Abstract
Decorin, a member of the small leucine-rich proteoglycan gene family, impedes tumor cell growth by down-regulating the epidermal growth factor receptor. Decorin has a complex binding repertoire, thus, we predicted that decorin would modulate the bioactivity of other tyrosine kinase receptors. We discovered that decorin binds directly and with high affinity (Kd = ∼1.5 nM) to Met, the receptor for hepatocyte growth factor (HGF). Binding of decorin to Met is efficiently displaced by HGF and less efficiently by internalin B, a bacterial Met ligand. Interaction of decorin with Met induces transient receptor activation, recruitment of the E3 ubiquitin ligase c-Cbl, and rapid intracellular degradation of Met (half-life = ∼6 min). Decorin suppresses intracellular levels of β-catenin, a known downstream Met effector, and inhibits Met-mediated cell migration and growth. Thus, by antagonistically targeting multiple tyrosine kinase receptors, decorin contributes to reduction in primary tumor growth and metastastic spreading.
Introduction
The extracellular matrix and its multiple constituents play both a structural and signaling role by interacting with surface receptors that ultimately affect gene expression, cell phenotypes, development, and cancer (Ramirez and Rifkin, 2003; Weigelt and Bissell, 2008). Decorin, a member of the small leucine-rich proteoglycan gene family that harbors one chondroitin/dermatan sulfate side chain at its N terminus, was originally named because of its ability to “decorate” collagen fibrils, thereby regulating fibrillogenesis, a key mechanism of matrix assembly and homeostasis (Schaefer and Iozzo, 2008). It was soon discovered that decorin regulates the TGF-β signaling pathway and also inhibits the growth of a variety of tumor cells (Iozzo, 1998) by down-regulating the EGF receptor (EGFR; Iozzo et al., 1999b) and other members of the ErbB family of receptor tyrosine kinase (RTK; Goldoni and Iozzo, 2008). Decorin suppresses tumor cell–mediated angiogenesis by inhibiting the endogenous production of vascular endothelial cell growth factor (Grant et al., 2002) similar to neutralizing antibodies directed toward EGFR (Petit et al., 1997). Genetic deficiency of decorin causes intestinal tumor formation through disruption of intestinal cell maturation (Bi et al., 2008), whereas mice with a double deficiency of decorin and p53 succumb prematurely to aggressive lymphomas (Iozzo et al., 1999b). Together, these observations indicate that lack of decorin is permissive for in vivo tumorigenesis.
Ectopic expression of decorin induced by stable transgenic systems, viral vectors, or inducible promoters attenuates the growth of tumor xenografts with diverse histogenetic origin (Santra et al., 1995, 2000; Csordás et al., 2000; Reed et al., 2002, 2005; Tralhão et al., 2003; Biglari et al., 2004; Seidler et al., 2006). Decorin slows the growth of squamous cell and breast carcinomas by inducing a sustained down-regulation of the EGFR (Csordás et al., 2000) and ErbB2 (Santra et al., 2000), a process that leads to a p21WAF1-mediated growth suppression and enhanced cytodifferentiation of mammary carcinoma cells (Santra et al., 2000). The basic mechanism has been partially elucidated and includes direct binding to the EGFR followed by protracted internalization of the receptor via caveolar-mediated endocytosis (Zhu et al., 2005) and the triggering of apoptosis via caspase-3 activation (Seidler et al., 2006). Moreover, decorin inhibits myeloma cell growth (Li et al., 2008b), and systemic delivery of decorin reduces pulmonary metastases in two animal models (Goldoni et al., 2008; Shintani et al., 2008). Notably, decorin-induced growth inhibition in osteosarcoma MG63 cells is overcome by a constitutive activation of EGFR (Zafiropoulos et al., 2008).
Because of the complex binding capabilities of decorin toward multiple targets (Brandan et al., 2008; Schaefer and Iozzo, 2008) and its dramatic antioncogenic effects (Reed et al., 2002, 2005; Goldoni et al., 2008), we predicted a role for decorin in modulating the bioactivity of other RTK. We discovered that decorin binds directly to the Met receptor, also known as hepatocyte growth factor (HGF) receptor, an established mediator of malignant transformation, invasion, and metastasis (Danilkovitch-Miagkova and Zbar, 2002; Birchmeier et al., 2003; Knudsen and Vande Woude, 2008). Our findings indicate that decorin is a novel antagonistic ligand of the Met receptor. Apart from HGF, decorin is the only mammalian ligand known to date. Interaction between decorin and the extracellular domain of Met leads to receptor down-regulation through a combination of enhanced ectodomain shedding and internalization. Decorin-induced inhibition of Met activity results in suppression of key biological events. Notably, decorin induces a marked proteasome-dependent degradation of the transcription factor β-catenin and inhibits Met-dependent cell motility. Collectively, our findings point to decorin as a novel inhibitor of the Met receptor. The ability of decorin to antagonize multiple receptors, including Met, EGFR, and ErbB2/ErbB4, suggests that this leucine-rich proteoglycan might have therapeutic value in treatment of cancers in which several RTKs are coactivated.
Results
Decorin down-regulates the Met receptor
To discover new pathways affected by decorin, we used an antibody array system that simultaneously examines the relative Tyr phosphorylation level of 42 different RTKs. After a 15-min exposure of quiescent (serum starved) HeLa cells to 100 nM recombinant decorin, there was a rapid phosphorylation of the EGFR (Fig. 1 A) in agreement with our previous experiments (Iozzo et al., 1999b). In addition, a novel target was found in the Met receptor, which showed a decorin-evoked increase in phosphorylation when the cells were quiescent (Fig. 1 A) and a marked suppression when the cells were cultured in full serum (Fig. 1 B). Note that under the latter conditions, Tyr phosphorylation of EGFR, ErbB2, and ErbB4 receptors was markedly down-regulated by decorin in full agreement with our previous studies (Santra et al., 2000; Zhu et al., 2005), thereby validating our approach.
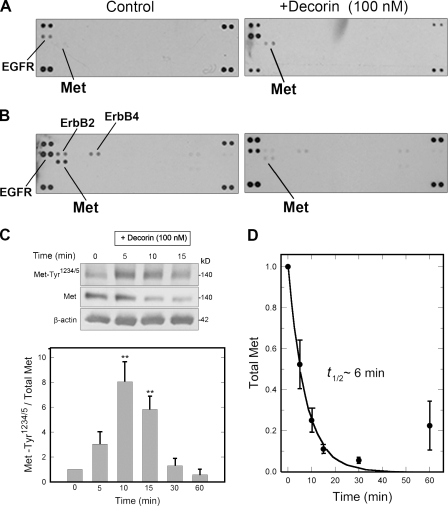
Figure 1.
Decorin affects Met receptor signaling and turnover. (A) Phospho-RTK arrays. HeLa cells were treated with decorin for 15 min. RTK membranes were incubated with cell lysates. The duplicate dots at each corner represent phospho-Tyr positive controls. (B) The same experiment as in A using nonquiescent cells. (C, top) Representative immunoblot of a short decorin time course showing phosphorylation of the Met receptor at Tyr1234/5, total Met, and β-actin. (bottom) Quantification of immunoblots similar to those shown in the top from three independent experiments performed in triplicate. Values represent the mean ± SEM (**, P < 0.01). (D) Best-fit plot of Met receptor degradation over time. Relative values were obtained by scanning densitometry (chemiluminescence) of blots as in C and represent means ± SEM from three independent experiments performed in triplicate.
Next, we performed dose-response and time course experiments to investigate the effects of decorin on Met phosphorylation kinetics. We used a phosphoantibody specific for the two Tyr residues located within the Met catalytic domain, Tyr1234 and Tyr1235. Decorin treatment of serum-starved cells evoked a transient phosphorylation of these residues (Fig. 1 C, top). In several experiments, we found a significant peak in phosphorylation at ∼10 min followed by pronounced down-regulation (Fig. 1 C, bottom). Interestingly, decorin induced a marked decrease in steady-state levels of Met, as detected by immunoblotting (Fig. 1 C, top). It is important to note that Met kinase activity was required for decorin-evoked down-regulation of Met, as tested by using SU11274, a specific Met tyrosine kinase inhibitor (Berthou et al., 2004; unpublished data). Remarkably, the levels of total Met receptor declined very rapidly with a t1/2 of ∼6 min (Fig. 1 D) and partially recovered at ∼60 min after treatment. However, even after 24 h of continuous decorin treatment, the Met levels were only ∼50% of control values (unpublished data). The kinetics of decorin-evoked Met phosphorylation were similar to those published for HGF in HeLa cells (Hammond et al., 2003), with a peak between 5 and 10 min of stimulation. In contrast, the kinetics of total Met degradation induced by HGF were much slower than those of decorin, showing a comparable down-regulation only after a 60-min treatment (Hammond et al., 2003), although those experiments were performed in full serum. These data suggest a role for decorin as a partial agonist insofar as it activates the Met kinase domain but with an outcome different from that evoked by HGF.
