Abstract
The aqueous extraction process of the leaves of Rubus suavissimus often brings in a large amount of non-active polysaccharides as part of the constituents. To purify this water extract for potential elevated bioactivity, alcohol precipitation (AP) consisting of gradient regimens was applied, and its resultants were examined through colorimetric and HPLC analyses. AP was effective in partitioning the aqueous crude extract into a soluble supernatant and an insoluble precipitant, and its effect varied significantly with alcohol regimens. Generally, the higher the alcohol concentration, the purer was the resultant extract. At its maximum, approximately 36% (w/w) of the crude extract, of which 23% was polysaccharides, was precipitated and removed, resulting in a purified extract consisting of over 20% bioactive marker compounds (gallic acid, ellagic acid, rutin, rubusoside, and steviol monoside). The removal of 11% polysaccharides from the crude water extract by using alcohol precipitation was complete at 70% alcohol regimen. Higher alcohol levels resulted in even purer extracts, possibly by removing some compounds of uncertain bioactivity. Alcohol precipitation is an effective way of removing polysaccharides from the water extract of sweet tea plant and could be used as an initial simple purification tool for many water plant extracts that contain large amounts of polysaccharides.
Keywords: Rubus suavissimus, Chinese sweet leaf tea, alcohol precipitation, purification
INTRODUCTION
Rubus suavissimus S. Lee (Rosaceae) is a perennial shrub widely abundant in Guangxi and Guizhou province of China. The leaf of R. suavissimus is the material to make beverage leaf tea by the local residents. Due to its intensely sweet flavor, it is better known as “tiancha” in Chinese, or “Chinese sweet tea”. The sweet taste from the leaf is attributed to the presence of diterpene glucosides, dominated by the major sweet principle rubusoside (1). Rubusoside has a slightly bitter aftertaste, but it is about 115 times sweeter than sucrose at a concentration of 0.025%, making it a good candidate for a natural sweetener (2, 3). Other diterpene glucosides contributing to the sweetness and bitterness of the leaf include the sweet glycosides, suavioside A (4), suaviosides B, G, H, I, and J, as well as the bitter glycoside, suaviosides C1, D2, and F (3).
In addition to the use of rubusoside as a natural sweetener, Chinese sweet leaf has also been used as a folk medicine to treat various diseases. For example, in southern China, it is used as a traditional remedy for alleviating hypertension, diabetes, atherosclerosis, and maintaining healthy kidneys as well as to relieve coughs (5). Recent studies have also demonstrated that sweet leaf exhibits anti-inflammatory, anti-allergic (6, 7), and anti-angiogenic activities (8). As a potential natural inhibitor of angiogenesis, sweet leaf tea extract has been reported to be capable of reducing corneal neovascularization in experimental rodents (9). Furthermore, the ability of sweet leaf to inhibit the transcription factor NF-κB (10) and α-amylase activity (11) may also prevent certain metabolic diseases such as diabetes and obesity.
Traditionally, the sweet leaf as beverage or folk medicine was prepared using boiling water or decoction. This preparation recovered bioactive compounds such as gallic acid, rutin, ellagic acid, rubusoside, and steviol monoside as well as other yet-to-be identified compounds (unpublished data) in a water extract of the sweet leaf. These bioactive compounds may play an important role in the development of pharmaceutical and food products. For instance, gallic acid is one of the active compounds that has potent anti-angiogenic (8) and alpha-glucosidase inhibitory (12) activities. Ellagic acid and rutin, on the other hand, are strong antioxidants (13, 14), and both compounds may also be responsible for anti-inflammatory activities (15, 16). In addition, ellagic acid also possesses potent α-amylase inhibitory (11) and anti-cancer (17, 18) properties. Although this preparation method may have extracted the majority of the bioactive components from the leaf material, it also pulls out a large amount of water-soluble polysaccharides and possibly other macromolecules such as proteins that are not bioactive, resulting in a crude leaf extract that has room for additional purification.
A purified extract with potentially improved bioactivity is highly desirable in many ways, including but not limited to reaching an effective dose range in a practical dosage. The first line of purification is often associated with the removal of polysaccharides that are not bioactive yet are highly extractable by boiling water. Alcohol precipitation is often used to achieve this initial purification of an aqueous extract. This alcohol precipitation method is simple, rapid, easily scalable, and cost effective in the removal of polysaccharides. Conventional applications of alcohol precipitation methods are mainly seen during the purification of plant DNA and RNA (19, 20) as well as isolation of biologically active polysaccharides (21–23). However, employing alcohol precipitation to purify active components from plant extracts is less common. Alcohol precipitation of crude plant extracts could separate macromolecules and polymers from small molecules including those of 1000 Daltons or less such as gallic acid, ellagic acid, rutin, and rubusoside. Therefore, it is hypothesized that during the precipitation process, most of the polymers, such as polysaccharides and proteins, will precipitate while the small molecules will stay in the supernatant solution. In the present study, this hypothesis was tested through a series of experiments and quantitative analyses. Qualitative and quantitative analyses of the purified extracts were performed using high performance liquid chromatography (HPLC) with the focus on the five bioactive components as adopted from Chou et al. (24). Total polysaccharide in the precipitated extracts was measured by the phenol-sulfuric acid colorimetric method. It is hoped that this simple but effective method could be demonstrated for the sweet leaf tea extract and used to achieve satisfactory degrees of purification of many other bioactive botanical extracts.
MATERIALS AND METHODS
The sweet leaf material
The sweet leaf tea plants (Rubus suavissimus S. Lee; Rosaceae) used in this study grew on a farm in Guizhou province, China. A voucher specimen was obtained and deposited in the Herbarium of the Louisiana State University. Fresh sweet leaves from the sweet leaf tea plants were harvested in June and air-dried.
Preparation of the crude extract
The crude extract was prepared by the industry. The air-dried leaves were extracted with distilled water at approximately 1:15 w/v ratio. After soaking for 1 h, the decoction was brought to boil for 60 min. The liquid extract was separated from the solids by filtration with an approximately 100-µm filter screen and cloth, and by still precipitation. In the industry operation, filtration and still gravity centrifugation were more cost effective than other separation methods such as centrifugation. Thus, the particular procedure was chosen in the preparation of crude extract. Later, the filtered supernatant liquid extract was concentrated and subsequently spray-dried to powder and designated as the crude extract RUS (batch RUS20040306).
