Abstract
trans-4-Hydroxynonenal (HNE) is a major peroxidation product of ω-6 polyunsaturated fatty acids. The reaction of HNE with DNA produces four diastereomeric 1,N2-γ-hydroxypropano adducts of deoxyguanosine (HNE-dG); background levels of these adducts have been detected in tissues of animals and humans. There is evidence to suggest that these adducts are mutagenic and involved in liver carcinogenesis in patients with Wilson’s disease and in other human cancers. Here, we present biochemical evidence that in human cell nuclear extracts the HNE-dG adducts are repaired by the nucleotide excision repair (NER) pathway. To investigate the recognition and repair of HNE-dG adducts in human cell extracts, we prepared plasmid DNA substrates modified by HNE. [32P]-Postlabeling/HPLC determined that the HNE-dG adduct levels were ~1200/106 dG of plasmid DNA substrate. We used this substrate in an in vitro repair-synthesis assay to study the complete repair of HNE-induced DNA adducts in cell-free extracts. We observed that nuclear extracts from HeLa cells incorporated a significant amount of α[32P]dCTP in DNA that contained HNE-dG adducts by comparison with UV-irradiated DNA as the positive control. Such repair synthesis for UV damage or HNE-dG adducts did not occur in XPA cell nuclear extracts that lack the capacity for NER. However, XPA cells complemented with XPA protein restored repair synthesis for both of these adducts. To verify that HNE-dG adducts in DNA were indeed repaired, we measured HNE-dG adducts in the post-repaired DNA substrates by the [32P]-postlabeling/HPLC method, showing that 50–60% of HNE-dG adducts were removed from the HeLa cell nuclear extracts after 3 h at 30 °C. The repair kinetics indicated that the excision rate is faster than the rate of gap-filling/DNA synthesis. Furthermore, the HNE-dG adduct isomers 2 and 4 appeared to be repaired more efficiently at early time points than isomers 1 and 3.
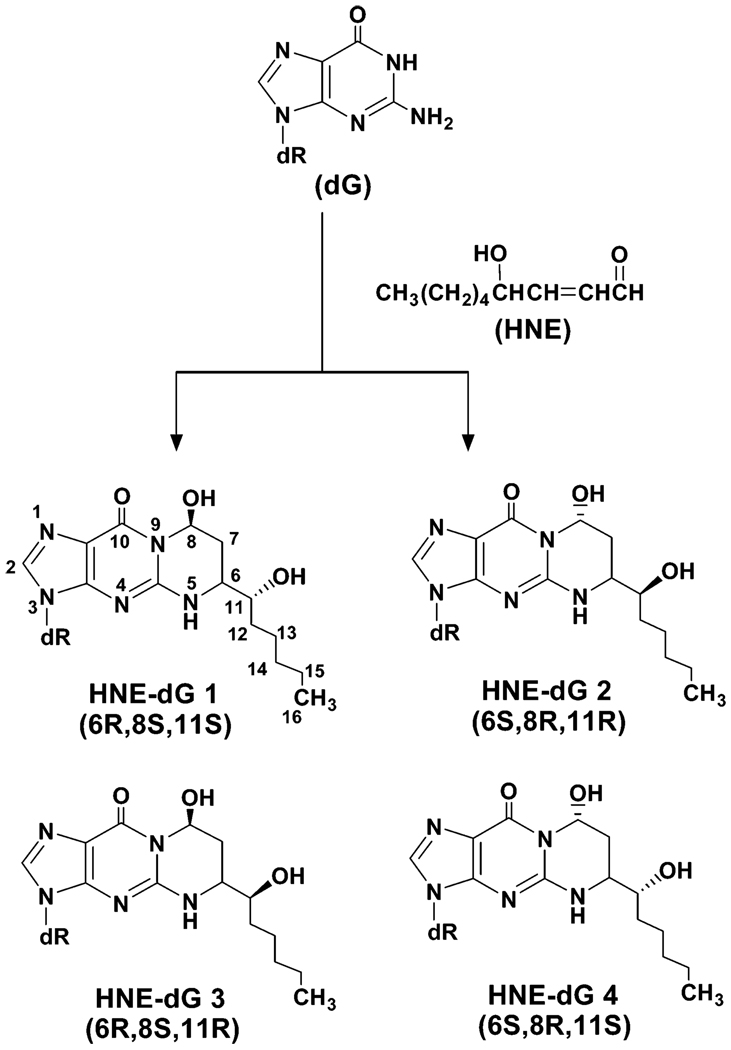
Reactive oxygen species (ROS1, e.g., O2•−, OH•, and H2O2) are generated as byproducts in mitochondrial oxidative phosphorylation by ionizing radiation and in oxyradical overload diseases such as Wilson’s disease and hemochromatosis and also by antitumor drugs such as bleomycin. In addition to their genotoxic effects, ROS also react with cellular membrane lipids and form reactive unsaturated aldehydes or enals via lipid peroxidation (1). There is increasing evidence that lipid peroxidation is involved in carcinogenesis (2–5). Rats receiving a choline-deficient diet or treated with peroxisome proliferators that elicit lipid peroxidation developed liver cancer (6, 7). Among enals, trans-4-hydroxynonenal (HNE; Figure 1) is one of the most abundant and also cytotoxic compounds (8). HNE is formed mainly by oxyradical-initiated degradation of ω-6 polyunsaturated fatty acids that are relatively abundant in human tissues (8, 9). HNE at physiological concentration stimulates phospholipase C and protein kinase C, both of which actively control cell division and proliferation (8). It also modulates gene expression via cell signaling, including activating oncogene c-jun in human hepatic stellate cells (10) and thus plays a role in carcinogenesis. HNE is also genotoxic and produces bulky 1,N2-propano-2′-deoxyguanosine adducts (HNE-dG, Figure 1), while its epoxy products, generated after further oxidative metabolism, can react with DNA by forming etheno adducts of adenosine, guanosine, and cytidine (11, 12). Furthermore, the reaction of HNE with deoxyguanosine (dG) generates three new chiral centers in the nucleoside (13), resulting in the formation of four stereoisomers (6R, 8S, 11R), (6S, 8R, 11S), (6R, 8S, 11S), and (6S, 8R, 11R) with 8-hydroxyl and 6-(1-hydroxyhexyl) in the trans configuration (ref 14; Figure 1).
FIGURE 1.
Chemical structures of HNE, guanosine, and four diastereoisomers of HNE-dG adducts.
The enal-derived cyclic DNA adducts have been detected as background lesions in tissues of rodents and humans (11, 15–18). Levels of HNE-dG adducts increased significantly in the livers of rats treated with carbon tetrachloride, which induces lipid peroxidation and liver carcinogenesis (17, 19). A recent study showed that liver DNA from patients with Wilson’s disease, characterized by hepatic deposition of copper and iron that increases the risk of liver cancer, had a significantly higher frequency of p53 hotspot mutations of G to T transversions at codon 249 (20). The same study also showed that exposing TK-6 lymphoblastoid cells (wild-type p53) to HNE increased predominantly the G to T mutation at p53 codon 249, suggesting that HNE-dG adducts may play a role in p53 mutation (20). The G at the third base of codon 249 represents a mutation hotspot in human cancer, particularly hepatocellular carcinomas (21). Recently, using the Escherichia coli nucleotide excision repair enzyme complex UVrABC nuclease with ligation-mediated PCR, Hu et al. mapped HNE-dG adducts in the human p53 gene modified with HNE. They showed that HNE-dG adducts were preferentially formed at the third base of codon 249 of the p53 gene (22). Taken together, these findings suggest that HNE and HNE-dG adducts in DNA play a critical role in mutagenesis and likely also in human carcinogenesis.
