Abstract
Dendritic cells can prime naïve CD4+ T cells, however we demonstrate that DC-mediated priming is insufficient for the development of TH2 cell-dependent immunity. We identify basophils as a dominant cell population that coexpressed MHC class II and Il4 message following helminth infection. Basophilia was promoted by thymic stromal lymphopoietin (TSLP) and depletion of basophils impaired immunity to helminth infection. In vitro, basophils promoted antigen-specific CD4+ T cell proliferation and IL-4 production and transfer of basophils augmented the expansion of helminth-responsive CD4+ T cells in vivo. Collectively, these studies suggest that MHC class II-dependent interactions between basophils and CD4+ T cells promote TH2 cytokine responses and immunity against helminth infection.
Keywords: Th2 cells, basophils, MHC class II, helminth infection
Since the demonstration of specification of CD4+ T helper (TH) cell fates1, substantial advances have been made in delineating the regulatory mechanisms that promote distinct modules of CD4+ T cell-dependent immunity and inflammation2. TH2 cell differentiation is dependent on interleukin 4 receptor (IL-4R) and the transcription factors STAT6 and GATA3 and their signature cytokine profile is characterized by expression of IL-4 http://www.signaling-gateway.org/molecule/query?afcsid=A001262, IL-5, IL-9 and IL-133. The hallmarks of TH2 cytokine-dependent inflammation at barrier surfaces such as the skin, airway and intestine include the recruitment of CD4+ TH2 cells, eosinophils, mast cells and basophils, coupled with goblet cell hyperplasia, mucus production and increased smooth muscle contractility4. Type 2 inflammatory responses are required for immunity and tissue repair following exposure to helminth parasites. However, TH2 cytokine responses can also promote pathological changes observed in the context of asthma and allergic diseases5.
While the sequelae of type 2 immunity and inflammation in peripheral tissues are well characterized, the innate responses that promote TH2 cell development, including the nature of the antigen-presenting cell (APC) involved, the host-microbial receptor-ligand interactions and the APC-derived factors required to initiate and sustain TH2 cell differentiation remain less well defined6. DCs are the only APC thought to prime naive T cells and the current paradigm suggests that recognition of conserved pathogen-associated molecular patterns via distinct pattern recognition receptors expressed on DCs promote appropriate pathogen-specific CD4+ TH cell responses7. Activation of DCs can result in increased surface expression of MHC class II and costimulatory molecules such as CD40, CD80 and CD86 as well as expression of factors that can shape the nature of the developing adaptive immune response8. However, the critical DC-derived signals responsible for driving TH2 cell responses in vivo remain undefined9. In vitro studies indicate that the requirements for DC-mediated TH2 cell differentiation include differential expression of the Notch ligand jagged 10 and up-regulation of the costimulatory molecules CD4011 and OX40L12. However whether these pathways are sufficient for DCs to promote CD4+ TH2 cell differentiation in vivo is unclear.
The recruitment and activation of mast cells, eosinophils and basophils are hallmarks of TH2 cytokine-dependent inflammation in peripheral tissues and earlier studies suggest that these granulocyte populations may function as accessory cells in the initiation of CD4+ TH2 cell responses. For example, mast cells, eosinophils and basophils are competent to produce and secrete IL-4 from intracellular stores, implicating these populations as early sources of IL-4 that could promote CD4+ TH2 cell differentiation13–15. In addition, mast cells and eosinophils can express MHC class II, and eosinophils have been implicated as potential APCs in both airway inflammation and helminth infection16–18. Basophil frequencies are increased following exposure to allergens and helminth parasites and previous work demonstrated that basophils are a dominant source of IL-4 and IL-13 following helminth infection and contribute to protective immunity19‐21. Although basophilia is a common feature of TH2 cytokine-mediated inflammation, little is known about how these cells are activated and recruited to peripheral tissues. A conserved mechanism for basophil-mediated recognition of parasite products and allergens through protease-dependent activation was recently proposed22. In that study, basophils were recruited to the draining lymph node early following allergen exposure and were essential for the generation of TH2 cytokine responses elicited following papain immunization. However, the potential accessory cell functions of basophils during CD4+ TH2 cell development remain unknown. Collectively, the inability of DCs to express IL-4 and the lack of defined mechanisms through which DCs promote TH2 cell differentiation have provoked a reassessment of the relative contribution of DCs in promoting TH2 cytokine responses in vivo.
In this study, we demonstrate that DC-restricted expression of MHC class II was insufficient for the generation of protective CD4+ TH2 cytokine-dependent immunity to the gastrointestinal helminth Trichuris muris. Basophils were identified as a dominant accessory cell population that expressed Il4 message and MHC class II. In vitro studies showed that basophils could promote MHC class II-dependent antigen-specific CD4+ T cell proliferation and TH2 cell differentiation. Depletion of basophils in vivo resulted in impaired protective immunity to T. muris, while adoptive transfer of primary wild-type basophils augmented CD4+ T cell proliferation in response to Schistosoma mansoni egg injection. Taken together, these studies suggest a previously unrecognized role for basophils in MHC class II-dependent cognate interactions with CD4+ T cells that promote parasite-specific TH2 cytokine responses and host protective immunity.
RESULTS
CD11c-restricted MHC II is insufficient for type 2 immunity
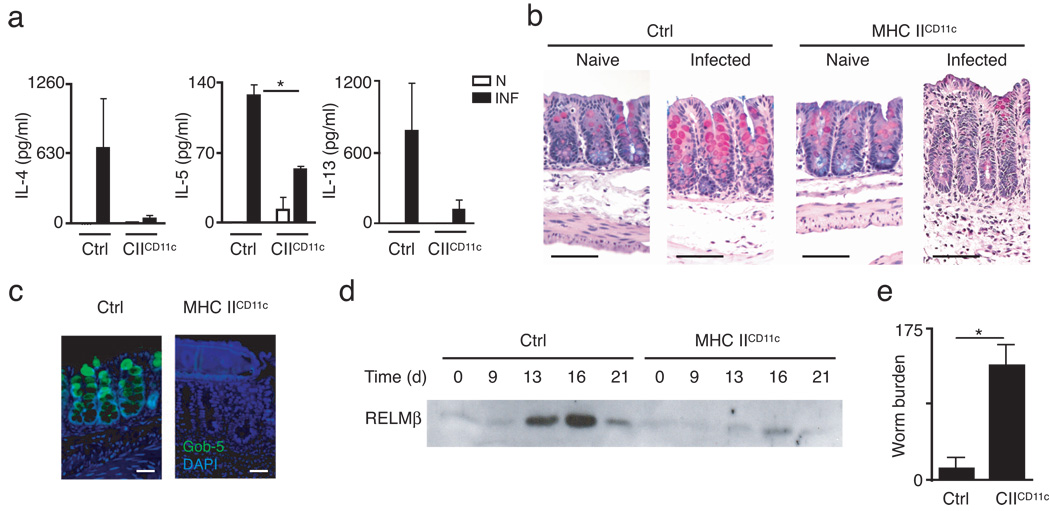
To test whether antigen presentation by CD11c+ DCs was sufficient to promote CD4+ TH2 cell-dependent immunity in vivo, mice in which MHC class II expression is restricted to CD11c+ cells (MHC IICD11c mice, Supplementary Fig. 1 online) were infected with the intestinal helminth parasite T. muris. Expulsion of T. muris and protective immunity is dependent on CD4+ TH2 cells while parasite-specific IFN-γ production promotes chronic infection23–25. T. muris infection provides a well-characterized in vivo model of TH2 cytokine-dependent immunity that offers a functional read-out of the magnitude of the host TH2 cytokine response. Since MHC IICD11c mice lack MHC class II expression on the thymic epithelium26 and therefore are unable to positively select CD4+ T cells in the thymus, mice were given fetal thymic grafts eight weeks prior to infection to provide an endogenous CD4+ T cell population. Following infection with T. muris, littermate control mice developed pathogen-induced TH2 cytokine responses characterized by production of IL-4, IL-5 and IL-13 by mesenteric lymph node (mLN) cells (Fig. 1a). In contrast, MHC IICD11c mice exhibited minimal infection-induced production of TH2 cytokines (Fig. 1a). Histological analysis of intestinal tissues in infected control mice revealed hallmarks of type 2 inflammation including goblet cell hyperplasia and increased mucin production (Fig. 1b), and expression of Gob5, also known as chloride channel calcium activated 3 (mCLCA3) (Fig. 1c), a goblet cell-specific marker regulated by TH2 cytokines and associated with type 2 inflammation27. Consistent with decreased TH2 cytokine production, infected MHC IICD11c mice exhibited a marked absence of goblet cells and goblet cell-derived proteins (Fig. 1b,c). TH2 cytokine-dependent expression and luminal secretion of goblet cell-derived resistin-like molecule beta (RELMβ) in resistant mice provides a non-invasive indicator of the kinetics of TH2 cytokine responses in the intestinal microenvironment28. As previously reported, luminal RELMβ protein peaked in resistant control mice between days 12 and 18 post-infection28, coincident with worm expulsion (Fig. 1d), while luminal secretion of RELMβ in infected MHC IICD11c mice was severely reduced in magnitude (Fig. 1d). Associated with a polarized TH2 cytokine response, littermate control mice also displayed increased titers of immunoglobulins IgG1 and IgE (data not shown). However, since MHC IICD11c mice lack MHC class II expression on B cells26, no antigen-specific class-switched antibody was detected in infected MHC IICD11c mice (data not shown). Critically, the defect in TH2 cytokine responses in MHC IICD11c mice resulted in susceptibility to infection in mice on a normally genetically resistant background (Fig. 1e). Taken together, these data demonstrate that restriction of MHC class II-dependent antigen presentation to CD11c+ cells was insufficient to promote CD4+ TH2 cell-dependent immunity following intestinal helminth infection.