Alcohol precipitation (AP)
The crude extract (RUS) prepared above was converted to aqueous solutions first by re-constituting the extract in deionized water at 1:4 w/v ratio, with the assistance of heat and stirring as needed. This aqueous solution was also used as a control regimen expressed as 0% AP (AP-0) subjected to no alcohol precipitation. For other regimens, appropriate volumes of ethanol (EtOH) were then added to the water extracts to achieve final ethanol concentrations of 10% (RUS-10), 20% (RUS-20), 30% (RUS-30), 40% (RUS-40), 50% (RUS-50), 60% (RUS-60), 70% (RUS-70), 80% (RUS-80), 90% (RUS-90), and 95% (RUS-95), respectively. These AP solutions were sealed with parafilm to avoid contaminations and minimize evaporation. Then, the solution was let stand for an hour at 4°C. Supernatant and precipitant were separated through centrifugation. The supernatant was removed and the precipitant was rinsed five times, each time with approximately 20 mL of appropriate ethanol regimens. Supernatant solutions were combined and filtered with filter papers (Whatman#4) (Whatman, Maidstone, Kent ME14 2LE, UK), then concentrated to remove ethanol, and freeze-drying to powder. The precipitants, on the other hand, were subjected to freeze-dry to yield powdered samples. Dry weights of supernatant and precipitant samples from each regimen were obtained and the yield (% w/w) was calculated respectively. Each regimen was done in five replicates.
Measurement of polysaccharide in the precipitants by phenol-sulfuric colorimetric method
Development of a Standard Curve
The phenol-sulfuric colorimetric method was a modified method adapted from Gao et al. (25), and glucose was used as a standard in the determination of total polysaccharides (PSAC) in the precipitant samples. A glucose stock solution was prepared at a concentration of 0.04 mg/mL. Subsequently, 0.2 mL, 0.4 mL, 0.6 mL, 0.8 mL, 1.0 mL, 1.2 mL, 1.4 mL, or 1.6 mL of the glucose stock solution was transferred to a test tube and brought up to 2 mL with deionized water, respectively. A blank solution was prepared with 2 mL of deionized water without glucose. These preparations resulted in a range of glucose concentrations from 0.008 mg/mL to 0.064 mg/mL. Then, 0.5 mL of 6% phenol solution (Sigma-Aldrich, St. Louis, MO) was added into each test tube, followed by addition of 5.5 mL of 98% sulfuric acid. Each tube was mixed well and placed at room temperature for 30 minutes. The optimum absorbance of the reacted solution was measured at 490 nm using the Ultraviolet (UV)-Visible Spectrophotometer (Beckman DU 720, Fullerton, CA). A standard curve was obtained by three replications.
Precipitant Sample Purification
For the purification process, 20 mg of precipitant sample was dissolved in absolute EtOH at a ratio of 1:20 w/v. The solution was sonicated for 30 min and centrifuged at 2060 × g for 10 min. The supernatant was discarded and the precipitant (containing polysaccharides) was dried. The dried precipitant was then dissolved in a 25-mL volumetric flask with deionized water. The solution was centrifuged to remove excessive insoluble residue, if any. This was the sample stock solution.
Determination of the Glucose-Equivalent Polysaccharide Content
Pipetted 0.1 mL of the sample stock solution prepared above into a test tube and brought up to 2 mL using deionized water. A blank solution was prepared with 2 mL of deionized water without sample stock solution. Then, 0.5 mL of 6% phenol solution was added into each test tube followed by the addition of 5.5 mL of 98% sulfuric acid. Each tube was mixed well and placed at room temperature for 30 min. The absorbance of reacted solution was measured at 490 nm using the UV-Visible Spectrophotometer. Glucose concentration and amount were obtained based on the standard curve. Polysaccharides content was expressed as glucose-equivalent polysaccharide in percentage.
Recovery Rate
Dissolve 10 mg of a precipitant sample with a known amount of glucose-equivalent polysaccharide in deionized water at a ratio of 1: 20 w/v. Then, 0.1 mL of the above solution was pipetted into a test tube followed by an addition of glucose in the amount equivalent to that found in the respective precipitant sample. The solution was then brought to 2 mL with deionized water and 0.5 mL of 6% phenol was added into each test tube followed by an addition of 5.5 mL of 98% sulfuric acid. The contents of each tube were mixed well and placed at room temperature for 30 min. The absorbance of reacted solution was measured at 490 nm using the UV-Visible Spectrophotometer. Recovery rate was calculated based on the following formula with five replications: Recovery rate (%): [(Total Polysaccharides (mg) − Known Polysaccharides (mg) from precipitant sample) / Added Glucose (mg)] × 100
HPLC analysis of the purified supernatant sample
Reference Standards
Gallic acid (GA; Purity > 98%), rutin (RUT; Purity > 95%), and ellagic acid (EGA; Purity > 95%) were purchased from Sigma Chemical Company (St. Louis, MO). The reference standards of rubusoside (RUB) and steviol monoside (SM) were isolated by our own lab and identified by spectral data (UV, MS, 1H NMR, 13C NMR and 2D-NMR). Both RUB and SM have purities greater than 98% by HPLC–PDA analyses based on a peak-area normalization method.
HPLC Conditions
An HPLC system consisting of a Waters (Milford, MA) 600 pump, a 717 auto-sampler, and a UV/Vis Photodiode Array (PDA) 2996 Detector was used for all analyses. Chromatographic separations were carried out on an Alltech Prevail C18 column (250 mm × 4.6 mm, 5 µm) with a C18 Guard column (7.5 mm×4.6 mm, 5 µm). The mobile phase consisted of solvent A (0.17% phosphoric acid in acetonitrile) and solvent B (0.17% phosphoric acid in water). The elution profile for A was: 0–65 min, linear gradient of 5–30%; 65–85 min, linear gradient of 30–60%; 85–90 min, linear gradient of 60–70%; and 90–100 min, isocratic 70%. A pre-equilibration period of 20 min was used between individual runs. Column temperature was set at 25°C. The flow rate was 1.0 mL/min and the injection volume was 10 µL. All compounds were detected at dual wavelengths of 254 (for GA, RUT, and EGA) and 205 (for RUB and SM) to derive combined chromatograms.
