Abstract
Vascular endothelial protein tyrosine phosphatase (VE-PTP) is an endothelial-specific receptor-type tyrosine phosphatase that associates with Tie-2 and VE-cadherin. VE-PTP gene disruption leads to embryonic lethality, vascular remodeling defects, and enlargement of vascular structures in extraembryonic tissues. We show here that antibodies against the extracellular part of VE-PTP mimic the effects of VE-PTP gene disruption exemplified by vessel enlargement in allantois explants. These effects require the presence of the angiopoietin receptor Tie-2. Analyzing the mechanism we found that anti–VE-PTP antibodies trigger endocytosis and selectively affect Tie-2–associated, but not VE-cadherin–associated VE-PTP. Dissociation of VE-PTP triggers the activation of Tie-2, leading to enhanced endothelial cell proliferation and enlargement of vascular structures through activation of Erk1/2. Importantly, the antibody effect on vessel enlargement is also observed in newborn mice. We conclude that VE-PTP is required to balance Tie-2 activity and endothelial cell proliferation, thereby controlling blood vessel development and vessel size.
Introduction
The formation of the blood vessel system during embryonic development requires a multitude of receptors and adhesion molecules that regulate the creation of the first primitive vascular plexus (vasculogenesis) and the various remodeling processes that lead to the establishment of the mature vascular system. Several of the receptors involved in these processes represent tyrosine kinases such as the receptors for VEGF and the Tie-2 receptor. Whereas VEGFR-2 is essential for vasculogenesis and sprouting of nascent blood vessels, Tie-2 is important for subsequent remodeling processes. Tie-2 is a receptor for the angiopoietins, of which Ang1 promotes vascular remodeling, maturation, and stabilization of the vasculature. Tie-2 knock-out mouse embryos die by E10.5 due to endocardial defects, hemorrhaging, and impaired vascular network formation (Dumont et al., 1994; Sato et al., 1995), similar to the defects of Ang1-null mice that die around E12.5, showing comparable deficits in vascular remodeling, maturation, and stabilization of blood vessels (Suri et al., 1996). In contrast, overexpression of the Tie-2 ligand Ang2 mimics the defects caused by Ang1 and Tie-2 ablation (Maisonpierre et al., 1997). This argues for an antagonistic function of Ang2 and illustrates the need to precisely balance the activation level of the Tie-2 receptor system during embryonic development.
Tyrosine phosphatases are obvious candidates for signaling molecules that counteract the activation of tyrosine kinase receptors. Very few receptor-type protein tyrosine phosphatases (RPTPs) are known as regulators of angiogenesis. A mutated form of density-enhanced phosphatase (DEP-1, CD148), with the phosphatase domain being replaced by the chromophore GFP caused embryonic lethality due to vascular malformations (Takahashi et al., 2003), and DEP-1 was found to be involved in arterial/venous specification in zebrafish (Rodriguez et al., 2008). Surprisingly, DEP-1 gene ablation in mice does not cause obvious defects during embryonic angiogenesis or embryonic lethality (Trapasso et al., 2006; Zhu et al., 2008).
In contrast to DEP-1, the vascular endothelial protein tyrosine phosphatase (VE-PTP) is an endothelial-specific RPTP (Fachinger et al., 1999). Deletion of its cytoplasmic phosphatase domain, the transmembrane region, and the most membrane-proximal extracellular fibronectin type III-like repeat causes embryonic lethality shortly before 10 d of gestation, accompanied by dramatically enlarged blood vessels in the yolk sac, which form large cavities (Baumer et al., 2006). Formation of the vascular plexus was generally not affected throughout the embryo, yet remodeling was defective. Explants of allantois tissue developed large endothelial sacs instead of the usual tubular vascular network. In addition, heart development was defective (Baumer et al., 2006). Defects essentially identical to the VE-PTP truncation mutants were observed in mice carrying a null allele of the VE-PTP gene (Dominguez et al., 2007).
The molecular and cellular mechanisms that cause the observed angiogenesis defects in VE-PTP mutant mice are unknown. VE-PTP was found to associate with two endothelial cell surface membrane proteins essential for angiogenesis. The first one was Tie-2, which was found to bind to the cytoplasmic phosphatase domain of VE-PTP. Co-expression with VE-PTP in transfected cells reduced tyrosine phosphorylation of Tie-2 (Fachinger et al., 1999; Saharinen et al., 2008). Interestingly, no such interactions were found between VE-PTP and VEGFR-2. Whether physiological functions of Tie-2 in angiogenesis are affected by VE-PTP has not been analyzed previously.
A second association partner of VE-PTP is the endothelial-specific VE-cadherin (Nawroth et al., 2002). This association is mediated via the extracellular domains of both membrane proteins. We have shown that induction of VE-PTP expression in cells cotransfected with VE-cadherin enhances the adhesive function of VE-cadherin in these cells (Nawroth et al., 2002). Recently, we found that silencing of VE-PTP expression in endothelial cells indeed strongly reduced the adhesive function of VE-cadherin, demonstrating the importance of VE-PTP in regulating VE-cadherin function in endothelial cells. (Nottebaum et al., 2008).
Here we show that antibodies against the extracellular domains of VE-PTP cause vessel enlargement in allantois explants resembling the defects in vascular remodeling caused by VE-PTP gene disruption. These effects required Tie-2, as they were completely abolished by lack of Tie-2. We show that mechanistically, this is due to the anti–VE-PTP antibodies triggering selective endocytosis of only the Tie-2–associated, but not the VE-cadherin–associated VE-PTP protein fraction. VE-PTP dissociation from Tie-2 led to activation of Tie-2, thus enhancing endothelial cell proliferation and enlargement of vascular structures via activation of Erk1/2. Importantly, the antibody effect on vessel enlargement could be confirmed in vivo in 1–2-wk-old mice. Our results show that VE-PTP is required to control Tie-2 activity and endothelial cell proliferation during vascular remodeling in the embryo and in newly born mice.