Decorin binds directly to the Met receptor: functional and biochemical evidence
We have previously shown that decorin binds directly to the EGFR, initiating a cohort of cellular responses (Iozzo et al., 1999b). Receptor cross talk is prevalent in cancer progression, and Met and EGFR are no exception, with many studies showing a link between the two either through direct interaction or by convergence of downstream signaling (Jo et al., 2000; Birchmeier et al., 2003; Li et al., 2008a; Reznik et al., 2008). To assess whether the observed effects on Met could be indirectly attributed to decorin/EGFR binding, we used two different EGFR-blocking strategies: either the blocking monoclonal antibody mAb425 (Rodeck et al., 1987) or AG1478, a specific EGFR tyrosine kinase inhibitor (Levitzki and Gazit, 1995). Preincubation for 1 h with mAb425 was sufficient to abrogate EGFR activation as demonstrated by a complete lack of phosphorylation in response to EGF (Fig. 2 A). Blocking EGFR kinase activity with AG1478 gave similar results (unpublished data). Even in the absence of EGFR activity, decorin evoked a rapid activation of the Met catalytic domain (Fig. 2 B) with no change in overall kinetics and a concurrent down-regulation of total Met. We conclude that Met receptor activation by decorin is independent of the EGFR.
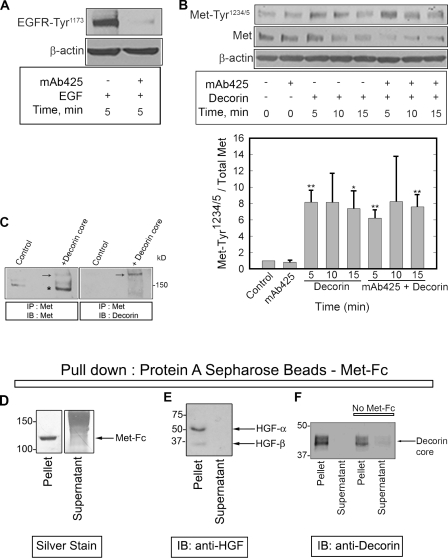
Figure 2.
Decorin interacts with the Met receptor. (A) 1 µg/ml mAb425, an EGFR-specific blocking antibody, was tested before experiments in combination with decorin by evaluating its effect in inhibiting EGF-dependent (16 nM) EGFR phosphorylation. (B, top) Immunoblot of a short decorin (100 nM) time course showing phosphorylation of the Met receptor at Tyr1234/5, total Met, and β-actin in the presence or absence of 1 µg/ml mAb425. (bottom) Quantification of immunoblots similar to those shown in the top panel. Values represent the mean ± SEM from three independent experiments performed in triplicate (*, P < 0.05; **, P < 0.01). (C) Immunoblots detecting Met (left) and decorin (right) in cells treated with decorin protein core for 15 min, cross-linked with 500 nM S-SMPB for 20 min at 37°C, and immunoprecipitated with an anti–C terminus Met antibody. Arrows point to a high Mr complex of Met and decorin protein core (∼190 kD). The asterisk indicates Met monomer (∼140 kD). (D) Silver-stained gel. Notice that the entire Met-Fc is bound to the protein A–Sepharose beads. Smear is the carrier proteins. (E) Immunoblotting (IB) of HGF after pull-down with protein A beads–Met-Fc. (F) Immunoblotting of decorin after pull-down with either protein A beads–Met-Fc or beads alone. Note the absence of HGF or decorin in the supernatants, indicating that essentially all of the ligands were bound. IP, immunoprecipitation. (D–F) Values shown are given in kiloDaltons.
This observation led us to hypothesize that decorin may act through a direct interaction with the Met receptor. To explore this possibility, we used a noncleavable impermeable cross-linker, S-SMPB (sulfo-succinimidyl-4-(p-maleimidophenyl)-butyrate). After cross-linking, the Met receptor was immunoprecipitated with an antibody specific for the intracellular C-terminal domain, and immunoblotting was performed to detect Met and decorin. We found that decorin protein core coimmunoprecipitated with Met in a complex of ∼190 kD (Fig. 2 C, arrows). The size of the complex suggests a 1:1 stoichiometry between decorin protein core (∼50 kD) and the β chain of the receptor (∼140 kD; Fig. 2 C, asterisk).
Next, the physical interaction of decorin with Met was established in pull-down experiments using protein A–linked Sepharose beads, which efficiently bound a Met-Fc chimera comprised of the extracellular domain of the Met fused to the Fc region of human IgG (Fig. 2 D). By this approach, we were able to efficiently pull down both HGF (Fig. 2 E) and decorin protein core (Fig. 2 F). Some decorin bound nonspecifically to the beads, but the presence of Met-Fc led to a significant enrichment in decorin binding.
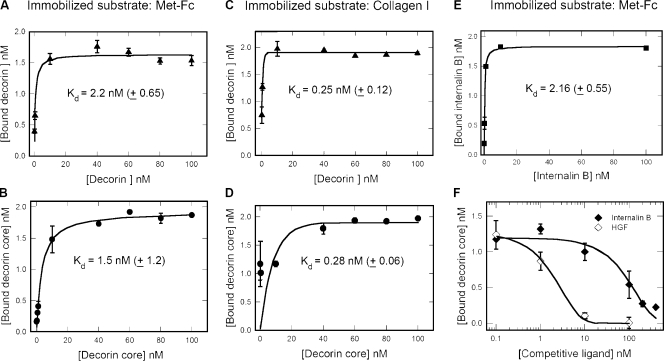
Next, we determined the binding affinity of decorin to immobilized Met-Fc chimera using solid-phase assays. Both decorin and decorin protein core bound to Met-Fc in a saturable manner (Fig. 3, A and B) with Kd of 2.2 nM and 1.5 nM, respectively. The biological activity of decorin and decorin protein core was tested by using fibrillar collagen type I, a known ligand for decorin. In this case, decorin and decorin core bound in a saturable manner with Kd of 0.25 nM and 0.28 nM, respectively (Fig. 3, C and D). In our assay, the binding of HGF alone to Met-Fc showed a Kd of 0.95 nM ± 0.47 (Fig. S1 A). As a negative control, we used a mouse monoclonal antibody as immobilized substrate, and no significant binding to decorin was observed (Fig. S1 B), ruling out the possibility that decorin binds nonspecifically to the Fc portion of the Met-Fc chimera. In addition, LG3 (the C-terminal portion of perlecan; Iozzo, 2005) did not interact with Met-Fc (Fig. S1 C). This rules out a role for the His tag in the binding insofar as LG3 has a His tag as decorin, it is a protein of similar size to decorin, and is expressed in the same eukaryotic cell system (293-EBNA cells).
Figure 3.
Affinity interaction between decorin and the Met receptor. (A–E) Ligand-binding assays using decorin, decorin protein core, or internalin B as soluble ligands and Met-Fc or fibrillar collagen I as immobilized substrates. (F) Competition experiments using constant amounts of decorin core (10 nM) and increasing amounts of internalin B or HGF as indicated. Notice that only at high molar ratios (20:1 and 40:1), internalin B significantly (∼70%) reduces decorin protein core binding to the Met (IC50 = ∼180 nM). In contrast, HGF is much more efficient (IC50 = ∼2.5 nM) in displacing decorin core. Values represent the mean ± SEM.
Two ligands of Met have been previously identified: the mammalian HGF and a bacterial leucine-rich repeat surface protein called internalin B. HGF plays key roles in promoting epithelial cell motility, growth, and differentiation (Birchmeier et al., 2003). Internalin B activates Met, leading to internalization of the bacterial pathogen Listeria monocytogenes into host cells (Shen et al., 2000; Ireton, 2007; Disson et al., 2008). Recent structural studies have shown that internalin B binds to the first Ig-like domain of Met (Niemann et al., 2007; Niemann et al., 2008). In contrast, HGF binds with high affinity to the Met terminal Ig3-4 (Basilico et al., 2008) and with lower affinity to the semaphorin domain (Stamos et al., 2004). To determine whether decorin binds to regions within the Met ectodomain that overlap with those used by HGF or internalin B, we performed competitive binding assays. First, we found that internalin B bound with high affinity (Kd = 2.16 nM) to Met-Fc (Fig. 3 E). Note that the Kd for Met/internalin B was previously reported to be 20–30 nM (Machner et al., 2003). A possible explanation for this discrepancy in the observed affinity could be that the Met-Fc used in our study is a dimer and fully glycosylated, whereas the Met used in the referenced study was a monomer and produced in glycosylation-deficient cells. HGF very effectively (50% inhibitory concentration [IC50] = ∼2.3 nM) competed with decorin protein core binding to Met-Fc (Fig. 3 F). In comparison, internalin B was ∼52-fold less efficient (IC50 = ∼120 nM) than HGF (Fig. 3 F). Because the overall affinity constants for decorin, internalin B, and HGF are relatively close (0.95–2.16 nM) in our assays, the conclusions from the competition experiments can be assessed as differential binding sites on the Met ectodomain for these ligands.