Statistical analysis
Data were analyzed with Statistical Analysis System (SAS, Cary, NC). Regression analysis was performed to examine the correlation between the response yield and AP regimen. Statistical significance of all tests was set at P ≤ 0.05.
RESULTS
Aqueous sample preparations prior to alcohol precipitation
Prior to performing alcohol precipitation of the aqueous extract samples, various extract-to-water ratios ranging from 1:4 w/v to 1:8 w/v were tested to determine the amount of precipitant (thus the reciprocal amount of the purified extract) caused by extract solubility itself. It was found that at the ratio of 1:4 w/v (i.e., 250 mg/ml), approximately 11% of the extract had already precipitated in water prior to the addition of alcohol. The yield of precipitant showed a constant rate of insignificant decrease of 0.2% w/w between the ratios of 1:4 w/v and 1:6 w/v, then leveled off at 10.6% w/w. Based on these results, the partitioning of each sample due to alcohol precipitation was adjusted and normalized by eliminating the insoluble effect averaging at 11%.
Yields of the purified and precipitant extracts in response to alcohol precipitation
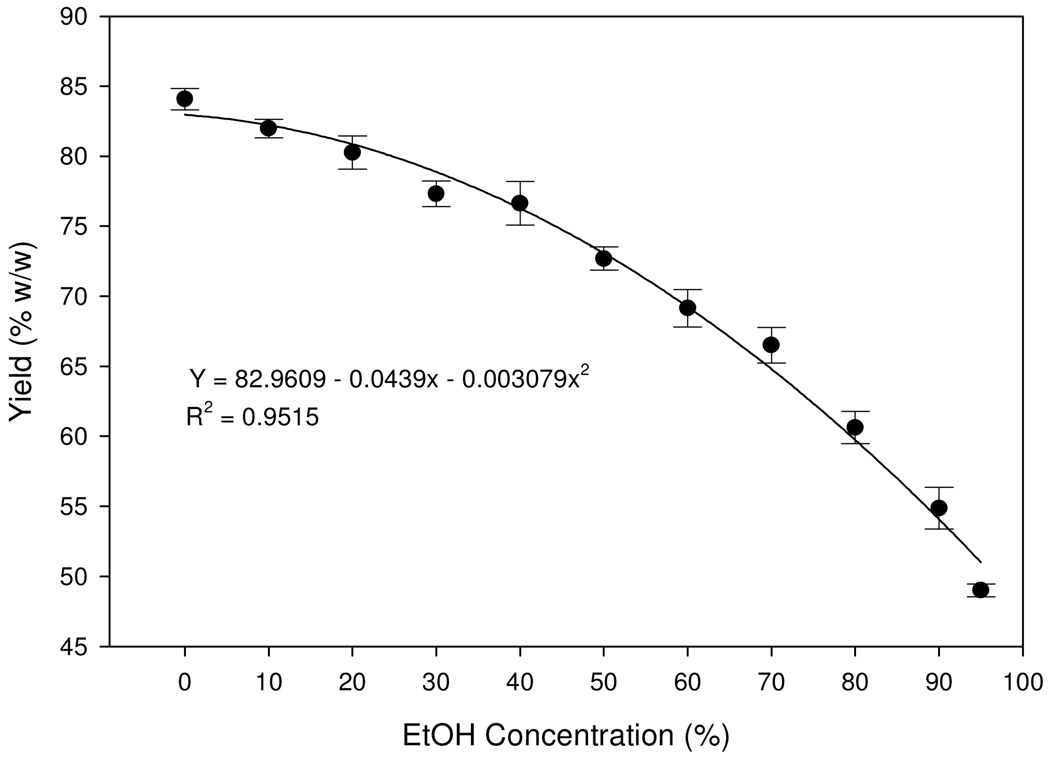
AP was successful in partitioning the crude extract into a soluble supernatant (the purified extract) and insoluble precipitant. The yields of the purified extracts in the form of supernatants decreased exponentially from 94% to 55% as the aqueous ethanol concentrations increased from 0% (control) to 95% (Fig. 1). The highest effect of alcohol precipitation occurred at the 95% AP regimen, which precipitated 36% of the crude extract mass as impure components, leaving 55% of the weight in the supernatant solution, which was later dried to become the purified extract. The unaccounted 9% was a loss during the process of filtration, concentration, or freeze-drying.
Fig. 1.
Yield of the purified extract (supernatant) as a result of alcohol precipitation (AP) regimens (n=5). Quadratic regression model was fitted. Values are expressed as mean ± standard error (vertical line; n=5).
Changes of marker compounds in the purified extract to alcohol precipitation
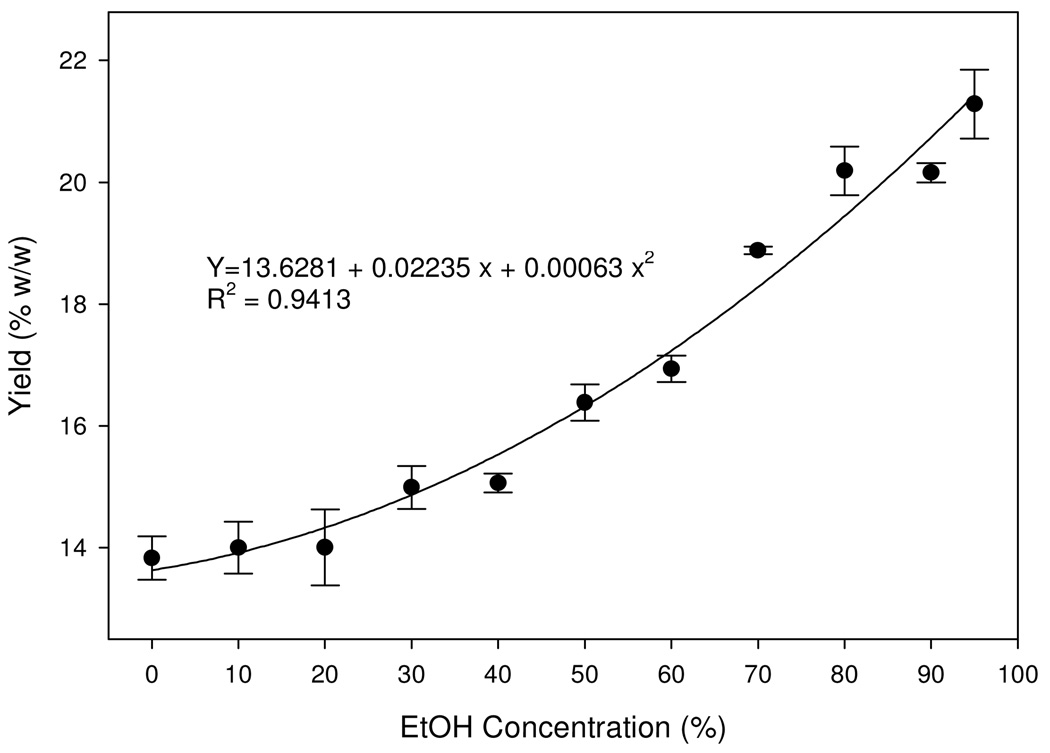
As the ethanol concentrations increased in the aqueous solutions, the contents of the combined five marker compounds increased in the solutions (Fig. 2). In the 95% aqueous ethanol solution where the content of the five markers was highest, the marker compounds accounted for over 20% by weight of the purified extract, a significant 8% increase from the 10% AP regimen.
