Abstract
Short stereoselective syntheses of various cyclitols, including the derivatives of conduritol B, conduritol F, myo-inositol and chiro-inositol, have been accomplished. The key steps in the syntheses are a ring-closing metathesis process and a diastereodivergent organometallic addition to a D-xylose-derived alde-hyde.
Keywords: conduritol, inositol, Grignard, stereochemistry model, chelation, Felkin-Anh, cyclophellitol, latent symmetry
The realization that inositols (hexahydroxycyclohexanes), conduritols (tetrahydroxycyclohexenes) and their numerous derivatives play important biological roles has made their study an important endeavor in health-related sciences.1 Thus, myo-inositol phosphates have been intensively investigated for their role in intracellular signal transduction and calcium mobilization.1d,2 Both myo- and chiro-inositols have been studied as components of inositolphosphoglycans (IPGs), believed to be important in insulin signaling.3 It was discovered that various conduritols act as modulators of insulin release4 and possess antifeedant, antibiotic, anticancer, and growth-regulating activities.5 Conduritol epoxides, and more prominently fungal metabolite cyclophellitol, are potent glycosidase inhibitors and are under investigation as inhibitors of HIV infection and cancer metastasis.6
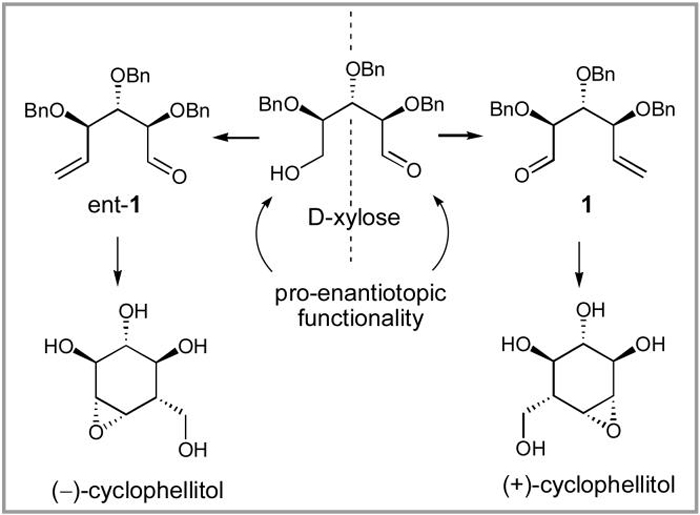
These and related research activities have generated considerable synthetic effort directed at developing practical preparations of the numerous biologically important cyclitols and their derivatives. Commercially available, inexpensive myo-inositol has been a common starting point in the syntheses of myo-inositol derivatives,7 while significantly more expensive naturally occurring methylated chiro-inositols, pinitol and quebrachitol, have been utilized to prepare cyclitols with D- and L-chiro-inositol stereochemical configurations respectively.8 However, labor-intensive selective hydroxyl protection/deprotection strategies and the necessity of optical resolution of racemic myo-inositol derivatives have led to utilization of chiral pool starting materials for cyclitol syntheses. Of these, carbohydrates represent a logical choice due to their availability in optically pure form and stereochemically complex oxygenation patterns that can be relayed to their target destinations in the desired cyclitols. Thus, D-glucose,9 D-galactose,10 D-mannitol,11 and L-iditol,12 among others, have served as starting points in efficient syntheses of cyclitol derivatives. One of our research groups has recently reported an enantiodiver-gent synthesis of (+)- and (-)-cyclophellitol from D-xylose.13 Utilizing the latent plane of symmetry present in the starting carbohydrate, aldehydes 1 and ent-1 were prepared as key intermediates for this enantiodivergent strategy (Scheme 1). In this paper we show that this chemistry has a much broader scope and provide a full account of an investigation resulting in the development of synthetic pathways to diverse cyclitols starting from D-xylose and utilizing a ring-closing metathesis reaction for carbocycle formation.14,15
Scheme 1.