The levels and persistence of the HNE-dG adducts in tissues may be determined by several factors: the rate of formation, the rate of repair, and the rate of DNA replication. Cyclic adducts in DNA are repaired either by base excision repair (BER) or nucleotide excision repair (NER) mechanisms. For example, the repair of etheno adducts is mainly mediated by the BER pathway, which is initiated by a specific DNA glycosylase, N-methylpurine DNA-glycosylase, present throughout the phylogeny including the humans (23–28). Other human proteins capable of removing etheno adducts have also been characterized (29). However, 1,N2-propanodG (PdG), a model for the bulky cyclic propano adducts, is not excised by the glycosylases and is actually repaired by the NER pathway (30). It appears that different repair mechanisms are operative for cyclic adducts depending on their structures. Acrolein (Acr-) or other enal-induced DNA adducts have recently been shown to be repaired by NER in E. coli (31, 32). Evidence from our initial studies also indicates that HNE-dG adducts are repaired by the NER pathway (33); however, the details of the repair mechanism of the cyclic propano adducts in mammalian cells are yet to be elucidated.
On the basis of our preliminary observations, we investigated the detailed characteristics of repair mechanism of HNE-dG adducts in nuclear extracts of human cells. Here, we demonstrate that HNE-dG adducts, the potentially important endogenous adducts, are repaired by NER proteins in a dose- and time-dependent manner and also describe the kinetics of this repair as well as the influence of the stereochemistry of HNE-dG adducts on their repair. The relatively efficient in vitro removal of all four isomers of HNE-dG adducts may partially explain their relatively low level as background DNA lesions in rodents and humans.
EXPERIMENTAL PROCEDURES
Cell Culture
NER-proficient HeLa (ATCC) and NER-deficient human XPA cells (kindly provided by Dr. Randy Legarski, MD Anderson Cancer Center, Houston, TX) were grown in Minimum Essential Medium (MEM; Gibco/Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum and penicillin/streptomycin (Gibco/Invitrogen, Carlsbad, CA) in a 5% CO2 humidified incubator. XAN1 cells, derivative of XPA cells stably transfected with XPA minigene (ref 34; kindly provided by Dr. J. Christopher States, University of Louisville School of Medicine, Louisville, KY), were grown in α Minimum Essential Medium (α MEM; Gibco/Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum and penicillin/streptomycin.
Preparation of Nuclear Extracts for the in Vitro NER Assay
The nuclear extracts were prepared from HeLa, XPA, and XAN1 cells following a published procedure (35). Briefly, cells (6–7 × 106) from 6–7 of the 10 cm2 plates were washed thoroughly with phosphate buffered saline (PBS), suspended in chilled buffer A (10 mM HEPES–KOH pH 7.9, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, and protease inhibitor cocktail [Roche Molecular Biochemicals, Indianapolis, IN]), and allowed to swell on ice for 15 min. The swelled pellet was then mixed with 0.6% Nonidet P-40 and vigorously vortexed for 10s. The homogenate was centrifuged for 30 s to recover the nuclear pellet, which was then resuspended in buffer B (20 mM HEPES–KOH pH 7.9, 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, and protease inhibitor cocktail), and shaken in a rocking shaker for 15 min at 4 °C. The nuclear extract was then clarified by centrifugation and stored at −80 °C in small aliquots. The typical yield was 3–4.5 mg of protein, and the concentration ranged from 4 to 6 mg/mL. Each aliquot was thawed only once for the in vitro NER activity assay to avoid inactivation due to repeated freeze–thaw cycles.
Preparation of Plasmid Substrates Containing HNE-dG
HNE was synthesized according to the method previously described (36). The pBluescript (pBSII) plasmid DNA was received from Recombinant DNA Laboratory core facility, UTMB, Galveston, TX, and used for subsequent HNE-modification and UV-irradiation. The plasmid DNA was modified with HNE as described by Hu et al. (22). Briefly, purified pBSII (10 µg) in TE buffer (10 mM Tris, 1 mM EDTA, pH 7.0) was incubated with a final concentration of 30 mg/mL of HNE (stock solution, 100 mg/mL in methanol) at 37 °C for 20 h. Control pBSII was treated with methanol only and used as HNE-untreated substrate in subsequent NER assays. The unreacted HNE was removed by repeated phenol/ chloroform extraction. The treated plasmids were then precipitated with ethanol, dissolved in TE buffer (pH 7.0), and used to quantify the HNE-dG adduct level by [32P]-postlabeling/HPLC assay.
In Vitro Repair Reactions. Repair Synthesis Assay by [32P] Incorporation/Agarose Gel Electrophoresis
In typical 20 µL reaction mixtures, 300 ng each of HNE-modified pBSII, or UV-irradiated pBSII (ref 37; ~1.0 adduct per molecule; provided by Dr. Yue Zou, East Tennessee State University, TN), or untreated pBSII closed circular plasmids was incubated at 30 °C for stipulated times with HeLa, XPA, or XAN1 cell nuclear extracts (60 µg) in the presence of 74 kBq of α-[32P] dCTP (110 TBq/mmol; Amersham Pharmacia Biotech, Piscataway, NJ); 13 mM HEPES–KOH (pH 7.9); 50 mM KCl; 7.4 mM MgCl2; 1 mM DTT; 2 mM ATP; 50 µM each dGTP, dATP, dTTP, and 10 µM dCTP; 40 mM creatine phosphate; 0.5 µg of creatine phosphokinase (Type I; Sigma-Aldrich Co., St. Louis, MO); and 6.4 µg of BSA as described (38). The reaction was stopped by adding 25 mM EDTA, subjected to digestion with RNAse and Proteinase K as described (38), and extracted with phenol/chloroform before the DNA was recovered by precipitation with ethanol in the presence of Pellet Paint Pink (Novagen, Madison, WI). The purified plasmid DNA was then linearized with EcoRI, electrophoresed on 1% agarose gel containing 0.5 µg/mL ethidium bromide, dried on DE-81 filter paper, and exposed to PhosphorImager (Amersham Pharmacia Biotech, Piscataway, NJ) for visualization and quantification of radioactivity in the bands.
Repair Excision Assay by [32P] Incorporation/Agarose Gel Electrophoresis
A typical 20 µL reaction mixture contained 300 ng each of HNE-modified pBSII or untreated pBSII closed circular plasmids. These mixtures were incubated at 30 °C for stipulated times with HeLa cell nuclear extracts (60 µg) and the reaction buffer as stated previously except that dNTPs were omitted and 4.5 µM aphidicoline was included as described in the literature (39). The reaction was terminated with 25 mM EDTA, and the DNA was recovered as described previously. The incubation of DNA at 20 °C was adjusted to 5 min and then performed in a 10 µL reaction mixture containing 50 mM Tris, pH 8.0; 10 mM MgCl2; 74 kBq of α-[32P] dCTP (110 TBq/mmol; Amersham Pharmacia Biotech, Piscataway, NJ); 1 mM DTT; 50 µM each dGTP, dATP, and dTTP; 5 µM dCTP; and 1 U of E. coli DNA polymerase I large fragment (New England Biolabs, Beverley, MA). The reaction was terminated by adding 50 mM EDTA and 1 mM dCTP. The mixture was treated with RNase before purification of DNA by extraction with phenol/ chloroform and precipitation with ethanol in the presence of Pellet Paint Pink (Novagen, Madison, WI) as described previously. The purified plasmid DNA was then linearized with EcoRI, electrophoresed on 1% agarose gel containing 0.5 µg/mL ethidium bromide, dried on DE-81 filter paper, and exposed to PhosphorImager (Amersham Pharmacia Biotech, Piscataway, NJ) for visualization and quantification of radioactivity in the bands.