Fig. 2.
Changes of contents of the total five marker compounds in the purified extract in response to alcohol precipitation (AP). Quadratic regression model was fitted. Values are expressed as mean ± standard error (vertical line; n=3).
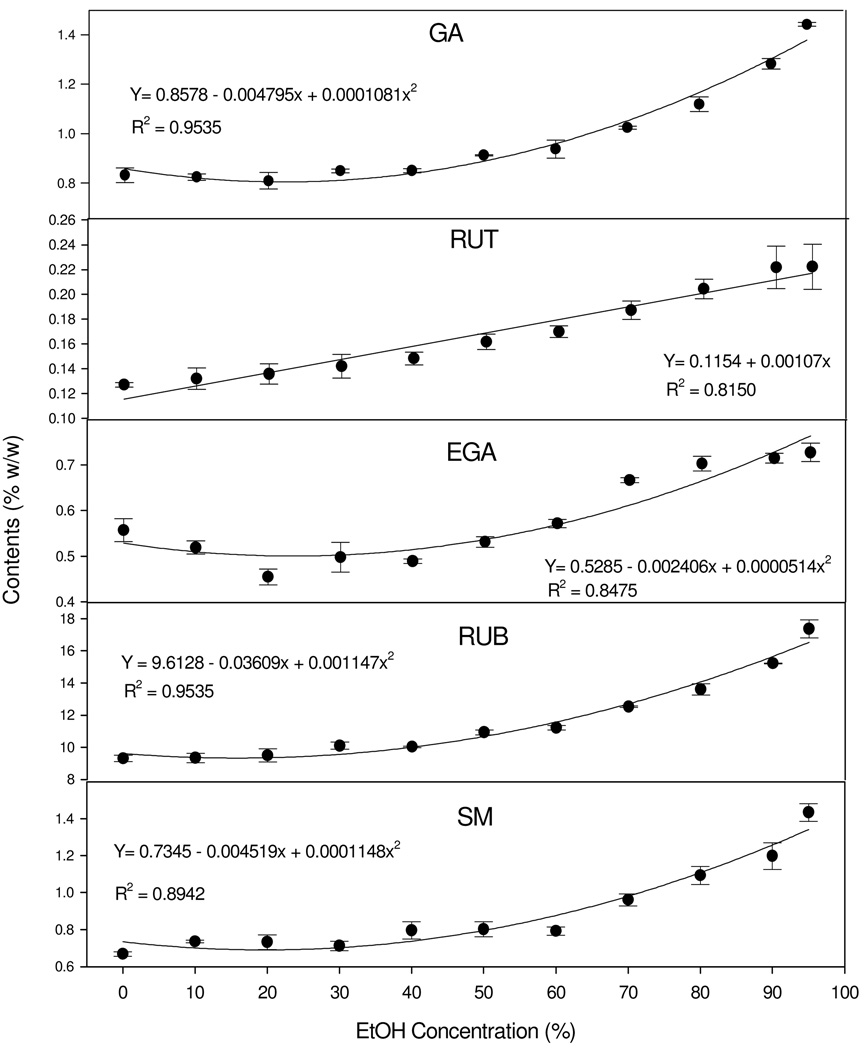
To further illustrate the changing trend of each individual compound, regression models were used to determine the relationships. Generally, all markers were continuously concentrated by increasing AP levels, but the pace of and maximum change differed among the markers (Fig. 3). Among them, GA, EGA, RUB, and SM had quadratic relationships (P < 0.001) characteristic of a slow or nearly zero rate of increase at lower alcohol concentrations followed by a rapid rate of increase. For example, the contents of GA, EGA, RUB, and SM in the purified extract seldom changed up to 40% AP regimens, but increased linearly and significantly after this point to a maximum at 95% AP regimen. In contrast, the relationship of RUT and AP regimens was linear (P <0.001). RUT content increased at a constant rate as AP regimens moved upward.
Fig. 3.
Changes of each marker compound in the purified extract (supernatant) corresponding to ethanol concentrations in the AP experiments. A simple linear regression model was fitted for RUT. Quadratic linear models were fitted for the contents of GA, EGA, RUB, or SM. All values are expressed as mean ± standard error (n=3).
