Abstract
Zinc, an essential micronutrient, is involved in wound healing. The hypozincemia seen with chronic aldosteronism is associated with enhanced fecal and urinary excretory Zn losses while its tissue distribution is less certain. Herein, we monitored tissue 65Zn distribution in uninephrectomized rats at wks 1 and 4 of aldosterone/salt treatment (ALDOST). Plasma and tissue total radionucleotide uptake was determined by calculating its mean radioactivity at 1, 4, 8, 24, and 48 h after intravenous 65Zn administration and where respective area under the concentration-time curves (AUC) were determined by the linear trapezoidal rule and expressed as a tissue:plasma AUC ratio. Tissues examined included: injured heart and kidney in response to ALDOST and incised skin; noninjured liver, skeletal muscle, and spleen, sites of stress-linked Zn uptake; and bone, a major storage and release site when Zn homeostasis is threatened. Compared to age-/gender-matched controls, with wk 1 and 4 ALDOST we found: reduced plasma 65Zn; an accumulation of 65Zn in heart and kidneys, where a well-known vasculopathy involves intramural vessels, and in incised skin at wk 1; an organ-specific increase in tissue 65Zn in liver, in keeping with upregulated metallothionein expression, skeletal muscle, and spleen; and a fall in bone and skin 65Zn at wk 4. Thus a wide-ranging disturbance in Zn homeostasis appears during ALDOST to include its translocation from plasma to injured heart, kidneys and skin and noninjured liver, skeletal muscle and spleen, together with a resorption of stored Zn in bone at wk 4. Zinc dyshomeostasis is an integral feature of chronic aldosteronism.
Keywords: zinc radioactivity, aldosteronism, injured tissue
Introduction
Zinc is an essential micronutrient whose biologic actions are integral to tissue repair, de novo protein synthesis, and crosslinking. This includes its role in regulating the activity of metalloproteases (e.g., angiotensin-converting enzyme, matrix metalloproteinases, and Cu/Zn-superoxide dismutase), the initiation of transcription and gene expression, immune cell function, and cell differentiation, each of which contribute to wound healing.1–4 In response to an acute myocardial infarction, burn injury, or hypothermia there appears to be an activation of the hypothalamic-pituitary-adrenal axis, featuring the release of such hormones as adrenocorticotropin (ACTH), catecholamines, cortisol and aldosterone, that is coupled to a fall in plasma Zn, the extent of which correlates with the degree of injury.4–10 This acute hypozincemia is associated with alterations in Zn metabolism that favors Zn uptake at the site of tissue injury and simultaneous increase in excretory Zn losses. In the case of severe burns, the reduction of plasma Zn relates to its rapid uptake at sites of injured skin together with the Zn lost in wound exudate and urine. Collectively, these responses threaten Zn homeostasis, which is rescued by Zn resorption from bone.5 During the relatively short-lived neurohormonal activation seen with bodily injury, an upregulation of metallothionein (MT), a Zn-binding protein, facilitates a redistribution of Zn to selective tissues that include the liver. This acute-phase response further perturbs Zn homeostasis.11, 12 However, responses in tissue Zn metabolism that accompany a proinflammatory phenotype seen with chronic neurohormonal activation remain uncertain.
A persistent acute-phase response, as suggested by chronic elevations in C-reactive protein and accompanying hypozincemia, are found in patients with heart failure, tuberculosis, and malignancy.13–15 In patients with heart failure, the chronic activation of the renin-angiotensin-aldosterone system is a potential mediator of the hypozincemia found at the time of hospital admission for worsening symptomatic heart failure.16 Increased Zn excretion, together with hypozincemia, are found in patients with secondary aldosteronism.17, 18
To simulate chronic, inappropriate (relative to dietary Na+) elevations in plasma aldosterone (ALDO), by raising plasma ALDO levels to those found in human heart failure, uninephrectomized rats are treated with aldosterone/salt (ALDOST) for 1 and 4 weeks. In this experimental model of aldosteronism, we found a marked increase in both fecal and urinary Zn excretion, as well as a redistribution of Zn from plasma to the injured myocardium, each of which contribute to the appearance of hypozincemia.19, 20 This study was undertaken to further elucidate tissue Zn metabolism in rats with chronic aldosteronism. We evaluated 65Zn distribution in plasma and tissues at wks 1 and 4 of ALDOST. Tissues were selected based on the following: a) tissue injury in heart and kidneys that appear during ALDOST,20, 21 and skin injury that occurs with surgical incisions; b) noninjured tissues, liver, skeletal muscle and spleen, which are stress-linked sites of Zn uptake based on upregulated expression of MT12; and c) bone, a major storage site for Zn and source of Zn during bone resorption that accompanies chronic Zn losses.22
METHODS
Animal Model
These experiments in rats were approved by the University of Tennessee Health Science Center Institutional Animal Care and Use Committee and were performed in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals. Eight-week-old male Sprague-Dawley rats (Harlan, Indianapolis, IN) were used in this study. Three groups of animals (n=20 animals per group) were evaluated: 1) one week of aldosterone/salt treatment (ALDOST); 2) four weeks of ALDOST; and 3) unoperated, untreated, age-matched controls. The ALDOST rat model used in our laboratory has been extensively characterized23–28 and uses uninephrectomized rats that receive ALDO (0.75 µg/h) by implanted osmotic minipump (Alzet, Cupertino, CA), together with 1% NaCl/0.4% KCl in drinking water.20 The animals had free access to drinking water and received standard laboratory chow containing 78.84 mg/kg elemental Zn (Harlan Teklad 2215 Rodent Diet, Madison, Wisconsin).