Figure 1. MHC class II expression restricted to CD11c+ DC is insufficient to promote type 2 immunity to intestinal helminth infection.
(a–e) Littermate control and MHC IICD11c mice were infected with T. muris eggs and sacrificed on day 21 post-infection. (a) mLN cells from naïve (N, open bars) and infected (INF, filled bars) mice were cultured in vitro for 48 h and supernatants were assayed by ELISA for IL-4, IL-5, and IL-13 secretion. *P = 0.003 (b) Cecal sections from naïve and infected control or MHC IICD11c mice were stained with Alcian blue/periodic-acid Schiff reagent to detect mucins. Bar = 20 µm (c) Cecal sections from day 21 infected control and MHC IICD11c mice were stained by immunofluoresence for Gob-5 (green) and DAPI (blue). (d) Protein extracted from pooled fecal pellets of control and MHC IICD11c mice collected on the indicated days post-infection was analyzed by immunoblot to assess luminal secretion of RELMβ. (e) Cecal worm burdens from infected control and MHC IICD11c mice were determined microscopically at day 21 post-infection. *P = 0.0006 Results are representative of three independent experiments with n = 3–5 mice per group (a–e). Graphs represent mean ± SEM of n = 3–5 mice per group (a,e).
Th1 cell differentiation is intact in MHC IICD11c mice
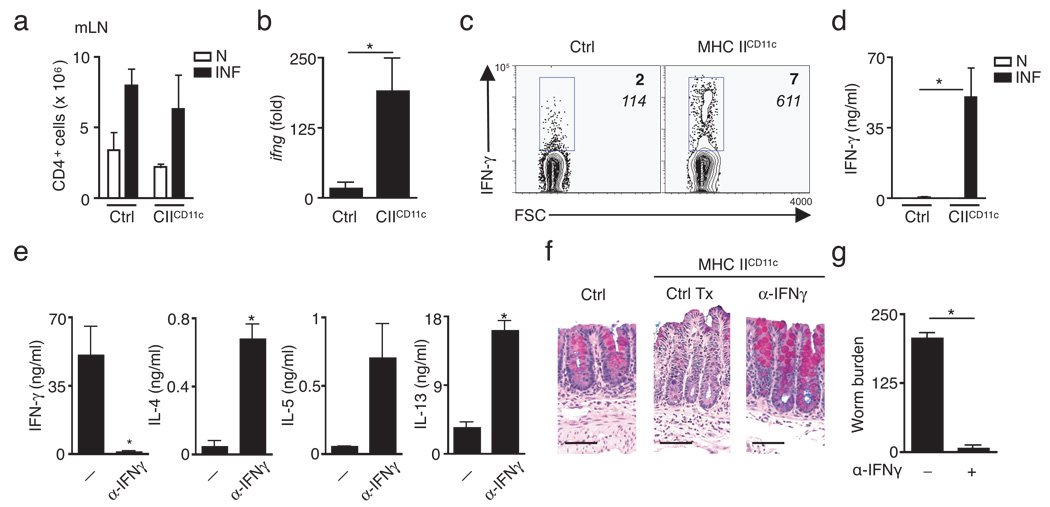
The extensive physical and biochemical barriers between antigenic material in the enteric space and lymphocytes in the underlying lymphoid follicles and lamina propria of the intestine create unique challenges in antigen sampling and presentation29. Therefore the impaired TH2 cytokine responses in T. muris-infected MHC IICD11c mice may indicate that additional APCs are required for either the sampling of T. muris antigens or the provision of signals necessary for the priming, proliferation and differentiation of pathogen-specific CD4+ T cells. However, following T. muris infection both littermate control mice and MHC IICD11c mice exhibited an infection-induced increase in total CD4+ T cell numbers in the draining mLNs (Fig. 2a), suggesting that DC-restricted antigen-presentation was sufficient for promoting proliferation of CD4+ T cells following infection. To determine whether CD4+ T cells in infected MHC IICD11c mice were non-responsive or had received signals for alternative differentiation, mRNA was isolated from mLNs of naive and infected control or MHC IICD11c mice and analyzed for expression of IL-10, IL-17, and IFN-γ to assess the magnitude of Treg, TH-17 and TH1 responses. While there was little to no induction of IL-10 and IL-17 expression in infected control and MHC IICD11c mice (Supplementary Fig. 2 online), Ifng mRNA was selectively and significantly induced in infected MHC IICD11c mice compared to controls (Fig. 2b). Consistent with elevated Ifng mRNA expression, the frequency of mLN CD4+ T cells producing IFN-γ (Fig. 2c; bold) as well as the amount of IFN-γ made per cell (Fig. 2c; italics) were increased in infected MHC IICD11c mice compared to control mice. Secretion of IFN-γ was also significantly elevated following in vitro stimulation of mLN cells isolated from infected MHC IICD11c mice compared to control mice (Fig. 2d). Thus, following intestinal infection, cognate interactions between antigen-specific CD4+ T cells and CD11c+ DCs alone were sufficient to promote the priming and expansion of CD4+ T cells as well as to provide signals necessary for TH1 cell differentiation but were insufficient for the development of TH2 cytokine-dependent immunity. These data suggested that CD11c+ cells may not be required for TH2 cytokine-dependent immunity. To determine the relative contribution of CD11c+ cells in immunity to T. muris we utilized CD11c-diphtheria toxin receptor (DTR) mice in which delivery of diphtheria toxin to littermate controls has no effect while similar administration to CD11c-DTR mice results in the selective apoptosis of CD11c+ cells. To avoid the toxicity associated with long-term diphtheria toxin treatment of intact CD11c-DTR mice, we employed bone marrow chimeras of either wild-type or CD11c-DTR donor bone marrow into wild-type recipients. While transient depletion of CD11c+ cells (Supplementary Fig. 3a online) throughout the course of T. muris infection resulted in a significant reduction in mLN CD4+ T cell numbers (Supplementary Fig. 3b), there was no effect on production of TH2 cytokines or worm burdens (Supplementary Fig. 3c,d). Together these data suggest that CD11c+ cells may not be essential for protective immunity to T. muris and that another APC may be required for the development of TH2 cytokine-dependent immunity in vivo.
Figure 2. Blockade of IFNγ in MHC IICD11c mice recovers Th2 cytokine-dependent immunity to T. muris infection.
(a) mLN cells from naïve (N, open bars) or T. muris-infected (INF, filled bars) littermate control and MHC IICD11c mice were counted and total numbers of CD4+ T cells determined by flow cytometry. Values are mean ± SEM of three mice per group representative of three independent experiments of n = 3–5 mice per group. (b) Ifng mRNA expression in mLN was determined by quantitative real-time PCR. Values represent mean ± SEM of the fold-increase over naïve controls. *P = 0.03 (c) Flow cytometry of intracellular IFN-γ staining of mLN cells from infected control or MHC IICD11c mice. Plots are gated on CD4+ T cells. Numbers indicate cells within the gated area. IFN-γ+, italics = mean fluorescence intensity. Plots representative of three independent experiments of n = 3–5 mice per group. (d) IFN-γ secretion by mLN cells isolated from naïve (N, open bars) and infected (INF, filled bars) mice was examined by ELISA. *P = 0.03. (e) Cytokine production by stimulated mLN cells isolated from control-treated (Tx) or anti-IFNγ-treated MHC IICD11c mice was examined by ELISA. IFN-γ *P = 0.03, IL-4 *P = 0.002, IL-13 *P = 0.001. (f) Cecal sections from littermate control, control-treated or anti-IFN-γ treated MHC IICD11c mice stained for mucins with Alcian blue-periodic-acid Schiff. Images representative of n = 3–4 mice per group. (g) Worm burdens from infected control-treated or anti-IFN-γ treated MHC IICD11c mice at day 21 post-infection. *P < 0.001. Results are presented as mean ± SEM n = 3–4 mice per group (e,g).