Repair Excision Assay by Detection of HNE-dG Adducts in Plasmid DNA via the [ 32P]-Postlabeling/HPLC Method
An improved 32P-postlabeling/SPE/HPLC method was applied to directly measure HNE-dG adducts in plasmid DNA before and after repair–synthesis assay. To yield sufficient DNA for the postlabeling assay, the repair synthesis was carried out essentially as described earlier, except under proportionally scaled-up conditions. The assay was performed with 3 µg of HNE-dG containing pBSII DNA, or untreated DNA, 250 µg of HeLa, or XPA cell nuclear extracts and similar concentrations of dNTPs (no α-[32P] dCTP) and other essential ingredients described earlier. The plasmid DNA from repair reactions was purified up to electrophoresis on agarose gel as before and then analyzed for remaining HNE-dG adducts by the [32P]-postlabeling/HPLC method.
This method was somewhat modified from the previously published procedures (40). The four diastereomers of HNE-dG adducts were detected and quantified using the synthetic HNE-dG adduct standards described previously (17, 40). Briefly, the DNA samples were digested with micrococcal nuclease and spleen phosphodiesterase and applied onto a C18 solid-phase extraction cartridge. The cartridge was first washed with 50 mM ammonium formate (pH 7.0), and the eluent containing the unmodified nucleotides was used to quantify dG 3′-monophosphates in each sample. The HNE-dG 3′-monophosphates was eluted with 50% methanol and labeled with [γ-32P]ATP. The labeled mixture was purified with SPE and sequential reverse-phase and ion-pairing HPLC systems. The purified HNE-dG 3′,5′-bisphosphates were converted into the corresponding ring-opened derivatives by the ring-opening/reduction reaction and finally quantified with a HPLC system with β-Ram radio-flow detectors.
RESULTS
Repair of HNE-Induced DNA Adducts by NER Pathway
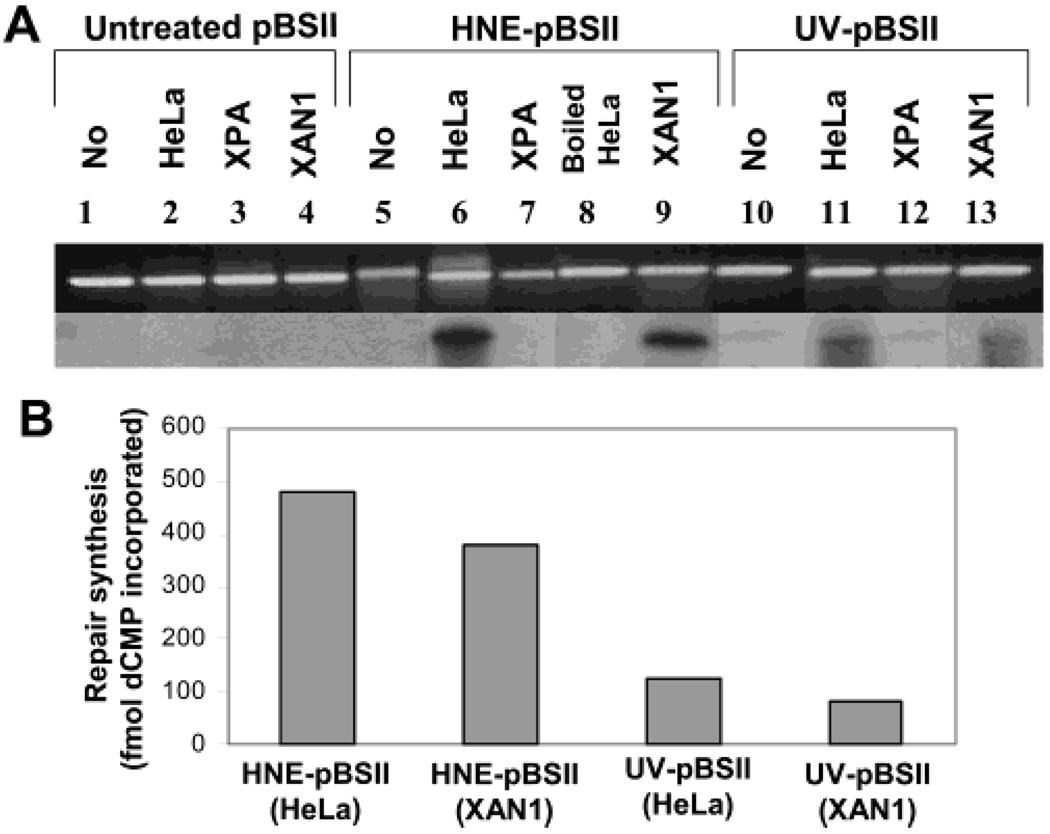
To address whether the mutagenic HNE-dG adducts in human cells are repaired, we assumed they, like the lower homologue cyclic adduct, acrolein-dG (Acr-dG), are likely to be repaired by the NER pathway. We chose HeLa cells as the NER-proficient cells and XPA cells as the NER-deficient cells because the extracts from these two cell lines have been extensively characterized for NER of bulky DNA adducts of diverse chemical structures. The plasmid DNA substrate (pBSII; 1000–1500/106 dG or ~1.7 adduct/molecule) modified with HNE was prepared for an in vitro repair–synthesis assay with human HeLa cell nuclear extract. In this assay, the repair–synthesis of HNE-dG adducts was measured by monitoring the incorporation of α[32P] dCTP into covalently closed circular plasmid DNA, separated on agarose gel. Our repair assay conditions differed from the classical assay (38, 41) in two ways: (1) rather than using a larger size untreated plasmid DNA as a control, along with the substrate DNA (smaller in size) in the same reaction, we intentionally chose the control DNA and substrate DNA to be of identical size and sequence, thus avoiding possible effects caused by sequence differences in repair reactions. Therefore, we incubated the control DNA (untreated pBSII), identical in size and sequence to HNE-treated DNA, in a separate reaction tube and (2) we optimized the repair assays by using nuclear extracts (60 µg of protein maximum) instead of 150 µg of whole cell extracts, as described in the classical protocol, to avoid any significant background incorporation of 32P in the control DNA. Other repair conditions were identical to those in the classical repair synthesis protocol described initially by R. Wood and later by other investigators (38, 41). Our results show that nuclear extracts of HeLa cells incorporated a significant amount of α[32P] dCTP in DNA that contained HNE-dG adducts (Figure 2A) when compared with UV-irradiated DNA (~1 adduct per molecule) as the positive control and untreated plasmid DNA substrates as the negative control. Under the same repair reaction conditions, a 2.4-fold higher amount of α[32P] dCTP was incorporated in pBSII that contained HNE-dG adducts than in UV-irradiated pBSII DNA (Figure 2B) after normalization of adduct levels, suggesting an efficient repair synthesis of HNE-dG adducts in human HeLa cells. Notably, the specific activity of dCMP incorporation (~1 fmol/µg of cell extract/300 ng of DNA) in UV-irradiated pBSII during repair synthesis is consistent with that reported by others (39). To ensure that there is no spontaneous loss of adducts during the repair reaction in the incubation and that the repair is mediated by enzymes, we incubated HNE-treated pBSII DNA with boiled HeLa nuclear extracts; this confirmed the absence of [32P]-incorporation or repair (Figure 2A). Moreover, the repair-synthesis activity was not evident in assays for DNA that contained HNE-dG adducts or had UV damage (Figure 2A), when we used the nuclear extracts from XPA cells that are deficient in NER capacity. However, the nuclear extracts from XAN1 cells, derivative of XPA cells stably transfected with XPA minigene and expressing active XPA protein (34), restored the repair synthesis of HNE dG adducts (Figure 2A,B). Taken together, we conclude that HNE-induced DNA adducts are repaired in human cells by NER pathway.