Collectively, our results demonstrate that decorin is a high affinity ligand of the Met receptor insofar as it shows saturable kinetics of binding and displacement by two established Met ligands. Moreover, the more efficient displacement by HGF suggests that decorin and HGF bind to overlapping sites on the Met ectodomain and further suggests that the decorin’s antagonistic effects might be the result of a unique mode of binding within the Met receptor.
Decorin evokes differential tyrosine phosphorylation of the Met receptor
In response to decorin binding, the kinase domain of Met is phosphorylated (Fig. 1 C and Fig. 2 B). In addition to Tyr1234/5, several other Tyr residues in the cytoplasmic tail of the Met receptor are known to undergo agonist-induced phosphorylation and play key roles in downstream signaling (Fig. 4 A; Birchmeier et al., 2003). For example, phosphorylation of Tyr1349 and Tyr1356 recruit the adaptor proteins Gab1 and Grb2, respectively, which are responsible for mediating most of the complex cellular responses (motility, growth, and differentiation; Birchmeier et al., 2003). Conversely, phosphorylation of Tyr1003 is involved in negative regulation of the receptor via recruitment of the E3 ubiquitin ligase c-Cbl, which is responsible for Met polyubiquitination and subsequent degradation in the proteasome (Petrelli et al., 2002).
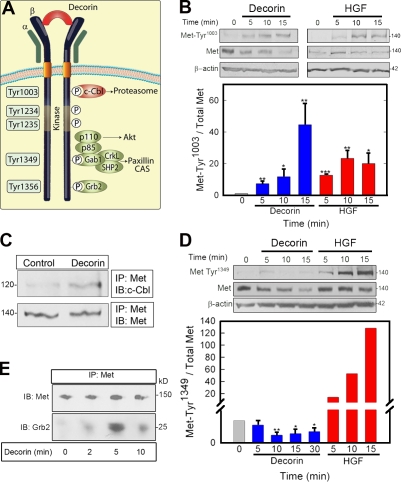
Figure 4.
Decorin induces differential and selective phosphorylation of Met Tyr residues. (A) Diagram of the main Met receptor Tyr phosphorylation sites and adaptor proteins. CAS, Crk-associated substrate; P, phosphate. (B, top) Representative immunoblots of a short decorin (100 nM) time course showing phosphorylation of the Met receptor at Tyr1003 and total Met amount vis à vis 1.5 nM HGF. (bottom) Quantification of immunoblots from three independent experiments. (C) Coimmunoprecipitation of c-Cbl and Met using an antibody directed toward the intracellular domain of Met. 100 nM decorin treatment was performed for 10 min. (D, top) Representative immunoblot showing phosphorylation of the Met receptor at Tyr1349 and total Met after 100 nM decorin treatment vis à vis 1.5 nM HGF. (bottom) Quantification of immunoblots from three independent experiments performed in triplicate. (E) Recruitment of Grb2 to the Met receptor mediated by 100 nM decorin. Coimmunoprecipitation of the Met receptor and Grb2 using an anti-Met C terminus antibody for the immunoprecipitation (IP) and either the same antibody or an anti-Grb2 monoclonal antibody for the immunoblotting (IB). Values represent the mean ± SEM (*, P < 0.05; **, P < 0.01; ***, P < 0.001). All of the relative values were obtained by scanning densitometry (chemiluminescence). (B–E) Values shown are given in kiloDaltons.
Decorin promoted phosphorylation of Tyr1003 (Fig. 4 B, top), which was slightly delayed in comparison to HGF, with a peak at 15 min (Fig. 4 B, bottom). Like the phosphorylation of residues 1234/1235 (Fig. 1 C and Fig. 2 B), phosphorylation of Tyr1003 in response to decorin was transient, with levels diminishing thereafter (not depicted). In support of this result, c-Cbl was recruited to the Met receptor after decorin treatment (Fig. 4 C).
Interestingly, the C-terminal Tyr1349 failed to be activated in response to decorin, whereas cells robustly responded to HGF (Fig. 4 D). This finding was unexpected, given that decorin induces efficient activation of Met, as assessed by phosphorylation of the catalytic Tyr1234/5 (Fig. 1 C and Fig. 2 B). Moreover, decorin induced robust recruitment of Grb2 to the Met receptor (Fig. 4 E). Because phosphorylated Tyr1356 serves as docking site for Grb2, this finding strongly suggests that decorin induces efficient phosphorylation of this Tyr residue. Notably, Tyr1356 is essential for receptor internalization, and Grb2 also indirectly recruits c-Cbl, leading to Met degradation (Li et al., 2007). Unfortunately, we were unable to directly assess phosphorylation of Tyr1356 because of the fact that phospho-specific antibodies recognizing this residue are not commercially available.
Collectively, our results show that decorin differentially affects key Tyr residues involved in Met signaling and homeostasis, inducing efficient phosphorylation of Tyr1356 and Tyr1003 while inhibiting phosphorylation of Tyr1349, the sole Tyr associated with downstream signaling events. Decorin and HGF activate the receptor in subtly different ways, perhaps by inducing different receptor conformations. This ability may be responsible for the more efficient down-regulation of Met caused by decorin (Fig. 4, B and D, top) and the lack of downstream signaling (Fig. 4 D, bottom).
Decorin causes Met down-regulation by inducing both ectodomain shedding and internalization
It is known that Met can be down-regulated not only via Cbl-mediated ubiquitination and degradation in the proteasome, but also by shedding of its ectodomain (Nath et al., 2001; Athauda et al., 2006; Petrelli et al., 2006). Specifically, the shedding is induced by a monoclonal Met-blocking antibody (Petrelli et al., 2006), which is effective in inhibiting primary tumor growth and metastastic spreading. Thus, we tested whether decorin could use a similar mechanism of action. Media conditioned by cells treated with decorin contained higher levels of shed Met ectodomain than controls (Fig. S2 A).
It has been reported that the Met ectodomain can be released from the plasma membrane through activation of the EGFR, a process that is mediated by a TIMP-3–sensitive pathway (Nath et al., 2001). Accordingly, we tested TIMP-2 and TIMP-3 ability to prevent decorin-dependent Met down-regulation. Both matrix metalloproteinase inhibitors were effective in blocking decorin activity on the Met receptor (Fig. S2 B, top). We observed that the full-length Met levels in cells incubated with the inhibitors were slightly higher than in control cells (Fig. S2 B, bottom). This suggests the existence of a basal level of receptor shedding, which is inhibited by TIMP-2 and TIMP-3. To test this possibility, we determined the amount of Met receptor shed into the media conditioned by cells treated with decorin in the presence or absence of the inhibitors. The results showed that both TIMP-2 and TIMP-3 reduced the amount of Met shedding (Fig. S2 C). Note that the control medium (Fig. S2 C) was conditioned for 24 h, showing a significant level of basal Met shedding (compare with Fig. S2 A).
To verify the contribution of Met internalization to Met down-regulation upon decorin binding, HeLa cells were treated for 5 and 30 min, subjected to immunostaining with a Met N terminus antibody, and analyzed by fluorescence microscopy (Fig. S2 D). Over time, decorin induced receptor relocation from the plasma membrane to intracellular compartments and perinuclear regions. This evidence was corroborated by biochemical data showing the presence of Met upon decorin treatment followed by trypsin digestion (Fig. S2 E). This assay is based on the fact that only internalized Met is not accessible to digestion. Collectively, our results provide a novel mechanism of action for decorin: inhibition of the Met receptor biological activity via a dual activity comprising enhanced shedding and intracellular degradation.
Decorin down-regulates β-catenin and induces apoptosis via the Met receptor
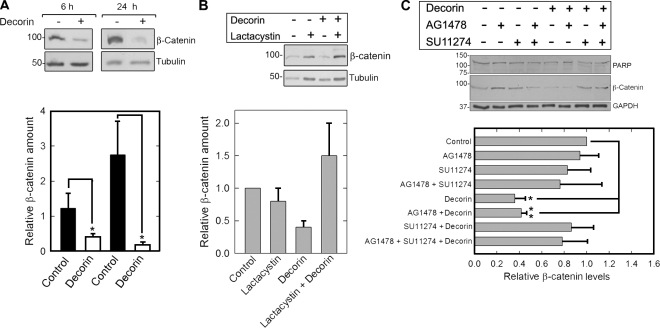
Next, we investigated whether decorin-evoked Met down-regulation could impair the β-catenin pathway, a known downstream effector of Met (Monga et al., 2002; Herynk et al., 2003; Rasola et al., 2007). After decorin treatment, β-catenin levels declined by ∼70% and ∼90% after 6 h and 24 h, respectively (Fig. 5 A). This degradation occurred via the proteasome, the main degradation pathway for β-catenin (Aberle et al., 1997), insofar as it was completely blocked by the proteasome inhibitor lactacystin (Fig. 5 B).