To determine whether alterations in the cytokine milieu could restore immunity in MHC IICD11c mice, T. muris-infected MHC IICD11c mice were treated with a monoclonal anti-IFN-γ blocking antibody during the course of infection. Consistent with previous findings (Fig. 1a, Fig. 2d) stimulated T cells isolated from the mLNs of infected MHC IICD11c mice exhibited a robust IFN-γ response with low concentrations of IL-4, IL-5 and IL-13 (Fig. 2e). Associated with the lack of TH2 cytokines, control-treated MHC IICD11c mice exhibited decreased goblet cell responses and susceptibility to T. muris infection (Fig. 2f,g). Anti-IFN-γ treatment of MHC IICD11c mice resulted in a significant reduction in IFN-γ production and the emergence of a TH2 cytokine response characterized by significantly increased IL-4, IL-5 and IL-13 production by mLN cells, goblet cell hyperplasia and recovery of immunity to infection (Fig. 2e–g). Taken together, these data suggest that following blockade of a non-protective TH1 cytokine response, CD11c+ cells alone can provide the antigen-specific interactions to drive CD4+ TH2 cell differentiation and protective immunity. However, in the presence of endogenous IFN-γ signals, non-DC populations are required for the development of protective TH2 cytokine responses in vivo.
Basophils express MHC class II and Il4 mRNA
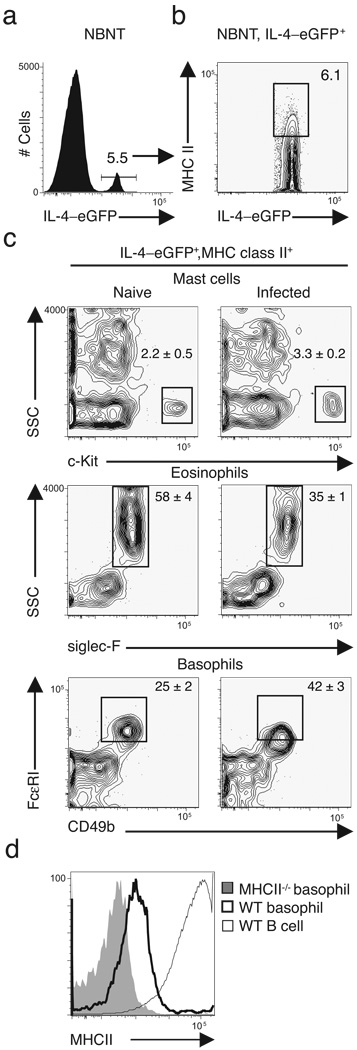
In addition to DC, macrophages and B cells are professional APCs involved in the development of adaptive CD4+ T cell-dependent immunity. However, clodronate-loaded liposome depletion of macrophages had no effect on cytokine-dependent inflammation or worm expulsion (Supplementary Fig. 4a–c online). Previous work demonstrated that adoptive transfer of CD4+ T cells alone into mice lacking both B and T cells was sufficient to restore immunity to T. muris30. Mice deficient in B cells (µMT) also exhibited intact TH2 cytokine-dependent goblet cell responses and protective immunity (Supplementary Fig. 4d–f). Collectively, these data suggest that while macrophages and B cells may contribute to immunity to T. muris in a physiologic setting, they do not have essential non-redundant roles in host protective immunity. We therefore focused on the identification of innate immune cells that could both express MHC class II and provide an innate source of IL-4 following T. muris infection. We previously employed IL-4–eGFP (4-get) reporter mice to track emerging CD4+ TH2 responses following T. muris infection31. 4-get mice contain an internal ribosomal entry site (IRES)-enhanced green fluorescent protein (eGFP) element within the Il4 locus allowing direct ex vivo analysis of cells competent to express IL-432. We utilized the same in vivo approach to identify non-B non-T cells that co-expressed Il4 mRNA and MHC class II molecules. Gating on non-B non-T cells, we identified an IL-4–eGFP+ cell population (Fig. 3a) that expressed MHC class II (Fig. 3b). Previous studies have shown that mast cells and eosinophils can express MHC class II16–18 and are competent to produce IL-413,14. However, classical mast cells (c-Kit+ SSChi) were not found following infection with T. muris and frequencies of siglec-F+ SSChi eosinophils were decreased after infection (Fig. 3c). In contrast, CD49b+ FcεRI+ basophils emerged as a dominant cell type expressing both Il4 mRNA and MHC class II following T. muris infection (Fig. 3c), consistently comprising 40% of IL-4–eGFP+ MHC class II+ cells. Although MHC class II was not expressed as abundantly as in professional APCs such as B cells, basophils expressed intermediate amounts of MHC class II compared to MHC class II-deficient basophils (Fig. 3d). While there have been previous reports of MHC class II expression on eosinophils17,18, this is the first report we are aware of demonstrating MHC class II expression on basophils and suggests a potential accessory cell function for this cell population during helminth infection.
Figure 3. FcεRI+ CD49b+ basophils co-express MHC class II and IL-4/eGFP.
Flow cytometry of splenocytes isolated from naïve and T. muris-infected 4-get mice at day 14 post-infection. (a) IL-4–eGFP+ cells were identified from a non-B non-T (NBNT) cell gate (CD3−B220−CD19−) and (b) analyzed for co-expression of MHC class II and IL-4–eGFP. (c) IL-4–eGFP+, MHC class II+ NBNT cells were characterized for expression of c-Kit, siglec-F, FcεRI, and CD49b. Plots representative of n = 3 mice per group (a–c); numbers are mean ± SEM (c). (d) MHC class II expression on WT basophils (heavy line), MHC class II−/− basophils (grey filled), or WT B cells (light line) isolated from naïve mice. Plots representative of n = 3 mice per group.
Basophils depletion impairs immunity to T. muris
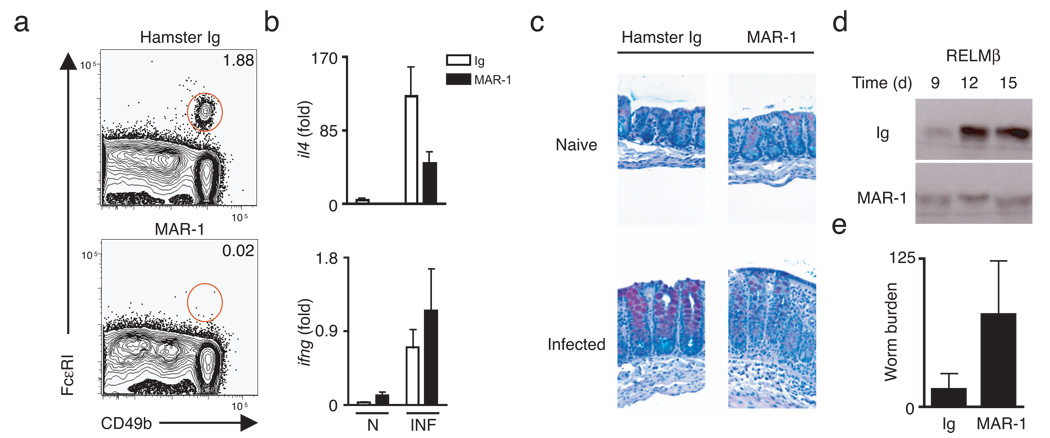
To determine whether basophils play a role in the development of TH2 cytokine-dependent protective immunity, wild-type C57BL/6 mice were infected with T. muris and treated with either control Ig or a monoclonal antibody against the FcεRI (MAR-1). Previous studies have demonstrated efficient depletion of basophils for up to 10 days following i.p. injection of MAR-133 and we observed greater than 90% depletion of basophils at day 21 post-infection (Fig. 4a) following MAR-1 treatment. Depletion of basophils in infected mice resulted in decreased Il4 mRNA expression (Fig. 4b), a marked reduction in TH2 cytokine-dependent goblet cell hyperplasia (Fig. 4c) and a decrease in luminal secretion of RELMβ in the intestine (Fig. 4d). Loss of basophils and impaired TH2 cytokine responses were associated with impaired expulsion of T. muris (Fig. 4e). Taken together, these data support a role for basophils in the development of protective type 2 immunity to intestinal helminth infection.
Figure 4. Depletion of FcεRI+ cells in vivo results in impaired immunity to Trichuris infection.
(a) Flow cytometric analysis of splenic basophils from control Ig-treated or MAR-1-treated mice at day 21-post-infection. Plots gated on CD3−B220−CD19− non-B non-T cells. (b) Real-time quantitative PCR of colon tissue from naïve and infected Ig-treated or MAR-1-treated mice at day 21-post-infection; results represented as fold increase over naïve Ig-treated controls. (c) Cecal sections from naïve and infected Ig-treated or MAR-1-treated mice at day 21-post-infection stained for mucins with Alcian blue/periodic-acid Schiff. (d) Immunoblot of protein extracted from pooled fecal pellets of Ig-treated or MAR-1-treated mice at indicated days post-infection and immunoblotted for RELMβ. (e) Cecal worm burdens at day 21 post-infection. Results are representative of two independent experiments of n = 3–4 mice per group (a–e).