Results
Antibodies against VE-PTP stimulate vessel enlargement in allantois explants
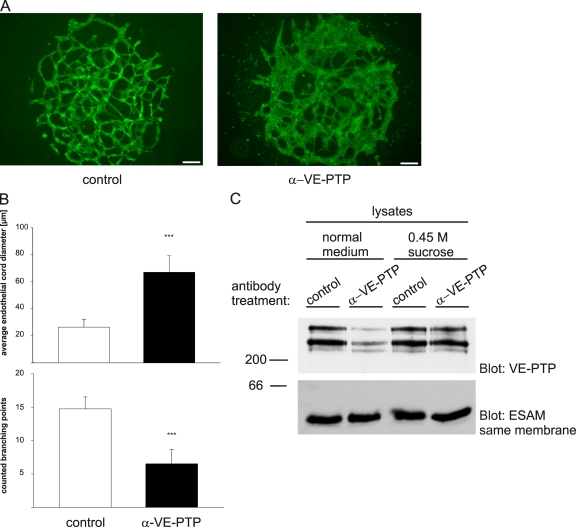
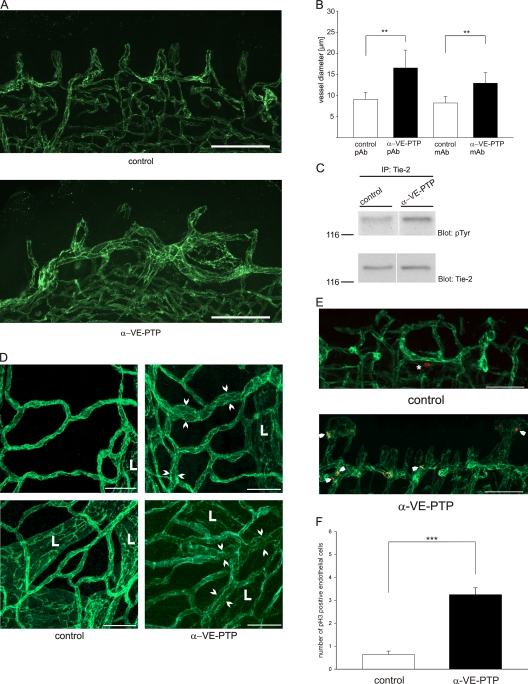
We have shown recently that disruption of the VE-PTP gene causes defects in vascular remodeling and leads to dramatically enlarged endothelial structures in allantois explants (Baumer et al., 2006). To elucidate the mechanism underlying this aberration, we tested whether antibodies against the extracellular domain of VE-PTP would be able to mimic this effect. Indeed, we found that such antibodies mimicked, when added for 22 h to cultured allantois explants derived from wild-type mice, the effect observed in explant cultures of VE-PTP mutant allantoides. As shown in Fig. 1 A, endothelial structures visualized by staining for VE-cadherin were enlarged upon incubation with anti–VE-PTP antibodies, resulting in a more than 2.5-fold increase in average diameter of endothelial cords compared with allantoides treated with preimmune antibodies (Fig. 1 B). Determining cord diameters is explained in LaRue et al. (2003). The number of branching points was reduced by more than twofold (Fig. 1 B). Endothelial cell contacts were not affected, as demonstrated by staining for the junctional proteins VE-cadherin and endothelial cell selective adhesion molecule (ESAM) (Fig. S1). The rat monoclonal antibody (mAb) 109.1 against mouse VE-PTP had a similar effect, whereas no effect was seen with a control mAb against the endothelial antigen ESAM (Fig. S1).
Figure 1.
Antibodies against VE-PTP trigger vessel enlargement in allantois explants and down-regulate VE-PTP. (A) Allantois explants from E8.5 wild-type embryos were cultured on gelatin-coated ultrathin glass slides in the presence of polyclonal antibodies against VE-PTP (α-VE-PTP) or preimmune antibodies (control) for 22 h and subsequently stained by indirect immunofluorescence with a monoclonal antibody for VE-cadherin. Bar, 50 µm. (B) Average endothelial cord diameters (top) and branching points (bottom) were determined for 5 control and 5 anti–VE-PTP treated allantois explants, with 30 randomly chosen vessels per explant (as shown in A); ***, P < 0,001. (C) Confluent mouse bEnd.5 cells were treated with polyclonal antibodies against VE-PTP or preimmune antibodies for 1 h either in normal culture medium or 0.45 M sucrose containing medium (as indicated) to block endocytosis. Aliquots of cell lysates with identical protein content were immunoblotted for VE-PTP and equal loading was controlled by blotting for the endothelial antigen ESAM (as indicated on the right). Molecular weight markers are indicated on the left.
Analyzing whether the antibodies against VE-PTP would affect the expression level of VE-PTP, we found that incubation of cultured mouse bEnd.5 endothelioma cells with antibodies against the extracellular part of VE-PTP for 1 h strongly reduced the amount of VE-PTP as tested in immunoblots with antibodies against the C terminus (Fig. 1 C). Blocking endocytosis by adding 0.45M sucrose to the culture medium while incubating with the antibodies prevented the reduction of VE-PTP protein, indicating that the anti–VE-PTP antibodies down-regulated the levels of VE-PTP by triggering endocytosis (Fig. 1 C).
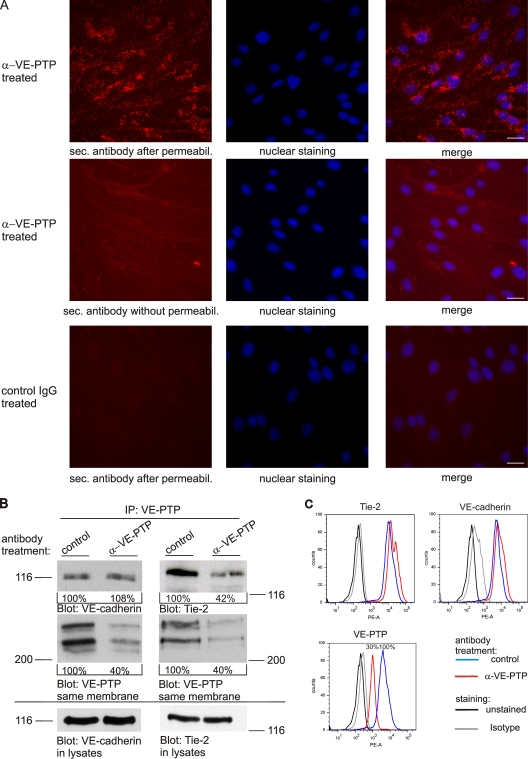
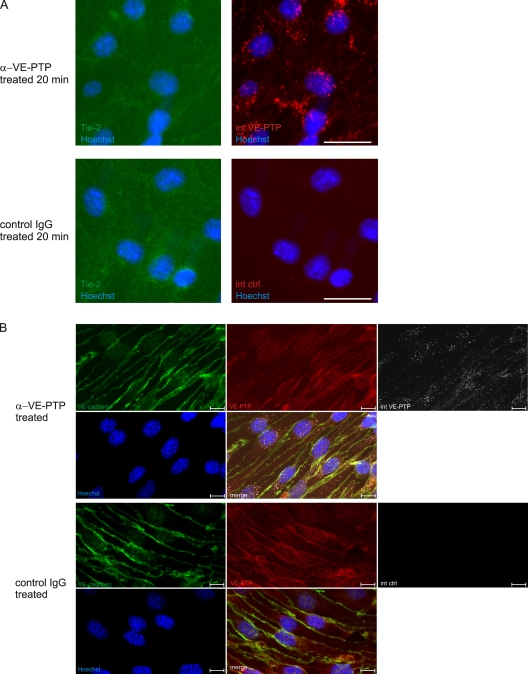
Endocytosis of VE-PTP induced by antibodies could be demonstrated by immunofluorescence. As shown in Fig. 2 A, preincubation of endothelioma cells with antibodies against the extracellular part of VE-PTP for 20 min followed by fixation, permeabilization, and incubation with a labeled secondary antibody indeed allowed to detect VE-PTP in granules inside the cells. No such staining was observed when cells were not permeabilized or when cells were preincubated with preimmune antibodies. After 10 min of antibody preincubation, VE-PTP colocalized partially with the early endosomal marker EEA1 (Fig. S2 A). After 90 min of preincubation time intracellular VE-PTP staining was no longer detectable (Fig. S2, B and C). We conclude that anti–VE-PTP antibodies trigger endocytosis of VE-PTP molecules. Endocytosis of VE-PTP was also observed in allantois explant cultures (Videos 5–7).