Figure 5.
Decorin down-regulates β-catenin via the Met receptor. (A, top) Representative immunoblots of HeLa cells treated with 100 nM decorin for the times indicated and probed for β-catenin. Tubulin was used as a loading control. (bottom) Quantification of immunoblots as those presented in the top panel from three independent experiments. (B, top) β-Catenin levels after treatment with 100 nM decorin for 30 min in the presence or absence of 10 µM of the proteasome inhibitor lactacystin. Cells were preincubated with lactacystin for 1 h before adding decorin. (bottom) Quantification of immunoblots as those presented in the top panel from three independent experiments. (C, top) β-Catenin and PARP immunoblots after treatment with 100 nM decorin for 6 h in the presence or absence of the tyrosine kinase inhibitors AG1478 and SU11274 (both 1 µM) as indicated. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as loading control. (bottom) Quantification of immunoblots as those presented in the top panel from three independent experiments performed in triplicate. Values represent the mean ± SEM (*, P < 0.05; **, P < 0.01). All of the relative values were obtained by scanning densitometry (chemiluminescence). Values shown in blots are given in kiloDaltons.
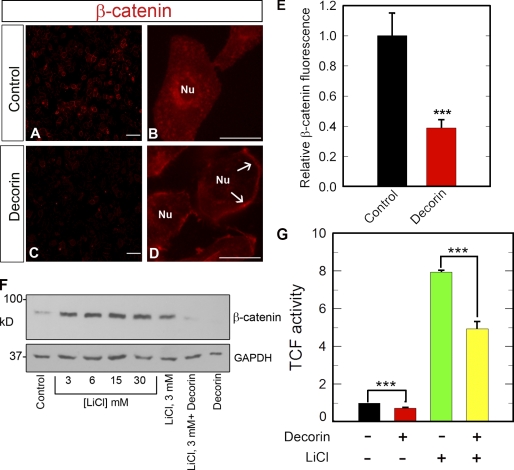
Next, we preincubated the cells with AG1478 and SU11274, EGFR and Met-specific tyrosine kinase inhibitors (Levitzki and Gazit, 1995; Berthou et al., 2004), respectively, in the presence or absence of decorin. AG1478 was not capable of inhibiting decorin effect on β-catenin levels, whereas SU11274 blocked decorin-evoked β-catenin degradation (Fig. 5 C). Importantly, the effect of decorin on the β-catenin pathway was direct and not the result of induction of apoptosis as proven by lack of poly ADP-ribose polymerase (PARP) cleavage after 6-h treatment (Fig. 5 C, top). In agreement with the biochemical data, the total levels of β-catenin were markedly reduced by decorin treatment as detected by qualitative and quantitative fluorescence microscopy (Fig. 6, A, C, and E). Notably, there was a marked displacement of β-catenin from perinuclear to plasmalemmal regions (Fig. 6, B and D). Finally, we tested whether decorin could cause β-catenin degradation in the presence or absence of LiCl, a known inhibitor of GSK3β (Klein and Melton, 1996). The results clearly showed that LiCl potently stabilized β-catenin levels in the absence of decorin but was not capable of inhibiting decorin-evoked β-catenin down-regulation (Fig. 6 F). These findings were corroborated by functional tests assessing β-catenin transcriptional activity. We performed transient cell transfection assays using the TopFlash reporter vector, which drives the expression of a luciferase reporter gene under the control of a T cell factor promoter, which is activated by endogenous β-catenin. Decorin significantly inhibited β-catenin activity in the presence or absence of LiCl (Fig. 6 G). The persistence of decorin activity in the presence of LiCl suggests that decorin evokes down-regulation of β-catenin independently of the canonical Wnt pathway, which requires GSK3β.
Figure 6.
Decorin attenuates β-catenin levels and transcriptional activity. (A–D) Representative β-catenin immunofluorescence images of HeLa cells after a 2-h incubation with or without 100 nM decorin. Notice the marked decline in β-catenin levels throughout the cytoplasm and perinuclear regions in the decorin-treated cells. In contrast, the plasma membrane localization of β-catenin increases (D, arrows). Nu, nucleus. Bars: (A and C) 50 µm; (B and D) 10 µm. (E) Quantification of the fluorescence intensity of images similar to those shown in A and C. The values represent the mean ± SEM of 12 images (∼120 cells/image) from four independent experiments. (F) Decorin inhibits β-catenin activity via a GSK3-β–independent mechanism. Representative β-catenin immunoblot of HeLa lysates treated with 100 nM decorin for 6 h in the presence or absence of 30 mM of the GSK3-β inhibitor LiCl. Cells were preincubated with LiCl for 1 h in full serum before decorin treatment. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. (G) HeLa cells were cotransfected with the TopFlash vector and a vector carrying the R. reniformis luciferase. 12 h after transfection, cells were treated with or without 100 nM decorin or LiCl for 6 h. Luciferase activity was measured after incubation with the cognate substrate luciferin. The values were normalized on R. reniformis luciferase activity. Cells preincubated with 30 mM LiCl, as in F, showed the same degree of reduction in luciferase activity when treated with decorin. Notice that LiCl enhances β-catenin activity (***, P < 0.001). The values represent the mean ± SEM of five independent experiments performed in triplicate. TCF, T cell factor.
Next, on the basis of our previous studies showing that decorin induces apoptosis of A431 (Seidler et al., 2006) and MTLn3 (Goldoni et al., 2008) cells, we tested whether the same would happen in HeLa cells and whether the Met receptor could be linked to decorin-evoked apoptosis. Decorin treatment stimulated significant PARP cleavage after 24 h, and this effect was blocked by SU11274 (Fig. S3 A). Moreover, decorin induced caspase-3/7 activity comparable with the levels induced by etoposide, an inhibitor of the topoisomerase II enzyme, and it did so in a Met-dependent manner (Fig. S3 B). VAD, a pancaspase inhibitor, was used to block decorin’s activity, and its effects can be compared to those evoked by the Met kinase inhibitor SU11274. These results suggest that Met phosphorylation is required for decorin-evoked apoptosis and reinforce our evidence that the Met receptor is a key player in decorin’s mechanism of action.
Decorin inhibits cell motility by a mechanism that involves both Met and EGFR
Deregulation of the Met receptor has been linked to the invasive behavior of tumor cells (Birchmeier et al., 2003). We have recently shown that decorin can prevent metastastic spreading to the lungs in a breast cancer model (Goldoni et al., 2008). In this study, we add an in vitro functional assay to support the relevance of our findings. HeLa cells were grown to confluency, “scratched” to allow motility, and treated with decorin for 24 h in the presence or absence of AG1478, H9786, a Met-blocking antibody, or the combination of both. Decorin significantly inhibited cell migration compared with control cells (Fig. S4). Both AG1478 and H9786, used alone, were effective but to a lesser extent than decorin. Interestingly, when decorin was used in combination with either inhibitor, it prevented wound closure even further than the individual compounds. When decorin was added in the presence of both inhibitors, it did not have any additional effect (Fig. S4, bottom). These data support the idea that both Met and EGFR are important to sustain cell migration and that decorin inhibits in vitro cell motility by a dual action on both receptors. Note that decorin is capable of down-regulating the Met receptor also in full-serum medium (Fig. S3 C), supporting the biological data regarding inhibition of the β-catenin pathway and cell migration, both performed in the presence of serum. In addition, once decorin is removed from the cells, Met expression is recovered over time, indicating that the cells are healthy and apoptosis is not occurring.
Discussion
The multifaceted ability of decorin to retard in vivo tumor growth and metastatic spreading has a mechanistic explanation in decorin’s ability to down-regulate multiple signaling pathways. We show for the first time that decorin is a novel endogenous antagonistic ligand of the Met receptor. Signaling mediated by HGF/Met axis promotes multiple biological activities, including survival, proliferation, motility/invasion, and angiogenesis (Trusolino and Comoglio, 2002; Birchmeier et al., 2003). Deregulation of the Met signaling pathway leads to uncontrolled growth and transformation, as shown by the TPR-Met, an oncogene that exhibits constitutive tyrosine kinase activation, and by activating mutations of Met intracellular domain in both hereditary and sporadic cancers (Gentile et al., 2008). Our results indicate that decorin is an inhibitor of multiple RTKs, insofar as it down-regulates the Met receptor as well as ErbB family members. The unique activity of decorin as a Met antagonist is manifested by a rapid induction of both Met receptor shedding and internalization with consequent downstream degradation of β-catenin, which is required for cell survival.