FIGURE 2.
DNA repair synthesis on HNE- and UV-damaged plasmids. HeLa and XPA cell nuclear extracts (60 µg per reaction) were incubated for 3 h at 30 °C with HNE- or UV-damaged plasmid DNA (pBSII) in repair synthesis reaction (details described in the Experimental Procedures). (A) Photograph of ethidium bromidestained gel (upper) and autoradiogram of the dried gel (lower). (B) The DNA repair synthesis value was calculated by subtraction of nonspecific dCMP incorporation into undamaged pBSII from dCMP incorporation into damaged pBSII and presented as the mean specific incorporation values with standard error derived from three independent experiments.
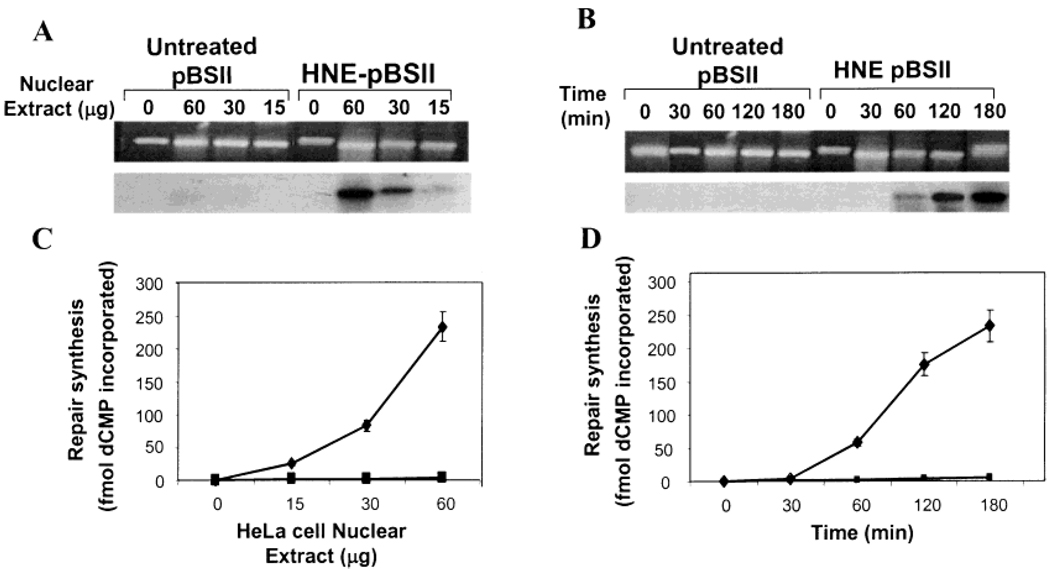
We also monitored the protein and time dependence in the repair of HNE-dG adducts. The α[32P] dCTP incorporation was detectable with as little as 15 ug of protein, and the extent of repair synthesis increased with increasing amount of proteins in HeLa nuclear extracts (Figure 3A,B). The repair kinetics showed that the 32P-incorporation was not detectable at or below 1/2 h, and the repair synthesis increased steadily as time progressed (Figure 3C,D). Thus, the repair of HNE-dG in human cell nuclear extracts is both protein concentration and time dependent. Furthermore, in both cases, the repair assay does not show any significant background 32P-incorporation in control pBSII DNA (Figure 3A,B), ensuring the specificity and reliability of the NER assay for HNE-dG adducts.
FIGURE 3.
DNA repair synthesis of HNE-dG adducts in HeLa cell nuclear extracts. Effect of protein concentration (A and C). The experimental conditions were similar to those described in the legend of Figure 2 with the exception of using increasing amounts of protein (0–60 µg). Time dependence (B and D). The experimental conditions were similar to those described in the legend of Figure 2 with the exception of increasing time (0–3 h). (A and B) Photograph of ethidium bromide-stained gel (top) and autoradiogram of the dried gel (bottom). (C and D) Mean value of dCMP incorporation into HNE-damaged (◆) and undamaged (■) pBSII derived from three independent experiments presented with standard errors.
Repair of All Four Stereoisomers of HNE-dG Adducts in Plasmid DNA by [32P]-Postlabeling/HPLC
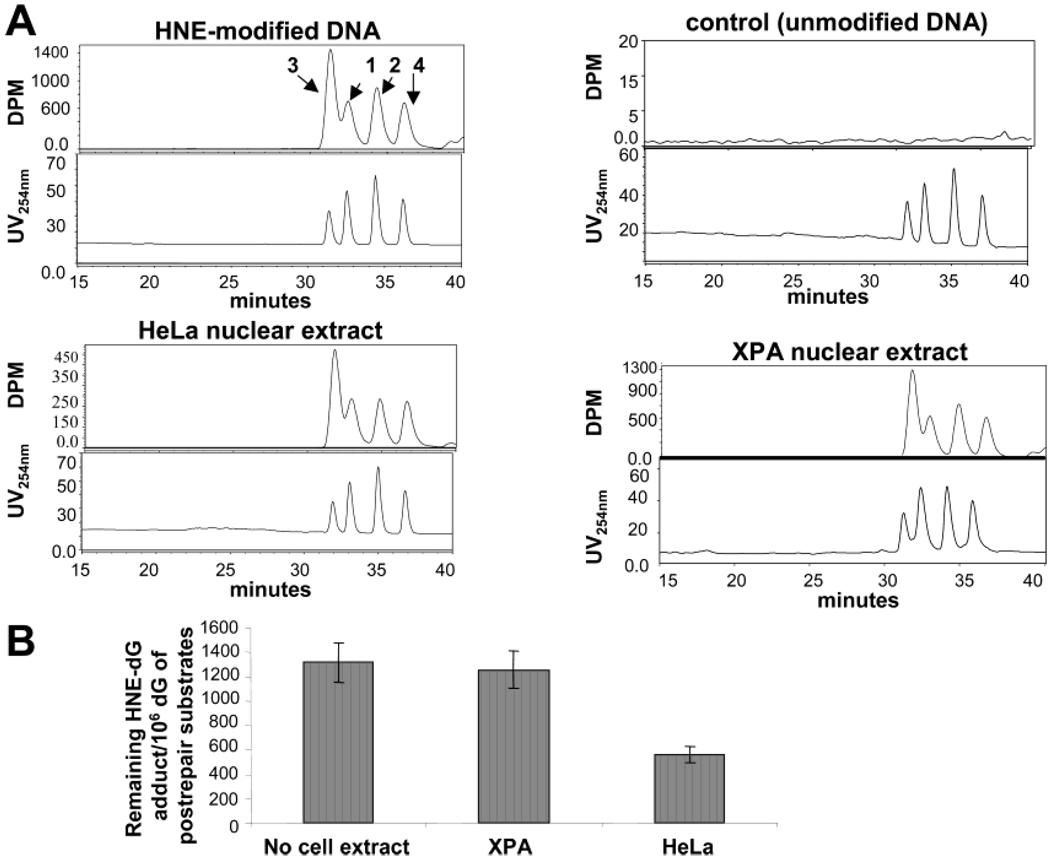
To determine whether HNE-dG adducts are repaired from the plasmid DNA, we measured their levels in DNA substrates before and after repair by the [32P]-postlabeling/HPLC method. This method determines the residual HNE-dG adducts after completion of repair or at any intermediate repair step after excision. We found that 50–60% of HNE-dG adducts at maximum were excised in nuclear extracts of HeLa cells within 3 h at 30 °C, whereas in nuclear extracts of XPA cells, HNE-dG adducts were not removed under similar conditions (Figure 4). It should be noted that earlier we found 90% of HNE-dG repaired by HeLa nuclear extracts (33), and this overestimation was due to the presence of free dGTP carry-over from repair reactions; after removal on agarose gel before [32P]-postlabeling/HPLC assays, we found in this study a consistent 50–60% repair as documented previously. Therefore, the results of the [32P]-postlabeling/HPLC assays corroborate those from gel electrophoresis and demonstrate that HNE-induced DNA damage is repaired in human cells by the NER pathway.