Figure 2.
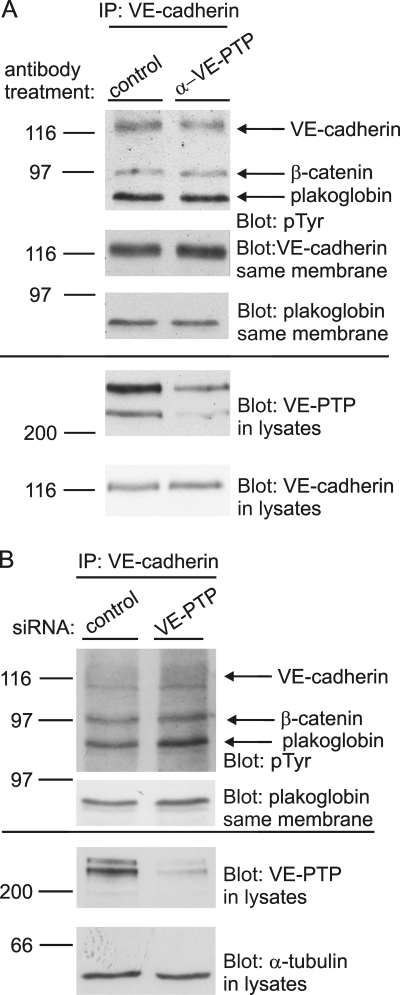
Antibodies against VE-PTP trigger endocytosis and down-regulation of Tie-2–associated but not VE-cadherin–associated VE-PTP. (A) Confluent bEnd.3 cells were treated with polyclonal antibodies against VE-PTP (α-VE-PTP) or preimmune antibodies (control IgG) for 20 min. Subsequently, cells were either fixed and permeabilized (top and bottom) or only fixed (middle) and stained with Alexa 568–conjugated secondary antibodies. Cell nuclei were counterstained with Hoechst. Bar, 20 µm. (B) bEnd.5 cells were treated with polyclonal antibodies against VE-PTP (α-VE-PTP) or preimmune antibodies (control) for 1 h. VE-PTP was immunoprecipitated from endothelial cells and analyzed by immunoblotting for coprecipitated VE-cadherin and Tie-2, respectively, or for VE-PTP (as indicated underneath). Aliquots of cell lysates with identical protein content were directly immunoblotted for VE-cadherin or Tie-2 (bottom). Quantified signal intensities are indicated. Molecular weight markers are indicated. (C) FACS analysis showing the surface expression of Tie-2, VE-cadherin, and VE-PTP of bEnd.5 cells after 1 h pretreatment with monoclonal antibodies against VE-PTP (red) or preimmune antibodies (blue). The mean fluorescence FACS signal for VE-PTP is indicated in percent (bottom).
Antibodies against VE-PTP selectively down-regulate the Tie-2–associated but not the VE-cadherin–associated VE-PTP protein fraction
VE-PTP associates with Tie-2 (Fachinger et al., 1999; Saharinen et al., 2008) and with VE-cadherin (Nawroth et al., 2002). We confirmed this by showing that VE-PTP and Tie-2, endogenously expressed in mouse and human endothelial cells, can indeed be coprecipitated (Fig. S3). To examine whether it would be VE-cadherin or Tie-2 that is involved in the anti–VE-PTP effect on the enlargement of vascular structures in allantois tissue, we tested whether anti–VE-PTP antibodies affected Tie-2 and VE-cadherin–associated VE-PTP molecules in the same way. To this end, we preincubated bEnd.5 cells with antibodies against the extracellular part of VE-PTP in order to reduce VE-PTP levels. Subsequently, cells were washed and lysed and residual VE-PTP was immunoprecipitated with antibodies against the C terminus of VE-PTP. Precipitates were analyzed in immunoblots for VE-PTP and for coprecipitated Tie-2 and VE-cadherin. To our surprise, we found that the amount of VE-cadherin coprecipitated with anti–VE-PTP antibodies was unchanged independent of whether cells had been pretreated with anti–VE-PTP or with control antibodies (Fig. 2 B). Thus, despite the strong down-regulation of VE-PTP, the amount of coprecipitated VE-cadherin was not reduced. In contrast, the amount of coprecipitated Tie-2 was reduced to a similar extent as the amount of VE-PTP that was still available for the immunoprecipitation (Fig. 2 B). Analyzing the cell surface expression of VE-PTP, Tie-2, and VE-cadherin on bEnd.5 cells by FACS revealed that cell surface levels of VE-PTP were indeed down-regulated by the preincubation with anti–VE-PTP antibodies, whereas the cell surface levels of Tie-2 and VE-cadherin were unchanged (Fig. 2 C). We conclude that anti–VE-PTP antibodies selectively displace VE-PTP from Tie-2 and trigger endocytosis of these VE-PTP molecules, whereas VE-PTP molecules that are associated with VE-cadherin stay unaffected.
In agreement with this interpretation, we found that Tie-2 was not endocytosed together with VE-PTP upon preincubation of living cells with anti–VE-PTP antibodies (Fig. 3 A). In addition, endocytosis of VE-PTP neither affected the expression of VE-cadherin nor that of VE-PTP at endothelial cell contacts (Fig. 3 B), and also the staining for the tight junction–associated ESAM was unaffected (Fig. S2 C).
Figure 3.