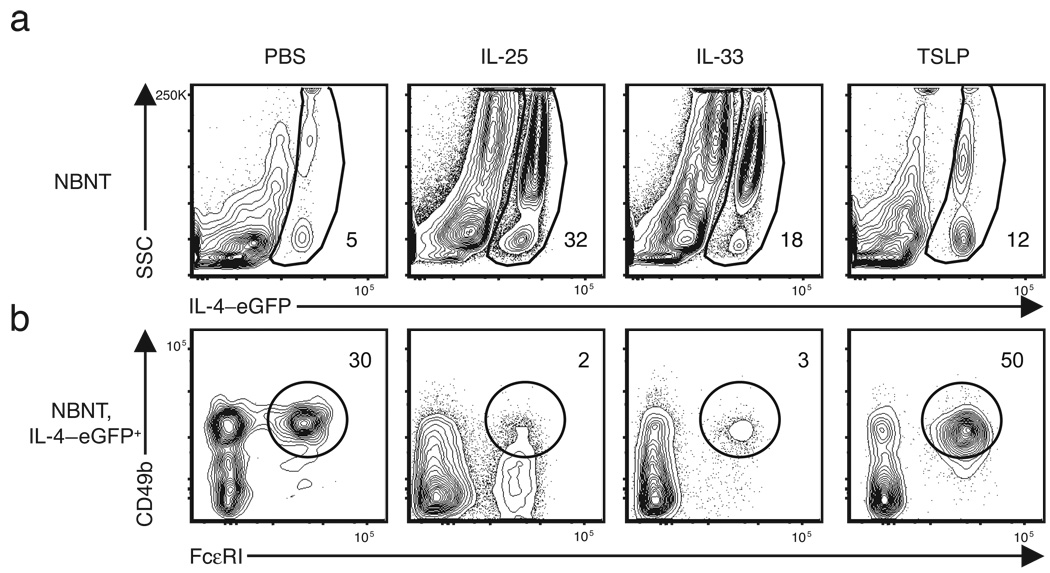
TSLP selectively elicits basophils
We recently identified essential functions for intestinal epithelial cell (IEC)-derived cytokines IL-2534 and TSLP27 http://www.signaling-gateway.org/molecule/query?afcsid=A002363 in the development of TH2 cytokine-dependent immunity to T. muris. In addition, IEC-derived IL-33 was shown to promote TH2 cytokine responses and worm expulsion35 and several studies have demonstrated that IL-33 treatment can directly stimulate cytokine and chemokine production from basophils and mast cells in vitro 36–38. To test whether IL-25, IL-33 or TSLP contributed to basophil responses in vivo, 4-get mice were injected with recombinant IL-25, IL-33 or TSLP and the peripheral basophil responses examined by flow cytometry. As previously reported, IL-25 treatment elicited a robust population of IL-4–eGFP+ SSChi cells39 (Fig. 5a). Treatment with IL-33 also resulted in a marked elevation in the frequency of IL-4–eGFP+ SSChi cells (Fig. 5a). However, phenotypic analysis of these IL-4–eGFP+ cells revealed two distinct cell populations selectively elicited by each cytokine. IL-25 treatment resulted in increased frequencies of a non-B non-T c-kit+ mast cell-like population while IL-33 treatment led to increases in the frequency of CCR3+ eosinophils (Supplementary Fig. 5 online). While administration of TSLP also resulted in a 3-fold increase in IL-4–eGFP+ cells over PBS-treated controls (Fig. 5a), unlike IL-25 and IL-33, TSLP treatment selectively elicited CD49b+ FcεRI+ basophils (Fig. 5b). These data suggest that while IEC-derived IL-25, IL-33 and TSLP promote the expansion of diverse innate cell populations competent to produce IL-4, only TSLP promotes basophil population expansion.
Figure 5. TSLP treatment selectively increases basophil frequencies in vivo .
Flow cytometric analysis of basophil frequencies in the blood of mice treated daily for four days with rIL-25, rIL-33 or rTSLP. (a) Non-B non-T cells from the peripheral blood were analyzed for expression of IL-4–eGFP. Numbers indicate frequency of gated population. (b) Basophil frequencies in IL-4–eGFP+ non-B non-T cell populations. Numbers indicate frequency of gated population. Results representative of at least two independent experiments with n = 3 mice per group.
Basophils promote CD4+ Th2 cell differentiation
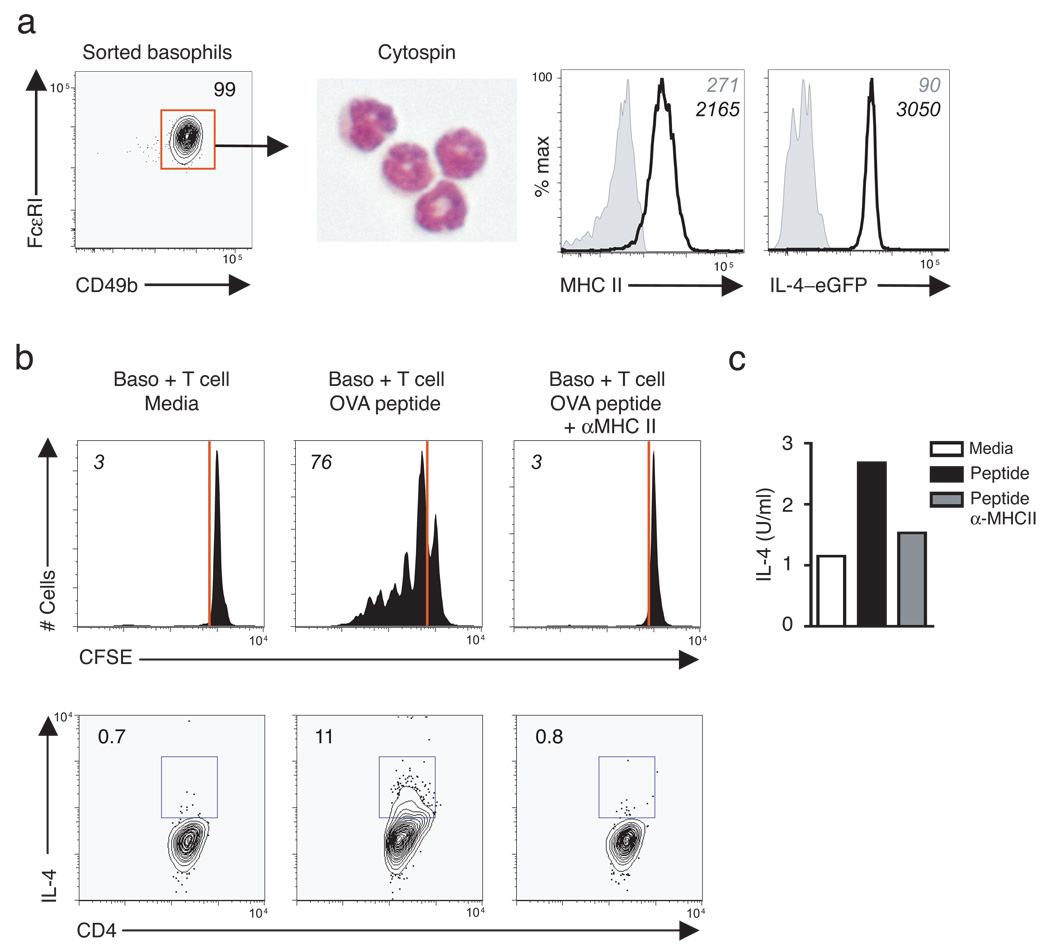
Demonstration that depletion of basophils resulted in impaired immunity to T. muris (Fig. 4) coupled with the co-expression of MHC class II and Il4 mRNA (Fig. 3) suggested that they may also participate in MHC class II-dependent cognate interactions with CD4+ T cells to promote TH2 cell differentiation. To test whether basophils could present antigen, an in vitro co-culture system was adopted in which antigen-pulsed purifed basophils were activated with recombinant IL-3, to provide survival signals and promote IL-4 production, and co-cultured with purified CFSE-labeled ovalbumin (OVA)-specific DO11.10 CD4+ T cells. Sorted basophils exhibited characteristic multi-lobed nuclei and expressed both MHC class II and IL-4-eGFP (Fig. 6a). While minimal proliferation was detected in the absence of OVA peptide, approximately 75% of CD4+ T cells co-cultured in the presence of antigen-pulsed basophils had diluted CFSE, consistent with proliferation (Fig. 6b). Basophil-induced CD4+ T cell proliferation was dependent on MHC class II expression as addition of a blocking antibody against MHC class II abrogated these responses (Fig. 6b). To determine whether basophils could influence CD4+ TH2 cell differentiation following antigen-specific stimulation of T cells, intracellular cytokine staining for IL-4 was performed (Fig. 6b) and IL-4 secretion measured in supernatants from co-cultured cells (Fig. 6c). Supernatants from basophil-T cell co-cultures in the absence of antigen contained basal amounts of IL-4 (Fig. 6c) and upon addition of OVA peptide, there was a 2- to 3-fold increase in secreted IL-4 that was abrogated in the presence of anti-MHC class II (Fig. 6c). Therefore, MHC class II-dependent cognate interactions between basophils and CD4+ T cells can promote antigen-specific TH2 cell differentiation in vitro.
