Abstract
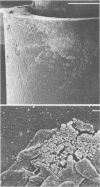
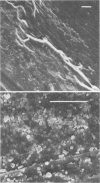
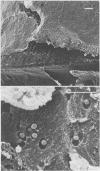
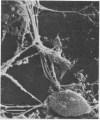
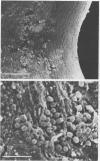
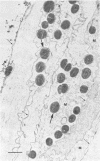
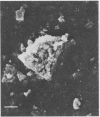
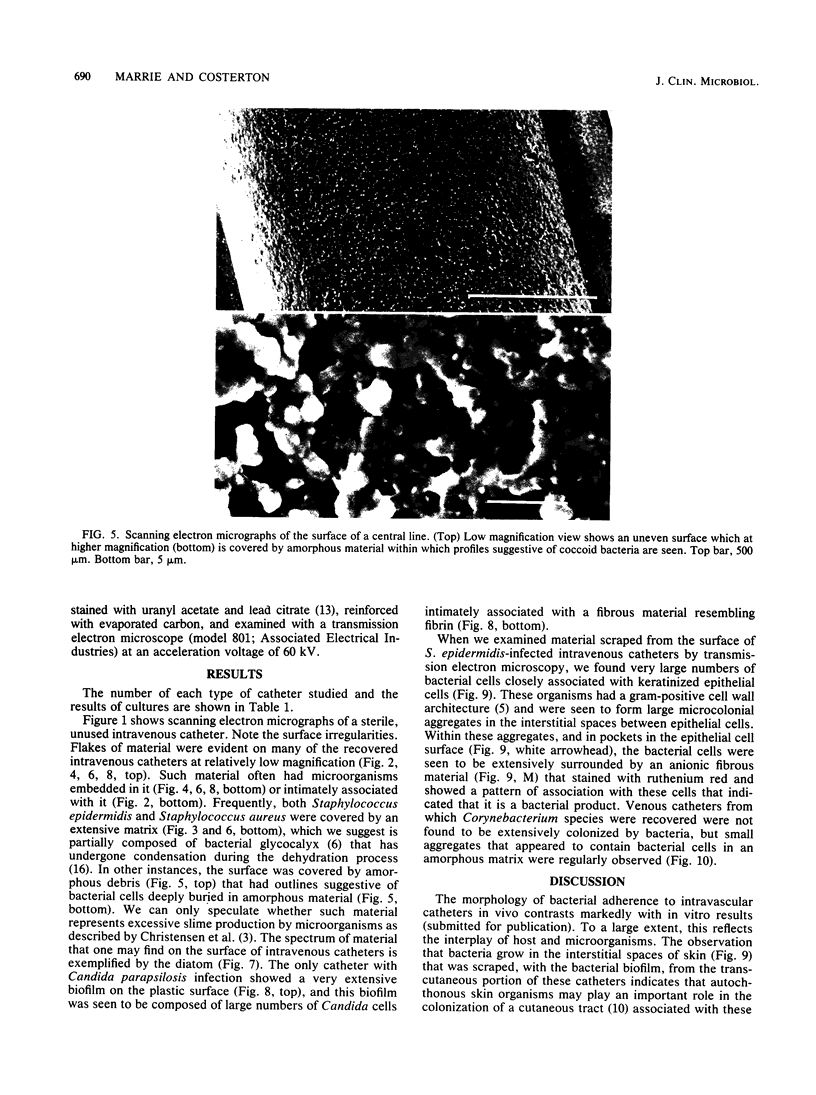
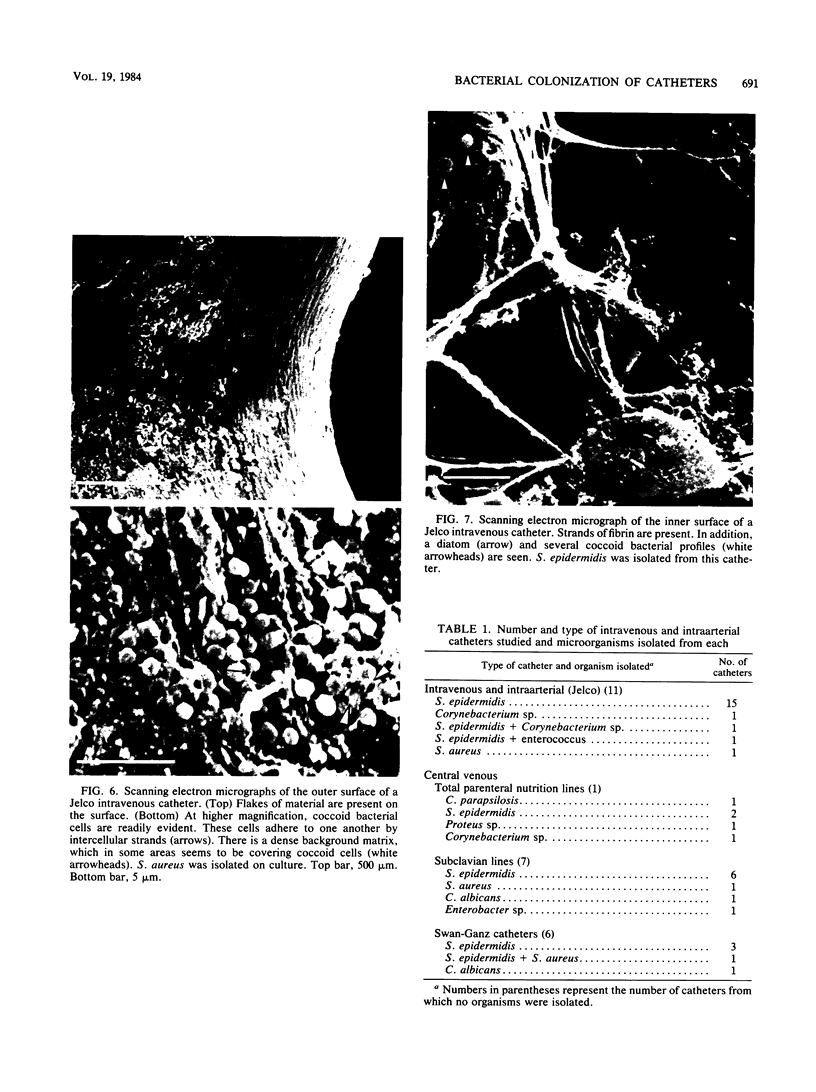
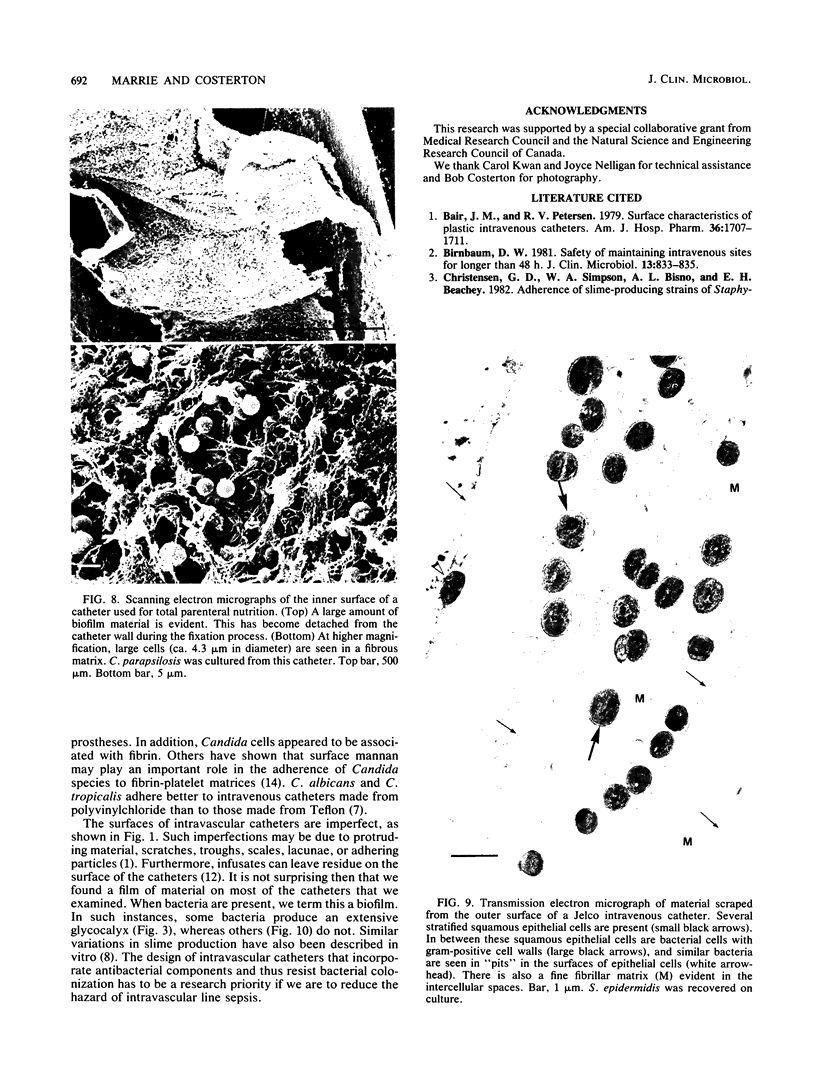
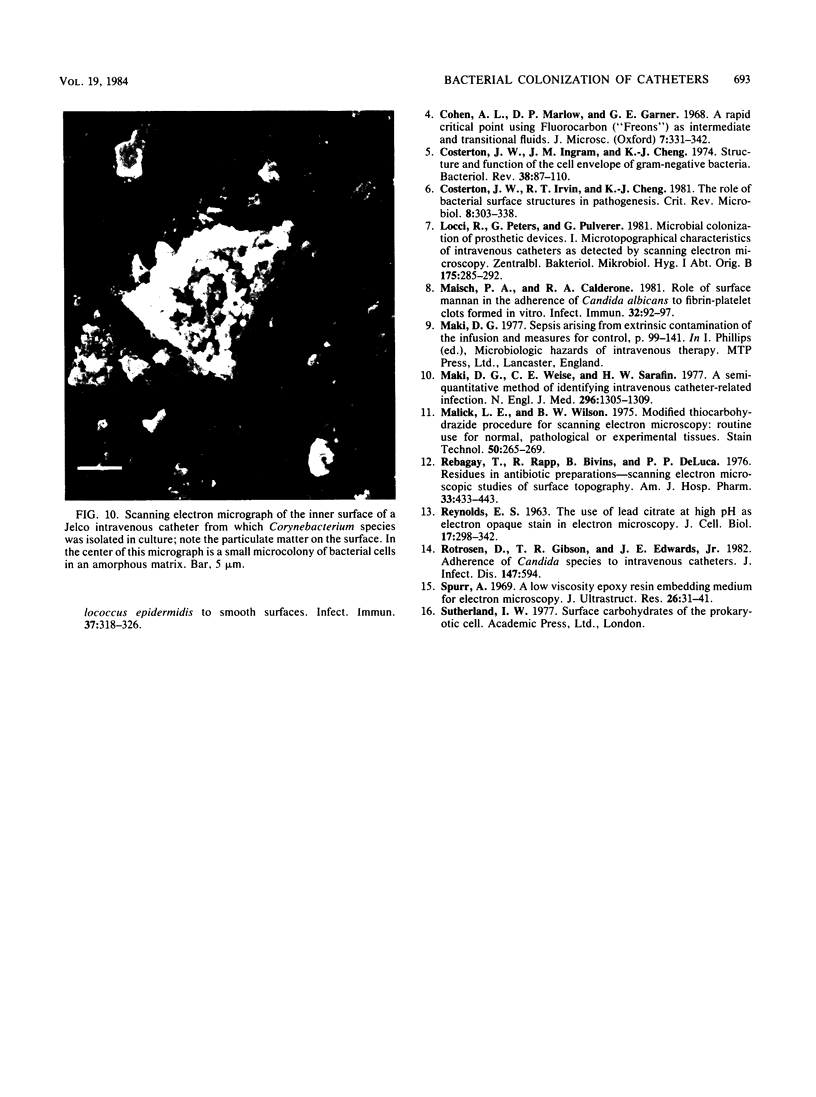
Intravenous and intraarterial catheters were examined microbiologically and morphologically. Bacteria or yeasts were recovered from 38 of the 63 catheters examined, and Staphylococcus epidermidis was present on 29 of the 38 colonized catheters. Examination of unused Teflon catheters ( Jelco ; Surgikos , Inc., Peterborough , Ontario, Canada) showed surface irregularities, and the examination of colonized intravascular catheters recovered from patients showed very extensive amorphous accretions on both their lumenal and external plastic surfaces. Detailed scanning electron microscope examination of the accretions on