FIGURE 4.
DNA repair excision activity in HNE-damaged plasmid. HeLa and XPA cellnuclear extracts (250 µg per reaction) were incubated for 3 h at 30 °C with HNE-treated pBSII as described in Experimental Procedures, and the levels of HNE-dG adducts were measured in pBSII before and after the repair reaction by the [32P]-postlabeling/HPLC method. (A) HPLC chromatograms were obtained from [32P]-postlabeling of the enzymatic hydrolysate of the pBSII plasmid DNA recovered from repair reactions. The upper panel in each set of chromatograms shows the [32P]-labeled adducts detected in the HNE-treated or untreated pBSII before repair reaction or HNE-treated pBSII after repair reaction with HeLa or XPA nuclear extracts. Two pairs of diastereoisomers (1–4) of ring-opened derivatives were detected by HPLC. The lower panels show the HNE-dG standards detected by UV (254 nm) absorption. (B) Quantification of HNE-dG adducts in pBSII pre- and post-repair. The values are shown after normalizing per 106 dG as a mean of three independent experiments and presented with standard errors.
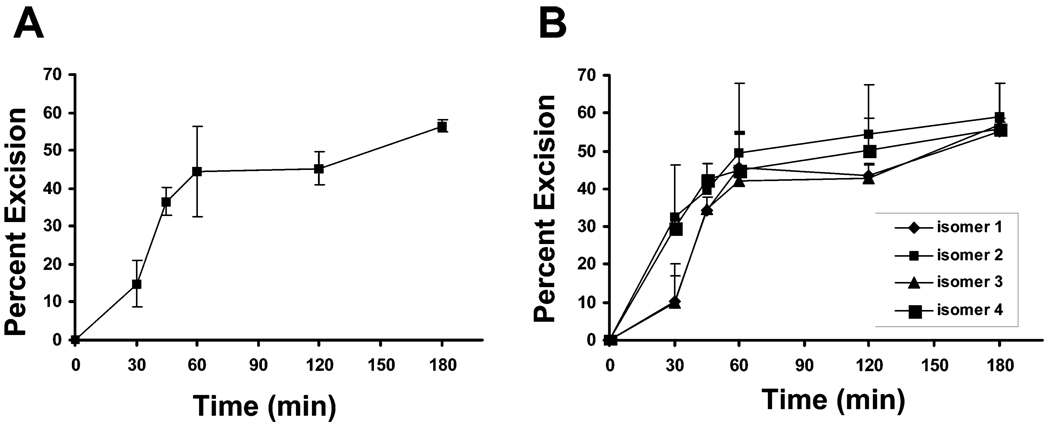
Following similar assay conditions, we determined the repair kinetics by measuring the residual HNE-dG adducts at various time points. The results show that the rate of excision of total HNE-dG adducts attained a maximum at 1 h and was not significantly altered at 2 and 3 h (Figure 5A). On the other hand, the total repair synthesis at 1 h was at least one-quarter of that at the 3 h time point (Figure 3B,D).
FIGURE 5.
Kinetics of DNA repair excision of HNE-dG adducts by HeLa cell nuclear extracts. The level of HNE-dG adducts in pBSII was measured before and after repair reaction with HeLa cell nuclear extracts by [32P]-postlabeling/HPLC method as described in Figure 5 with the exception that the repair reaction was performed with increasing time (0–3 h). (A) Kinetics of excision of total HNE-dG. The amount of excised HNE-dG was determined by subtracting the residual adduct from the total amount of initial adducts in pBSII. (B) Kinetics of excision for four isomers of HNE-dG. The amount of excision of each isomer was evaluated similarly to those described for total HNE-dG adducts. The data are the mean value derived from two independent experiments and presented with standard errors.
Moreover, the [32P]-postlabeling/HPLC method enabled us to directly quantify the repair characteristics of four stereoisomers of HNE-dG adducts. The repair kinetics showed that although all four isomers were excised at somewhat similar rates after 1 h of incubation in the HeLa cell nuclear extracts, HNE-dG isomers 2 and 4 appeared to be excised at greater efficiency than isomers 1 and 3 at an early phase of the repair process.
Rate of Excision of HNE-dG Adducts Is Faster than the Rate of Its Complete Repair Synthesis
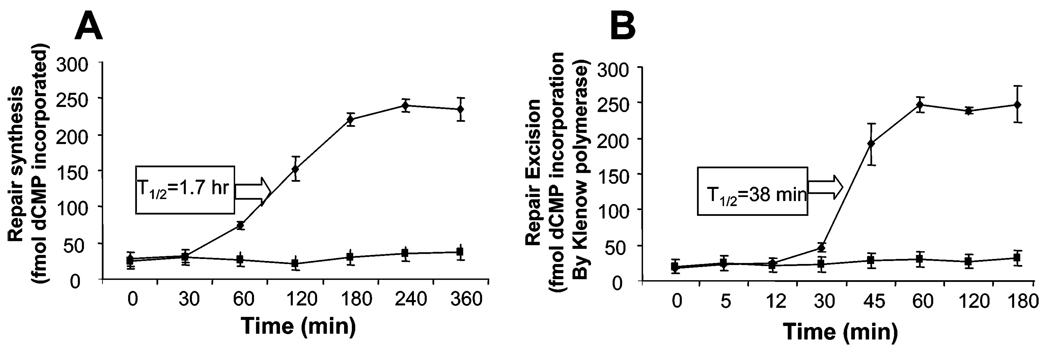
The rate of excision of HNE-dG (Figure 5A) seemed to be faster than the rate of complete repair synthesis (Figure 3D). It is in contrast to observations with the UV-induced DNA adducts as the classical NER substrates in which the damage recognition/excision step was found to be rate-limiting and hence had the slowest rate (41). We compared the rates of HNE-dG excision and complete repair synthesis using a methodology of [32P] incorporation/agarose gel electrophoresis similar to that described by Barett et al. (39). We found that the repair synthesis reached a plateau at about 4 h with a T1/2 of 1.7 h (Figure 6A), whereas the excision of HNE-dG attained a maximum at about 1 h with a T1/2 of 0.6 h (Figure 6B). The excision kinetics obtained from the [32P] incorporation/agarose gel electrophoresis study is similar to those obtained from the [32P]-postlabeling/HPLC method (Figure 5A). These studies indicate that the rate of excision of the HNE-dG adduct is ~2.8-fold faster than that of its complete repair synthesis.
FIGURE 6.