Experimental Procedures
Each group of animals received 1.0 µCi 65ZnCl2 (0.029 mCi/µg, Oak Ridge National Laboratories, Oak Ridge, TN) in 0.1 mL of 0.9% saline solution intravenously via the tail vein. Four animals from each group were sacrificed at each of the following time points after 65Zn administration: 1, 4, 8, 24, and 48 hours. At each time point, rats were anesthetized with isofluorane and blood was collected by cardiac puncture. The blood was placed into heparinized tubes and plasma obtained by centrifugation. At the time of sacrifice, the following whole organs were collected: heart, kidney, and bone (tibia). Samples were also harvested from other organs/tissues, i.e., liver, skeletal muscle, and skin. All tissue samples were collected, weighed, and tissue and plasma samples were stored at −20°C until analysis using standard procedures.
Sample Analysis
Whole organ and tissue samples were solubilized by placing each specimen in 2 mL of Solvable® (PerkinElmer, Boston, MA) solution and incubated overnight in a 50–60°C water bath. The total radioactivity in each tissue and plasma sample was determined by counting an aliquot of each specimen for 10 minutes in a gamma scintillation counter (Packard Cobra II, Meriden, CT). Plasma and tissue concentrations were expressed as counts per minute (cpm)/mL or cpm/g of plasma or tissue, respectively.
The total radioactivity in plasma and tissues at each time point was determined by calculating the mean radioactivity from the animals sacrificed at each respective point. Area under the concentration time curves (AUC) for plasma and tissue were calculated by the linear trapezoidal rule using the mean radioactivity at each previously specified time point. Because rats were sacrificed at each time point, repeated samples from the same animal were not possible. A well-established standard procedure (i.e., destructive sampling strategy), described elsewhere,29 was therefore used. For each group of animals, only one AUC for plasma and each tissue could be determined and are summarized in one mean concentration-time profile without variability measures. Thus, no standard errors could be provided for any AUC data. The tissue to plasma concentration AUC ratio in each group of rats was calculated as tissue AUC÷plasma AUC. Differences in plasma and tissue radioactivity between the groups were determined by analysis of variance. If significant differences were detected, Dunnett’s multiple comparison test was then used to compare the 1 week and 4 week ALDOST groups to the control group. Statistical significance was defined as p≤0.05.
Metallothionein
Metallothionein (MT)-I protein levels in the liver were measured by Western blot analysis. Briefly, left ventricles were dissected free and homogenized in lysis buffer, then MT was separated by 12% SDS-PAGE. After electrophoresis, samples were transferred to polyvinylidene fluoride membranes and incubated with monoclonal antibody against metallothionein. Blots were subsequently incubated with peroxidase-conjugated secondary antibody. After washing, the blots were developed with the enhanced chemiluminescence method. The amount of protein detected by the antibody was measured by a computer image analysis system.
RESULTS
Plasma Zn Concentrations
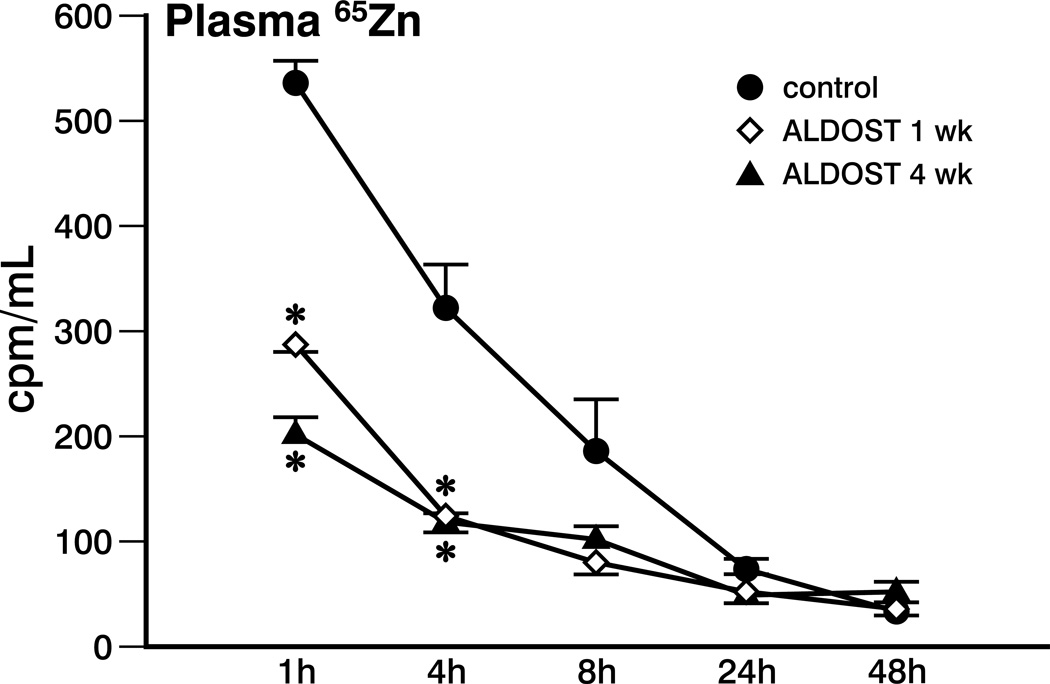
The plasma radioactive Zn activity concentration-time profile is shown in Figure 1. At both the 1 hour and 4 hour time points after 65Zn administration, radioactivity was significantly higher in the control group compared to animals treated with either 1 or 4 weeks of ALDOST. The 65Zn plasma AUC in the control animals was 5932 cpm hr/mL which was reduced by 40–45% to 3283 and 3442 cpm·hr/mL after 1 week and 4 weeks of ALDOST, respectively.
Figure 1.
Plasma Zn radioactivity vs. time for controls and rats receiving aldosterone/salt treatment (ALDOST) for 1 and 4 wks. *p<0.05 ALDOST vs. controls.