Changes of chemical components in the precipitant to alcohol precipitation
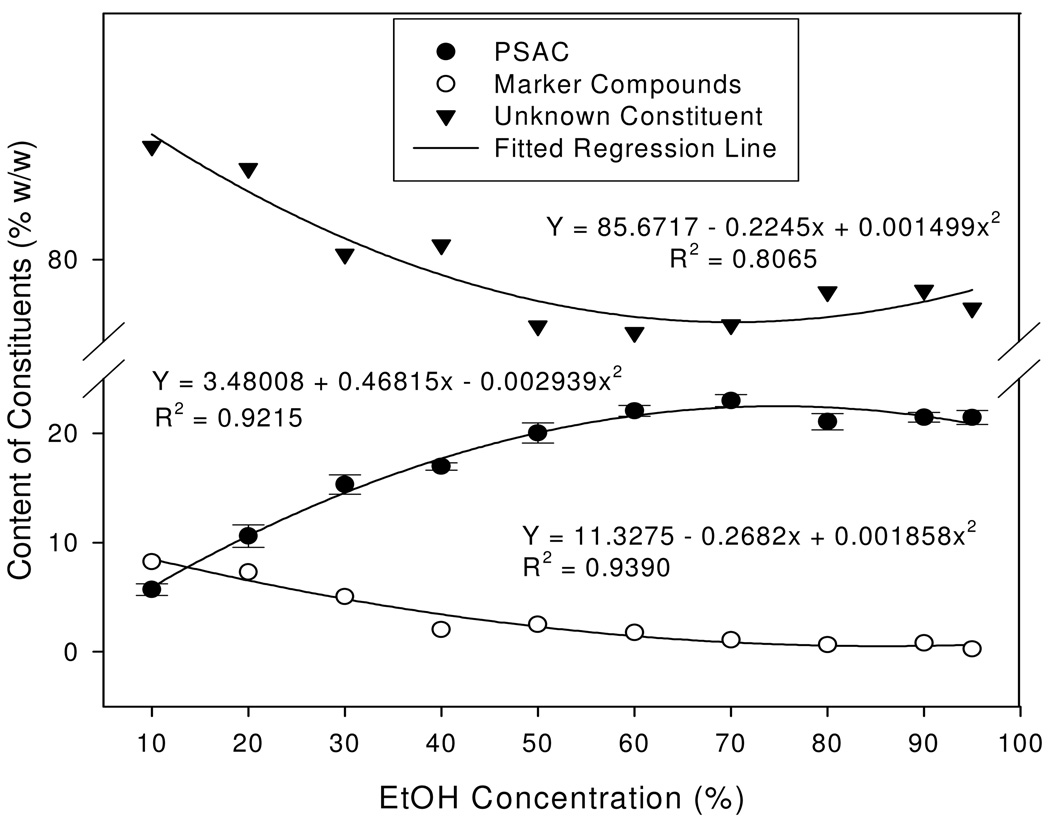
Marker compounds were minor components in the precipitant to begin with. At a low alcohol concentration of 10%, marker compounds accounted for approximately 8% (w/w). As the alcohol concentrations increased from 10% to 50%, the contents of the marker compounds decreased at nearly a linear rate to 2.8%, reflecting an obviously higher affinity of marker compounds with ethanol than with water. Further increases in alcohol concentrations of 60% or higher caused an additional 1.2% decrease of the marker compounds, to rest at a final 1.6% in the precipitant (Fig. 4).
Fig. 4.
Changes of contents of the five marker compounds, polysaccharides (PSAC), and unknown constituents in the precipitant in response to alcohol precipitation (AP). Quadratic regression model was fitted. Values are expressed as mean ± standard error (vertical line; n=3).
Polysaccharide (PSAC), on the other hand, was a major component in the precipitant, a target component to be removed by alcohol precipitation. The total polysaccharides content was measured using the phenol-sulfuric acid colorimetric method. A standard curve (n=3) was developed and validated using glucose as a standard compound. The R2 was 0.993 and the recovery rate was 98.79% with a relative standard deviation (RSD) of 2.74. All polysaccharides measurements were expressed as glucose-equivalent polysaccharide. Polysaccharides content in the precipitant linearly increased fourfold but stopped at 70% alcohol concentration when maximal saturation was reached (Fig. 4). At that point, polysaccharides accounted for 22.97% of the precipitant and approximately 11% of the crude extract by weight. Leveling off of polysaccharide content at the 70% AP regimen was an indication of complete removal of polysaccharides. The observed continued slight increases in the precipitant yield were accompanied by the slight re-bounce of the unknown components. These components analyses, hence, demonstrated the positive effect of AP in purifying the bioactive compounds (markers) in the sweet tea extract through the removal of polysaccharides. The other unknown constituents in the precipitant followed the similar response patterns to the marker compounds except at 80% AP or higher, where there was a slight re-bounce (Fig. 4).
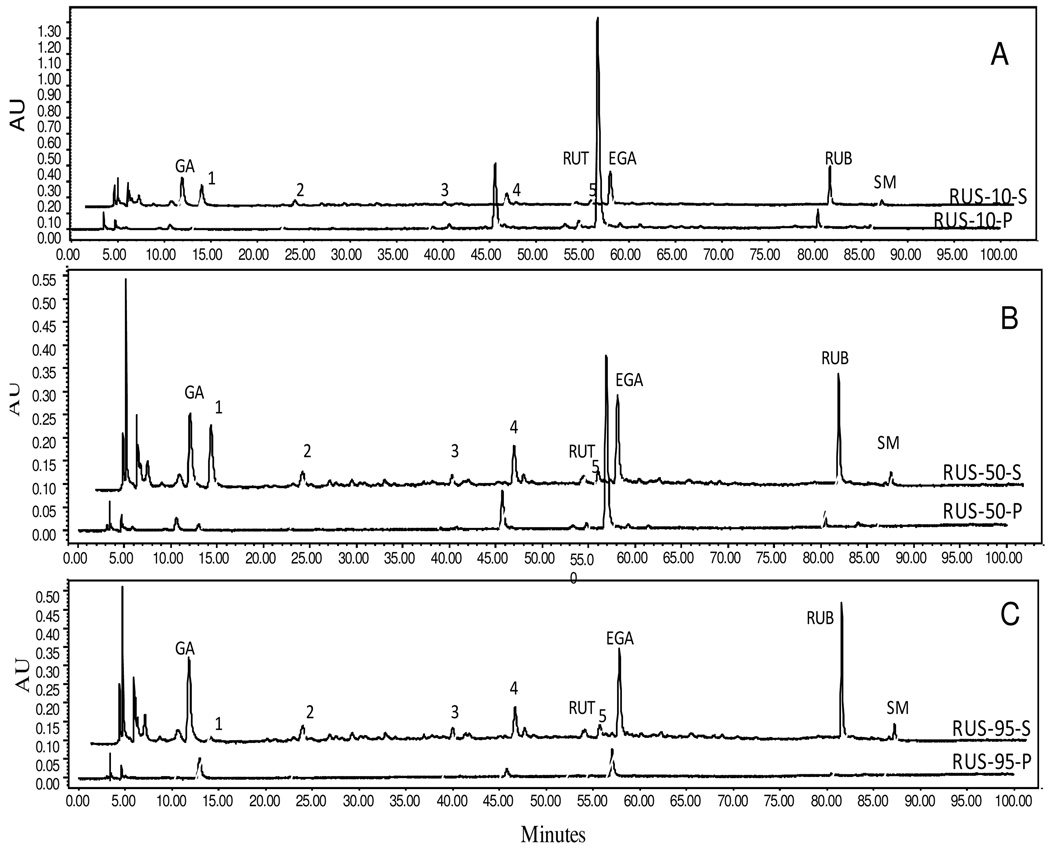
Chromatographic fingerprints of the purified and precipitated extracts
Fingerprints were developed for the purified and precipitated samples to gain insight into the differences in composition benchmarked by the five marker compounds and unknown but characteristic peaks (1–5) that responded noticeably to alcohol precipitation.