Comparison of kinetics of DNA repair synthesis and excision of HNE-dG adducts by HeLa cell nuclear extracts using a [32P]-incorporation/agarose gel electrophoresis method. (A) Kinetics of DNA repair synthesis of HNE-dG adducts. The repair reaction conditions were similar to those described in Figure 4. (B) Kinetics of DNA repair excision of HNE-dG adducts. The details are described in Experimental Procedures. T1/2 denotes the time required to complete 50% of the maximum extent of DNA repair synthesis and excision under our reaction conditions, and its values (shown) are deduced from the synthesis and excision rate curves. The symbols (◆) and (■) denote the HNE-damaged and undamaged pBSIIs. The data are the mean value derived from three independent experiments and presented with standard errors.
DISCUSSION
A growing body of evidence indicates that endogenous DNA damage induced by lipid peroxidation may play an important role in carcinogenesis (2–5). The enal-induced cyclic DNA adducts which include HNE-dG and ethenoadenine are mutagenic and have been detected as background lesions in tissues from rodents and humans (11, 15–18). HNE-induced mutation frequency has recently been shown to be increased significantly in NER-deficient cells (42). This observation suggests that the NER pathway is involved in the repair of HNE-dG adducts. However, direct evidence for the repair of the HNE-dG adduct by NER is still lacking. In this study, we demonstrated that in human nuclear extracts HNE-dG adducts are repaired by the NER pathway. We also showed that the repair of HNE-dG in human cell extracts proceeds at least 2.4-fold more efficiently than the repair of UV-induced DNA lesions under similar conditions. NER has been shown to repair not only UV-damaged DNA but also a variety of structurally diverse bulky and even small DNA oxidative adducts, 8-oxoguanine and thymine glycol (43). However, many of those adducts are repaired at significantly lower efficiency by comparison to UV-damage (43, 44). Notably, although our study shows that HNE-dG is repaired by NER at ~2.4-fold higher efficiency, other studies have documented that the extent of cleavage of the cyclobutane dimer (a UV photoproduct)-containing duplex by NER proteins was similar to that of propanodeoxyguanosine (PdG; a model substrate of HNE-dG)-containing duplex (30). Thus, HNE-dG repair via NER is preferred to repair of UV lesions or a variety of other known substrates including the model substrate (PdG). Our studies to understand the mechanism of this difference showed that the recognition and excision of HNE-dG is in fact about three times more efficient than that of its complete repair synthesis. Particularly, the recognition/excision step was found to be the rate-limiting and slowest for NER of UV adducts (41). It is conceivable that the rate of gap–synthesis after excision of the adducts could be similar for HNE-dG and UV adducts. Thus, the higher rate of recognition/excision of HNE-dG over that of UV adducts may be the major reason for the faster repair of HNE-dG by NER. The lack of three-dimensional structure of HNE-dG adducted DNA limits the explanation as to why HNE-dG is better recognized and/or excised than the UV-induced adducts or its own model adduct (PdG). An unusually long tail at the core propane adduct, which is not seen in other bulky adducts, may play a critical role in recognition/excision by NER proteins. There is also emerging evidence for different protein requirements in NER among structurally diverse DNA lesions. For example, Xeroderma pigmentosum (complementation group C) protein has recently been shown to have different roles in the repair of cyclobutane pyrimidine dimer (CPD) versus 6–4 photo products (45). Therefore, involvement of different proteins in the repair of HNE-dG versus UV-induced adduct may also be attributed to differences in NER efficiency.
Stereoisomers of various bulky adducts may be repaired by the NER system at different rates. The isomers of tamoxifen-DNA adducts are repaired at different rates (44) while the isomers of benzo (a) pyrene diol epoxide (BPDE)-DNA adducts are repaired at similar rates by the NER pathway (46). Our in vitro kinetic data show that the extent of repair of all four isomers of HNE-dG by the NER pathway is comparable at the 1 h point or beyond (Figure 5B). These results may explain that relatively low levels of these adducts exist as background endogenous adducts in humans (17, 18). It is notable that 50–60% of HNE-dG adducts were removed at 1–3 h. Little or no further excision was observed after 1 h (Figure 5). Since the pBSII DNA was globally modified by HNE, it is possible that HNE-dG adducts were formed at various sequence contexts. It is also possible that the repair was affected by sequence differences resulting in little or no repair of adducts at some sequences. Although it was unexpected, we found that at earlier time points isomers 2 and 4 were excised with at least 3 times greater efficiency than their counterpart isomers 1 and 3. Repeated measurements at 30 min showed this difference consistently. This suggests the importance of the stereo configuration of the 8-hydroxyl group with regard to the initial recognition and excision properties of NER proteins. Recently, it was also shown that the mutagenicity of the HNE-dG adducts depends on their stereochemistry, as isomers 3 and 4 were found to be more mutagenic than isomers 1 and 2 (47). However, mutagenicity cannot necessarily be correlated with repair efficiency. It is possible that the individual stereoisomers of HNE-dG adducts affect the DNA helical structure in different ways; consequently, they are recognized and excised at different rates. Indeed, the lesion recognition by NER proteins does not depend only on the chemical structure of the DNA lesion itself; the alterations in DNA helical structure induced by these lesions also play a critical role in the recognition step. These structural alterations include local unwinding of a few DNA bases around the damaged site. This unwinding also energetically favors bending of the DNA, resulting in further unwinding by NER enzymes. On the other hand, the DNA lesions that are good substrates for NER even become better substrates when they are superimposed on a mismatch DNA (48).
The levels of adducts detected in tissue DNA reflect the steady-state of adduct generation and removal; thus, if HNE-dG and/or other exocyclic adducts are removed efficiently by NER, their contribution to endogenous DNA damage is significantly higher than one would estimate by determination of absolute adduct levels. Nevertheless, the effects of oxidative stress and free radicals on the NER system and consequent changes of HNE-dG and other exocyclic DNA adduct levels need to be determined and warrant the investigation of their direct link, if any, to carcinogenesis in future studies.
ACKNOWLEDGMENT
We thank Drs. Randy Legarski of MD Anderson Cancer Center, Houston, TX and J. Christopher States of University of Louisville School of Medicine, Louisville, KY for generously providing the XPA and XAN1 cell lines. We thank Dr. Yue Zou of East Tennessee State University, TN for UV-irradiated pBSII DNA substrate, and also thank Ms. Ilse Hoffmann at the Institute for Cancer Prevention for editing the manuscript.
Footnotes
Supported in part by the NIH RO1 Grants CA 80917 (R.R.) and CA 43159 (F.L.C.) and NCI Cancer Center Grant P30 CA 17613.
Abbreviations: Acr, acrolein; Cro, crotonaldehyde; dG, deoxyguanosine; eA, 1,N6-ethenoadenine, HNE, trans-4-hydroxynonenal; NER, nucleotide excision repair; pBSII, pBluescript II SK− plasmid DNA; ROS, reactive oxygen species; XPA, Xeroderma pigmentosum complementation group A.