Enantiodivergent strategy utilized in the synthesis of (+)-and (-)-cyclophellitol from D-xylose
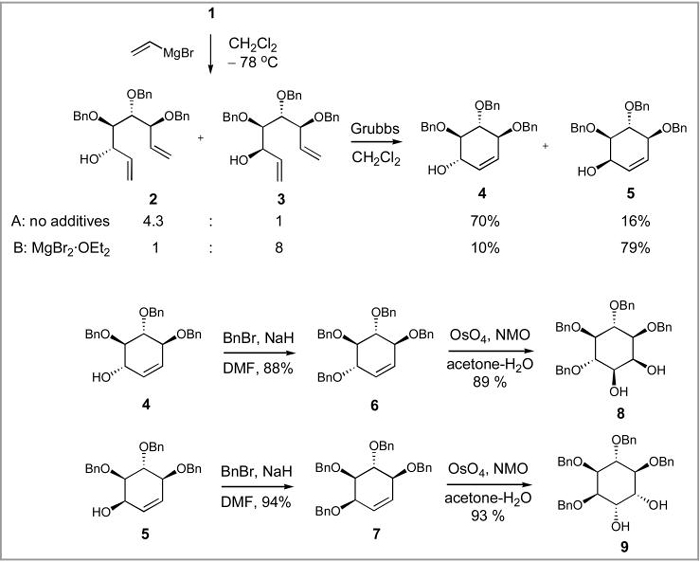
Transformation of aldehyde 1 to biologically important conduritols and inositols can be achieved using a short synthetic sequence that starts with a Grignard addition of a vinyl metal reagent to generate an inseparable epimeric mixture of alcohols 2 and 3 (Scheme 2). The ratio of the two is highly dependent on the nature of the vinyl metal reagent, solvent and the presence of chelating salts. The highest selectivity for syn alcohol 2 (2:3 = 4.3:1) is attained using vinylMgBr in CH2Cl2 at -78 °C,16 whereas a preponderance of anti alcohol 3 is observed in the presence of 3 equiv. of MgBr2·OEt2 under the otherwise similar conditions (2:3 =1:8).
Scheme 2.
Syntheses of benzylated derivatives of conduritol B, conduritol F, myo-inositol and chiro-inositol
The stereochemical assignment of the syn and anti addition products was confirmed by their conversion to the corresponding tetrabenzyl ethers, whose NMR analysis revealed the symmetry of the syn alcohol-derived compound. Although 2 and 3 can in principle be separated, for example by converting them into corresponding TIPS ethers and then desilylating,17 direct treatment of their mixture with the first generation Grubbs' ruthenium catalyst gives chromatographically separable conduritols B (4) and F (5). The facility of the metathesis process is remarkable. TLC monitoring of the reaction progress reveals complete conversion seconds after the catalyst is added to the reaction mixture. The overall yields of the conduritol mixtures from aldehyde 1 are in the range of 85-90%, but the individual yields vary depending on the conditions used to perform the Grignard reaction and they are dependent on the ratio of intermediate alcohols 2 and 3.
Conduritols 4 and 5 are further benzylated and dihydroxylated to give myo- and chiro-inostiol derivatives 8 and 9 in good yields. While the C2-symmetry of 6 accounts for the formation of only one possible cis dihydroxy compound 8, the facial preference of the dihydroxylation reaction leading to the exclusive formation of 9 is noteworthy. Evidently, the two vicinal benzyloxy substituents flanking the olefin in 7 provide a strong steric bias resulting in the observed stereochemical outcome. The NMR spectra of 8 and 9 are consistent with those previously reported for these compounds by us18 and others.19
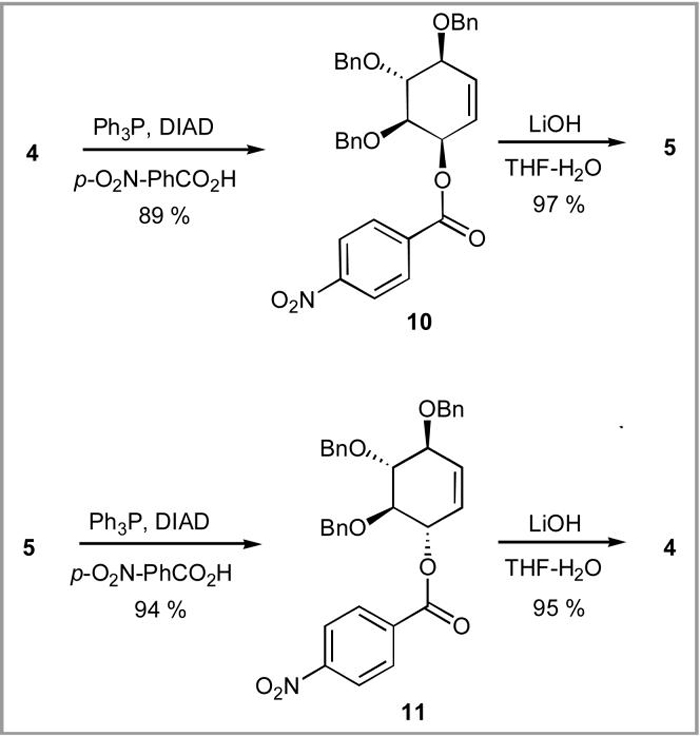
We also found that conduritols 4 and 5 are excellent substrates for Mitsunobu inversion. Thus, the yield of a desired conduritol, regardless of whether it is 4 or 5, can be further improved by treating the minor unwanted epimer with Ph3P, p-nitrobenzoic acid and diisopropylazadicarboxylate in ether to afford p-nitrobenzoates 10 and 11, which are smoothly hydrolyzed to 5 and 4 respectively (Scheme 3).