65Zn Distribution in Injured Tissues (Heart, Kidney and Skin)
At 48 hours after administration of 65Zn, radioactivity in the heart was significantly (p<0.05) greater in animals that received 4 weeks of ALDOST than in controls (966±128 cpm/g vs. 424±92 cpm/g, mean±SEM). Although statistically insignificant, a trend toward higher activity in the heart compared to control was observed in the 1-week ALDOST group (722±88 cpm/g). In the kidney, no differences in tissue radioactivity between the control group and either ALDOST group were found at any time point. In the skin, no overall differences were observed between the control group (247±59 cpm/g) and the ALDOST groups, although a trend at the 48-hour time point toward increased accumulation in the 1-week ALDOST group (332±32 cpm/g) and reduced accumulation in the 4-week group (149±29 cpm/g) was seen.
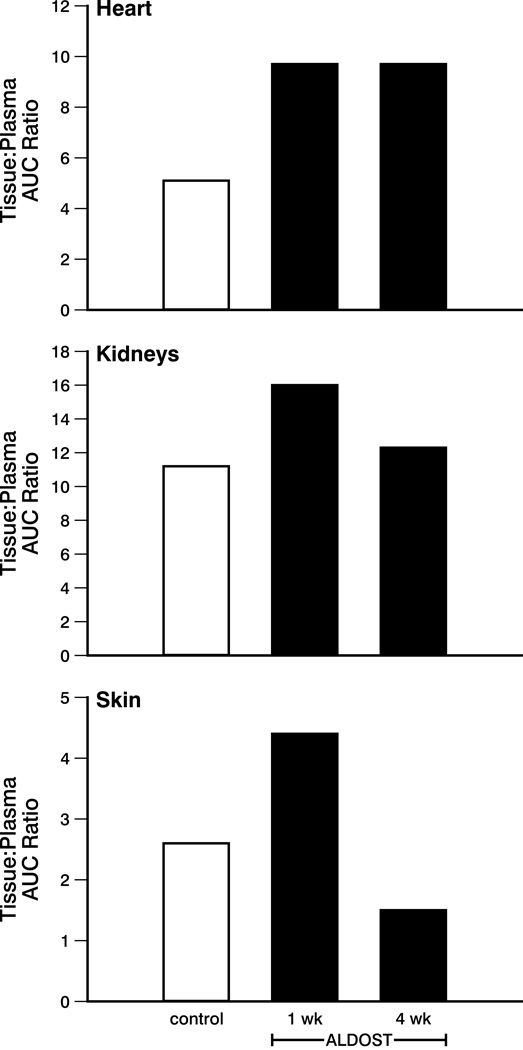
The tissue:plasma AUC ratio for these tissues following 65Zn administration is shown in Figure 2. Note that because of the use of destructive sampling strategy, where rats were killed at each time point, serial samples from the same animal could not be collected (see Methods). One mean concentration-time profile is presented without variability measures. Hence no standard errors could be calculated for the AUC data.29 Overall accumulation of 65Zn activity in the heart was increased nearly 2-fold in each of the ALDOST groups compared to control. In the kidney, 1 week of ALDOST increased accumulation by approximately 40% compared to control. However, after 4 weeks of ALDOST, the kidney:plasma AUC ratio approached that of the control group. Accumulation of radioactivity in the skin was increased by nearly 70% in the 1-week ALDOST group compared to control. After 4 weeks of ALDOST, skin:plasma AUC ratio was over 40% lower than the control group.
Figure 2.
The tissue:plasma AUC ratio for heart, kidneys, and skin of controls and at wks 1 and 4 of ALDOST. See text. Note that because of the destructive sampling strategy, variability in the AUC data were not calculated.
65Zn Translocation in Noninjured Tissues (Liver, Skeletal Muscle and Spleen)
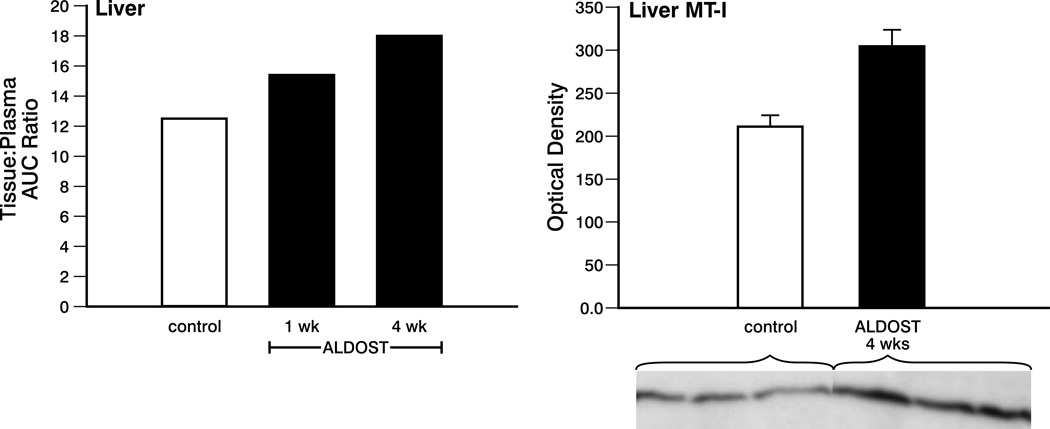
As seen in Figure 3, left panel, a progressive increase in liver:plasma AUC was observed in the 1-week ALDOST (23%), and 4-week ALDOST groups (44%) compared to control, which was in keeping with increased MT-I protein seen by Western blot at week 4 ALDOST (see right panel, Figure 3).
Figure 3.