Antibodies against VE-PTP do not trigger endocytosis of Tie-2 and leave VE-cadherin and VE-PTP at endothelial cell contacts unaffected. (A) Confluent bEnd.3 cells were treated with polyclonal antibodies against VE-PTP (α-VE-PTP) or preimmune antibodies (control IgG) for 20 min. Subsequently, fixed and permeabilized cells were stained with Alexa 568–conjugated secondary antibodies (internalized VE-PTP, internalized control) and for Tie-2 (Tie-2). An anti–Tie-2 staining control is shown in Fig. S5. Cell nuclei were counterstained with Hoechst. Bar, 25 µm. (B) Confluent bEnd.3 cells were treated with monoclonal antibodies against VE-PTP (α-VE-PTP) or control antibodies (control IgG) for 30 min. Subsequently, fixed and permeabilized cells were stained with Alexa 568–conjugated secondary antibodies (internalized VE-PTP, internalized control) and for VE-cadherin and VE-PTP. Cell nuclei were counterstained with Hoechst. Internalized VE-PTP was not detected with new antibodies against VE-PTP, probably because epitopes were masked by the antibodies that had triggered endocytosis. Bar, 10 µm.
Antibodies against VE-PTP activate tyrosine phosphorylation of Tie-2
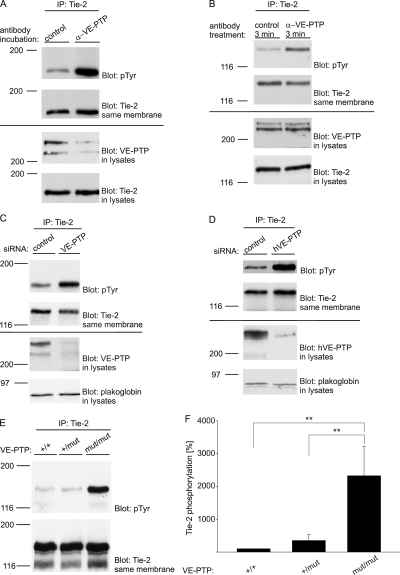
The selective effect of the anti–VE-PTP antibodies on the Tie-2–associated fraction of VE-PTP molecules prompted us to test whether these antibodies stimulate tyrosine phosphorylation of Tie-2. Incubating bEnd.5 cells for 1 h with antibodies against VE-PTP resulted in a strong increase in tyrosine phosphorylation of Tie-2 (Fig. 4 A). This effect was rapid, because even anti–VE-PTP antibody incubations as short as 3 min triggered Tie-2 phosphorylation (Fig. 4 B). Interestingly, anti–VE-PTP antibodies did not enhance tyrosine phosphorylation of VE-cadherin, or associated β-catenin or plakoglobin (Fig. 5 A), although silencing of VE-PTP by siRNA enhanced plakoglobin tyrosine phosphorylation (Fig. 5 B and Nottebaum et al., 2008). Again, this suggests that the anti–VE-PTP antibodies did not affect the VE-cadherin–associated population of VE-PTP molecules.
Figure 4.
VE-PTP expression inhibited by either antibodies, siRNA, or gene disruption triggers Tie-2 tyrosine phosphorylation in endothelial cells. (A) bEnd.5 cells were treated with polyclonal antibodies against VE-PTP or preimmune antibodies for 1 h and subsequently immunoprecipitated for Tie-2, followed by immunoblotting with anti-phosphotyrosine antibodies (pTyr) and antibodies against Tie-2. Aliquots of cell lysates with identical protein content were directly immunoblotted for VE-PTP and Tie-2 (bottom). (B) Similar as in A, except that antibodies were only incubated for 3 min (C) bEnd.5 cells were either transfected with control siRNA or with siRNA directed against VE-PTP. 24 h later, Tie-2 was immunoprecipitated and immunocomplexes (top) or cell lysates (bottom) were analyzed by immunoblotting with antibodies against phosphotyrosine (pTyr), Tie-2, VE-PTP, and plakoglobin (as indicated). (D) HUVECs instead of bEnd.5 cells were analyzed as in C. (E) Embryonic endothelioma cells established either from wild-type (+/+), heterozygous (+/mut), or homozygous (mut/mut) VE-PTP mutant embryos were subjected to immunoprecipitations with antibodies against Tie-2. Immunocomplexes were analyzed by immunoblotting with antibodies against phosphotyrosine or Tie-2 (as indicated). (F) Quantification of Tie-2 tyrosine phosphorylation (±SD) in wild-type (+/+; n = 2 cell lines), heterozygous (+/mut; n = 3 cell lines), or homozygous (mut/mut; n = 3 cell lines) VE-PTP mutant endothelioma, analyzed as in E. **, P < 0,01.
Figure 5.
Down-regulation of VE-PTP by siRNA, but not by anti–VE-PTP antibodies, enhances tyrosine phosphorylation of VE-cadherin–associated plakoglobin. (A) bEnd.5 cells were treated with polyclonal antibodies against VE-PTP (α-VE-PTP) or preimmune antibodies (control) for 1 h and subsequently immunoprecipitated for VE-cadherin, followed by immunoblotting with antibodies against phosphotyrosine (pTyr), VE-cadherin, or plakoglobin. Aliquots of cell lysates with identical protein content were directly immunoblotted for VE-PTP and VE-cadherin (bottom). (B) Similar as in A, except that antibody preincubation of cells was replaced by transfection with either control siRNA or VE-PTP siRNA.
In agreement with the anti–VE-PTP antibody effects on Tie-2 activation, similar effects were obtained after silencing VE-PTP by siRNA in bEnd.5 (Fig. 4 C) and in HUVEC (Fig. 4 D). In addition, we found that VE-PTP gene disruption leads to tyrosine phosphorylation of Tie-2, as was analyzed with endothelioma cells generated from gene-disrupted mice (Baumer et al., 2006) expressing a truncated form of VE-PTP lacking the trans-membrane region and the cytoplasmic tail with its phosphatase domain (Fig. 4 E). Results were analyzed with three independent cell lines for each genotype and quantified (Fig. 4 F).