Scheme 3.
Mitsunobu inversion interconverting conduritols 4 and 5
The diastereodivergence of this synthetic pathway arises from the stereocontrol in the Grignard reaction of aldehyde 1 with vinyl magnesium bromide. Before the conditions favoring the formation of 2 over 3 and vice versa were found, extensive experimentation had been performed and the observed general trends warrant a discussion. Factors controlling the stereochemistry of organometallic addition reactions with α-alkoxy, and even α,β-dialkoxy, aldehydes have been investigated in some detail.20 However, such processes are considerably more complicated in the case of carbohydrate-derived aldehydes, which may contain additional alkoxy groups capable of chelation.
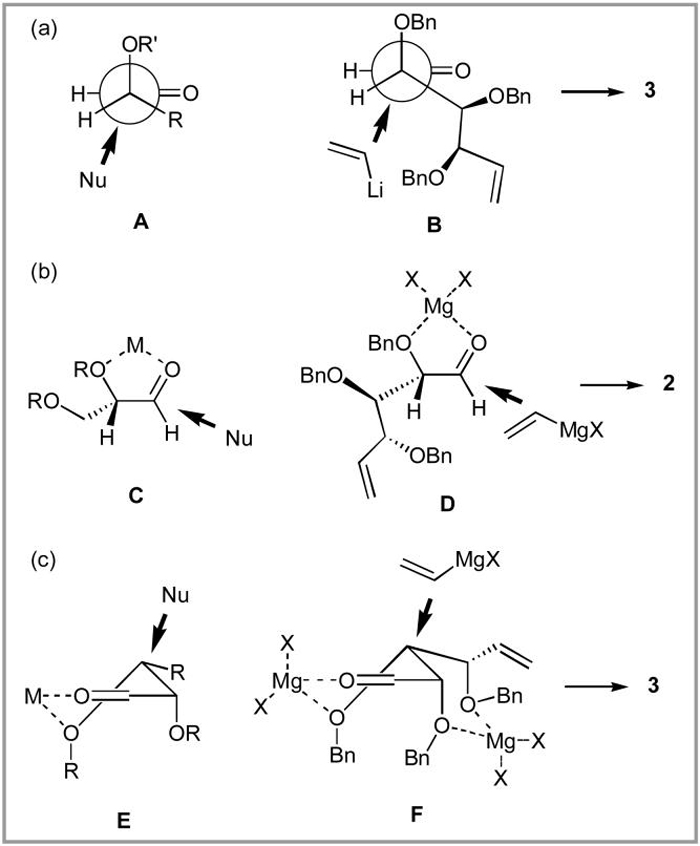
Generally, researchers have interpreted the stereochemical outcomes of such reactions in terms of Felkin-Anh (Figure 1a, transition state A), α-chelation (Figure 1b, transition state C), and β-chelation (Figure 1c, transition state E) models.20a When applied to aldehyde 1, these models will be represented by transition states B, D and F, which will lead to the formation of anti, syn and anti (3, 2, and 3) alcohols, correspondingly.
Figure 1.
Possible transition states governing the stereocontrol in vinyl metal addition to aldehyde 1
It appears that the reaction can be channeled through B, D or F by adjusting the chelating power of the reaction medium.21 Non-chelating reagents would be expected to react through the Felkin-Anh transition state B, leading to the selective formation of anti alcohol 3. Our results with vinylLi (Table 1, entries 1-4) are consistent with this interpretation and can be explained by the low chelating ability of organolithium reagents in ethereal solvents. Macdonald, Reetz, and more recently Evans and co-workers, have reached similar conclusions in their investigations of Li- and Ti-based organometallic addition reactions to α,β-dialkoxy aldehydes.20b,22 Literature reports indicate that the selectivity can be switched from anti to syn by replacing organolithium reagents with their organomagnesium counterparts.20b,23 Due to the higher chelating ability of Mg2+ the operative transition state for these addition reactions should be D and the results of our experiments with aldehyde 1 (entries 5,6) are consistent with this proposal. The replacement of Lewis basic ethereal solvents with CH2Cl2 should further strengthen the metal coordination in transition state D and this is also well-precedented in the literature.22a,24 Indeed complete removal of THF from the commercial vinylMgBr reagent and its substitution by CH2Cl2 gives the highest syn selectivity we have been able to attain (entry 9).16
Table 1.