Left panel. The tissue:plasma AUC ratio for liver of controls and at wks 1 and 4 of ALDOST. Note again, variability in the AUC data were not calculated. Right panel. Metallothionein (MT)-I protein levels in the liver for controls and at 4 wks ALDOST. Western blot for MT-I found in liver of controls and 4 wks ALDOST is presented as an insert. See text.
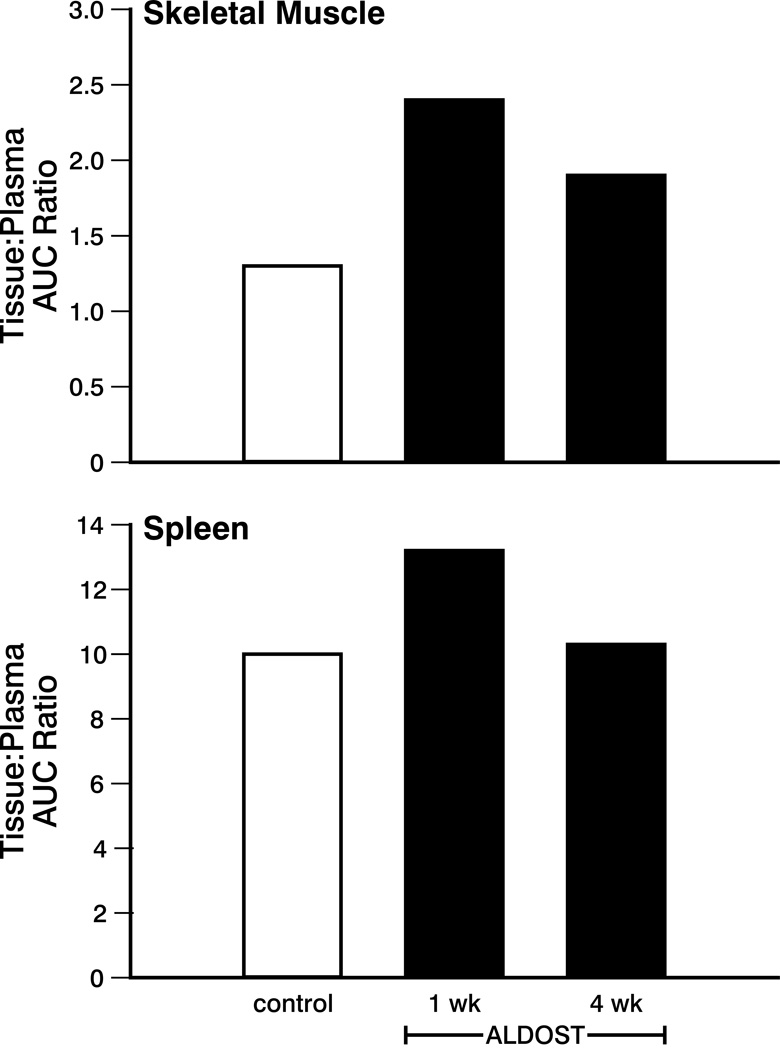
65Zn uptake in skeletal muscle increased nearly 2-fold after 1 week of ALDOST compared to control (see Figure 4). After 4 weeks of ALDOST, a nearly 50% increase in accumulation in skeletal muscle versus control was seen. The spleen:plasma AUC ratio was slightly increased compared to control after 1 week of ALDOST—this increase was no longer present after 4 weeks of ALDOST.
Figure 4.
Tissue:plasma AUC ratio for skeletal muscle and spleen for controls and wks 1 and 4 of ALDOST. See text. Variability in the AUC data were not calculated.
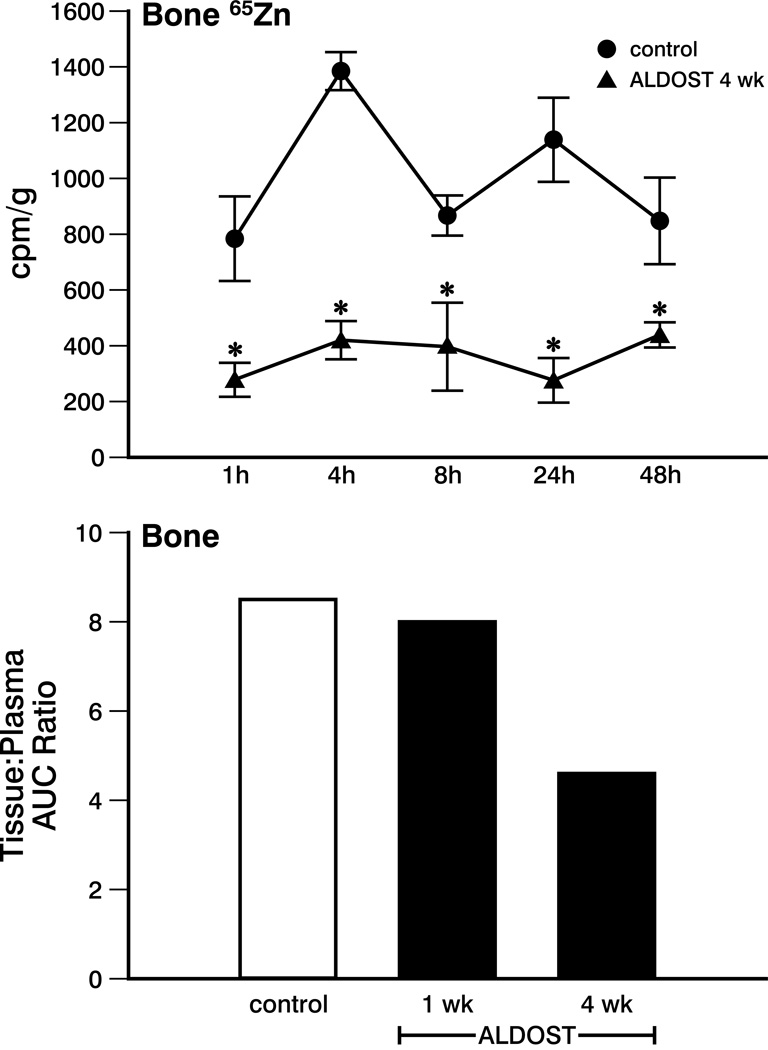
65Zn Storage and Resorption (Bone and Skin)
In the 4-week ALDOST group, 65Zn translocation to bone was significantly less than control at all sampling time points (see upper panel, Figure 5). There was no change in the bone:plasma AUC ratio between the control and the 1-week ALDOST group (not shown). However, after 4 weeks of ALDOST, 65Zn uptake was reduced compared to control as demonstrated by the 46% decrease in the AUC ratio (see lower panel, Figure 5).