vascular catheters from which S. epidermidis had been isolated showed (ca. 0.8 micron) coccoid bacteria within confluent biofilms , in which they were enveloped by amorphous material. Transmission electron microscope examination of these same accretions revealed coccoid cells (ca. 0.8 micron) with a gram-positive cell wall structure living in fibrous matrix-enclosed microcolonies in spaces between squamous epithelial cells. Staphylococcus aureus biofilms were seen to contain coccoid cells (ca. 1 micron) in a very extensive amorphous matrix, and a Candida parapsilosis biofilm contained very large numbers of large coccoid cells (ca. 4.3 microns) in a fibrous matrix resembling fibrin. Cells of a Corynebacterium species appeared to form much less extensive matrix-enclosed microcolonies on the colonized plastic surface. These data indicate bacteria and yeasts colonize intravascular catheters by an adherent biofilm mode of growth on these plastic surfaces.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bair J. N., Petersen R. V. Surface characteristics of plastic intravenous catheters. Am J Hosp Pharm. 1979 Dec;36(12):1707–1711. [PubMed] [Google Scholar]
- Birnbaum D. W. Safety of maintaining intravenous sites for longer than 48 H. J Clin Microbiol. 1981 May;13(5):833–835. doi: 10.1128/jcm.13.5.833-835.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Ingram J. M., Cheng K. J. Structure and function of the cell envelope of gram-negative bacteria. Bacteriol Rev. 1974 Mar;38(1):87–110. doi: 10.1128/br.38.1.87-110.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Irvin R. T., Cheng K. J. The role of bacterial surface structures in pathogenesis. Crit Rev Microbiol. 1981;8(4):303–338. doi: 10.3109/10408418109085082. [DOI] [PubMed] [Google Scholar]
- Locci R., Peters G., Pulverer G. Microbial colonization of prosthetic devices. I. Microtopographical characteristics of intravenous catheters as detected by scanning electron microscopy. Zentralbl Bakteriol Mikrobiol Hyg B. 1981;173(5):285–292. [PubMed] [Google Scholar]