To date, there is only one known mammalian ligand of the Met receptor (i.e., HGF) and one bacterial protein, internalin B, which is synthesized and partly secreted by Lysteria monocytogenes. Internalin B, a protein containing seven leucine-rich tandem repeats with homology to decorin, mimics HGF-induced receptor trafficking (Li et al., 2005) and causes sustained activation of the Met receptor (Shen et al., 2000), leading to bacterial internalization into host cells (Shen et al., 2000; Ireton, 2007; Disson et al., 2008). The 213–amino acid leucine-rich repeat portion of internalin B is sufficient for entry into mammalian cells (Braun et al., 1999). The recent cocrystallization of internalin B with the ectodomain of the Met receptor has shown that internalin B complexes with the first Ig domain of the receptor (Niemann et al., 2007; Niemann et al., 2008). This interaction keeps Met in an active configuration while maintaining the flexibility in the Met semaphorin domain, where HGF binds with low affinity (Stamos et al., 2004). The interaction interface includes the concave part of the leucine-rich domain of internalin B and a loop that protrudes from the first Ig-like domain of the Met receptor (Niemann et al., 2008). Notably, several key aromatic amino acids within the concave face of internalin B are required for Met binding and internalization of the bacteria (Machner et al., 2003). These results have been confirmed in the aforementioned cocrystallization study (Niemann et al., 2007). Mutation of each of these residues (Fig. S5 A) abolishes binding to the Met receptor (Machner et al., 2003). Very importantly, a specific sequence of internalin B, encoding Y170 (required for Met binding) and surrounding residues, is highly analogous to a sequence of mammalian decorin (Fig. S5 B). This highly conserved motif suggests that both proteins have evolved to fulfill a common function, i.e., interacting with the Met receptor, albeit with divergent outcomes.
In contrast to internalin B, HGF binds with low affinity to the Met semaphorin domain (Stamos et al., 2004) and with high affinity to the terminal Ig3-4 (Basilico et al., 2008). This is in agreement with early biochemical experiments demonstrating that internalin B and HGF do not substantially compete for receptor occupancy (Shen et al., 2000). We discovered that decorin is readily displaced by HGF (IC50 = ∼2.3 nM) from binding to the immobilized Met ectodomain fused to the dimerizing Fc fragment. In contrast, internalin B was much less efficient in displacing decorin binding to Met-Fc because it required >50-fold higher concentrations (IC50 = ∼120 nM). These findings suggest that decorin binds to a similar location of the Met ectodomain where HGF binds with additional secondary sites overlapping with internalin B binding.
In spite of the fact that decorin mode of binding to the Met ectodomain is apparently similar to that of HGF, decorin evokes a profound antagonistic effect on the receptor signaling by inducing both physical and functional receptor down-regulation and by triggering apoptosis via induction of caspase-3/7 activity. Moreover, decorin causes Met-mediated down-regulation of β-catenin levels and transcriptional activity. It is well established that the Met receptor not only physically interacts with β-catenin on the cell surface but upon HGF binding, also phosphorylates β-catenin and triggers its translocation into the nucleus and consequent transcription of genes vital for cell proliferation and migration (Monga et al., 2002; Müller et al., 2002; Herynk et al., 2003; Ishibe et al., 2006; Rasola et al., 2007). Importantly, the Met receptor and β-catenin are engaged in a positive feedback loop that sustains tumor growth and invasion, where β-catenin drives Met receptor expression (Rasola et al., 2007). β-Catenin is a key player in Wnt signaling and plays a central role in cancer development (Clevers, 2006). For instance, β-catenin regulates both differentiation and proliferation of intestinal epithelial cells by enhancing the expression of genes, such as cyclin D1 and D4, associated with tumor progression. The ability of exogenous decorin to suppress β-catenin levels and transcriptional activity, coupled with the decorin-evoked translocation of β-catenin from the perinuclear to plasmalemmal compartments, suggests that decorin signaling affects the β-catenin pathway. Our data show that this effect is mediated through the Met pathway. A recent study using decorin-deficient mice has shown that ∼30% of these mutant mice develop intestinal tumors, a process that is accelerated and amplified when the decorin-deficient animals are subjected to a high risk diet (Bi et al., 2008). Notably, the endogenous β-catenin levels were markedly increased in the intestinal epithelium of the decorin-null mice, suggesting that lack of decorin is permissive for tumorigenesis, as we hypothesized previously (Iozzo et al., 1999a), thereby providing in vivo evidence that β-catenin might be regulated by extracellular signaling events evoked by decorin.
How does decorin induce protracted Met degradation? In the case of the EGFR, EGF but not TGF-α induces efficient receptor internalization and degradation. EGF remains closely linked to its receptor during clathrin-dependent endocytosis, whereas TGF-α rapidly dissociates from the receptor in the acidic microenvironment of early endosomes, resulting in receptor recycling (Schlessinger, 2000). Decorin causes a caveolar-mediated endocytosis of the EGFR, and even after 30 min, decorin and EGFR colocalize within late endocytic compartments and subsequently within lysosomes (Zhu et al., 2005). This mechanism might explain the lower levels of EGFR after decorin treatment due in part to a reduced receptor recycling to the surface. A similar scenario could occur with the Met receptor, although we have not formally shown that Met internalization and degradation occur via a caveolar-mediated endocytosis. This idea is supported by a recent study, which has shown that both internalin B and the leucine-rich repeats of internalin B, the region that shares analogy with decorin, are properly internalized and remain associated with Met during transit through early and late endosomes when provided as soluble ligands to HeLa cells (Gao et al., 2009). Thus, one possibility is that HGF/internalin B, as agonistic ligands for Met, are internalized via a clathrin-mediated pathway and in analogy with EGF/EGFR, clathrin-mediated internalization has been shown to be essential for sustained receptor signaling (Sigismund et al., 2008). In contrast, antagonistic ligands such as decorin could induce internalization via a caveolar-mediated pathway, leading to attenuated signaling and intracellular proteolysis of the receptor.
The ability of decorin to differentially phosphorylate Met receptor Tyr residues is fascinating. More investigation into this novel decorin mechanism of action will be needed in the future, and most likely, more information regarding the peculiar Met conformation induced by decorin binding will shed light onto the phosphorylation events described in this study. Notably, coactivation of RTKs affects the response of tumor cells to targeted therapies (Stommel et al., 2007), and amplification of the Met-encoding gene promotes drug resistance in ErbB-driven cancers (Engelman et al., 2007). Although in the past main efforts were aimed at developing highly specific inhibitors acting on single RTKs, more recently there has been a general consensus that molecules interfering simultaneously with multiple RTKs might be more effective than single target agents (Knudsen and Vande Woude, 2008). In this perspective, the activity of decorin, and perhaps of other molecules harboring leucine-rich repeats, might represent a novel therapeutic modality against metastatic cancer.
Materials and methods
Cell culture and materials
HeLa cells were obtained from American Type Culture Collection and maintained in DME (Mediatech) supplemented with 10% FBS (PAA Laboratories, Inc). Dulbecco’s phosphate buffer saline was purchased from Mediatech. Cell culture supplies were purchased from Thermo Fisher Scientific. The following antibodies were used: monoclonal mouse anti-Grb2, anti–β-catenin, antiphosphotyrosine HRP conjugated (BD), anti–β-actin (Sigma-Aldrich), anti-PARP (BD), monoclonal rabbit against EGFR-Tyr1173, Met-Tyr1234/5, Met-Tyr1003, Met-Tyr1349 (Cell Signaling Technology), polyclonal against Met C terminus (Santa Cruz Biotechnology, Inc.), Met N terminus (H9786 [Sigma-Aldrich] and AF276 [R&D Systems]), and HGF (Abcam). Lactacystin was purchased from Sigma-Aldrich. Anti–human decorin antibody (LC-001b) was provided by LifeCell Corporation. EGFR-blocking antibody mAb425 was provided by U. Rodeck (Thomas Jefferson University, Philadelphia, PA). The EGFR tyrosine kinase inhibitor, AG1478, was obtained from EMD. HRP-conjugated donkey anti–rabbit and sheep anti–mouse were purchased from Jackson ImmunoResearch Laboratories. Protein G– and A–Sepharose beads were obtained from GE Healthcare. S-SMPB and SuperSignal West Pico chemiluminescence substrate were purchased from Thermo Fisher Scientific. HGF, TIMP-2, and TIMP-3 were purchased from R&D Systems. Rat tail collagen type I was obtained from BD. Human recombinant decorin was expressed and purified as described previously (Zhu et al., 2005). Decorin harbors one glycosaminoglycan side chain and is fully glycosylated. Decorin protein core was obtained by expressing a mutant at the glycosaminoglycan chain attachment site. Decorin protein core is expressed in a mammalian cell system and is fully glycosylated.