Figure 5.
Upper panel. Zn radioactivity in bone for controls and at wk 4 ALDOST. *p<0.05 vs. controls. Lower panel. Tissue:plasma AUC ratio for bone in controls and wks 1 and 4 of ALDOST. See text. Variability in the AUC data were not calculated because of the destructive sampling strategy.
DISCUSSION
In a previous study, we found an early (wk 1) and persistent (wk 4) increase in fecal (in mg/24 h) and urinary (in µg/24 h) Zn excretion during ALDOST, which contributes to a fall in plasma Zn at these time points.19 In the present study we monitored 65Zn translocation in injured and noninjured tissues obtained from rats at 1 and 4 wks of ALDOST. Herein, we made several observations on the temporal distribution patterns of tissue Zn in response to chronic aldosteronism.
First, aldosteronism is associated with a translocation of Zn from plasma to selected tissues. The tissue-specific distribution of Zn included injured skin at wk 1, which appeared in response to the surgical incisions involved with the implementation of this model, and injured heart and kidneys that appear with ALDOST.21 At wk 1 ALDOST, translocation of Zn to skin was seen after it had been incised twice: first to perform uninephrectomy; and second for the subcutaneous implantation of the minipump. In a rat incisional skin wound model, Lansdown et al.30 found an increase in Zn2+, Ca2+, and Mg2+ concentrations at the site of skin injury, which peaked on day 5 coincident with inflammatory cell invasion, granulation tissue formation, and epidermal cell proliferation. The appearance of MT protein was evident on days 1 through 5 in keeping with the increased metalloenzyme requirements needed for inflammatory and fibrogenic phases of repair. Translocation of Zn2+ in the tissue subsided when healing was completed. Wound healing in mice is likewise characterized by an early upregulation and synthesis of MT necessary to increase Zn2+ binding in injured skin and where increased intracellular Zn accumulation is found in proliferating cells.31 At 4 wks ALDOST, when wound healing at the incision site had been completed, skin Zn2+ was reduced from that found in controls and when skin releases its stored Zn2+ to sustain homeostasis during ongoing Zn2+ losses seen with ALDOST (vida infra).
We found tissue Zn levels to be increased in the heart and kidneys at wks 1 and 4 of ALDOST. A vasculopathy involving the intramyocardial coronary vasculature of the right and left heart, and intramural renal arterial circulation, accompanies chronic mineralocorticoid excess.21, 32, 33 These lesions include the invasion of the perivascular space by ED1+ monocytes/macrophages and CD4+ lymphocytes, together with the upregulation of adhesion molecules and chemokines, and the appearance of oxidative stress with activation of NADPH oxidase and redox-sensitive nuclear transcription factor (NF)-κB, as well as the proinflammatory genes it encodes.20, 32, 33 Cardiac Zn levels are similarly increased during the inflammatory phase of tissue repair that follows myocardial injury, except in necrotic cardiomyocytes where Zn uptake is no longer possible.34 Toxin-induced renal injury is accompanied by an early increase in tissue Zn.35 The increased uptake of Zn in injured tissues is invariably associated with upregulation of MT, where reactive oxygen species regulate MT expression.36–38 In this study, we did not address the cellular localization of MT expression at sites of injury, but would expect this to occur in viable parenchymal cells.39, 40 Using an immunohistochemical approach, Sato et al.39 found MT to be confined to hepatocytes of the cirrhotic rat liver (vis-à-vis areas of fibrosis).