At the 10% alcohol concentration, more GA, Peak 1, Peak 2, and RUB were in the supernatant solution than in the precipitant; less Peak 3, Peak 4, RUT, and Peak 5 were in the solution than in the precipitant; much less EGA was in the solution than in the precipitant; and SM was equal in the solution and precipitation (Fig. 5A). At the 50% alcohol concentration, the majority of GA, Peak 1, Peak 2, Peak 3, and RUB were in the supernatant solution; Peak 4, RUT, and Peak 5 were equally split between the solution and precipitant; more EGA was in the precipitant than in the solution; and SM was all in solution (Fig. 5B). When the alcohol concentration increased to 95%, some obvious shifts of partitioning patterns were observed. GA, Peak 3, RUT, Peak 4, Peak 5, and RUB were almost completely in solutions, similar to SM already started at the 50% AP regimen (Fig. 5C). Peak 2 remained unchanged. Conversely, Peak 1 and EGA changed to opposite directions relative to their responses to 50% alcohol concentration. Most of Peak 1 (or the component it represented) went to the precipitant at the 95% AP regimen, whereas most of it stayed in the solution at the 50% AP regimen. While more EGA was in the precipitant at the 50% AP regimen, it was apparently much less at the 95% AP regimen.
Fig. 5.
Chromatographic fingerprints of the Chinese sweet leaf tea (Rubus suavissimus) extracts subjected to alcohol precipitation (AP). Selected chromatograms demonstrate the partitioning of the five marker compounds between the supernatant (S) and precipitant (P) fractions as results of 0% EtOH (RUS-0), 50% EtOH (RUS-50), or 95% EtOH (RUS-95) treatments. Presence of the five marker compounds is indicated as gallic acid (GA), rutin (RUT), ellagic acid (EGA), rubusoside (RUB), and steviol monoside (SM). Peaks 1– 5 are unknown compounds. NOTE: Alignment may be slightly off for some fingerprints due to graphic demonstration purpose.
DISCUSSION
Alcohol precipitation allows the separation of small molecules from polymers, and therefore is a useful initial purification procedure for desired chemical compounds. This procedure has been applied in many studies to separate biologically active polysaccharides (21–23) from other components in botanical samples. In our current study, however, we found that the bioactive compounds in the crude extract of the Chinese sweet tea plant are secondary metabolites with low molecular weight, rather than those of macromolecules such as polysaccharides. Thus, in order to eliminate polysaccharides or other polymers in the crude extract prepared by boiling water, a simple yet effective method was sought to purify the bioactive small molecules in the crude extract. In this study, the alcohol precipitation method, commonly used to obtain pure polysaccharides, was examined for its effectiveness of removing rather than purifying polysaccharides from the crude water extract of the Chinese sweet leaf tea. Schmourla et al. (22) demonstrated that there was a clear difference of antifungal activity in medicinal and food plants between the supernatant and precipitant phases using AP as a purification procedure. The separation which resulted from their respective study did enhance the antifungal activity of certain plants.
Another study on the honeysuckle flowers (26) also clearly illustrated the improvement of chlorogenic acid purity from 31.62% to 37.72% after the precipitation of crude extract with 60% ethanol. Our current finding that the contents of selected bioactive marker compounds were significantly increased in a concentration-dependant manner was similar. Using the alcohol precipitation method, the crude extract of sweet tea was purified by one-fold through the removal of 11% polysaccharides and other yet-to-be identified components. The separation of mass to this magnitude by a single step shows the efficiency and cost-effectiveness of alcohol precipitation in obtaining purer botanical samples.
Most interestingly, the degree of purity of the finished product can be readily controlled so that the purified extract contains the levels of desired components. For example, to safely remove all polysaccharides without removing unnecessary unknown components, 70% alcohol concentrations would be sufficient and appropriate, and alcohol strength higher than 70% would cause additional precipitation of unknown components, such as that represented by Peak 1. Although the exact mechanism is unknown, it may be due to the intrinsic affinity nature of the compound itself in Peak 1. While Peak 1 was found highest in the supernatant at 70% ethanol, either too high (e.g., 95%) or too low (e.g., 10%) returned this compound to the precipitant. Apparently, this compound has higher affinity to water than to the ethanol. Another example in our study was EGA (ellagic acid) where the absorption peak was found highest in the precipitant at 10% alcohol. In fact, as the concentration of ethanol increased from 10% to 95%, the EGA content decreased by almost 15-fold in the precipitant. On the other hand, in the supernatant solution, the yield of EGA increased with the alcohol regimens and then reached a plateau at 80% AP. These phenomena clearly suggested greater affinity of EGA to ethanol than water. Liu (27) also reported that most starch, protein, polysaccharides, inorganic salts, or other polymers can be removed by AP when using 80% ethanol. Due to the concern of losing small molecules with high alcohol concentrations (e.g., higher than 80%), 60% to 75% ethanol is usually recommended to avoid additional loss of bioactive metabolites (27) Therefore, our detailed quantitative and qualitative fingerprint analyses over the fractionated extracts as a result of alcohol precipitation provide the best understanding of the partitioning behaviors of each known component as well as some unknown compounds so specific alcohol precipitation regimens can be adopted for the desired extract purity and composition.
The crude extract of the Chinese sweet leaf prepared by boiling water contained approximately 11% w/w of polysaccharides as determined by the phenol-sulfuric acid colorimetric method. Phenol-sulfuric assay is a simple, convenient, and sensitive method to measure the concentration of polysaccharides in plant extracts. The reaction mechanisms may involve the condensation with phenol after the parallel dehydrations of carbohydrates with sulfuric acid (28). Under a proper condition, the phenol-sulfuric colorimetric method has approximately ±2% of accuracy (29) and has also been applied in microplate format due to its simplicity and sensitivity (30). Our study indicated that the recovery rate of the phenol-sulfuric acid colorimetric method was 98.79% with an RSD of 2.74. These data confirm the validity of this method in measuring the content of polysaccharides in complex botanical samples.