Phospho-RTK arrays, time course experiments, and blocking experiments
Arrays were purchased from R&D Systems. Array membranes were incubated with cell lysates and processed as recommended by the manufacturer’s protocol using a Phospho-Tyr–specific antibody. Approximately 8 × 106 HeLa cells were either serum starved or maintained in full serum overnight and treated with 100 nM decorin for 15 min or left untreated. After decorin incubation, cells were washed with ice-cold PBS and lysed with a buffer containing 1% NP-40, 20 mM Tris-HCl, pH 8.0, 137 mM NaCl, 10% glycerol, 2 mM EDTA, 1 mM Na3VO4, 10 µg/ml aprotinin, and 10 µg/ml leupeptin for 30 min. Protein assay (Bio-Rad Laboratories) was performed before incubating the lysates with the RTK membranes. HeLa cells were serum starved overnight before treatment with 100 nM decorin for 5, 10, 15, 30, and 60 min. Cells were washed with ice-cold PBS and lysed in RIPA buffer. 10 µg/ml EGFR-blocking antibody mAb425 or 1 µM AG1478 were incubated with or without decorin. HGF was used at 1.5 nM. Lysates were resolved on an 8% SDS-PAGE. Cells were preincubated with mAb425 or AG1478 for 1 h before decorin treatment. Efficiency of mAb425 and AG1478 was measured by testing their ability to block EGFR phosphorylation evoked by EGF (16 nM for 5 min).
Cross-linking and immunoprecipitation
Cells were treated with or without 220 nM decorin protein core for 15 min and incubated with 0.5 mM of the noncleavable cross-linker S-SMPB for 20 min at 37°C (Zhu et al., 2005). At the end, reactions were quenched with a 90-mM glycine solution. Cells were washed with ice-cold PBS and lysed with an NP-40–containing buffer (as described in Phospho-RTK arrays, time course…). Cell extracts were subjected to immunoprecipitation with an anti–C terminus Met receptor antibody, separated on a 6% SDS-PAGE, and immunoblottings for Met and decorin were performed. Met was also immunoprecipitated to examine c-Cbl and Grb2 recruitment to the receptor before and after decorin treatment. Approximately 3 × 106 HeLa cells were serum starved overnight for this purpose.
Pull-down– and solid-phase–binding assays
Human Met-Fc chimera (Sigma-Aldrich) was bound to protein A–Sepharose beads (GE Healthcare). 2 µg human Met/Fc chimera was added to 20 µl protein A–Sepharose beads. After an overnight incubation with rotation at 4°C, the beads were extensively washed with PBS and resuspended in 400 µl of serum-free medium containing Complete Mini protease inhibitor (Roche). The mixture was incubated with equimolar amounts of various ligands at 37°C for 3 h. The beads were collected by centrifugation, extensively washed with a buffer containing 0.1% Triton X-100, boiled in reducing sample buffer, and subjected to electrophoresis on an 8% SDS-PAGE. In these experiments, antibodies against human HGF (Abcam) and decorin (Santa Cruz Biotechnology, Inc.) were used. ELISAs were performed following a standard protocol. The substrates, either Met-Fc (100 ng/well) or neutralized fibrillar collagen type I (1 mg/ml; 50 µl/well), were allowed to adhere to the wells (BD) overnight at room temperature in the presence of carbonate buffer, pH 9.6. Plates were washed with PBS and incubated for 3 h with serial dilutions of decorin or decorin core. In the competition experiments, decorin core was kept at constant concentration (10 nM) and incubated with increasing concentrations of either internalin B or HGF. After ligand incubation, plates were extensively washed with PBS, blocked with 1% BSA solution in PBS, and incubated with primary and HRP-conjugated secondary antibodies. Signal was developed using Sigma-Fast tablets (Sigma-Aldrich) and read at 450-nm OD. To correct for antibody affinity, the values obtained were converted to bound ligand (nanomolars) by performing separate ELISA experiments using increasing amounts of each ligand and extrapolating from the generated standard curves.
Ectodomain shedding and slot blot
HeLa cells were serum starved overnight and treated with 100 nM decorin for 5–30 min. Conditioned media from decorin-treated cells and controls were collected, slot blotted, and probed for the N-terminal domain of the Met receptor. For the inhibition of shedding experiments, 1 µM TIMP-2 and TIMP-3 were incubated for 30 min before decorin treatment (100 nM for 30 min). Both lysates and media were collected and analyzed by Western analysis and slot blot, respectively. Lysates were probed with a Met antibody recognizing the intracellular domain of the receptor, whereas media with an antibody raised against the Met extracellular domain.
Met internalization experiments
Cells for immunofluorescence were grown on chamber glass slides, treated with decorin, washed with PBS, fixed in ice-cold methanol for 5 min, and stained according to standard procedures. To detect Met, the AF276 antibody (R&D Systems) raised against the N terminus domain of the receptor was used followed by an FITC-conjugated donkey anti–goat antibody (Santa Cruz Biotechnology, Inc.). Images were acquired on a laser-scanning confocal microscopy system (LSM 510 META; Carl Zeiss Inc.) driven by imaging software (LSM 510; Carl Zeiss, Inc.). 63× magnification was used with a 1.25 objective lens aperture. Confocal image processing, including z stacks, was performed with ImageJ (National Institutes of Health). Contrast enhancement was applied uniformly to all panels. A microscope (BX51; Olympus) driven by SPOT Advanced imaging software (version 4.0.9; Diagnostic Instruments, Inc.) was used to acquire fluorescence images with 40× magnification and 0.75 aperture. FITC signal was acquired at 25°C. Vectashield mounting medium was purchased from Vector Laboratories. Approximately 8 × 106 HeLa cells were serum starved for 1 h before decorin treatment (100 nM for 5 and 30 min). Cells were trypsinized with 0.2% trypsin (Cellgro) for 5 min at 37°C and pelleted by centrifugation at 300 g for 5 min. The pellet was dissolved in RIPA buffer, and samples were run on SDS-PAGE.
β-Catenin experiments and migration assays
For the β-catenin experiments, subconfluent HeLa cells in DME full serum were used. Cells were treated with 100 nM decorin from 30 min to 24 h. For the GSK3-β inhibition experiments, cells were incubated with 30 mM LiCl for 1 h before decorin treatment. Cells for immunofluorescence were grown on chamber slides, treated with decorin, fixed in ice-cold methanol for 5 min, and stained according to standard procedures. To detect the β-catenin signal, a rhodamine-conjugated goat anti–mouse antibody was used (Santa Cruz Biotechnology, Inc.). To study the effect of decorin on β-catenin transcriptional activity, we used the TopFlash luciferase reporter vector (Addgene). TopFlash vector was provided by M. Pacifici (Thomas Jefferson University, Philadelphia, PA). Subconfluent HeLa cells in 12-well plates were transfected overnight with TopFlash and a Renilla reniformis luciferase reporter vector (phRL-TK; Promega) as transfection control in the ratio 10:1 (TopFlash:R. reniformis) using Lipofectamine2000 (Invitrogen). The next day, media were changed and cells were treated with or without 30 mM LiCl for 1 h before decorin (100 nM) stimulation for 6 h. Cells were lysed, and the luciferase activity was measured using a dual luciferase assay kit (Promega). The FopFlash mutant was used vis à vis the TopFlash vector as negative control to evaluate the background signal. No luciferase activity was observed with the FopFlash vector.
For migration assays, HeLa cells were grown to confluency in 12-well plates and scratched with a pipette tip. Cells were incubated for 24 h with or without 100 nM decorin, 1 µM AG1478, and 2 µg/ml Met-blocking antibody H9786 in full serum. Blocking agents were incubated for 1 h before decorin treatment. An inverted phase-contrast microscope (IM; Olympus) with 10× magnification and 0.25 aperture was used. Pictures were taken over time with a digital microscope camera (DP12; Olympus).
Quantification and statistical analysis
Immunoblots were quantified by scanning densitometry using Scion Image software (National Institutes of Health). Graphs were generated using SigmaStat (version 3.10; Systat Software, Inc.). Significance of the differences was evaluated by Student’s t test. Fluorescence intensity was quantified by measuring pixels with ImageJ software. In the scratch assay, wound closure was measured with ImageJ. The mean of three linear distances between the two edges of the wound was measured. Three wounds per condition were analyzed. Three independent experiments were run. All data presented were collected from three independent experiments run in triplicates or quadruplicates.
Online supplemental material
Fig. S1 shows a representative HGF/Met-Fc solid-phase binding curve and negative controls for the binding experiments, decorin/IgG, and LG3/Met-Fc. Fig. S2 shows decorin-induced Met ectodomain shedding and Met internalization after decorin binding. Fig. S3 shows induction of apoptosis downstream of decorin-evoked Met phosphorylation and down-regulation of Met by decorin treatment in full serum followed by recovery of Met expression upon decorin withdrawal. Fig. S4 shows a motility assay. Fig. S5 presents the 3D structure of internalin B leucine-rich repeats and a key portion of the sequence alignment with decorin. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200901129/DC1.
Acknowledgments
We thank U. Rodeck for providing the mAb425 and M. Pacifici for the TopFlash vector. We also thank M. Sobkowiak and A. Kelsey for valuable help, George Purkins with 3D modeling, and A. McQuillan for help with the graphics.
This work was supported in part by the National Institutes of Health grants (RO1 CA39481, RO1 CA47282, and RO1 CA120975 to R.V. Iozzo) and a Canadian Institutes of Health Research grant (MT-15497 to K. Ireton).