Collectively, the preferential translocation of Zn to injured tissues and its enhanced excretory losses during ALDOST contribute to the fall in plasma Zn. This is not unlike the hypozincemia seen with burn injury and where losses include urinary excretion, wound exudate, and uptake in injured skin.5 To promote tissue healing and to improve recovery in the setting of such profound and acute Zn2+ losses, supplemental Zn is provided to patients with burn injury, often administered intravenously.5, 41 In this connection, treatment with exogenous Zn or a Zn ionophore (e.g., pyrrolidine dithiocarbamate) promotes healing, thus reducing potential cardiac and renal injury in models of tissue injury that include ALDOST, angiotensin II infusion, catecholamine administration, and ischemia/reperfusion.20, 33, 37, 42, 43
Our second major finding was the redistribution of plasma Zn to liver, skeletal muscle, and spleen, where its uptake was increased in the absence of tissue injury. As we found herein with ALDOST, the expression of MT-I during hormonal stress is upregulated consequent to an acute phase response to promote Zn2+ uptake in these tissues.12, 44 Radioactive 65Zn monitoring in patients with severe burn injury revealed a rapid uptake in liver.45 The Zn bound to liver MT is derived directly from plasma and indirectly from bone, delivered via the circulation.12 As also reported by Dunn & Cousins12 for cAMP treatment, we found Zn2+ uptake to be increased in the spleen during ALDOST, which is involved in the production and storage of lymphomyeloid cells involved in host defense mechanisms. These cells contribute to the inflammatory phase of tissue repair that occurs in the injured heart and kidneys during ALDOST.20, 21 Serum levels of C-reactive protein, an acute phase response biomarker, are increased in patients with heart failure and are related to its clinical severity and RAAS activation and portend a poor prognosis.15
Our third major finding is the reduction in Zn stored in bone and healed skin at wk 4 of ALDOST. Bone Zn represents 30% of its bodily stores and provides a rich source of Zn in an attempt to preserve Zn homeostasis during chronic Zn losses.22 In burn injury, bone Zn levels are reduced.45 Zinc derived by bone resorption, together with increased intestinal Zn absorption, are fundamental compensatory homeostatic responses when Zn uptake is suboptimal or Zn losses are persistent (e.g., via breast milk in lactating mothers).22 During periods of stress associated with marked Zn losses, such as seen with ALDOST or streptozotocin-induced diabetes, the mobilization of bone Zn stores may not be adequate to obviate the need for Zn supplementation.46 In keeping with this ongoing bone resorption that accompanies chronic ALDOST is a progressive reduction in bone Ca2+ and Mg2+, which is consequential to the appearance of secondary hyperparathyroidism that occurs in response to falling plasma-ionized Ca2+ and Mg2+ because of their increased fecal and urinary losses.24, 27
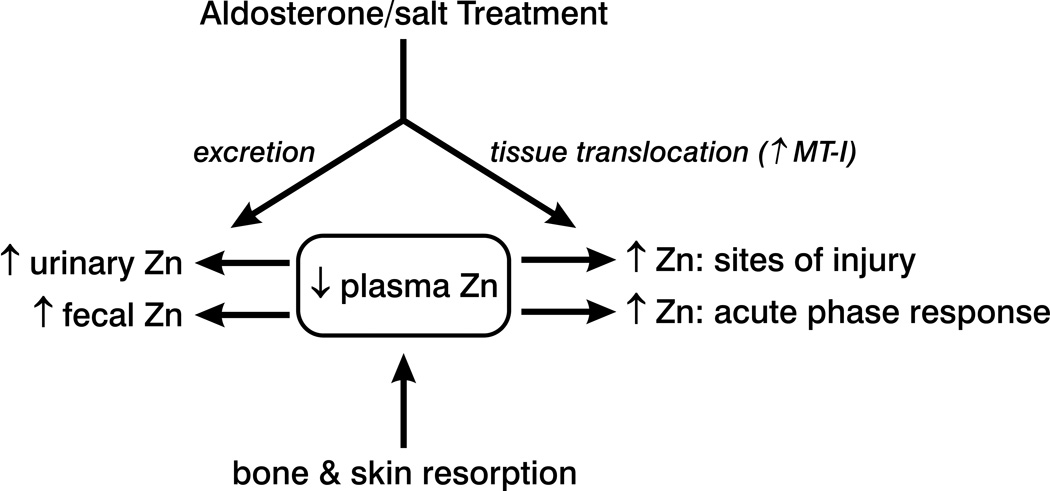
Zinc is an essential micronutrient because of its vital functions that are integral to a host of cellular responses involved in wound healing and cellular regeneration. In states of chronic neurohormonal activation, such as occurs in rats with ALDOST, a preferential translocation of Zn from plasma to selected tissues occurs based on the appearance of injury and an acute phase regenerative response. In the presence of persistent excretory Zn losses, such as the fecal and urinary Zn losses seen with ALDOST, a translocation of Zn appears to be coupled to its resorption from storage sites in bone and skin. Supplemental sources of Zn, under these circumstances, may have the therapeutic potential to sustain Zn homeostasis.
Figure 6.
A schematic overview of zinc translocation that occurs in rats receiving aldosterone/salt treatment and which leads to reductions in plasma zinc, or hypozincemia. These include its increased urinary and fecal excretion and its migration to tissue sites where an upregulated expression of a zinc-binding protein, metallothionein (MT)-1, takes place. These include sites of tissue injury and tissues involved in the acute phase response to stress, such as the liver. A resorption of zinc from storage sites in bone and skin also occurs in an attempt to restore extracellular zinc homeostasis.
Acknowledgement
We wish to acknowledge Richard A. Parkinson, MEd, for editorial assistance and scientific illustrations.