Stereoselectivities of vinyl metal addition to aldehyde 1
| vinyl metal reagent | solvent | additive | chelation | anti:syn 3:2 | |
|---|---|---|---|---|---|
| 1 | vinylLi | THF | 12-crown-4 | low | 1.5:1 |
| 2 | vinylLi | THF | none | low | 2.2:1 |
| 3 | vinylLi | THF | Me2S | low | 2.6:1 |
| 4 | vinylLi | ether | none | low | 3.5:1 |
| 5 | vinylMgBr | THF-ether 1:1 | none | medium | 1:2 |
| 6 | vinylMgBr | THF | none | medium | 1:2 |
| 7 | vinylMgBr | CH2Cl2-THF 1:1 | none | medium | 1:2 |
| 8 | vinylMgBr | CH2Cl2-THF 5:1 | none | medium | 1:3 |
| 9 | vinylMgBr | CH2Cl2 | none | medium | 1:4.3 |
| 10 | vinylMgBr | CH2Cl2 | MgBr2·OEt2 1 equiv. | high | 1:1 |
| 11 | vinylMgBr | CH2Cl2 | MgBr2·OEt2 2 equiv. | high | 2:1 |
| 12 | vinylMgBr | CH2Cl2 | MgBr2·OEt2 3 equiv. | high | 8:1 |
Finally, we propose that the syn to anti switch that occurs with increasing amounts of MgBr2·OEt2 (entries 10-12), should be interpreted in terms of the β-chelation controlled transition state E. Here, the second metal coordination event with the participation of α- and γ-alkoxyl groups leads to reactive conformer F, in which the perpendicular geometry of Cα-OBn bond and coordination of this α-alkoxyl to the metal would enhance the “Anh effect.” This hypothesis is in agreement with the results reported by Martin and co-workers, who found that the selectivity of organometallic addition to α,β,γ-trialkoxyaldehyde was crucially dependent on the protecting group on the γ-oxygen.25 The reaction stereochemistry was completely reversed when the nonchelating γ-TBDPS ether was replaced by the benzyloxy moiety, arguing in favor of an α- to β-chelation switch similar to the one proposed in this work.
Although we found that the highest selectivities favoring both syn and anti alcohols 2 and 3 are obtained in CH2Cl2, the practicality of these procedures, especially performed on a large scale, are somewhat undermined by the side-reaction of the Grignard reagent with the solvent and, therefore, the necessity to use a large excess of the reagent (20 equiv.). The procedures involving the use of vinylLi in ether (2:3 = 1:3.5) and vinylMgBr in CH2Cl2-THF 5:1 (2:3 = 3:1) may be recommended for large-scale preparations.
In conclusion, a short synthetic route to a diverse group of cyclitol derivatives has been developed. The synthesis is enantiodivergent and allows for the preparation of various derivatives of conduritols B and F as well as myo- an chiro-inositols in both enantiomeric series. In addition, conduritol B derivative ent-4 served as a penultimate intermediate in the synthesis of (+)-cyclophellitol by Trost and co-workers.26 Therefore, our route provides another strategy for an enantiodivergent synthesis of this intensively researched anti-HIV and antimetastic agent and, more importantly, a library of its analogues in both enantiomeric series. Finally, we believe that our studies of the stereochemical outcome of the vinyl metal addition to a carbohydrate-derived aldehyde shed more light on these generally poorly understood processes.
Unless otherwise noted all commercially obtained reagents were used without purification. THF was distilled from sodium benzophenone ketyl prior to use. Dichloromethane was distilled from calcium chloride. Reactions were carried out under a nitrogen atmosphere in oven-dried glassware using standard syringe, cannula and septa techniques. Reactions were monitored by TLC (Silica Gel 60 F254, 250 μm) and visualized with UV light and ceric ammonium molybdate solution. Flash chromatography was performed on silica gel (32-63 μm). Optical rotations were measured with an Autopol III automatic polarimeter. 1H and 13C NMR spectra were recorded on JEOL 300 MHz spectrometer.