Figure 6. Basophils promote MHC class II-dependent antigen-specific CD4+ T cell proliferation and Th2 cytokine production in vitro .
(a) Sorted TSLP-elicited basophils were subjected to cytospin and stained with DiffQuick or examined by flow cytometry for expression of MHC class II and IL-4–eGFP (solid line) over fluorescence minus one (FMO) controls (shaded histrograms). Numbers in italics are mean fluorescence intensity. Plots are representative of four independent experiments with n = 5–10 mice pooled per experiment. (b) CFSE-dilution of DO11.10 CD4+ T cells following 4 day co-culture with basophils in media, OVA peptide, or OVA peptide plus MHC class II blocking antibody (M5114), top. Lower panel indicates frequencies of IL-4+ CD4+ T cells by intracellular cytokine staining. (c) Supernatants from basophil-CD4+ T cell co-cultures in were analyzed for IL-4 secretion by ELISA. Results are representative of two independent experiments (b,c).
S. mansoni eggs recruit MHC class II+ basophils to LN
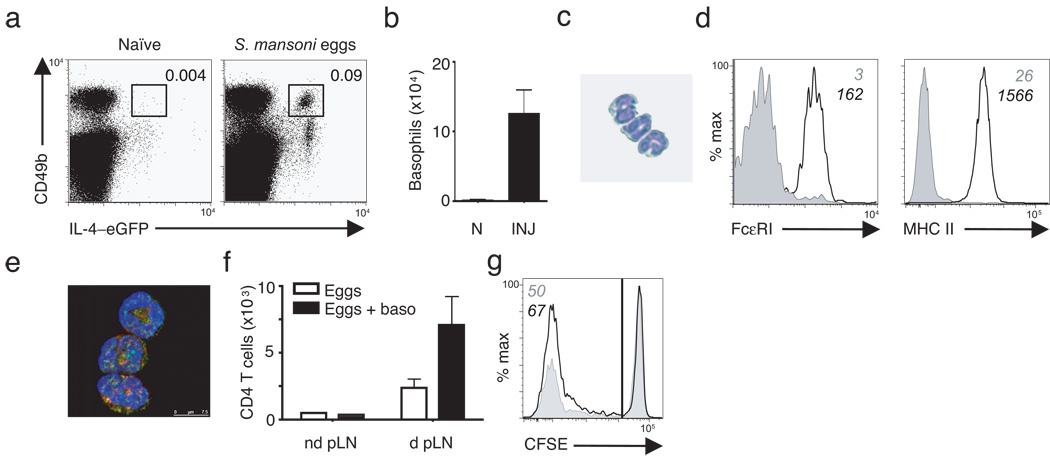
We sought to determine whether the recruitment of IL-4–eGFP+ MHC class II+ basophils was unique to T. muris infection or whether they were common events following exposure to other helminth parasites. To test this, we employed footpad injection of Schistosoma mansoni eggs whereby delivery of S. mansoni eggs results in an acute and synchronous TH2 cytokine responses in the draining popliteal lymph node (pLN). In previous studies we have demonstrated robust proliferation of CD4+ T cells following egg injection with greater than 40% of pLN CD4+ T cells becoming BrdU+ and 20% expressing IL-4–eGFP40 providing a powerful in vivo model to examine helminth-induced innate and adaptive responses. S. mansoni eggs were delivered into the footpad of 4-get mice and pLN harvested at various time points post-injection. A transient recruitment of basophils to the draining pLN occurred by day 2 post-injection with a greater than 20-fold increase in frequency (Fig. 7a) and number (Fig. 7b) that was absent by day 5 (data not shown). Sorted IL-4–eGFP+ basophils from S. mansoni egg-injected mice exhibited characteristic multi-lobular nuclei by cytospin (Fig. 7c), were FcεRI+, and expressed MHC class II by both flow cytometry (Fig. 7d) and immunofluorescence (Fig. 7e). We next investigated whether helminth-elicited basophils could influence CD4+ T cell proliferation in vivo. To address this, CFSE-labeled CD4+ T cells were adoptively transferred into MHC IICD11c mice. We then utilized the S. mansoni egg injection model to assess whether adoptive transfer of basophils influenced helminth-induced proliferation of CD4+ T cells in the draining pLN. Sorted wild-type basophils from S. mansoni egg-injected mice were adoptively transferred into naive MHC IICD11c recipients. We have previously observed that in the absence of additional antigen stimulation in recipient mice, transfer of basophils alone does not induce the recruitment of antigen-specific T cells to the pLN (data not shown). We therefore challenged MHC IICD11c mice that had received both T cells and basophils with S. mansoni eggs in the footpad. Following egg injection, MHC IICD11c mice that received eggs alone exhibited a four- to five-fold increase in total CD4+ T cell numbers in the draining pLN compared to the non-draining pLN (Fig. 7f) and 50% of pLN CD4+ T cells were CFSElo (Fig. 7g). In contrast, proliferation of CD4+ T cells was substantially augmented in MHC IICD11c mice that had received activated basophils. At day 4 following delivery of S. mansoni eggs, there was a greater than 14-fold increase in total pLN CD4+ T cells (Fig. 7f) and nearly 70% of CD4+ T cells were CFSElo (Fig. 7g). This proliferation was consistent with the magnitude of CD4+ T cell responses we previously observed in egg-injected wild-type mice40. In addition, in earlier studies we found that unlike adoptively transferred T cells in Rag1 −/− or Rag2 −/− recipient mice, donor CD4+ T cells delivered into MHC IICD11c mice do not undergo homeostatic proliferation, likely due to the fact that MHC IICD11c mice have a normal CD8+ T cell compartment26,41. Collectively, these data demonstrate that MHC class II+ basophils are rapidly recruited to the lymph node following exposure to helminth antigens and suggest potential cooperation between basophils and DCs in the efficient expansion of helminth-responsive CD4+ T cells in vivo.
Figure 7. IL-4–eGFP+ MHC class II+ basophils are recruited to the draining LN following exposure to Schistosoma mansoni eggs and augment CD4+ T cell proliferation in vivo .
(a) Flow cytometry of basophil frequencies in popliteal LN from naïve or S. mansoni egg-injected 4-get mice day 2 post-injection. (b) Total numbers of basophils in the lymph node of naïve (N) or S. mansoni egg injected (INJ) mice. (c) Cytospin of sorted S. mansoni-elicited basophils stained by Diffquick. (d) Flow cytometry of sorted S. mansoni-elicited basophils stained for FcεRI or MHC class II. Grey histograms are expression on CD3+ cells, black lines are sorted basophils. Numbers indicate mean fluorescence intensity. (e) Confocal microscopy of sorted S. mansoni-elicited basophils. GFP (green); MHC class II (red); DAPI (blue). Results are representative of five independent experiments with n = 3–5 mice per group (a–e). (f) Total numbers of CD4+ cells in the non-draining (nd pLN) versus draining (d pLN) popliteal lymph nodes of MHC CIICD11c that received either S. mansoni eggs alone or in combination with basophils. (g) Flow cytometry showing CFSE-dilution of donor CD4+ T cells from MHC CIICD11c that received either S. mansoni eggs alone (grey histogram) or with basophils (black line histogram). Numbers in italics refers to percent of CFSElo cells. Results represent one experiment with n = 2 mice per group (f,g).