References
- 1.Hambidge M. Human zinc deficiency. J Nutr. 2000;130(5S Suppl):1344S–1349S. doi: 10.1093/jn/130.5.1344S. [DOI] [PubMed] [Google Scholar]
- 2.Sun Y, Cleutjens JPM, Diaz-Arias AA, et al. Cardiac angiotensin converting enzyme and myocardial fibrosis in the rat. Cardiovasc Res. 1994;28:1423–1432. doi: 10.1093/cvr/28.9.1423. [DOI] [PubMed] [Google Scholar]
- 3.Cleutjens JPM, Kandala JC, Guarda E, et al. Regulation of collagen degradation in the rat myocardium after infarction. J Mol Cell Cardiol. 1995;27:1281–1292. doi: 10.1016/s0022-2828(05)82390-9. [DOI] [PubMed] [Google Scholar]
- 4.Agay D, Anderson RA, Sandre C, et al. Alterations of antioxidant trace elements (Zn, Se, Cu) and related metallo-enzymes in plasma and tissues following burn injury in rats. Burns. 2005;31:366–371. doi: 10.1016/j.burns.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Voruganti VS, Klein GL, Lu HX, et al. Impaired zinc and copper status in children with burn injuries: need to reassess nutritional requirements. Burns. 2005;31:711–716. doi: 10.1016/j.burns.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 6.Tan IK, Chua KS, Toh AK. Serum magnesium, copper, and zinc concentrations in acute myocardial infarction. J Clin Lab Anal. 1992;6:324–328. doi: 10.1002/jcla.1860060513. [DOI] [PubMed] [Google Scholar]
- 7.Arnaud J, Faure H, Bourlard P, et al. Longitudinal changes in serum zinc concentration and distribution after acute myocardial infarction. Clin Chim Acta. 1994;230:147–156. doi: 10.1016/0009-8981(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 8.Lekakis J, Kalofoutis A. Zinc concentrations in serum as related to myocardial infarction. Clin Chem. 1980;26:1660–1661. [PubMed] [Google Scholar]
- 9.Altekin E, Coker C, Sisman AR, et al. The relationship between trace elements and cardiac markers in acute coronary syndromes. J Trace Elem Med Biol. 2005;18:235–242. doi: 10.1016/j.jtemb.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Sahin E, Gümüslü S. Stress-dependent induction of protein oxidation, lipid peroxidation and anti-oxidants in peripheral tissues of rats: comparison of three stress models (immobilization, cold and immobilization-cold) Clin Exp Pharmacol Physiol. 2007;34:425–431. doi: 10.1111/j.1440-1681.2007.04584.x. [DOI] [PubMed] [Google Scholar]
- 11.Cousins RJ, Dunn MA, Leinart AS, et al. Coordinate regulation of zinc metabolism and metallothionein gene expression in rats. Am J Physiol. 1986;251:E688–E694. doi: 10.1152/ajpendo.1986.251.6.E688. [DOI] [PubMed] [Google Scholar]
- 12.Dunn MA, Cousins RJ. Kinetics of zinc metabolism in the rat: effect of dibutyryl cAMP. Am J Physiol. 1989;256:E420–E430. doi: 10.1152/ajpendo.1989.256.3.E420. [DOI] [PubMed] [Google Scholar]
- 13.Koyanagi A, Kuffo D, Gresely L, et al. Relationships between serum concentrations of C-reactive protein and micronutrients, in patients with tuberculosis. Ann Trop Med Parasitol. 2004;98:391–399. doi: 10.1179/000349804225003424. [DOI] [PubMed] [Google Scholar]
- 14.Mayland C, Allen KR, Degg TJ, et al. Micronutrient concentrations in patients with malignant disease: effect of the inflammatory response. Ann Clin Biochem. 2004;41:138–141. doi: 10.1258/000456304322880032. [DOI] [PubMed] [Google Scholar]
- 15.Windram JD, Loh PH, Rigby AS, et al. Relationship of high-sensitivity C-reactive protein to prognosis and other prognostic markers in outpatients with heart failure. Am Heart J. 2007;153:1048–1055. doi: 10.1016/j.ahj.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 16.Arroyo M, LaGuardia SP, Bhattacharya SK, et al. Micronutrients in African-Americans with decompensated and compensated heart failure. Transl Res. 2006;148:301–308. doi: 10.1016/j.trsl.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Zozaya JL. Nutritional factors in high blood pressure. J Hum Hypertens. 2000;14 Suppl 1:S100–S104. doi: 10.1038/sj.jhh.1000995. [DOI] [PubMed] [Google Scholar]
- 18.Pironi L, Miglioli M, Ruggeri E, et al. Nutritional status of patients undergoing ileal pouch-anal anastomosis. Clin Nutr. 1991;10:292–297. doi: 10.1016/0261-5614(91)90009-2. [DOI] [PubMed] [Google Scholar]
- 19.Thomas M, Vidal A, Bhattacharya SK, et al. Zinc dyshomeostasis in rats with aldosteronism. Response to spironolactone. Am J Physiol Heart Circ Physiol. 2007;293:H2361–H2366. doi: 10.1152/ajpheart.00200.2007. [DOI] [PubMed] [Google Scholar]
- 20.Sun Y, Zhang J, Lu L, et al. Aldosterone-induced inflammation in the rat heart. Role of oxidative stress. Am J Pathol. 2002;161:1773–1781. doi: 10.1016/S0002-9440(10)64454-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun Y, Zhang J, Zhang JQ, et al. Local angiotensin II and transforming growth factor-β1 in renal fibrosis of rats. Hypertension. 2000;35:1078–1084. doi: 10.1161/01.hyp.35.5.1078. [DOI] [PubMed] [Google Scholar]
- 22.Moser-Veillon PB. Zinc needs and homeostasis during lactation. Analyst. 1995;120:895–897. doi: 10.1039/an9952000895. [DOI] [PubMed] [Google Scholar]
- 23.Gerling IC, Sun Y, Ahokas RA, et al. Aldosteronism: an immunostimulatory state precedes the proinflammatory/fibrogenic cardiac phenotype. Am J Physiol Heart Circ Physiol. 2003;285:H813–H821. doi: 10.1152/ajpheart.00113.2003. [DOI] [PubMed] [Google Scholar]
- 24.Chhokar VS, Sun Y, Bhattacharya SK, et al. Hyperparathyroidism and the calcium paradox of aldosteronism. Circulation. 2005;111:871–878. doi: 10.1161/01.CIR.0000155621.10213.06. [DOI] [PubMed] [Google Scholar]
