. [Google Scholar]
- 46.Lange KWL, Young AW, Bullmore ET, et al. Task instructions modulate neural responses to fearful facial expressions. Biol Psychiatry. 2003;53:226–232. doi: 10.1016/s0006-3223(02)01455-5. [DOI] [PubMed] [Google Scholar]
- 47.Lundqvist D, Flykt A, Ohman A. The Karolinska directed emotional faces – KDEF. Department of Clinical Neuroscience PS. Karolinska Institute; Stockholm: 1998. [Google Scholar]
- 48.Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox. NeuroImage. 2002;16:A497. [Google Scholar]
- 49.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 50.Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 51.Almeida JRC, Akkal D, Hassel S, et al. Reduced gray matter volume in ventral prefrontal cortex but not amygdala in bipolar disorder: significant effects of gender and trait anxiety. Psychiatry Res: Neuroimaging. 2008 doi: 10.1016/j.pscychresns.2008.02.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62:10–17. [PubMed] [Google Scholar]
- 53.Davis JM, Chen N. Dose response and dose equivalence of antipsychotics. J Clin Psychopharmacol. 2004;24:192–208. doi: 10.1097/01.jcp.0000117422.05703.ae. [DOI] [PubMed] [Google Scholar]
- 54.Green MJ, Cahill CM, Malhi GS. The cognitive and neurophysiological basis of emotion dysregulation in bipolar disorder. J Affect Disord. 2007;103:29–42. doi: 10.1016/j.jad.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 55.Davidson RJ, Irwin W. The Functional Neuroanatomy of Emotion and Affective Style. Trends Cogn Sci. 1999;3:11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- 56.O'Doherty JP, Buchanan TW, Seymour B, Dolan RJ. Predictive neural coding of reward preferences involves dissociable responses in human ventral midbrain and ventral striatum. Neuron. 2006;49:157–166. doi: 10.1016/j.neuron.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 57.Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage. 2003;18:263–272. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- 58.Strakowski SM, Adler CM, Holland SK, Mills NP, DelBello MP. A preliminary fMRI study of sustained attention in euthymic unmedicated bipolar disorders. Neuropsychopharmacology. 2004;29:1734–1740. doi: 10.1038/sj.npp.1300492. [DOI] [PubMed] [Google Scholar]