REFERENCES
- 1.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde, and related aldehydes. Free Radical Biol. Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 2.Bartsch H. Keynote address: exocyclic adducts as new risk markers for DNA damage in man. In: Singer B, Bartsch H, editors. Exocyclic DNA Adducts in Mutagenesis and Carcinogenesis. Lyon, France: IARC Sci Publ.; 1999. pp. 1–16. No. 150. [PubMed] [Google Scholar]
- 3.Ames BN, Gold LS. Endogenous mutagens and the causes of aging and cancer. Mutat. Res. 1991;250:3–16. doi: 10.1016/0027-5107(91)90157-j. [DOI] [PubMed] [Google Scholar]
- 4.Christians FC, Newcomb TG, Loeb LA. Potential sources of multiple mutations in human cancers. Prev. Med. 1995;24:329–332. doi: 10.1006/pmed.1995.1054. [DOI] [PubMed] [Google Scholar]
- 5.Marnett LJ, Plastaras JP. Endogenous DNA damage and mutation. Trends Genet. 2001;17:214–221. doi: 10.1016/s0168-9525(01)02239-9. [DOI] [PubMed] [Google Scholar]
- 6.Goel SK, Lalwani ND, Reddy JK. Peroxisome proliferation and lipid peroxidation in rat liver. Cancer Res. 1986;46:1324–1330. [PubMed] [Google Scholar]
- 7.Rushmore TH, Lim YP, Farber E, Ghohal AK. Rapid lipid peroxidation in the nuclear fraction of rat liver induced by a diet deficient in choline and methionine. Cancer Lett. 1984;24:251–255. doi: 10.1016/0304-3835(84)90020-x. [DOI] [PubMed] [Google Scholar]
- 8.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde, and related aldehydes. Free Radical Biol. Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 9.Schneider C, Tallman KA, Porter NA, Brash AR. Two distinct pathways of formation of 4-hydroxynonenal. Mechanisms of nonenzymatic transformation of the 9-and 13-hydroperoxides of linoleic acid to 4-hydroxyalkenals. J. Biol. Chem. 2001;276:20831–20838. doi: 10.1074/jbc.M101821200. [DOI] [PubMed] [Google Scholar]
- 10.Dianzani MU. 4-Hydroxynonenal and cell signaling. Free Radical Res. 1998;28:553–560. doi: 10.3109/10715769809065811. [DOI] [PubMed] [Google Scholar]
- 11.Chung FL, Chen HJ, Nath RG. Lipid peroxidation as a potential endogenous source for the formation of exocyclic DNA adducts. Carcinogenesis. 1996;17:2105–2111. doi: 10.1093/carcin/17.10.2105. [DOI] [PubMed] [Google Scholar]
- 12.Nath RG, Chung FL. Detection of exocyclic 1,N2-propanodeoxyguanosine adducts as common DNA lesions in rodents and humans. Proc. Natl. Acad. Sci. U.S.A. 1994;91:7491–7495. doi: 10.1073/pnas.91.16.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yi P, Zhan D, Samokyszyn VM, Doerge DR, Fu PP. Synthesis and 32P-postlabeling/high-performance liquid chromatography separation of diastereomeric 1,N2-(1,3-propano)-2′-deoxyguanosine 3′-phosphate adducts formed from 4-hydroxy-2-nonenal. Chem. Res. Toxicol. 1997;10:1259–1265. doi: 10.1021/tx970100r. [DOI] [PubMed] [Google Scholar]
- 14.Wang H, Kozekov ID, Harris TM, Rizzo CJ. Site-specific synthesis and reactivity of oligonucleotides containing stereochemically defined 1,N2-deoxyguanosine adducts of the lipid peroxidation product trans-4-hydroxynonenal. J. Am. Chem. Soc. 2003;125:5687–5700. doi: 10.1021/ja0288800. [DOI] [PubMed] [Google Scholar]
- 15.Chung F-L, Nath RG, Nagao M, Nishikawa A, Zhou G-D, Randerath K. Endogenous formation and significance of 1,N2-propanodeoxyguanosine adducts. Mutat. Res. 1999;424:71–81. doi: 10.1016/s0027-5107(99)00009-3. [DOI] [PubMed] [Google Scholar]
- 16.Wacker M, Schuler D, Wanek P, Eder E. Development of a (32)P-postlabeling method for the detection of 1,N2-propanodeoxyguanosine adducts of trans-4-hydroxy-2-nonenal in vivo. Chem. Res. Toxicol. 2000;13:1165–1173. doi: 10.1021/tx000058r. [DOI] [PubMed] [Google Scholar]
- 17.Chung FL, Nath RG, Ocando J, Nishikawa A, Zhang L. Deoxyguanosine adducts of t-4-hydroxy-2-nonenal are endogenous DNA lesions in rodents and humans: detection and potential sources. Cancer Res. 2000;60:1507–1511. [PubMed] [Google Scholar]
- 18.Chung FL, Zhang L, Ocando J, Nath RG. Role of 1,N2-propanodeoxyguanosine adducts as endogenous DNA lesions in rodents and humans. In: Singer B, Bartsch H, editors. Review in Exocyclic DNA Adducts in Mutagenesis and carcinogenesis. Lyon, France: IARC Sci Publ.; 1999. pp. 45–54. No 150. [PubMed] [Google Scholar]
- 19.Wacker M, Wanek P, Eder E. Detection of 1,N2-propanodeoxyguanosine adducts of trans-4-hydroxy-2-nonenal after gavage of trans-4-hydroxy-2-nonenal or induction of lipid peroxidation with carbon tetrachloride in F344 rats. Chem. Biol. Interact. 2001;137:269–283. doi: 10.1016/s0009-2797(01)00259-9. [DOI] [PubMed] [Google Scholar]
- 20.Hussain SP, Raja K, Amstad PA, Sawyer M, Trudel LJ, Wogan GN, Hofseth LJ, Shields PG, Billiar TR, Trautwein C, Hohler T, Galle PR, Phillips DH, Markin R, Marrogi AJ, Harris CC. Increased p53 mutation load in nontumorous human liver of Wilson disease and hemochromatosis: oxyradical overload diseases. Proc. Nat. Acad. Sci. U.S.A. 2000;97:12770–12775. doi: 10.1073/pnas.220416097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenblatt MS, Bennett WP, Hollstein M, Harris CC. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res. 1994;54:4855–4878. [PubMed] [Google Scholar]
- 22.Hu W, Feng Z, Eveleigh J, Iyer G, Pan J, Amin S, Chung FL, Tang MS. The major lipid peroxidation product, trans-4-hydroxy-2-nonenal, preferentially forms DNA adducts at codon 249 of human p53 gene, a unique mutational hotspot in hepatocellular carcinoma. Carcinogenesis. 2002;23:1781–1789. doi: 10.1093/carcin/23.11.1781. [DOI] [PubMed] [Google Scholar]
- 23.Singer B, Antoccia A, Basu AK, Dosanjh MK, Fraenkel-Conrat H, Gallagher PE, Kusmierek JT, Qiu Z-H. Both purified human 1,N6-ethenoadenine-binding protein and purified human 3-methyladenine-DNA glycosylase act on 1,N6-ethenoadenine and 3-methyladenine. Proc. Natl. Acad. Sci. U.S.A. 1992;89:9386–9390. doi: 10.1073/pnas.89.20.9386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dosanjh MK, Chenna A, Kim E, Fraenkel-Conrat H, Samson L, Singer B. All four known cyclic adducts formed in DNA by the vinyl chloride metabolite chloroacetaldehyde are released by a human DNA glycosylase. Proc. Natl. Acad. Sci. U.S.A. 1994;91:1024–1028. doi: 10.1073/pnas.91.3.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dosanjh MK, Roy R, Mitra S, Singer B. 1,N6-ethenoadenine is preferred over 3-methyladenine as substrate by a cloned human N-methylpurine-DNA glycosylase (3-methyladenine-DNA glycosylase) Biochemistry. 1994;33:1624–1628. doi: 10.1021/bi00173a002. [DOI] [PubMed] [Google Scholar]