- 25.Ahokas RA, Sun Y, Bhattacharya SK, et al. Aldosteronism and a proinflammatory vascular phenotype. Role of Mg2+, Ca2+ and H2O2 in peripheral blood mononuclear cells. Circulation. 2005;111:51–57. doi: 10.1161/01.CIR.0000151516.84238.37. [DOI] [PubMed] [Google Scholar]
- 26.Ahokas RA, Warrington KJ, Gerling IC, et al. Aldosteronism and peripheral blood mononuclear cell activation. A neuroendocrine-immune interface. Circ Res. 2003;93:e124–e135. doi: 10.1161/01.RES.0000102404.81461.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chhokar VS, Sun Y, Bhattacharya SK, et al. Loss of bone minerals and strength in rats with aldosteronism. Am J Physiol Heart Circ Physiol. 2004;287:H2023–H2026. doi: 10.1152/ajpheart.00477.2004. [DOI] [PubMed] [Google Scholar]
- 28.Vidal A, Sun Y, Bhattacharya SK, et al. The calcium paradox of aldosteronism and the role of the parathyroid glands. Am J Physiol Heart Circ Physiol. 2006;290:H286–H294. doi: 10.1152/ajpheart.00535.2005. [DOI] [PubMed] [Google Scholar]
- 29.Mager H, Göller G. Resampling methods in sparse sampling situations in preclinical pharmacokinetic studies. J Pharm Sci. 1998;87:372–378. doi: 10.1021/js970114h. [DOI] [PubMed] [Google Scholar]
- 30.Lansdown AB, Sampson B, Rowe A. Sequential changes in trace metal, metallothionein and calmodulin concentrations in healing skin wounds. J Anat. 1999;195(Pt 3):375–386. doi: 10.1046/j.1469-7580.1999.19530375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iwata M, Takebayashi T, Ohta H, et al. Zinc accumulation and metallothionein gene expression in the proliferating epidermis during wound healing in mouse skin. Histochem Cell Biol. 1999;112:283–290. doi: 10.1007/s004180050449. [DOI] [PubMed] [Google Scholar]
- 32.Mervaala EM, Müller DN, Park JK, et al. Monocyte infiltration and adhesion molecules in a rat model of high human renin hypertension. Hypertension. 1999;33(1 Pt 2):389–395. doi: 10.1161/01.hyp.33.1.389. [DOI] [PubMed] [Google Scholar]
- 33.Muller DN, Dechend R, Mervaala EM, et al. NF-κB inhibition ameliorates angiotensin II-induced inflammatory damage in rats. Hypertension. 2000;35(1 Pt 2):193–201. doi: 10.1161/01.hyp.35.1.193. [DOI] [PubMed] [Google Scholar]
- 34.Yarom R, Wisenberg E, Peters PD, et al. Zinc distribution in injured myocardium. EMMA-4 examinations of dogs' hearts after coronary ligation. Virchows Arch B Cell Pathol. 1977;23:65–77. [PubMed] [Google Scholar]
- 35.Scibior A, Zaporowska H. Effects of vanadium(V) and/or chromium(III) on L-ascorbic acid and glutathione as well as iron, zinc, and copper levels in rat liver and kidney. J Toxicol Environ Health A. 2007;70:696–704. doi: 10.1080/15287390601187906. [DOI] [PubMed] [Google Scholar]
- 36.Song Y, Wang J, Li Y, et al. Cardiac metallothionein synthesis in streptozotocin-induced diabetic mice, and its protection against diabetes-induced cardiac injury. Am J Pathol. 2005;167:17–26. doi: 10.1016/S0002-9440(10)62949-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karagulova G, Yue Y, Moreyra A, et al. Protective role of intracellular zinc in myocardial ischemia/reperfusion is associated with preservation of protein kinase C isoforms. J Pharmacol Exp Ther. 2007;321:517–525. doi: 10.1124/jpet.107.119644. [DOI] [PubMed] [Google Scholar]
- 38.Andrews GK. Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem Pharmacol. 2000;59:95–104. doi: 10.1016/s0006-2952(99)00301-9. [DOI] [PubMed] [Google Scholar]
- 39.Sato S, Shimizu M, Hosokawa T, et al. Distribution of zinc-binding metallothionein in cirrhotic liver of rats administered zinc. Pharmacol Toxicol. 2000;87:292–296. doi: 10.1034/j.1600-0773.2000.pto870609.x. [DOI] [PubMed] [Google Scholar]
- 40.Wang ZH, Iguchi H, Ohshio G, et al. Increased pancreatic metallothionein and glutathione levels: protecting against cerulein- and taurocholate-induced acute pancreatitis in rats. Pancreas. 1996;13:173–183. doi: 10.1097/00006676-199608000-00009. [DOI] [PubMed] [Google Scholar]
- 41.Berger MM. Antioxidant micronutrients in major trauma and burns: evidence and practice. Nutr Clin Pract. 2006;21:438–449. doi: 10.1177/0115426506021005438. [DOI] [PubMed] [Google Scholar]
- 42.Singal PK, Kapur N, Dhillon KS, et al. Role of free radicals in catecholamine-induced cardiomyopathy. Can J Physiol Pharmacol. 1982;60:1390–1397. doi: 10.1139/y82-207. [DOI] [PubMed] [Google Scholar]
- 43.Wang J, Song Y, Elsherif L, et al. Cardiac metallothionein induction plays the major role in the prevention of diabetic cardiomyopathy by zinc supplementation. Circulation. 2006;113:544–554. doi: 10.1161/CIRCULATIONAHA.105.537894. [DOI] [PubMed] [Google Scholar]
- 44.Cho K, Adamson L, Jeong J, et al. Alterations in the levels of metallothionein and metals in the liver, and unique serum liver enzyme response in metallothionein knock-out mice after burn injury. Pathobiology. 2004;71:223–230. doi: 10.1159/000078677. [DOI] [PubMed] [Google Scholar]
- 45.van Rij AM, Hall MT, Bray JT, et al. Zinc as an integral component of the metabolic response to trauma. Surg Gynecol Obstet. 1981;153:677–682. [PubMed] [Google Scholar]
- 46.Uchiyama S, Yamaguchi M. Alteration in serum and bone component findings induced in streptozotocin-diabetic rats is restored by zinc acexamate. Int J Mol Med. 2003;12:949–954. [PubMed] [Google Scholar]