Footnotes
Abbreviations used in this paper: EGFR, EGF receptor; HGF, hepatocyte growth factor; IC, inhibitory concentration; PARP, poly ADP-ribose polymerase; RTK, receptor tyrosine kinase.
References
- Aberle H., Bauer A., Stappert J., Kispert A., Kemler R. 1997. β-catenin is a target for the ubiquitin-proteasome pathway.EMBO J. 16:3797–3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athauda G., Giubellino A., Coleman J.A., Horak C., Steeg P.S., Lee M.-J., Trepel J., Wimberly J., Sun J., Coxon A., et al. 2006. c-Met ectodomain shedding rate correlates with malignant potential.Clin. Cancer Res. 12:4154–4162 [DOI] [PubMed] [Google Scholar]
- Basilico C., Arnesano A., Galuzzo M., Comoglio P.M., Michieli P. 2008. A high affinity hepatocyte growth factor-binding site in the immunoglobulin-like region of Met.J. Biol. Chem. 283:21267–21277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthou S., Aebersold D.M., Schimdt L.S., Stroka D., Heigl C., Streit B., Stalder D., Gruber G., Liang S., Howlett A.R., et al. 2004. The Met kinase inhibitor SU11274 exhibits a selective inhibition pattern toward different receptor mutated variants.Oncogene. 23:5387–5393 [DOI] [PubMed] [Google Scholar]
- Bi X., Tong C., Dokendorff A., Banroft L., Gallagher L., Guzman-Hartman G., Iozzo R.V., Augenlicht L.H., Yang W. 2008. Genetic deficiency of decorin causes intestinal tumor formation through disruption of intestinal cell maturation.Carcinogenesis. 29:1435–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biglari A., Bataille D., Naumann U., Weller M., Zirger J., Castro M.G., Lowenstein P.R. 2004. Effects of ectopic decorin in modulating intracranial glioma progression in vivo, in a rat syngeneic model.Cancer Gene Ther. 11:721–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchmeier C., Birchmeier W., Gherardi E., Vande Woude G.F. 2003. MET, metastasis, motility and more.Nat. Rev. Mol. Cell Biol. 4:915–925 [DOI] [PubMed] [Google Scholar]
- Brandan E., Cabello-Verrugio C., Vial C. 2008. Novel regulatory mechanisms for the proteoglycans decorin and biglycan during muscle formation and muscular dystrophy.Matrix Biol. 27:700–708 [DOI] [PubMed] [Google Scholar]
- Braun L., Nato F., Payrastre B., Mazié J.-C., Cossart P. 1999. The 213-amino-acid leucine-rich repeat region of the Listeria monocytogenes lnlB protein is sufficient for entry into mammalian cells, stimulation of Pl 3-kinase and membrane ruffling.Mol. Microbiol. 34:10–23 [DOI] [PubMed] [Google Scholar]
- Clevers H. 2006. Wnt/β-catenin signaling in development and disease.Cell. 127:469–480 [DOI] [PubMed] [Google Scholar]
- Csordás G., Santra M., Reed C.C., Eichstetter I., McQuillan D.J., Gross D., Nugent M.A., Hajnóczky G., Iozzo R.V. 2000. Sustained down-regulation of the epidermal growth factor receptor by decorin. A mechanism for controlling tumor growth in vivo.J. Biol. Chem. 275:32879–32887 [DOI] [PubMed] [Google Scholar]
- Danilkovitch-Miagkova A., Zbar B. 2002. Dysregulation of Met receptor tyrosine kinase activity in invasive tumors.J. Clin. Invest. 109:863–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disson O., Grayo S., Huillet E., Nikitas G., Langa-Vives F., Dussurget O., Ragon M., Le Monnier A., Babinet C., Cossart P., Lecuit M. 2008. Conjugated action of two species-specific invasion proteins for fetoplacental listeriosis.Nature. 455:1114–1118 [DOI] [PubMed] [Google Scholar]
- Engelman J.A., Zejnullahu K., Mitsudomi T., Song Y., Hyland C., Park J.O., Lindeman N., Gale C.-M., Zhao X., Christensen J., et al. 2007. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling.Science. 316:1039–1043 [DOI] [PubMed] [Google Scholar]
- Gao X., Lorinczi M., Hill K.S., Brooks N.C., Dokainish H., Ireton K., Elferink L.A. 2009. Met receptor tyrosine kinase degradation is altered in response to the leucine-rich repeat of the Lysteria invasion protein internalin B.J. Biol. Chem. 284:774–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile A., Trusolino L., Comoglio P.M. 2008. The Met tyrosine kinase receptor in development and cancer.Cancer Metastasis Rev. 27:85–94 [DOI] [PubMed] [Google Scholar]
- Goldoni S., Iozzo R.V. 2008. Tumor microenvironment: modulation by decorin and related molecules harboring leucine-rich tandem motifs.Int. J. Cancer. 123:2473–2479 [DOI] [PubMed] [Google Scholar]
- Goldoni S., Seidler D.G., Heath J., Fassan M., Baffa R., Thakur M.L., Owens R.A., McQuillan D.J., Iozzo R.V. 2008. An anti-metastatic role for decorin in breast cancer.Am. J. Pathol. 173:844–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant D.S., Yenisey C., Rose R.W., Tootell M., Santra M., Iozzo R.V. 2002. Decorin suppresses tumor cell-mediated angiogenesis.Oncogene. 21:4765–4777 [DOI] [PubMed] [Google Scholar]
- Hammond D.E., Carter S., McCullough J., Urbé S., Van Woude G., Clague M.J. 2003. Endosomal dynamics of Met determine signaling output.Mol. Biol. Cell. 14:1346–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herynk M.H., Tsan R., Radinsky R., Gallick G.E. 2003. Activation of c-Met in colorectal carcinoma cells leads to constitutive association of tyrosine-phopshorylated β-catenin.Clin. Exp. Metastasis. 20:291–300 [DOI] [PubMed] [Google Scholar]
- Iozzo R.V. 1998. Matrix proteoglycans: from molecular design to cellular function.Annu. Rev. Biochem. 67:609–652 [DOI] [PubMed] [Google Scholar]
- Iozzo R.V. 2005. Basement membrane proteoglycans: from cellar to ceiling.Nat. Rev. Mol. Cell Biol. 6:646–656 [DOI] [PubMed] [Google Scholar]
- Iozzo R.V., Chakrani F., Perrotti D., McQuillan D.J., Skorski T., Calabretta B., Eichstetter I. 1999a. Cooperative action of germline mutations in decorin and p53 accelerates lymphoma tumorigenesis.Proc. Natl. Acad. Sci. USA. 96:3092–3097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iozzo R.V., Moscatello D., McQuillan D.J., Eichstetter I. 1999b. Decorin is a biological ligand for the epidermal growth factor receptor.J. Biol. Chem. 274:4489–4492 [DOI] [PubMed] [Google Scholar]
- Ireton K. 2007. Entry of the bacterial Listeria monocytogenes into mammalian cells.Cell. Microbiol. 9:1365–1375 [DOI] [PubMed] [Google Scholar]
- Ishibe S., Haydu J.E., Togawa A., Marlier A., Cantley L.G. 2006. Cell confluence regulates hepatocyte growth factor-stimulated cell morphogenesis in a β-catenin-dependent manner.Mol. Cell. Biol. 26:9232–9243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo M., Stolz D.B., Esplen J.E., Dorko K., Michalopoulos G.K., Strom S.C. 2000. Cross-talk between epidermal growth factor receptor and c-Met signal pathways in transformed cells.J. Biol. Chem. 275:8806–8811 [DOI] [PubMed] [Google Scholar]
- Klein P.S., Melton D.A. 1996. A molecular mechanism for the effect of lithium on development.Proc. Natl. Acad. Sci. USA. 93:8455–8459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen B.S., Vande Woude G. 2008. Showering c-MET-dependent cancers with drugs.Curr. Opin. Genet. Dev. 18:87–96 [DOI] [PubMed] [Google Scholar]
- Levitzki A., Gazit A. 1995. Tyrosine kinase inhibition: an approach to drug development.Science. 267:1782–1788 [DOI] [PubMed] [Google Scholar]
- Li N., Xiang G.-S., Dokainish H., Ireton K., Elferink L.A. 2005. The Listeria protein internalin B mimics hepatocyte growth factor-induced receptor trafficking.Traffic. 6:459–473 [DOI] [PubMed] [Google Scholar]
- Li N., Lorinczi M., Ireton K., Elferink L.A. 2007. Specific Grb2-mediated interactions regulate clathrin-dependent endocytosis of the cMet-tyrosine kinase.J. Biol. Chem. 282:16764–16775 [DOI] [PubMed] [Google Scholar]