DISCUSSION
Basophils are rare circulating cells that make up less than 0.5% of total blood leukocytes yet are evolutionarily conserved across all vertebrate species and can accumulate in peripheral tissues in multiple settings associated with type 2 inflammation. Although basophils were first described 130 years ago42, their scarcity, coupled with a paucity of reagents, has made it difficult to study their function in vivo. Availability of new reagents has revealed distinct non-redundant roles for basophils in augmenting CD4+ TH2 cytokine responses22,43, in providing B cell help for IgE class-switch recombination and enhanced humoral immune responses33,44 and in the initiation and maintenance of chronic allergic inflammation45. To these functions, the results of the present study add a previously unrecognized role for basophils as accessory cells that can promote CD4+ TH2 cell differentiation in part through MHC class II-dependent cognate interactions as indicated by basophil-CD4+ T cell co-culture experiments. A critical question that emerges from these findings is where functional basophil-T cell cognate interactions occur in vivo. Basophils are readily found in the blood and spleen but have been reported to be excluded from lymph nodes, where CD4+ T cell priming is likely to take place46. However, basophils have recently been shown to be transiently recruited to draining lymph nodes following allergen exposure22. In this study we demonstrate that basophils are rapidly recruited to the lymph node following exposure to S. mansoni eggs. Basophils that accumulated in lymph nodes co-expressed MHC class II and il4 mRNA, suggesting that they have the capacity to directly interact with naive T cells in peripheral lymphoid tissues. Consistent with a role in the development of TH2 cell responses, in vivo depletion of basophils resulted in impaired expression of TH2 cytokines and host protective immunity while adoptive transfer of basophils augmented helminth-induced CD4+ T cell proliferation.
In addition to a potential role in the initial priming of naive CD4+ T cells in the lymph node, basophils may act as accessory cells at the site of inflammation, where activated T cells may require additional cognate interactions to promote or maintain TH2 cell differentiation and effector function. Supporting this notion, a previous report utilizing cytokine reporter mice demonstrated that cytokine mRNA and protein expression are uncoupled following priming and expansion of naive T cells, suggesting that additional activation at the site of infection may be required to license effector function47. Depletion of basophils also suggests that these cells may provide chemotactic factors, either directly or indirectly, that are required for the recruitment of eosinophils to peripheral tissues48. Microarray analyses of basophils sorted from the lung during Nippostrongylus brasiliensis infection also revealed high expression of the chemokines CCL3 (MIP1α), CCL4, (MIP1β), CCL6 (C10), and CCL17 (TARC), supporting a role for basophils in the recruitment of activated CD4+ T cells to the site of infection21. Therefore, identifying the factors that regulate basophil proliferation and recruitment could be an important target for modulating early events in the generation of CD4+ TH2 cell-dependent immunity and inflammation.
Previous reports identified T cell-derived IL-3 as a critical cytokine for basophilia during intestinal helminth infection49. However, whether other innate cell-derived cytokines contribute to early basophil responses is unclear. We recently identified a critical role for intestinal epithelial cell (IEC) activation in the generation of protective TH2 cytokine-dependent immunity to T. muris and demonstrated that TSLP is an important part of the IEC response required for immunity to infection27,50. Here we have shown that delivery of recombinant TSLP resulted in the selective accumulation of basophils in the periphery, identifying a previously unappreciated role for TSLP in promoting basophilia. TSLP has previously been implicated in the promotion of type 2 inflammation in the skin and lung through effects on both innate and adaptive immune cells51,52. Although no analysis of basophil responses were conducted in the TSLP-transgenic mice used in those earlier studies, it is tempting to speculate that a component of the enhanced type 2 inflammation observed could be a consequence of elevated basophil responses.
In addition to TSLP, the IEC-derived cytokines IL-25 and IL-33 have also been implicated in the promotion of type 2 inflammation and IL-33 can directly activate basophils34,36–38,53–56. However, we demonstrate that these cytokines, although capable of promoting the accumulation of IL-4-eGFP+ innate cells, do not promote basophilia in vivo. Rather, IL-25 promoted the proliferation and/or accumulation of c-Kit+ cells while IL-33 promoted peripheral eosinophilia. While TSLP appears to have a selective effect on basophil responses, the influence of TSLP, IL-25, and IL-33 in combination with other stimuli such as IL-3, IL-18, TLR ligation, and FcεRI crosslinking on basophil cytokine production, lymph node recruitment and APC function remains to be determined.
In addition to IEC-derived cytokines, there is evidence to suggest that basophils can be directly activated by either helminth-derived products or allergens that may act as ‘superallergens’ to stimulate FcεRI cross-linking in a non-antigen-specific manner57. For example, IPSEα1, a glycoprotein derived from S. mansoni eggs, has been shown to directly stimulate the production of IL-4 from basophils by an IgE-dependent but non-antigen-specific mechanism58. Thus, a combination of non-hematopoietic and innate immune cell-derived cytokines, coupled with direct stimulation by helminth products or allergens, may act together to elicit basophil proliferation and activation in vivo.
In addition to the identification of a role for basophils in MHC class II-dependent promotion of TH2 cell differentiation and immunity to T. muris infection, Medzhitov and colleagues found a critical role for basophils in the development of allergen-specific TH2 cytokine responses. In those studies, allergen-stimulated basophils expressed MHC class II, CIITA, the invariant chain and co-stimulatory molecules and promoted allergen-specific CD4+ TH2 cell differentiation (personal commnication, R. Medzhitov). Taken together, these findings indicate that basophil-mediated recognition of allergens and helminth-derived products, coupled with their MHC class II-dependent promotion of TH2 cell responses, may be an evolutionarily conserved pathway that plays a cardinal role in the development of type 2 inflammation at mucosal sites.
METHODS
Mice and parasites
6–8 week-old C57BL/6 mice and timed pregnant female B6.SJL mice were obtained from The Jackson Laboratories. MHC IICD11c (also known as CD11c-Aβ b; generated as previously described26), MHC class II-deficient (H2Ab1 −/−), 4-get–IL-4eGFP (C.129-IL4tm1Lky/J) (from M. Mohrs, Trudeau Institute), B cell-deficient (µMT), CD11c-DTR, and DO11.10 mice were bred and housed in specific pathogen-free conditions at the University of Pennsylvania. Littermate control mice were sham-grafted and MHC IICD11c mice were given subcutaneous thymic grafts from neonatal (0–2 days) B6.SJL mice at 4–6 weeks of age and allowed 8 weeks to reconstitute CD4+ T cells before experimental use. Bone marrow chimeras were generated by i.v. injection of 5 × 106 bone marrow cells from wild-type or CD11c-DTR mice into irradiated (2 times 500 rads) wild-type recipients. Recipient mice were on antibiotics for two weeks and allowed 8 weeks to reconstitute. All experiments were performed following the guidelines of the University of Pennsylvania Institutional Animal Care and Use Committee. T. muris was maintained in genetically susceptible mouse strains and eggs harvested as previously described31. Mice were infected by oral gavage with 200–300 embryonated T. muris eggs. S. mansoni eggs were prepared as previously described40. Mice were injected in the footpad with 2500 eggs in 50 µl PBS.
Polyclonal T cell stimulation
mLNs were harvested and single-cell suspensions prepared in complete media (DMEM supplemented with 10% heat-inactivated FBS, 2 mM glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, 25 mM HEPES, and 50 µM beta-mercaptoethanol). mLN cells were seeded in 48-well plates at 2.5 × 106 per well and incubated with either media or 1 µg/ml soluble anti-CD3 and anti-CD28 (eBioscience) for 48 h. Cell-free supernatants were harvested and cytokine production determined by sandwich ELISA (all antibody pairs purchased from eBioscience: clones AN-18, R4-6A2 (IFN-γ), 11B11, BVD6-24G2, (IL-4), TRFK5, TRFK4 (IL-5) and eBio13A, eBio 1316H (IL-13)).
Immunoblot
Fecal protein isolation was performed as previously described28. 30 µg of protein was loaded per sample for analysis by SDS-PAGE and immunoblotted for RELMβ using a polyclonal rabbit α-murine RELMβ (Peprotech).
Real-time PCR
RNA was isolated from intestinal tissues of mice using a TRizol extraction (Invitrogen) and from mLN cells using RNEasy Spin Columns (Qiagen). Tissues were disrupted in a tissue homogenizer (TissueLyzer, Qiagen) and cDNA synthesized using Superscript Reverse Transcriptase (Invitrogen). Quantitative real-time PCR was carried out on cDNA samples using commercial primer sets (Qiagen il4 QT00160678, ifng QT01038821, il10 QT00106169, il17a QT00103278) and SYBR Green chemistry. All reactions were run on an ABI 7500 Fast Real-Time PCR System (Applied Biosystems). Samples are normalized to naive controls unless otherwise stated.
Histology and immunofluorescence
Cecal tips were fixed in 4% paraformaldehyde (PFA) and embedded in paraffin. 5 µm sections were cut and stained for hematoxylin and eosin (H&E) or Alcian blue-periodic acid-Schiff. Unstained sections prepared on immunoslides were stained for Gob5 by immunofluoresence as previously described27. Briefly, samples were de-paraffinized by consecutive methanol and ethanol rinses, boiled in citric acid buffer and stained with an antibody against Gob5 at 4 °C overnight followed by staining with a Cy2-conjugated anti-goat antibody. Sorted basophils were subjected to cytospin and fixed in 2% PFA at 4 °C overnight. Slides were washed in PBS, permeablized in Triton-X, blocked with streptavidin and biotin, and stained with anti-MHC class II-biotin and anti-GFP at 4 °C overnight. Slides were then washed with PBS and stained with streptavidin-Cy3 and donkey anti-rabbit-Cy2 for 2 h at 25 °C, washed with PBS, and nuclei stained with DAPI.