Purification of crude plant extracts is widely used in botanical research to augment the initial observation of biological activities. Chou et al. (24) have presently developed a validated HPLC method in assessing the quality of the Chinese sweet leaf tea extract possessing multiple bioactivities. This would further help us to quantify and qualify the end products resulted from AP. Because the stability, safety, and effectiveness of AP depend on several factors such as the extract concentration, alcohol volume and concentration, reaction time length, temperature, stirring procedure during precipitation, and chemical and physical properties of raw material used in precipitation (27), it is important to verify and control the overall quality and thus the bioactivity of the extracts under the conditions of AP. In the study of antifungal activity, Schmourla et al. (22) demonstrated the lost activity in some medicinal plants after the separation of the plant extracts into precipitant and supernatant. The crude aqueous extract of the Chinese sweet leaf displayed potent antiangiogenesis activities due to the presence of gallic acid (8). Therefore, to purify the crude sweet leaf tea extract for increased antiangiogenesis activity, achieving an increased level of gallic acid is clearly desirable. On the other hand, since gallic acid is not the only compound that explained the overall antiangiogenic activity of the sweet leaf tea extract, it is imperative to retain other possible bioactive compounds until proven not useful. This also further explained the reasons we adopted a longer analytical time (100 min) to our HPLC methods which would help us optimize the separation of compounds, known or unknown, that may exhibit bioactivity (24). In addition to the quantity of each known marker compound, the ratios of these compounds are of great importance to us because of the prospect of concerted action potential. This argument might be exemplified by the observed lost activity in some medicinal plants after AP regimens (22), which could be a result of changes of the ratios of active compounds that could have modified the intrinsic synergistic or additive properties. Because the alcohol precipitation used in this study resulted in a whole spectrum of extracts differing in ratios, thus in composition, these samples warrant differential bioactivity examinations. For this purpose, detailed fingerprint analyses over the results of a simple purification procedure were proven worthy.
CONCLUSIONS
This is a first report on the purification of sweet leaf crude extract using alcohol precipitation. Our study confirmed a clear separation of selected marker components from polysaccharides in response to alcohol precipitation. The level of rubusoside, one of the marker compounds and the characteristic sweetening agent, was doubled in a 95% alcohol solution via a complete removal of 11% polysaccharides and other macromolecules or ethanol insoluble components. Overall, 70% to 80% EtOH was the best range for purifying the five markers without risking the loss of many unknown compounds. By employing this purification method, a significantly purer extract can be obtained for potential improved bioactivity. Alcohol precipitation, therefore, proves to be a useful tool in purifying polysaccharide-rich plant extracts such as the sweet leaf tea extract.
ACKNOWLEDGMENTS
The authors thank Dr. Shunjun Xu and Dr. Dong Liu for assistance in the HPLC analyses. Thanks also to Dr Paul Y. Burns for editorial assistance.
The project was partially supported by the McIntire-Stennis Cooperative Fund, the LSU Agricultural Center Technology Transfer Fund, and by Grant Number R21AT002882 from the National Center for Complementary & Alternative Medicine to Liu Z. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Complementary & Alternative Medicine or the National Institutes of Health.
ABBREVIATIONS USED
- AP
Alcohol precipitation
- GA
Gallic acid
- RUT
Rutin
- EGA
Ellagic acid
- RUB
Rubusoside
- SM
Steviol monoside
- PSAC
polysaccharides
LITERATURE CITED
- 1.Tanaka T, Kohda H, Tanaka O, Chen FH, Chou WH, Leu JL. Rubusoside (β-D-Glucosyl Ester of 13-O-β-D-Glucosyl-steviol), a Sweet Principle of Rubus chingii Hu (Rosaceae) Agric. Biol. Chem. 1981;45:2165–2166. [Google Scholar]
- 2.Ohtani K, Aikawa Y, Kasai R, Chou WH, Yamasaki K, Tanaka O. Minor Diterpene Glycosides from Sweet Leaves of Rubus suavissimus. Phytochemistry. 1992;31:1553–1559. [Google Scholar]
- 3.Sugimoto N, Sato K, Liu H, Kikuchi H, Yamazaki T, Maitani T. Analysis of Rubusoside and Related Compounds in Tenryocha Extract Sweetener. J. Food Hyg. Soc. Japan. 2002;43:250–253. doi: 10.3358/shokueishi.43.250. [DOI] [PubMed] [Google Scholar]
- 4.Hirono S, Chou WH, Kasai R, Tanaka O, Tada T. Sweet and Bitter Diterpene-Glucosides from Leaves of Rubus suavissimus. Chem. Pharm. Bull. 1990;38:1743–1744. [Google Scholar]
- 5.Huang P, Jiang S. Complex Utilization of Rubus suavissimus S. Lee. Guangxi Chemical Industry. 2002;31:24–25. [Google Scholar]
- 6.Ono Y. Anti-inflammatory and Anti-allergic Effects of Tiencha (Rubus suavissimus S. Lee) Allergy in Practice. 2004;317:380–385. [Google Scholar]
- 7.Kotaro U. Anti-allergic action of Rubus suavissimus. Shokuhin Kogyo. 1997;40:52–59. [Google Scholar]
- 8.Liu Z, Schwimer J, Liu D, Lewis J, Greenway FL, York DA, Woltering EA. Gallic Acid is Partially Responsible for the Antiangiogenic Activities of Rubus Leaf Extract. Phytother. Res. 2006;20:806–813. doi: 10.1002/ptr.1966. [DOI] [PubMed] [Google Scholar]