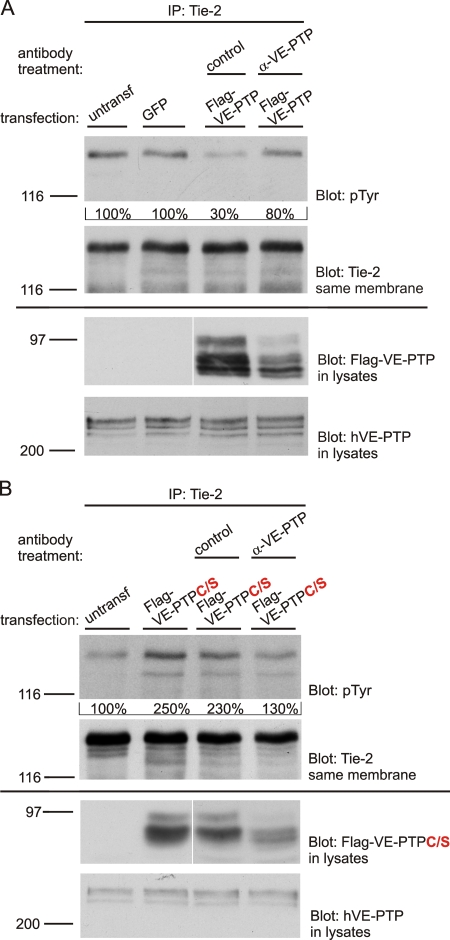
To obtain additional evidence for the role of VE-PTP in the regulation of Tie-2 activity, we combined the expression of a phosphatase-dead mutant of mouse VE-PTP in HUVEC (using adenovirus vectors) with the use of anti–VE-PTP antibodies. We expressed either mouse VE-PTP with an intact phosphatase domain or in a parallel experiment the phosphatase-dead mutant of mouse VE-PTP in HUVEC (using adenovirus) and tested the effect of antibodies against the extracellular part of mouse VE-PTP on Tie-2 activation in these cells. Importantly, the antibodies we used did not crossreact with human VE-PTP. As we expected, the expression of intact VE-PTP reduced tyrosine phosphorylation of Tie-2, whereas the dominant-negative mutant enhanced Tie-2 activity (Fig. 6, A and B). Incubation of the cells with antibodies against mouse VE-PTP induced activation of Tie-2 in cells that expressed intact mouse VE-PTP (Fig. 6 A). Intriguingly, the same antibodies had the opposite effect in HUVEC expressing the dominant-negative form of VE-PTP (Fig. 6 B). This clearly rules out indirect effects of the antibodies on Tie-2 activation and shows that it is the dissociation of the intact phosphatase VE-PTP from Tie-2 that leads to the activation of Tie-2, whereas the dissociation of the dominant-negative form of VE-PTP makes it accessible again for intact human VE-PTP, which then leads to the dephosphorylation of Tie-2.
Figure 6.
VE-PTP regulates Tie-2 phosphorylation via its active phosphatase. (A) HUVECs either untransfected, or expressing GFP or expressing mouse Flag-VE-PTP (as indicated above) were either untreated or treated with polyclonal antibodies against mouse VE-PTP or preimmune antibodies (as indicated above) for 1 h and subsequently immunoprecipitated for Tie-2, followed by immunoblotting with anti-phosphotyrosine antibodies (pTyr) and antibodies against Tie-2. Aliquots of cell lysates with identical protein content were directly immunoblotted for Flag-VE-PTP and hVE-PTP (bottom). Quantified signal intensities are indicated. (B) As in A with mouse Flag-VE-PTP being replaced by the corresponding inactive phosphatase mutant Flag-VE-PTP-C/S. White lines indicate that intervening lanes have been spliced out.
VE-PTP counterbalances activation of Tie-2 by Ang1
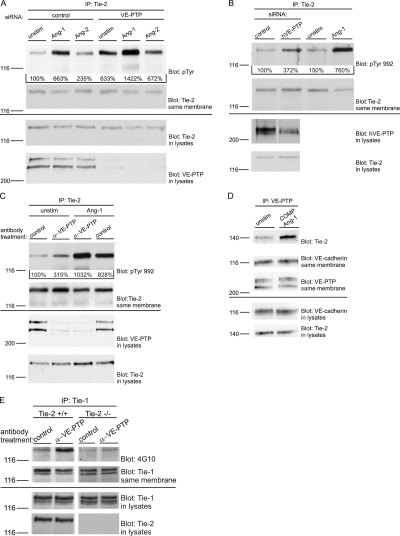
To determine whether VE-PTP indeed counterbalances the activation of Tie-2 by Ang1, we combined silencing of VE-PTP by siRNA in bEnd.5 cells with the stimulation with Ang1. As shown in Fig. 7 A, a combined treatment with Ang1 and VE-PTP siRNA stimulated Tie-2 phosphorylation to a greater extent than a combined treatment with Ang1 and control siRNA. This suggests that VE-PTP indeed counteracts the activation of Tie-2 by Ang1. Ang2 enhanced the phosphorylation of Tie-2 in endothelial cells only weakly and had no significant additive effect in combination with silencing of VE-PTP expression (Fig. 7 A). However, it cannot be ruled out that VE-PTP may also counteract small activation effects of Ang2.
Figure 7.
VE-PTP counterbalances Ang1 activation of Tie-2 and regulates Tie-1 tyrosine phosphorylation dependent on Tie-2. (A) bEnd.5 cells were either transfected with control siRNAs or with siRNAs directed against VE-PTP. 24 h later, cells were stimulated either with 600 ng/ml Ang1 or 600 ng/ml Ang2 for 10 min, or unstimulated (unstim). Immunoprecipitates of Tie-2 (top two panels) or cell lysates (bottom two panels) were immunoblotted for the indicated antigens. (B) HUVECs were either transfected with control siRNAs or with siRNAs directed against hVE-PTP. 24 h later, siRNA-transfected cells (left two lanes) and untransfected cells, stimulated either with 600 ng/ml Ang1 for 10 min or unstimulated (right two lanes), were subjected to immunoprecipitations for Tie-2, followed by immunoblotting with antibodies against the phosphorylated tyrosine 992 in the active loop of the kinase (pTyr 992) and Tie-2. Aliquots of cell lysates were immunoblotted directly for hVE-PTP and Tie-2 (bottom two panels). White line indicates that intervening lanes have been spliced out. (C) bEnd.5 cells were treated either with polyclonal antibodies against VE-PTP (α-VE-PTP) or preimmune antibodies (control) for 1 h, followed by either Ang1 stimulation for 10 min (Ang1) or no stimulation (unstim). Phosphorylation of tyrosine 992 was analyzed as described for B. Quantified signal intensities are indicated. (D) bEnd.5 cells were stimulated with 200 ng/ml COMP-Ang1 for 10 min or left untreated. VE-PTP immunoprecipitates (top three panels) and cell lysates (bottom two panels) were analyzed by immunoblotting for Tie-2, VE-cadherin, and VE-PTP as indicated on the right. (E) Embryonic endothelioma cells established either from wild-type (+/+) or Tie-2–deficient (−/−) embryos were antibody pretreated as indicated and subjected to immunoprecipitations with antibodies against Tie-1. Immunocomplexes were analyzed by immunoblotting with antibodies against phosphotyrosine or Tie-1 (as indicated). Aliquots of cell lysates were immunoblotted directly for Tie-1 and Tie-2 (bottom two panels).