- Li X., Huang Y., Jiang J., Frank S.J. 2008a. ERK-dependent threonine phosphorylation of EGF receptor modulates receptor downregulation and signaling.Cell. Signal. 20:2145–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Pennisi A., Yaccoby S. 2008b. Role of decorin in the antimyeloma effects of osteoblasts.Blood. 112:159–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machner M.P., Frese S., Schubert W.-D., Orian-Rousseau V., Gherardi E., Wehland J., Niemann H.H., Heinz D.W. 2003. Aromatic amino acids at the surface of InIB are essential for host cell invasion by Listeria monocytogenes.Mol. Microbiol. 48:1525–1536 [DOI] [PubMed] [Google Scholar]
- Monga S.P., Mars W.M., Pediaditakis P., Bell A., Mulé K., Bowen W.C., Wang X., Zarnegar R., Michalopoulos G.K. 2002. Hepatocyte growth factor induces Wnt-independent nuclear translocation of beta-catenin after MET-beta-catenin dissociation in hepatocytes.Cancer Res. 62:2064–2071 [PubMed] [Google Scholar]
- Müller T., Bain G., Wang X., Papkoff J. 2002. Regulation of epithelial cell migration and tumor formation by β-catenin signaling.Exp. Cell Res. 280:119–133 [DOI] [PubMed] [Google Scholar]
- Nath D., Williamson N.J., Jarvis R., Murphy G. 2001. Shedding of c-Met is regulated by crosstalk between a G-protein coupled receptor and the EGF receptor and is mediated by a TIMP-3 sensitive metalloproteinase.J. Cell Sci. 114:1213–1220 [DOI] [PubMed] [Google Scholar]
- Niemann H.H., Jäger V., Butler P.J.G., van den Heuvel J., Schmidt S., Ferraris D., Gherardi E., Heinz D.W. 2007. Structure of the human receptor tyrosine kinase Met in complex with the Listeria invasion protein lnlB.Cell. 130:235–246 [DOI] [PubMed] [Google Scholar]
- Niemann H.H., Petoukhov M.V., Härtlein M., Moulin M., Gherardi E., Timmins P., Heinz D.W., Svergun D.I. 2008. X-ray and neutron small-angle scattering analysis of the complex formed by the Met receptor and the Listeria monocytogenes invasion protein InIB.J. Mol. Biol. 377:489–500 [DOI] [PubMed] [Google Scholar]
- Petit A.M.V., Rak J., Hung M.-C., Rockwell P., Goldstein N., Fendly B., Kerbel R.S. 1997. Neutralizing antibodies against epidermal growth factor and Erb-2/neu receptor tyrosine kinases down-regulated vascular endothelial growth factor production by tumor cells in vitro and in vivo. Angiogenic implications for signal transduction therapy of solid tumors.Am. J. Pathol. 151:1523–1530 [PMC free article] [PubMed] [Google Scholar]
- Petrelli A., Gilestro G.F., Lanzardo S., Comoglio P.M., Migone N., Giordano S. 2002. The endophilin-CIN85-Cbl complex mediates ligand-dependent downregulation of c-Met.Nature. 416:187–190 [DOI] [PubMed] [Google Scholar]
- Petrelli A., Circosta P., Granziero L., Mazzone M., Pisacane A., Fenoglio S., Comoglio P.M., Giordano S. 2006. Ab-induced ectodomain shedding mediates hepatocyte growth factor receptor down-regulation and hampers biological activity.Proc. Natl. Acad. Sci. USA. 103:5090–5095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez F., Rifkin D.B. 2003. Cell signaling events: a view from the matrix.Matrix Biol. 22:101–107 [DOI] [PubMed] [Google Scholar]
- Rasola A., Fassetta M., De Bacco F., D’Alessandro L., Gramaglia D., Di Renzo M.G., Comoglio P.M. 2007. A positive feedback loop between hepatocyte growth factor receptor and β-catenin sustains colorectal cancer cell invasive growth.Oncogene. 26:1078–1087 [DOI] [PubMed] [Google Scholar]
- Reed C.C., Gauldie J., Iozzo R.V. 2002. Suppression of tumorigenicity by adenovirus-mediated gene transfer of decorin.Oncogene. 21:3688–3695 [DOI] [PubMed] [Google Scholar]
- Reed C.C., Waterhouse A., Kirby S., Kay P., Owens R.A., McQuillan D.J., Iozzo R.V. 2005. Decorin prevents metastatic spreading of breast cancer.Oncogene. 24:1104–1110 [DOI] [PubMed] [Google Scholar]
- Reznik T.E., Sang Y., Ma Y., Abounader R., Rosen E.M., Xia S., Laterra J. 2008. Transcription-dependent epidermal growth factor receptor activation by hepatocyte growth factor.Mol. Cancer Res. 6:139–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodeck U., Herlyn M., Herlyn D., Molthoff C., Atkinson B., Varello M., Steplewski Z., Koprowski H. 1987. Tumor growth modulation by a monoclonal antibody to the epidermal growth factor receptor: immunologically mediated and effector cell-independent effects.Cancer Res. 47:3692–3696 [PubMed] [Google Scholar]
- Santra M., Skorski T., Calabretta B., Lattime E.C., Iozzo R.V. 1995. De novo decorin gene expression suppresses the malignant phenotype in human colon cancer cells.Proc. Natl. Acad. Sci. USA. 92:7016–7020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santra M., Eichstetter I., Iozzo R.V. 2000. An anti-oncogenic role for decorin: downregulation of ErbB2 leads to growth suppression and cytodifferentiation of mammary carcinoma cells.J. Biol. Chem. 275:35153–35161 [DOI] [PubMed] [Google Scholar]
- Schaefer L., Iozzo R.V. 2008. Biological functions of the small leucine-rich proteoglycans: from genetics to signal transduction.J. Biol. Chem. 283:21305–21309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J. 2000. Cell signaling by receptor tyrosine kinases.Cell. 103:211–225 [DOI] [PubMed] [Google Scholar]
- Seidler D.G., Goldoni S., Agnew C., Cardi C., Thakur M.L., Owens R.A., McQuillan D.J., Iozzo R.V. 2006. Decorin protein core inhibits in vivo cancer growth and metabolism by hindering epidermal growth factor receptor function and triggering apoptosis via caspase-3 activation.J. Biol. Chem. 281:26408–26418 [DOI] [PubMed] [Google Scholar]
- Shen Y., Naujokas M., Park M., Ireton K. 2000. lnlB-dependent internalization of Listeria is mediated by the Met receptor tyrosine kinase.Cell. 103:501–510 [DOI] [PubMed] [Google Scholar]
- Shintani K., Matsumine A., Kusuzaki K., Morikawa J., Matsubara T., Wakabayashi T., Araki K., Satonaka H., Wakabayashi H., Lino T., Uchida A. 2008. Decorin suppresses lung metastases of murine osteosarcoma.Oncol. Rep. 19:1533–1539 [PubMed] [Google Scholar]
- Sigismund S., Argenzio E., Tosoni D., Cavallaro E., Polo S., Di Fiore P.P. 2008. Clathrin-mediated internalization is essential for sustained EGFR signaling but dispensable for degradation.Dev. Cell. 15:209–219 [DOI] [PubMed] [Google Scholar]
- Stamos J., Lazarus R.A., Yao X., Kirchhofer D., Wiesmann C. 2004. Crystal structure of the HGF β-chain in complex with the Sema domain of the Met receptor.EMBO J. 23:2325–2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stommel J.M., Kimmelman A.C., Ying H., Nabioullin R., Ponugoti A.H., Wiedemeyer R., Stegh A.H., Bradner J.E., Ligon K.L., Brennan C., et al. 2007. Coactivation of receptor tyrosine kinases affects the response of tumor cells to target therapies.Science. 318:287–290 [DOI] [PubMed] [Google Scholar]
- Tralhão J.G., Schaefer L., Micegova M., Evaristo C., Schönherr E., Kayal S., Veiga-Fernandes H., Danel C., Iozzo R.V., Kresse H., Lemarchand P. 2003. In vivo selective and distant killing of cancer cells using adenovirus-mediated decorin gene transfer.FASEB J. 17:464–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trusolino L., Comoglio P.M. 2002. Scatter-factor and semaphorin receptors: cell signalling for invasive growth.Nat. Rev. Cancer. 2:289–301 [DOI] [PubMed] [Google Scholar]
- Weigelt B., Bissell M.J. 2008. Unraveling the microenvironmental influences on the normal mammary gland and breast cancer.Semin. Cancer Biol. 18:311–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafiropoulos A., Nikitovic D., Katonis P., Tsatsakis A., Karamanos N.K., Tzanakakis G.N. 2008. Decorin-induced growth inhibition is overcome through protracted expression and activation of epidermal growth factor receptors in osteosarcoma cells.Mol. Cancer Res. 6:785–794 [DOI] [PubMed] [Google Scholar]
- Zhu J.-X., Goldoni S., Bix G., Owens R.A., McQuillan D., Reed C.C., Iozzo R.V. 2005. Decorin evokes protracted internalization and degradation of the EGF receptor via caveolar endocytosis.J. Biol. Chem. 280:32468–32479 [DOI] [PubMed] [Google Scholar]