Neutralizing and depleting antibodies and recombinant cytokines
Neutralizing monoclonal antibody against IFN-γ (XMG-6) was purified from ascites (grown by Harlan Bioscience) by ammonium sulfate precipitation and dialyzed against PBS. Mice were given 1 mg antibody i.p. every 3–5 days during the course of infection starting at day 0. Basophils were depleted by i.p. injection of 10 µg anti-FcεRI (MAR-1, eBioscience) on days 0, 1, 2 and 10, 11, 12 post-infection. Recombinant murine IL-25 (4 µg/ml), IL-33 (20 µg/ml), and TSLP (0.1 mg/ml) were all purchased from R&D Systems and 100 µl in PBS was injected i.p. once daily for four days.
Basophil isolation and CD4+ T cell co-culture
CD4+ T cells were isolated from spleens by negative selection via incubation with hybridoma supernatants (αB220, αFCR, αCD8, αMHCII) followed by magnetic bead purification (Qiagen). To obtain purified basophils, blood, spleen, and mLN cells were isolated from 4-get IL-4–eGFP reporter mice injected with 10 µg rTSLP (R&D System) i.p. once daily for 4 days to enrich for basophils, positively selected for CD49b expression by MACs column purification (Milltenyi) and stained with fluorochrome-conjugated mAbs against B220 (RA3-6B2), CD3ε (145-2C11), c-Kit (2B8), CD49b (HMα2), and FcεRI (MAR-1) (BD Bioscience and eBioscience). Basophils were sorted based on negative staining for B220, CD3 and c-kit, positive staining for CD49b, FcεRI and expression of IL-4–eGFP using a FACS Aria (BD Bioscience). Following purification, sorted basophils were resuspended at 1 × 105 cells/ml in complete medium. 100 µl of basophils were used for cytospin and stained by Diffquick to confirm cellular morphology. Between 5 × 103 − 1 × 104 basophils were co-cultured with 2 × 105 purified, CFSE-labeled DO11.10 CD4+ T cells with 10 ng/ml rIL-3 (RnD Systems) in the presence or absence of 1 µg per ml OVA peptide and 5 µg/ml blocking antibody for MHC class II (M5/114). After four days of culture, cells were stimulated with PMA, ionomycin, and brefeldin A for 4 h. Cells were pelleted at 485 g for 5 min. Supernatants were collected for ELISA and cells washed in FACS buffer, incubated with Fc Block (2.4G2 and rat IgG) for 10 min at 4 0C, stained with fluorochrome-conjugated monoclonal antibodies against CD4 (RM4-5) and fixed with 2% paraformaldehyde. Cells were permeabilzed with 0.4% saponin in FACS buffer and stained for intracellular cytokines using fluorochrome-conjugated monoclonal antibodies against IL-4 (11B11) and IL-13 (eBio13A) (eBioscience).
Adoptive transfer of basophils
C57BL/6 mice were injected with 2.5 × 103 S. mansoni eggs in each footpad, popliteal lymph nodes, spleen, and blood pooled two days later, and basophils purified by sequential CD49b enrichment and cell sorting as described above. Recipient MHC IICD11c mice were given 1 × 107 purified CFSE-labeled CD4+ T cells from naive C57BL/6 mice one day prior to egg injection. Sorted basophils were resuspended in a PBS plus S. mansoni egg suspension and each recipient MHC IICD11c mouse given either 5 × 104 basophils and 2.5 × 103 S. mansoni eggs or 2.5 × 103 S. mansoni eggs alone in the right footpad in a volume of 50 µl. Draining and non-draining pLN cells were isolated 4 days post-egg injection, stained with fluorochrome-conjugated monoclonal antibodies and analyzed by flow cytometry on a FACSCanto II (BD Biosciences).
Macrophage and dendritic cell depletion
PBS- or Clodronate-loaded liposomes were prepared as previously described59. 150 ul of liposomes were injected i.v. every 2 days during the course of infection. Clodronate was a gift from Roche Diagnostics GmBH (Mannheim, Germany). DC were depleted in the CD11c-DTR mice by injection of 100ng diphtheria toxin i.p. per mouse every 3 days during the course of infection.
Statistics
Results represent the mean ± SEM unless otherwise stated. Statistical significance was determined by the Student’s t test.
Supplementary Material
Acknowledgments
The authors would like to thank A. Troy for critical reading of this manuscript, E. Tait for performing the bone marrow chimeras, and the University of Pennsylvania Flow Cytometry Core and Center for Molecular Studies in Digestive and Liver Diseases Morphology Core for assistance with sorting and immunofluorescence staining. Research in the Artis lab is supported by the National Institutes of Health (NIH), AI61570, AI074878 (D.A.), NIH T32 Training Grant AI007532-08 (J.G.P.), NIH F31 Training Grant GM082187 (S.A.S.), the Burroughs Wellcome Fund (Investigator in Pathogenesis of Infectious Disease Award to D.A.), the Pilot Feasibility Program of the National Institute of Diabetes and Digestive Kidney Diseases (NIDDK) DK50306, the Crohns and Colitis Foundation of America’s William and Shelby Modell Family Foundation Research Award (D.A., M.G.N.), and pilot grants from the University of Pennsylvania (University Research Foundation Award, PGI Pilot Grant, and UPenn Center for Infectious Diseases Pilot Grant to D.A., Department of Medicine to D.A. and T.M.L.). Work in the Pearce lab is supported by NIH grants AI32573 and AI53825 Work in the Laufer lab is supported by the NIH. Michael Comeau is employed by Amgen.
References
- 1.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 2.Reiner SL. Development in motion: helper T cells at work. Cell. 2007;129:33–36. doi: 10.1016/j.cell.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 3.Mowen KA, Glimcher LH. Signaling pathways in Th2 development. Immunol Rev. 2004;202:203–222. doi: 10.1111/j.0105-2896.2004.00209.x. [DOI] [PubMed] [Google Scholar]
- 4.Anthony RM, Rutitzky LI, Urban JF, Jr., Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nat Rev Immunol. 2007;7:975–987. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol. 2004;22:789–815. doi: 10.1146/annurev.immunol.22.012703.104716. [DOI] [PubMed] [Google Scholar]
- 6.Perrigoue JG, Marshall FA, Artis D. On the hunt for helminths: innate immune cells in the recognition and response to helminth parasites. Cell Microbiol. 2008;10:1757–1764. doi: 10.1111/j.1462-5822.2008.01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 8.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3:984–993. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 9.MacDonald AS, Maizels RM. Alarming dendritic cells for Th2 induction. J Exp Med. 2008;205:13–17. doi: 10.1084/jem.20072665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amsen D, et al. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 2004;117:515–526. doi: 10.1016/s0092-8674(04)00451-9. [DOI] [PubMed] [Google Scholar]
- 11.MacDonald AS, Straw AD, Dalton NM, Pearce EJ. Cutting edge: Th2 response induction by dendritic cells: a role for CD40. J Immunol. 2002;168:537–540. doi: 10.4049/jimmunol.168.2.537. [DOI] [PubMed] [Google Scholar]
- 12.Ekkens MJ, et al. The role of OX40 ligand interactions in the development of the Th2 response to the gastrointestinal nematode parasite Heligmosomoides polygyrus. J Immunol. 2003;170:384–393. doi: 10.4049/jimmunol.170.1.384. [DOI] [PubMed] [Google Scholar]
- 13.Gessner A, Mohrs K, Mohrs M. Mast cells, basophils, and eosinophils acquire constitutive IL-4 and IL-13 transcripts during lineage differentiation that are sufficient for rapid cytokine production. J Immunol. 2005;174:1063–1072. doi: 10.4049/jimmunol.174.2.1063. [DOI] [PubMed] [Google Scholar]
- 14.Mohrs K, Wakil AE, Killeen N, Locksley RM, Mohrs M. A two-step process for cytokine production revealed by IL-4 dual-reporter mice. Immunity. 2005;23:419–429. doi: 10.1016/j.immuni.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Min B, et al. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. J Exp Med. 2004;200:507–517. doi: 10.1084/jem.20040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skokos D, et al. Mast cell-derived exosomes induce phenotypic and functional maturation of dendritic cells and elicit specific immune responses in vivo. J Immunol. 2003;170:3037–3045. doi: 10.4049/jimmunol.170.6.3037. [DOI] [PubMed] [Google Scholar]
- 17.MacKenzie JR, Mattes J, Dent LA, Foster PS. Eosinophils promote allergic disease of the lung by regulating CD4(+) Th2 lymphocyte function. J Immunol. 2001;167:3146–3155. doi: 10.4049/jimmunol.167.6.3146. [DOI] [PubMed] [Google Scholar]