Tyrosine phosphorylation analyzed in such experiments indeed represented proper Tie-2 activation because these results could be verified in HUVEC for tyrosine residue 992 in the activation loop of the human Tie-2 kinase domain, the first tyrosine phosphorylated upon activation (Shewchuk et al., 2000; Murray et al., 2001) (Fig. 7 B). Likewise, down-regulation of VE-PTP with antibodies also further enhanced Ang1 stimulated phosphorylation of tyrosine 992 of Tie-2 in mouse bEnd.5 cells (Fig. 7 C). We conclude that antibodies against VE-PTP enhance the activation of the kinase domain of Tie-2 by Ang1.
We tested whether VE-PTP is constitutively associated with Tie-2 or whether this would be enhanced upon activation of Tie-2. Interestingly, we found that COMP-Ang1 stimulation of Tie-2 in bEnd.3 cells clearly increased the association of VE-PTP with Tie-2 (Fig. 7 D). A similar effect was also seen with 600 ng/ml Ang1, but not with the same concentration of Ang2 (not depicted). This suggests that Ang1 stimulates activation of Tie-2 and simultaneously triggers a negative feed back process that limits activation by enhancing the association of Tie-2 with VE-PTP, and thus eventually balances Tie-2 receptor activity.
Interestingly, antibody-stimulated endocytosis of VE-PTP also triggered tyrosine phosphorylation of Tie-1 and this effect required the expression of Tie-2, as it was not observed in Tie-2–null endothelioma cells (Fig. 7 E). This shows that VE-PTP specifically regulates Tie-2, whereas Tie-1 is indirectly affected.
Vessel enlargement induced by anti–VE-PTP antibodies requires Tie-2
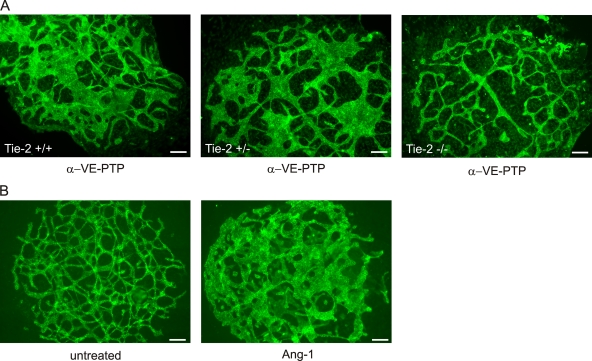
Despite the essential function of Tie-2 during embryonic vascular remodeling, we have found that allantois explants from Tie-2–null mice develop normal vascular networks (Baumer et al., 2006). However, the selective anti–VE-PTP antibody effect on Tie-2 activation suggested that VE-PTP deficiency, caused by either gene disruption or by antibody-mediated down-regulation of VE-PTP, might affect vascular remodeling in the allantois through hyper-activation of Tie-2. If this were the case, anti–VE-PTP antibodies should not affect vascular remodelling in Tie-2–null allantoides. Indeed, we found that antibodies against VE-PTP enlarged cord structures only in Tie-2+/+ and Tie-2+/−, but not in Tie-2−/− allantoides (Fig. 8 A). In agreement with this, the activating Tie-2 ligand Ang1 when added to wild-type allantois explants had a similar effect as the anti–VE-PTP antibodies (Fig. 8 B), further supporting the assumption that VE-PTP controls vascular remodeling by counteracting the activation of Tie-2.
Figure 8.
Vessel enlargement induced by anti–VE-PTP antibodies requires Tie-2. (A) Allantois explants from E8.5 Tie-2 +/+, Tie-2 +/−, or Tie-2 −/− embryos (as indicated) were cultured on gelatin-coated ultrathin glass slides in the presence of polyclonal antibodies against VE-PTP for 22 h. (B) Allantois explants from E8.5 embryos were cultured in medium (untreated) or in the presence of 600 ng/ml Ang1. Subsequently, endothelium was visualized in all samples in A and B by indirect immunofluorescence staining for VE-cadherin. Bars, 50 µm.
Anti–VE-PTP antibodies trigger vessel enlargement by enhancing endothelial cell proliferation via stimulating Tie-2 and Erk1/2
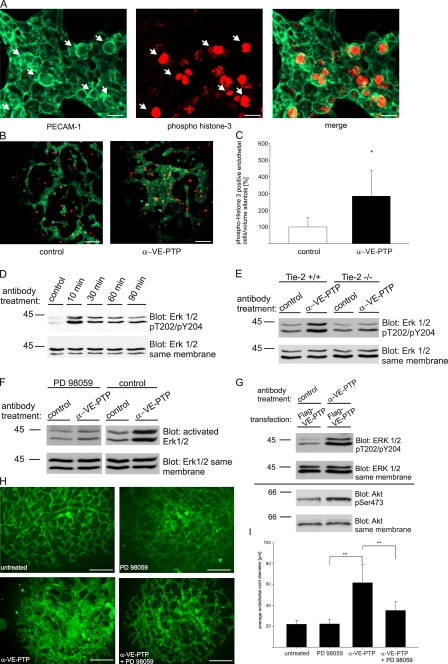
To determine how Tie-2 hyper-activation upon down-regulation of VE-PTP leads to the enlargement of endothelial structures in allantois explants, we directly visualized the formation of endothelial structures in the explants. To this end, we performed live imaging of allantois explants from mice expressing a VE-cadherin-GFP fusion protein via a cDNA construct knocked into the VE-cadherin locus, thereby replacing the endogenous VE-cadherin allele. Mice homozygous for the VE-cadherin-GFP allele developed normally and were viable (unpublished data). Allantois explants from these mice were cultured for 12 h and then incubated for another 12 h in the presence of either anti–VE-PTP antibodies, or preimmune antibodies or Ang1. As shown in the respective Videos (Videos 1–3), Ang1 and anti–VE-PTP antibodies, but not control antibodies, rapidly increased the area covered with endothelial structures and stimulated endothelial cell division. Dividing endothelial cells could clearly be identified as cells transiently rounding up. Staining of explants with phospho-Histone 3 antibodies subsequent to live imaging revealed that round cells still visible at the end of the observation period were indeed positive for the mitosis marker (Fig. 9, A and B). The stimulatory effect of anti–VE-PTP antibodies on endothelial proliferation was quantified by costaining allantois explants for PECAM-1 and phospho-Histone 3 and analyzing them by laser scanning confocal microscopy with the help of 3D software (Video 4), which allowed to unambiguously determine the endothelial nature of phospho-Histone 3 positive nuclei. Quantification revealed that anti–VE-PTP antibodies increased the number of mitotically active endothelial cells by 2.8-fold in comparison to allantoides cultured in the presence of control antibodies (Fig. 9 C). In agreement with this, the number of endothelial cells in a defined volume had increased by a factor of 4.4, although the number of sprouts was not increased. Thus, anti–VE-PTP antibodies stimulate endothelial cell proliferation within cord structures.