3(S),4(R),5(R)-Tribenzyloxy-6(S)-hydroxycyclohexene (4)
To a 1M solution of vinylmagnesium bromide in CH2Cl2 (23 mL) was added aldehyde 1 (0.5 g, 1.2 mmol) in CH2Cl2 (7 mL) dropwise during 30 min at -78 °C. The mixture was stirred at that temperature for 3 h and MeOH (2 mL) was added to quench the excess of the Grignard reagent. The mixture was warmed up to rt and washed with H2O (10 mL), aqueous 1M NH4Cl (10 mL), H2O (10 mL) and brine. The organic layer was dried over MgSO4 and evaporated to dryness. The residue consisted of a 4.3:1 (based on the integration of doublets at 2.64 and 3.25 ppm) mixture of 2 and 3, which was chromatographed (hexanes - ethyl acetate, 6:1). To a solution of 2 and 3 (0.46 g, 4.3:1) in CH2Cl2 (40 mL) was added (Cy3P)2RuCl2(CHPh) (56 mg, 0.068 mmol) at rt. The reaction mixture was stirred for 15 min and opened to the atmosphere for 4 h. The solvent was evaporated and the residue chromatographed (hexanes - ether, 1:1 → 1:2) to give 0.35 g (70%) of 4, followed by 80 mg (16%) of 5.
[α]D20: +123.3 (c 0.8, CHCl3).
1H NMR (CDCl3): δ = 7.25-7.36 (m, 15H), 5.70 (m, 2H), 5.03 (d, J = 11.3 Hz, 1H), 4.92-4.25 (m, 5H), 4.32-4.25 (m, 2H), 3.79 (dd, J = 7.4, 10.2 Hz, 1H), 3.53 (dd, J = 8.0, 10.1 Hz, 1H), 2.22 (d, J = 3.9 Hz, 1H).
13C NMR (CDCl3): δ = 138.7, 138.3, 129.5, 128.7, 128.6, 128.5, 128.1, 128.0, 127.9, 127.8, 127.1, 84.4, 83.4, 80.6, 75.4, 72.4, 72.0.
HRMS (ESI): m/z calcd for C27H28NaO4 (M+Na)+ 439.1885; found: 439.1884.
3(S),4(R),5(R)-Tribenzyloxy-6(R)-hydroxycyclohexene (5)
To a solution of aldehyde 1 (0.5 g, 1.2 mmol) in CH2Cl2 (11 mL) was added magnesium bromide etherate (0.92 g, 3.6 mmol) in one portion and the mixture was stirred at rt for 30 min. To a cold (-78 °C) mixture was added vinylmagnesium bromide in CH2Cl2 (24 mL of 1M solution, 24 mmol) over a period of 30 min. The reaction mixture was stirred at -78 °C for 3 h after which time methanol (10 mL) was added. The mixture was allowed to warm up to rt and treated with 1M NH4Cl (25 mL). The aqueous layer was extracted with additional CH2Cl2 (2 × 25 mL) and the combined organic extracts were washed with water (40 mL), brine (40 mL) and dried (MgSO4). The residue consisted of a 1:8.5 (based on the integration of doublets at 2.61 and 3.15 ppm) mixture of 2 and 3, which was chromatographed (hexanes - ethyl acetate, 6:1). To a solution of 2 and 3 (0.44 g, 1:8.5) in CH2Cl2 (40 mL) was added (Cy3P)2RuCl2(CHPh) (56 mg, 0.068 mmol) at rt. The reaction mixture was stirred for 15 min and opened to the atmosphere for 4 h. The solvent was evaporated and the residue chromatographed (hexanes - ether, 1:1 → 1:2) to give 0.39 g (79%) of 5, preceded by 50 mg (10%) of 4.
[α]D20: +39.3 (c 0.9, CHCl3).
1H NMR (CDCl3): δ = 7.35-7.25 (m, 15H), 5.88 (d, J = 1.9 Hz, 2H), 4.94-4.66 (m, 6H), 4.29 (m, 1H), 4.10 (d, J = 7.4 Hz, 1H), 4.50 (dd, J = 7.2, 9.7 Hz, 1H), 3.56 (dd, J = 4.1, 9.7 Hz, 1H), 2.71 (d, J = 2.5 Hz, 1H).
13C NMR (CDCl3): δ = 138.8, 138.6, 138.2, 131.0, 128.6, 128.5, 128.1, 128.0, 127.8, 127.7, 127.0, 79.7, 79.1, 79.0, 75.2, 73.2, 72.0, 65.7.
HRMS (ESI): m/z calcd for C27H28NaO4 (M+Na)+ 439.1885; found: 439.1885.