- 18.Padigel UM, et al. Eosinophils act as antigen-presenting cells to induce immunity to Strongyloides stercoralis in mice. J Infect Dis. 2007;196:1844–1851. doi: 10.1086/522968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voehringer D, Reese TA, Huang X, Shinkai K, Locksley RM. Type 2 immunity is controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells of the innate immune system. J Exp Med. 2006;203:1435–1446. doi: 10.1084/jem.20052448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohnmacht C, Voehringer D. Basophil effector function and homeostasis during helminth infection. Blood. 2008 doi: 10.1182/blood-2008-05-154773. [DOI] [PubMed] [Google Scholar]
- 21.Voehringer D, Shinkai K, Locksley RM. Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity. 2004;20:267–277. doi: 10.1016/s1074-7613(04)00026-3. [DOI] [PubMed] [Google Scholar]
- 22.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Else KJ, Finkelman FD. Intestinal nematode parasites, cytokines and effector mechanisms. Int J Parasitol. 1998;28:1145–1158. doi: 10.1016/s0020-7519(98)00087-3. [DOI] [PubMed] [Google Scholar]
- 24.Cliffe LJ, Grencis RK. The Trichuris muris system: a paradigm of resistance and susceptibility to intestinal nematode infection. Adv Parasitol. 2004;57:255–307. doi: 10.1016/S0065-308X(04)57004-5. [DOI] [PubMed] [Google Scholar]
- 25.Else KJ, Finkelman FD, Maliszewski CR, Grencis RK. Cytokine-mediated regulation of chronic intestinal helminth infection. J Exp Med. 1994;179:347–351. doi: 10.1084/jem.179.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lemos MP, Fan L, Lo D, Laufer TM. CD8alpha+ and CD11b+ dendritic cell-restricted MHC class II controls Th1 CD4+ T cell immunity. J Immunol. 2003;171:5077–5084. doi: 10.4049/jimmunol.171.10.5077. [DOI] [PubMed] [Google Scholar]
- 27.Zaph C, et al. Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis. Nature. 2007;446:552–556. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- 28.Artis D, et al. RELMbeta/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proc Natl Acad Sci U S A. 2004;101:13596–13600. doi: 10.1073/pnas.0404034101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008;8:411–420. doi: 10.1038/nri2316. [DOI] [PubMed] [Google Scholar]
- 30.Else KJ, Grencis RK. Antibody-independent effector mechanisms in resistance to the intestinal nematode parasite Trichuris muris. Infect Immun. 1996;64:2950–2954. doi: 10.1128/iai.64.8.2950-2954.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zaph C, et al. Persistence and function of central and effector memory CD4+ T cells following infection with a gastrointestinal helminth. J Immunol. 2006;177:511–518. doi: 10.4049/jimmunol.177.1.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15:303–311. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- 33.Denzel A, et al. Basophils enhance immunological memory responses. Nat Immunol. 2008;9:733–742. doi: 10.1038/ni.1621. [DOI] [PubMed] [Google Scholar]
- 34.Owyang AM, et al. Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J Exp Med. 2006;203:843–849. doi: 10.1084/jem.20051496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Humphreys NE, Xu D, Hepworth MR, Liew FY, Grencis RK. IL-33, a potent inducer of adaptive immunity to intestinal nematodes. J Immunol. 2008;180:2443–2449. doi: 10.4049/jimmunol.180.4.2443. [DOI] [PubMed] [Google Scholar]
- 36.Suzukawa M, et al. An IL-1 cytokine member, IL-33, induces human basophil activation via its ST2 receptor. J Immunol. 2008;181:5981–5989. doi: 10.4049/jimmunol.181.9.5981. [DOI] [PubMed] [Google Scholar]
- 37.Pecaric-Petkovic T, Didichenko SA, Kaempfer S, Spiegl N, Dahinden CA. Human basophils and eosinophils are the direct target leukocytes of the novel IL-1 family member IL-33. Blood. 2009;113:1526–1534. doi: 10.1182/blood-2008-05-157818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smithgall MD, et al. IL-33 amplifies both Th1-and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol. 2008;20:1019–1030. doi: 10.1093/intimm/dxn060. [DOI] [PubMed] [Google Scholar]
- 39.Fallon PG, et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006;203:1105–1116. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor JJ, Mohrs M, Pearce EJ. Regulatory T cell responses develop in parallel to Th responses and control the magnitude and phenotype of the Th effector population. J Immunol. 2006;176:5839–5847. doi: 10.4049/jimmunol.176.10.5839. [DOI] [PubMed] [Google Scholar]
- 41.Allenspach EJ, Lemos MP, Porrett PM, Turka LA, Laufer TM. Migratory and lymphoid-resident dendritic cells cooperate to efficiently prime naive CD4 T cells. Immunity. 2008;29:795–806. doi: 10.1016/j.immuni.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ehrlich P. Beitrage zur Kenntins der granulierten Bindegewebs zellen und der eosinophilen Leukocythen. Arch Anat Physiol Lpz. 1879;3:166–169. [Google Scholar]
- 43.Oh K, Shen T, Le Gros G, Min B. Induction of Th2 type immunity in a mouse system reveals a novel immunoregulatory role of basophils. Blood. 2007;109:2921–2927. doi: 10.1182/blood-2006-07-037739. [DOI] [PubMed] [Google Scholar]
- 44.Gauchat JF, et al. Induction of human IgE synthesis in B cells by mast cells and basophils. Nature. 1993;365:340–343. doi: 10.1038/365340a0. [DOI] [PubMed] [Google Scholar]
- 45.Obata K, et al. Basophils are essential initiators of a novel type of chronic allergic inflammation. Blood. 2007;110:913–920. doi: 10.1182/blood-2007-01-068718. [DOI] [PubMed] [Google Scholar]
- 46.Min B, Le Gros G, Paul WE. Basophils: a potential liaison between innate and adaptive immunity. Allergol Int. 2006;55:99–104. doi: 10.2332/allergolint.55.99. [DOI] [PubMed] [Google Scholar]
- 47.Scheu S, et al. Activation of the integrated stress response during T helper cell differentiation. Nat Immunol. 2006;7:644–651. doi: 10.1038/ni1338. [DOI] [PubMed] [Google Scholar]
- 48.Brown SJ, Galli SJ, Gleich GJ, Askenase PW. Ablation of immunity to Amblyomma americanum by anti-basophil serum: cooperation between basophils and eosinophils in expression of immunity to ectoparasites (ticks) in guinea pigs. J Immunol. 1982;129:790–796. [PubMed] [Google Scholar]
- 49.Shen T, et al. T cell-derived IL-3 plays key role in parasite infection-induced basophil production but is dispensable for in vivo basophil survival. Int Immunol. 2008;20:1201–1209. doi: 10.1093/intimm/dxn077. [DOI] [PubMed] [Google Scholar]
- 50.Taylor BC, et al. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J Exp Med. 2009;206:655–667. doi: 10.1084/jem.20081499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoo J, et al. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J Exp Med. 2005;202:541–549. doi: 10.1084/jem.20041503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou B, et al. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6:1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 53.Schmitz J, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 54.Angkasekwinai P, et al. Interleukin 25 promotes the initiation of proallergic type 2 responses. J Exp Med. 2007;204:1509–1517. doi: 10.1084/jem.20061675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Allakhverdi Z, Smith DE, Comeau MR, Delespesse G. Cutting edge: The ST2 ligand IL-33 potently activates and drives maturation of human mast cells. J Immunol. 2007;179:2051–2054. doi: 10.4049/jimmunol.179.4.2051. [DOI] [PubMed] [Google Scholar]
- 56.Saenz SA, Taylor BC, Artis D. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol Rev. 2008;226:172–190. doi: 10.1111/j.1600-065X.2008.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Falcone FH, Pritchard DI, Gibbs BF. Do basophils play a role in immunity against parasites? Trends Parasitol. 2001;17:126–129. doi: 10.1016/s1471-4922(00)01846-8. [DOI] [PubMed] [Google Scholar]
- 58.Schramm G, et al. Cutting edge: IPSE/alpha-1, a glycoprotein from Schistosoma mansoni eggs, induces IgE-dependent, antigen-independent IL-4 production by murine basophils in vivo. J Immunol. 2007;178:6023–6027. doi: 10.4049/jimmunol.178.10.6023. [DOI] [PubMed] [Google Scholar]
- 59.Van Rooijen N. The liposome-mediated macrophage 'suicide' technique. J Immunol Methods. 1989;124:1–6. doi: 10.1016/0022-1759(89)90178-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.