Figure 9.
Anti–VE-PTP antibody treatment of allantois explants stimulates endothelial cell proliferation and enlargement of endothelial cords through activation of Erk1/2. (A) Allantois explants of E8.5 embryos from knock-in mice expressing VE-cadherin-GFP from the VE-cadherin genetic locus were cultured on gelatin-coated ultrathin glass slides for 12 h and were then analyzed by live imaging during the next 12 h (see Video 2), while they were cultured in the presence of polyclonal antibodies against VE-PTP. Subsequently, allantoides were fixed and double stained for PECAM-1 and phospho-Histone 3. Arrows indicate proliferating endothelial cells with a characteristic round cell shape. Bar, 20 µm. (B) Same as in A, depicting larger areas of the explants. Bar, 100 µm. (C) Percentage of phospho-Histone 3–positive endothelial cells per volume tissue in E8.5 allantois explants cultured with polyclonal antibodies against VE-PTP or preimmune antibodies for 12 h. *, P < 0,05. (D) bEnd.5 cells were treated with mAb against ESAM (control) or for indicated time periods with a mAb against VE-PTP. Cell lysates were analyzed by immunoblotting with anti–phospho-Erk1/2–specific antibodies (pT202/pY204) and antibodies against Erk1/2. Treatment with mAb against ESAM gave a similar result as in the absence of antibodies (not depicted). (E) Endothelioma cells of wild-type genotype (Tie-2 +/+) or deficient for Tie-2 (Tie-2 −/−) were treated with monoclonal antibodies against ESAM (control) or against VE-PTP for 1 h, followed by immunoblotting cell lysates with anti–phospho-Erk1/2–specific antibodies (pT202/pY204) and antibodies against Erk1/2, as indicated on the right. (F) bEnd.5 cells were treated with 50 µM of the Erk1/2 inhibitor PD98059 (PD 98059) or DMSO only (control) for 30 min, followed by incubation with a control mAb (control) or mAb against VE-PTP (α-VE-PTP) in the presence of the inhibitor or DMSO for 20 min. Cell lysates were analyzed by immunoblotting with anti–phospho-Erk1/2–specific antibodies (pT202/pY204) and antibodies against Erk1/2. (G) Mouse Flag-VE-PTP expressing HUVECs were treated with polyclonal antibodies against VE-PTP or preimmune antibodies for 20 min and subsequently cell lysates were analyzed by immunoblotting with anti–phospho-Erk1/2–specific antibodies (pT202/pY204), anti–phospho-Akt–specific antibodies (Ser473) and antibodies against Erk1/2 and Akt. (H) Allantois explants from E8.5 wild-type embryos were cultured on gelatin-coated glass slides either in the presence of Erk1/2 inhibitor PD 98059 (PD 98059), polyclonal antibodies against VE-PTP (α-VE-PTP), or the combination of both (α-VE-PTP + PD 98059) or left untreated for 22 h. Endothelial structures were stained with a mAb against VE-cadherin by indirect immunofluorescence Bar, 100 µm. (I) Quantification of the experiment illustrated in H. Average endothelial cord diameters were determined for allantois explants that were left untreated (untreated, n = 3), cultured in the presence of Erk1/2 inhibitor PD 98059 (PD 98059, n = 3), polyclonal antibodies against VE-PTP (α-VE-PTP, n = 11), or the combination of both (α-VE-PTP + PD 98059, n = 7) for 22 h; **, P < 0,01.
Activation of Tie-2 feeds into various signaling pathways. Among those only Tie-2–dependent activation of Erk1/2 has been reported to trigger proliferation of cultured endothelial cells (Kanda et al., 2005). This prompted us to test whether anti–VE-PTP antibodies stimulate Erk1/2 in mouse endothelioma cells. Indeed, we found a rapid (10 min) and transient activation of Erk1/2 (Fig. 9 D). This activation was dependent on Tie-2 because it was only seen in endothelioma cells established from Tie-2–expressing mice, but not in endothelioma cells established from Tie-2−/− mice (Fig. 9 E). Similar results were found with three independently derived cell lines from wild-type and from Tie-2−/− mice, respectively.
Activation of Erk1/2 with anti–mouse VE-PTP antibodies was also seen in HUVEC that were transduced with mouse Flag-VE-PTP (Fig. 9 G). In addition, we found that the kinase Akt was phosphorylated at serine 473 (Fig. 9 G). Activation of Akt is a step in another signaling pathway known to be stimulated by Tie-2 (Brindle et al., 2006).
To test whether activation of Erk triggered by anti–VE-PTP antibodies would be involved in the anti–VE-PTP induced enlargement of endothelial structures in allantois explants, we coincubated explants with the antibodies together with 50 µM of the Erk inhibitor PD98059, a concentration that blocked the anti–VE-PTP effect on Tie-2 activation in bend.5 cells (Fig. 9 F). As shown in Fig. 9 H, the inhibitor could strongly reduce the antibody effect on enlargement of the vascular structures. Quantification of the effect revealed a 67% inhibitory effect of the Erk1/2 inhibitor (Fig. 9 I). Another inhibitor of Erk1/2, U0126, also inhibited the anti–VE-PTP effect on vessel enlargement in allantois explants (Fig. S4). In addition, this inhibitor reversed the stimulatory effect of the anti–VE-PTP antibodies on proliferation, as analyzed by counting phospho-Histone 3 positive endothelial nuclei (control antibodies 6.25 ± 0.35; anti–VE-PTP antibodies 14.8 ± 4.5; anti–VE-PTP antibodies plus PD98059 5.5 ± 1.24 mitotic endothelial nuclei per volume allantois; P < 0.001). In combination with the report by Kanda et al. (2005), these results strongly suggest that the down-regulation of VE-PTP by antibodies leads to the activation of Tie-2, which in turn stimulates endothelial proliferation via stimulating the MAP kinase Erk1/2.