3(S),4(R),5(R),6(S)-Tetrabenzyloxycyclohexene (6)19
To a solution of 4 (7 mg, 0.017 mmol) in DMF (0.6 mL) was added NaH (4 mg of 60% dispersion in mineral oil, 0.1 mmol) at 0 °C. The mixture was stirred for 15 min after which time BnBr (5 μL, 0.04 mmol) was added and the resulting solution was stirred overnight. Ether (3 mL) was added and the excess of NaH was quenched with 1M NH4Cl (2 mL). The organic layer was washed with water (2 × 2 mL), dried (MgSO4) and evaporated. The residue was subjected to a preparative chromatography plate (hexanes - ethyl acetate, 9:1) to give 7.5 mg (88%) of 6, whose 1H NMR spectrum was identical to that reported previously.19
3(S),4(R),5(R),6(R)-Tetrabenzyloxycyclohexene (7)27
To a solution of 5 (8 mg, 0.019 mmol) in DMF (0.6 mL) was added NaH (4 mg of 60% dispersion in mineral oil, 0.1 mmol) at 0 °C. The mixture was stirred for 15 min after which time BnBr (5 μL, 0.04 mmol) was added and the resulting solution was stirred overnight. Ether (3 mL) was added and the excess of NaH was quenched with 1M NH4Cl (2 mL). The organic layer was washed with water (2 × 2mL), dried (MgSO4) and evaporated. The residue was subjected to a preparative chromatography plate (hexanes - ethyl acetate, 9:1) to give 9 mg (94%) of 7, whose 1H NMR spectrum was identical to that reported previously.27
3,4,5,6-Tetra-O-benzyl-D-myo-inositol (8)19
To a solution of 6 (6 mg, 0.012 mmol) and NMO (2 mg, 0.017 mmol) in acetone-H2O (9:1, 0.6 mL) was added a catalytic amount of OsO4. The mixture was stirred for 3 days at rt and treated with ether (3 mL). The organic layer was washed with 10% Na2S2O3 (2mL), H2O (2 mL), dried (MgSO4) and evaporated. The residue consisted of 8, which was >98% pure by TLC and 1H NMR (5.8 mg, 89%). 1H NMR spectrum of 8 was identical to that reported previously.19
1,2,3,4-Tetra-O-benzyl-L-chiro-inositol (9)18
To a solution of 7 (6 mg, 0.012 mmol) and NMO (2 mg, 0.017 mmol) in acetone-H2O (9:1, 0.6 mL) was added a catalytic amount of OsO4. The mixture was stirred for 2 h at rt and treated with ether (3 mL). The organic layer was washed with 10% Na2S2O3 (2mL), H2O (2 mL), dried (MgSO4) and evaporated. The residue consisted of 9, which was >98% pure by TLC and 1H NMR (5.9 mg, 93%). 1H NMR spectrum of 9 was identical to that reported previously.18
Mitsunobu Inversion of Conduritols 4 and 5
To a stirred solution of 4 or 5 (30 mg, 0.072 mmol) in ether (1.25 mL) was added PPh3 (19 mg, 0.072 mmol) and p-nitrobenzoic acid (12 mg, 0.072 mmol) at rt. After the material dissolved, DIAD (17.7 μL, 0.074 mmol) was added to the reaction mixture. After stirring for 17 h at rt, the mixture was concentrated under reduced pressure and the residue was chromatographed (hexanes - ethyl acetate, 9:1) to yield pure 10 (37 mg, 89%) or 11 (39 mg, 94%).
(1R,4S,5R,6R)-4,5,6-Tris(benzyloxy)cyclohex-2-enyl 4-nitrobenzoate (10)
[α]D20: -114.3 (c 0.1, CHCl3).
1H NMR (CDCl3): δ = 8.28 (d, J = 8.5 Hz, 2H), 8.18 (d, J = 8.5, 2H), 7.36-7.23 (m, 15H), 6.04-5.87 (m, 3H), 4.99-4.67 (m, 6H), 4.16-4.04 (m, 2H), 3.73 (dd, J = 3.0, 9.9 Hz, 1H).
13C NMR (CDCl3): δ = 164.3, 150.6, 138.6, 138.2, 138.0, 135.7, 134.2. 131.0, 128.8, 128.6, 128.4, 128.2, 128.0, 127.8, 123.6, 123.1, 79.8, 78.4, 77.9, 77.3, 75.3, 72.8, 68.3.
HRMS (ESI): m/z calcd for C34H31NNaO7 (M+Na)+ 588.1998; found: 588.1982.
(1S,4S,5R,6R)-4,5,6-Tris(benzyloxy)cyclohex-2-enyl 4-nitrobenzoate (11)
[α]D20: +156.8 (c 0.05, CHCl3).