- 9.Oner FH, Bezerra Y, Peyman GA, Conway MD, Lewis JM, Liu Z, Greenway F, Woltering EA. Antiangiogenic Effect of a Chinese Sweet Leaf Tea Extract in Experimental Corneal Neovascularization. Pharm. Biol. 2007;45:45–47. [Google Scholar]
- 10.Liu D, Gao Z, Zhang J, Ye J, Liu Z. Bioassay-guided Fractionation of Rubus suavissimus Leaf Extracts Possessing NF-κB Inhibitory Activities and a Separable Cytotoxicity. Pharm. Biol. 2005;43:713–717. [Google Scholar]
- 11.Li H, Tanaka T, Zhang YJ, Yang CR, Kouno I, Rubusiviis A-F. Monomeric and Oligomeric Ellagitannins from Chinese Sweet Tea and Their α-amylase Inhibitory Activity. Chem. Pharm. Bull. 2007;55:1325–1331. doi: 10.1248/cpb.55.1325. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Lu Y, Su X, Li F, She Z, He X, Lin Y. A Norsesquiterpene Lactone and a Benzoic Acid Derivative from the Leaves of Cyclocarya paliurus and their Glucosidase and Glycogen Phosphorylase Inhibiting Activities. Planta Med. 2008;74:287–289. doi: 10.1055/s-2008-1034309. [DOI] [PubMed] [Google Scholar]
- 13.Kartika H, Li QX, Wall MM, Nakamoto ST, Iwaoka WT. Major Phenolic Acids and Total Antioxidant Activity in Mamaki leaves, Pipturus albidus. J. Food Sci. 2007;72:S696–S701. doi: 10.1111/j.1750-3841.2007.00530.x. [DOI] [PubMed] [Google Scholar]
- 14.Vukics V, Kery A, Bonn GK, Guttman A. Major Flavonoid Components of Heartsease (Viola tricolor L.) and their Antioxidant Activities. Anal. Bioanal. Chem. 2008;390:1917–1925. doi: 10.1007/s00216-008-1885-3. [DOI] [PubMed] [Google Scholar]
- 15.Rogerio AP, Fontanari C, Borducchi E, Keller AC, Russo M, Soares EG, Albuquerque DA, Faccioli LH. Anti-inflammatory Effects of Lafoensia pacari and Ellagic Acid in a Murine Model of Asthma. Eur. J. Pharmacol. 2008;580:262–270. doi: 10.1016/j.ejphar.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 16.Papoutsi Z, Kassi E, Chinou I, Halabalaki M, Skaltsounis LA, Moutsatsou P. Walnut Extract (Juglans regia L.) and its Component Ellagic Acid Exhibit Anti-Inflammatory Activity in Human Aorta Endothelial Cells and Osteoblastic Activity in the Cell Line KS483. Br. J. Nutr. 2008;99:715–722. doi: 10.1017/S0007114507837421. [DOI] [PubMed] [Google Scholar]
- 17.Bell C, Hawthorne S. Ellagic Acid, Pomegranate and Prostate cancer -- a mini review. J. Pharm. Pharmacol. 2008;60:139–144. doi: 10.1211/jpp.60.2.0001. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Seeram NP, Lee R, Feng L, Heber D. Isolation and Identification of Strawberry Phenolics with Antioxidant and Human Cancer Cell Antiproliferative Properties. J. Agric. Food Chem. 2008;56:670–675. doi: 10.1021/jf071989c. [DOI] [PubMed] [Google Scholar]
- 19.Wang D, Wang B, Li B, Duan C, Zhang J. Extraction of Total RNA from Chrysanthenum Containing High Level of Phenolic and Carbohydrates. Colloids and Surfaces B. 2004;36:111–114. doi: 10.1016/j.colsurfb.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 20.Gemini S, Umdu ES, Yaprak N, Ustok FI, Yener FYG, GÜÇbilmez ÇM, Altinkaya SA, Yemennicioglu A. Partial Purification of Hen Egg White Lysozyme by Ethanol Precipitation Method and Determination of the Thermal Stability of its Lyophilized Form. Turk J. Agric For. 2007;31:125–134. [Google Scholar]
- 21.Yang X, Zhao Y, Yang Y, Ruan Y. Isolation and Characterization of Immunostimulatory Polysaccharide from an Herb Tea, Gynostemma pentaphyllum Makino. J. Agric. Food Chem. 2008;56:6905–6909. doi: 10.1021/jf801101u. [DOI] [PubMed] [Google Scholar]
- 22.Schmourlo G, Mendonca-Filho RR, Alviano CS, Costa SS. Screening of Antifungal Agents using Ethanol Precipitation and Bioautography of Medicinal and Food Plants. J. Ethnopharmacol. 2005;96:563–568. doi: 10.1016/j.jep.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Wu Y, Cui SW, Tang J, Wang Q, Gu X. Preparation, Partial Characterization and Bioactivity of Water-Soluble Polysaccharides from Boat-fruited Sterculia Seeds. Carbohydrates Polymers. 2007;70:437–443. [Google Scholar]
- 24.Chou G, Xu SJ, Liu D, Koh GY, Zhang J, Liu Z. Quantitative and Fingerprint Analyses of Chinese Sweet Tea Plant (Rubus suavissimus S. Lee) J. Agric. Food Chem. 2009;57:1076–1083. doi: 10.1021/jf8029397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao LJ, Wang HZ, Cui JH, Su YY, Song ZQ, Wang JH. Measuring the Content of Polysaccharides in Radix cyanchum Bungei by the Method of Phenol-Vitril. J. Shandong Agricultural University. 2004;35:295–297. [Google Scholar]
- 26.Yang H, Chen F, Yan W. Effect of Ethanol Precipitation on Chlorogenic Acid from Flos Lonicerae Japonica. Zhongchengyao. 2006;28:1256–1259. [Google Scholar]
- 27.Liu M. Ethanol Precipitation of Chinese Drugs and its Equipment. Zhongchengyao. 2007;29:1202–1204. [Google Scholar]
- 28.Scherz H, Bonn G. Analytical Chemistry of Carbohydrates. Stuttgart, New York: Thieme Medical Publishers; 1998. Reaction with Acids; pp. 51–52. [Google Scholar]
- 29.Hodge JE, Hofreiter BT. Determination of Reducing Sugars and Carbohydrates. In: Whistler RL, Wolfrom MW, editors. Methods in Carbohydrate Chemistry. Vol. 1. New York City, New York: Academic Press; 1962. pp. 380–394. [Google Scholar]
- 30.Masuko T, Minami A, Iwasaki N, Majima T, Nishimura S, Lee YC. Carbohydrate Analysis by a Phenol-Sulfuric Acid Method in Microplate Format. Anal. Biochem. 2005;339:69–72. doi: 10.1016/j.ab.2004.12.001. [DOI] [PubMed] [Google Scholar]