- Maisch P. A., Calderone R. A. Role of surface mannan in the adherence of Candida albicans to fibrin-platelet clots formed in vitro. Infect Immun. 1981 Apr;32(1):92–97. doi: 10.1128/iai.32.1.92-97.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki D. G., Weise C. E., Sarafin H. W. A semiquantitative culture method for identifying intravenous-catheter-related infection. N Engl J Med. 1977 Jun 9;296(23):1305–1309. doi: 10.1056/NEJM197706092962301. [DOI] [PubMed] [Google Scholar]
- Malick L. E., Wilson R. B. Modified thiocarbohydrazide procedure for scanning electron microscopy: routine use for normal, pathological, or experimental tissues. Stain Technol. 1975 Jul;50(4):265–269. doi: 10.3109/10520297509117069. [DOI] [PubMed] [Google Scholar]
- Rebagay T., Rapp R., Bivins B., DeLuca P. P. Residues in antibiotic preparations, i: scanning electron microscopic studies of surface topography. Am J Hosp Pharm. 1976 May;33(5):433–443. [PubMed] [Google Scholar]
- Rotrosen D., Gibson T. R., Edwards J. E., Jr Adherence of candida species to intravenous catheters. J Infect Dis. 1983 Mar;147(3):594–594. doi: 10.1093/infdis/147.3.594. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]