1H NMR (CDCl3): δ = 8.24 (d, J = 8.8 Hz, 2H), 8.02 (d, J = 8.8 Hz, 2H), 7.36-7.10 (m, 15H), 5.83 (m, 2H), 5.64 (d, J = 10.5 Hz, 1H), 4.98-4.67 (m, 6H), 4.30 (dd, J = 2.8, 4.7 Hz, 1H), 3.93-3.85 (m, 2H).
13C NMR (CDCl3): δ = 164.1, 150.6, 138.5, 138.1, 135.3, 130.9, 130.0, 128.6, 128.5, 128.4, 128.1, 128.1, 127.9, 127.7, 125.4, 123.5, 83.8, 81.1, 79.8, 77.5, 75.8, 75.7, 72.7.
HRMS (ESI): m/z calcd for C34H31NNaO7 (M+Na)+ 588.1998; found: 588.1995.
Hydrolysis of Esters 10 and 11
To a stirred solution of 10 or 11 (20 mg, 0.035 mmol) in THF (0.5 mL) was added 1M LiOH (0.5 mL). The reaction mixture was stirred for 3 h at rt. The solvent was evaporated and the residue chromatographed (hexanes - ethyl acetate, 9:1) to yield pure 5 (14.1 mg, 97%) or 4 (13.8 mg, 95%).
Acknowledgment
A. K. would like to acknowledge the financial support from the US National Institutes of Health (RR-16480 and CA-99957) under the BRIN/INBRE and AREA programs. M. D. acknowledges the financial support from the National Institutes of Health (DK-44589 and GM-84819). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Dedicated to Professor E. J. Corey on the occasion of his 80th birthday.
References
- (1).(a) Posternak T. Chemistry of the Cyclitols - The Cyclitols. Hermann; Paris: 1965. [Google Scholar]; (b) Hudlicky T, Cebulak M. Cyclitols and Derivatives. A Handbook of Physical, Spectral and Synthetic Data. VCH; New York: 1993. [Google Scholar]; (c) Ferrier RJ, Middleton S. Chem. Rev. 1993;93:2779. [Google Scholar]; (d) Billington DC. The Inositol Phosphates - Chemical Synthesis and Biological Significance. VCH; Weinheim: 1993. [Google Scholar]; (e) Hudlicky T, Entwistle DA, Pitzer KK, Thorpe AJ. Chem. Rev. 1996;96:1195. doi: 10.1021/cr9403300. [DOI] [PubMed] [Google Scholar]; (f) Gültekin MS, Çelik M, Balci M. Curr. Org. Chem. 2004;8:1159. [Google Scholar]
- (2).Potter BVL, Lampe D. Angew. Chem. Int. Ed. Engl. 1995;34:1933. [Google Scholar]
- (3).See: Chakraborty N, d'Alarcao M. Bioorg. Med. Chem. 2005;13:6732. doi: 10.1016/j.bmc.2005.07.020. and references cited therein.
- (4).Billington DC, Perron-Sierra F, Beaubras S, Duhault J, Espinal J, Challal S. Bioorg. Med. Chem. Lett. 1994;4:2307. [Google Scholar]
- (5).(a) Balci M, Sütbeyaz Y, Seçen H. Tetrahedron. 1990;46:3715. [Google Scholar]; (b) Balci M. Pure Appl. Chem. 1997;69:97. and references cited therein. [Google Scholar]
- (6).(a) Legler G, Herrchen M. FEBS Lett. 1981;135:139. doi: 10.1016/0014-5793(81)80962-3. [DOI] [PubMed] [Google Scholar]; (b) Atsumi S, Iinuma H, Nosaka C, Umezawa K. J. Antibiot. 1990;43:1579. doi: 10.7164/antibiotics.43.1579. [DOI] [PubMed] [Google Scholar]; (c) Atsumi S, Umezawa K, Iinuma H, Naganawa H, Nakamura H, Iitaka I, Takeuchi T. J. Antibiot. 1990;43:49. doi: 10.7164/antibiotics.43.49. [DOI] [PubMed] [Google Scholar]
- (7).Sureshan KM, Shashidhar MS, Praveen T, Das T. Chem. Rev. 2003;103:4477. doi: 10.1021/cr0200724. [DOI] [PubMed] [Google Scholar]
- (8).Kiddle JJ. Chem. Rev. 1995;95:2189. [Google Scholar]
- (9).See, for example: Saito S, Shimazawa R, Shirai R. Chem. Pharm. Bull. 2004;52:727. doi: 10.1248/cpb.52.727.
- (10).See, for example: Dubreuil D, Cleophax J, de Almeida MV, Verre-Sebrié C, Liaigre J, Vass G, Géro SD. Tetrahedron. 1997;53:16747.