- 26.Roy R, Biswas T, Lee JC, Mitra S. Mutation of a unique aspartate residue abolishes the catalytic activity but not substrate binding of the mouse N-methylpurine-DNA glycosylase (MPG) J. Biol. Chem. 2000;275:4278–4282. doi: 10.1074/jbc.275.6.4278. [DOI] [PubMed] [Google Scholar]
- 27.Saparbaev M, Kleibl K, Laval J. Escherichia coli, Saccharomyces cerevisiae, rat and human 3-methyladenine DNA glycosylases repair 1,N6-ethenoadenine when present in DNA. Nucleic Acids Res. 1995;23:3750–3755. doi: 10.1093/nar/23.18.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matijasevic Z, Sekiguchi M, Ludlum DB. Release of N2,3-ethenoguanine from chloroacetaldehyde-treated DNA by Escherichia coli 3-methyladenine DNA glycosylase II. Proc. Natl. Acad. Sci. U.S.A. 1992;89:9331–9334. doi: 10.1073/pnas.89.19.9331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hang BH, Chenna A, Rao S, Singer B. 1,N6-ethenoadenine and 3,N4-ethenocytosine are excised by separate human DNA glycosylases. Carcinogenesis. 1996;17:155–157. doi: 10.1093/carcin/17.1.155. [DOI] [PubMed] [Google Scholar]
- 30.Johnson KA, Fink SP, Marnett LJ. Repair of propanodeoxyguanosine by nucleotide excision repair in vivo and in vitro. J. Biol. Chem. 1997;17:11434–11438. doi: 10.1074/jbc.272.17.11434. [DOI] [PubMed] [Google Scholar]
- 31.Yang I-Y, Hossain M, Miller H, Khullar S, Johnson F, Grollman A, Moriya M. Responses to the major acrolein-derived deoxyguanosine adduct in Escherichia coli. J. Biol. Chem. 2001;276:9071–9076. doi: 10.1074/jbc.M008918200. [DOI] [PubMed] [Google Scholar]
- 32.Van der Veen LA, Hashim MF, Nechev LV, Harris TM, Harris CM, Marnett LJ. Evaluation of the mutagenic potential of the principal DNA adduct of acrolein. J. Biol. Chem. 2001;276:9066–9070. doi: 10.1074/jbc.M008900200. [DOI] [PubMed] [Google Scholar]
- 33.Chung F-L, Pan J, Choudhury S, Roy R, Hu W, Tang M-S. Formation of trans-4-hydroxy-2-nonenal-and other enal-derived cyclic DNA adducts from omega-3 and -6 polyunsaturated fatty acids and their roles in DNA repair and human p53 gene mutation. Mutat. Res. 2003;531:25–26. doi: 10.1016/j.mrfmmm.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Myrand SP, Topping RS, States JC. Stable transformation of xeroderma pigmentosum group A cells with an XPA minigene restores normal DNA repair and mutagenesis of UV-treated plasmids. Carcinogenesis. 1996;17:1909–1917. doi: 10.1093/carcin/17.9.1909. [DOI] [PubMed] [Google Scholar]
- 35.Schreiber E, Matthias P, Muller MM, Schaffner W. Rapid detection of octamer binding proteins with miniextracts, prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Esterbauer H, Weger W. Uber die Wirkung Von Aldehyden auf gesunde und maligne Zellen, 3. Mitt: Synthese Von homologen 4-Hydroxy-2-alkenalen, II. Monatsh Chem. 1967;78:1994–2000. [Google Scholar]
- 37.Biggerstaff M, Wood RD. Assay for nucleotide excision repair protein activity using fractionated cell extracts and UV-damaged plasmid DNA DNA Repair Protocols Eukaryotic Systems. In: Henderson DS, editor. Methods in Molecular Biology. Vol. 113. Totowa, NJ: Humana Press; 1999. pp. 357–372. [DOI] [PubMed] [Google Scholar]
- 38.Kohno K, Shimamoto T. Nucleotide excision repair assay in Drosophila melanogaster using established cell lines. DNA Repair Protocols Eukaryotic Systems. In: Henderson DS, editor. Methods in Molecular Biology. Vol 113. Totowa, NJ: Humana Press; 1999. pp. 337–346. [DOI] [PubMed] [Google Scholar]
- 39.Barret J-M, Calsou P, Salles B. Deficient nucleotide excision repair activity in protein extracts from normal human lymphocytes. Carcinogenesis. 1995;16:1611–1616. doi: 10.1093/carcin/16.7.1611. [DOI] [PubMed] [Google Scholar]
- 40.Chung FL, Zhang L. Deoxyguanosine adducts of tert-4-hydroxy-2-nonenal as markers of endogenous DNA lesions. Methods Enzymol. 2002;353:523–536. doi: 10.1016/s0076-6879(02)53074-3. [DOI] [PubMed] [Google Scholar]
- 41.Aboussekhra A, Biggerstaff M, Shivji MK, Vilpo JA, Moncollin V, Podust VN, Protic M, Hubsc U, Egly JM, Wood RD. Mammalian DNA nucleotide excision repair reconstituted with purified protein components. Cell. 1995;80:859–868. doi: 10.1016/0092-8674(95)90289-9. [DOI] [PubMed] [Google Scholar]
- 42.Feng Z, Hu W, Amin S, Tang M-S. Mutational spectrum and genotoxicity of the major lipid peroxidation product, trans-4-hydroxy-2-nonenal, induced DNA adducts in nucleotide excision repair-proficient and -deficient human cells. Biochemistry. 2003;42:7848–7854. doi: 10.1021/bi034431g. [DOI] [PubMed] [Google Scholar]
- 43.Reardon JT, Bessho T, Kung HC, Bolton PH, Sancar A. In vitro repair of oxidative DNA damage by human nucleotide excision repair system: possible explanation for neurodegeneration in xeroderma pigmentosum patients. Proc. Natl. Acad. Sci. U.S.A. 1997;94:9463–9468. doi: 10.1073/pnas.94.17.9463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shibutani S, Reardon JT, Suzuki N, Sancar A. Excision of tamoxifen-DNA adducts by the human nucleotide excision repair system. Cancer Res. 2000;60:2607–2610. [PubMed] [Google Scholar]
- 45.Emmert S, Kobayashi N, Khan SG, Kraemer KH. The xeroderma pigmentosum group C gene leads to selective repair of cyclobutane pyrimidine dimers rather than 6–4 photoproducts. Proc. Natl. Acad. Sci. U.S.A. 2000;97:2151–2156. doi: 10.1073/pnas.040559697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang MS, Pierce JR, Doisy RP, Nazimiec ME, MacLeod MC. Differences and similarities in the repair of two benzo[a]pyrene diol epoxide isomers induced DNA adducts by uvrA, uvrB, and uvrC gene products. Biochemistry. 1992;31:8429–8436. doi: 10.1021/bi00151a006. [DOI] [PubMed] [Google Scholar]
- 47.Fernandes PH, Wang H, Rizzo CJ, Lloyd RS. Site-specific mutagenicity of stereochemically defined 1,N2-deoxyguanosine adducts of trans-4-hydroxynonenal in mammalian cells. EnViron. Mol. Mutagen. 2003;42:68–74. doi: 10.1002/em.10174. [DOI] [PubMed] [Google Scholar]
- 48.Hess MT, Schwitter U, Petretta M, Giese B, Naegeli H. Bipartite substrate discrimination by human nucleotide excision repair. Proc. Natl. Acad. Sci. U.S.A. 1997;94:6664–6669. doi: 10.1073/pnas.94.13.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]