- (11).See, for example: Chiara JL, Martín-Lomas M. Tetrahedron Lett. 1994;35:2969.
- (12).See, for example: Guidot JP, Le Gall T, Mioskowski C. Tetrahedron Lett. 1994;35:6671.
- (13).Kireev AS, Breithaupt AT, Collins W, Nadein ON, Kornienko A. J. Org. Chem. 2005;70:742. doi: 10.1021/jo048459p. [DOI] [PubMed] [Google Scholar]
- (14).For preliminary account of this work, see: Kornienko A, d'Alarcao M. Tetrahedron: Asymmetry. 1999;10:827.
- (15).For other examples of utilization of ring-closing metathesis in cyclitol synthesis, see: Verhelst SHL, Wennekes T, van der Marel GA, Overkleeft HS, van Boeckel CAA, van Boom JH. Tetrahedron. 2004;60:2813. Andersen TL, Skytte DM, Madsen R. Org. Biomol. Chem. 2004;2:2951. doi: 10.1039/B411021H. Heo JN, Holson EB, Roush WR. Org. Lett. 2003;5:1697. doi: 10.1021/ol034349d.Marco-Contelles J, de Opazo E. J. Org. Chem. 2002;67:3705. doi: 10.1021/jo0111107. Conrad RM, Grogan MJ, Bertozzi CR. Org. Lett. 2002;4:1359. doi: 10.1021/ol025680k. Boyer F-D, Hanna I, Nolan SP. J. Org. Chem. 2001;66:4094. doi: 10.1021/jo0155761. Jorgensen M, Iversen EH, Paulsen AL, Madsen R. J. Org. Chem. 2001;66:4630. doi: 10.1021/jo0101297. Nishikawa A, Saito S, Hashimoto Y, Koga K, Shirai R. Tetrahedron Lett. 2001;42:9195. Hanna I, Ricard L. Org. Lett. 2000;2:2651. doi: 10.1021/ol006182j. Marco-Contelles J, de Opazo E. J. Org. Chem. 2000;65:5416. doi: 10.1021/jo000110o. Marco-Contelles J, de Opazo E. Tetrahedron Lett. 2000;41:2439. Kapferer P, Sarabia F, Vasella A. Helv. Chim. Acta. 1999;82:645. Sellier O, Van de Weghe P, Eustache J. Tetrahedron Lett. 1999;40:5859. Seepersaud M, Al-Abed Y. Org. Lett. 1999;1:1463.
- (16).After our original disclosure of these results (ref. 14), Madsen and co-workers reported that performing this reaction with vinyl magnesium chloride in toluene leads to a higher (5:1) syn selectivity (ref. 15b).
- (17).See the next article in this issue.
- (18).Kornienko A, Marnera G, d'Alarcao M. Carbohydr. Res. 1998;310:141. doi: 10.1016/s0008-6215(98)00168-2. [DOI] [PubMed] [Google Scholar]
- (19).Yamauchi N, Kakinuma K. J. Antibiot. 1992;45:756. doi: 10.7164/antibiotics.45.756. [DOI] [PubMed] [Google Scholar]
- (20).(a) Reetz MT. Angew. Chem. Int. Ed. Engl. 1984;23:556. [Google Scholar]; (b) Mead K, Macdonald TL. J. Org. Chem. 1985;50:422. [Google Scholar]
- (21).Both the organometallic reagent and the reaction solvent are important since Lewis basic solvents such as THF or ether can diminish chelation by strongly coordinating to the metal species.
- (22).(a) Reetz MT, Kesseler K. J. Org. Chem. 1985;50:5436. [Google Scholar]; (b) Evans DA, Cee VJ, Siska SJ. J. Am. Chem. Soc. 2006;128:9433. doi: 10.1021/ja061010o. [DOI] [PubMed] [Google Scholar]
- (23).Mead KT. Tetrahedron Lett. 1987;28:1019. [Google Scholar]
- (24).(a) Keck GE, Andrus MB, Romer DR. J. Org. Chem. 1991;56:417. [Google Scholar]; (b) Guillarme S, Haudrechy A. Tetrahedron Lett. 2005;46:3175. [Google Scholar]
- (25).Martin SF, Chen H-J, Yang C-Y. J. Org. Chem. 1993;58:2867. [Google Scholar]
- (26).Trost BM, Hembre EJ. Tetrahedron Lett. 1999;40:219. [Google Scholar]
- (27).Ackermann L, Tom DE, Fürstner A. Tetrahedron. 2000;56:2195. [Google Scholar]