Antibodies against VE-PTP induce blood vessel enlargement in 1–2-wk-old mice
To test whether antibodies against VE-PTP can indeed affect vascular remodeling in the living animal, we injected 7-d-old mice daily for 5 d with 100 µg of anti–VE-PTP antibodies. Blood vessels were visualized in vibratome sections of the tongue by staining for PECAM-1. As shown in Fig. 10 A, vessels in the dermal papillae and draining venules were clearly enlarged. Quantification revealed that polyclonal antibodies against VE-PTP enlarged vessels by 82%, compared with tissue sections from mice treated with control antibody and the mAb 109.1 against VE-PTP, which increased vessel diameters by 57% (Fig. 10 B). These effects are reminiscent of what was reported for the influence of Ang1 on vessel enlargement in newborn mice (Thurston et al., 2005). Indeed, we could show that tyrosine phosphorylation of Tie-2 was enhanced in the animals upon anti–VE-PTP antibody injection, as analyzed in immunoblots of lung tissue of these animals (Fig. 10 C). This effect was accompanied with a fivefold increase of the number of phospho-Histone 3 positive endothelial nuclei (Fig. 10, E and F), suggesting that vessel enlargement was caused by hyperplasia. Similar as for tongue tissue, anti–VE-PTP antibodies also enlarged blood vessels of the trachea (Fig. 10 D). Collectively, our results suggest that VE-PTP is required to balance the activity of Tie-2 during vascular remodeling in young mice. In addition, our results demonstrate that the described anti–VE-PTP antibody effects on vascular remodeling and Tie-2 activity are not limited to explant cultures devoid of blood flow.
Figure 10.
Antibodies against VE-PTP induce blood vessel enlargement and endothelial proliferation in juvenile mice and lead to activation of Tie-2. (A) 7-d-old wild-type mice were injected daily i.p. with 100 µg antibodies against VE-PTP (α-VE-PTP) or preimmune antibodies (control) for a period of 7 d. 100-µm vibratome sections of tongues were immunostained for PECAM-1. Bar, 60 µm. (B) Average vessel diameter in the tongue of wild-type mice injected daily i.p. with 100 µg polyclonal (α-VE-PTP pAb), or monoclonal (a-VE-PTP mAb) antibodies against VE-PTP, preimmune antibodies (control pAb), or monoclonal antibodies against ESAM (control mAb), respectively, for a period of 7 d. In each case three animals were analyzed. (C) 7-d-old wild-type mice were treated as described for A. Lungs of mice were lysed and subjected to immunoprecipitation for Tie-2 and subsequent immunoblotting with antibodies either against phosphorylated tyrosine or against Tie-2 (as indicated). White line indicates that intervening lanes have been spliced out. (D) Mice were treated as in A and trachea whole mounts were immunostained for PECAM-1. Arrowheads indicate enlarged blood vessels. L, lymphatic vessels. Bar, 50 µm. (E) Mice were treated for 4 d as in A and vibratome sections of tongues were immunostained for PECAM-1 and phospho-Histone 3. Asterisk, nonendothelial nucleus; arrowheads, endothelial nuclei. Bar, 50 µm. (F) Quantification of the experiment illustrated in E with phospho-Histone 3–positive endothelial nuclei counted per volume tissue (38 × 200 × 500 µm). ***, P < 0,001.
Discussion
VE-PTP, the first known endothelial-specific receptor-type tyrosine phosphatase, associates with Tie-2 and VE-cadherin and is essential for vascular remodeling during embryonic development. In this study we show that VE-PTP controls vascular remodeling via regulating the ability of Tie-2 to drive endothelial cell proliferation. These mechanistic insights into the physiological function of VE-PTP were enabled by antibodies against VE-PTP, which selectively dissociate VE-PTP from Tie-2 but not from VE-cadherin. The antibodies stimulated Tie-2 activation, as documented by increased tyrosine phosphorylation of Tie-2 and activation of the downstream signaling target Erk1/2. In addition, we found that the anti–VE-PTP antibodies elicit enlargement of vascular structures in allantois explants and in 1–2-wk-old mice, accompanied by enhanced endothelial cell proliferation. Most importantly, enlargement of vascular structures by these antibodies was eliminated in the absence of Tie-2 and required activation of Erk1/2. Our results establish VE-PTP as an essential negative regulator of Tie-2 during blood vessel remodeling, providing a mechanism for how VE-PTP affects angiogenesis.
Precise balancing of positive and negative stimulation of Tie-2 is essential for vessel remodeling and angiogenesis. A lack of activation caused by deleting the Tie-2 gene itself or by disrupting the gene for the agonist Ang1 leads to embryonic vascular malformations and lethality (Dumont et al., 1994; Sato et al., 1995; Suri et al., 1996), a similar phenotype that is observed upon overexpression of the antagonistic ligand Ang2 (Maisonpierre et al., 1997). On the other hand, hyper-activation of Tie-2 via a missense mutation in the kinase domain leads to venous malformations in human patients (Vikkula et al., 1996), and deletion of the gene for the antagonist Ang2 affects remodeling in hyaloid vasculature in the eye of newborn mice and lymphatic patterning (Gale et al., 2002). Interestingly, defects in Ang2-deficient mice were limited to postnatal development, and no defects were found during embryonic vascular development in these mice. Thus, VE-PTP represents the first negative regulator of Tie-2 essential for embryonic angiogenesis. We can not, of course, rule out that VE-PTP gene disruption affects additional molecular mechanisms besides Tie-2 signaling, e.g., VE-cadherin or other still-unknown substrates. However, the vascular aberrations caused by our anti–VE-PTP antibodies in allantois explant cultures were strictly dependent on Tie-2 and therefore identify Tie-2 as an essential substrate for VE-PTP during embryonic vascular remodeling.
We assume that VE-PTP represents a negative feedback control mechanism that limits the activation of Tie-2. In agreement with this, stimulation of Tie-2 with Ang1 leads to increased association of Tie-2 and VE-PTP, enabling the agonist to trigger activation and at the same time to launch the negative mechanism that ensures that the signal is switched off again. Whether, in addition, a ligand for VE-PTP may exist that could induce uptake of VE-PTP and thereby indirectly could enhance Ang1-driven activation of Tie-2 is unknown, and may be an interesting hypothesis to test in the future.
The effects of our anti–VE-PTP antibodies on blood vessel enlargement in young mice are similar to the effects observed in mice either injected with Ang1 or COMP-Ang1 (Thurston et al., 2005; Kim et al., 2007) or overexpressing COMP-Ang1 via adenovirus vectors (Cho et al., 2005). However, as these studies were based on exogenously added large doses of Ang1, it could not be determined whether physiological levels of Ang1 would indeed play a role in the normal regulation of vessel size during perinatal development. Because the anti–VE-PTP antibodies simply dissociate a negative regulator from Tie-2, our results suggest that Tie-2–stimulating ligands are indeed acting in newborns to determine vessel size.
It is interesting that antibodies against VE-PTP also stimulated Tie-1 activation and that this only occurred in the presence of Tie-2. This is in agreement with other studies demonstrating that Tie-1 activation by Ang1 depends on Tie-2 (Saharinen et al., 2005; Yuan et al., 2007).
Tie-2 activation triggers various signaling pathways and biological activities, such as survival and protection from apoptosis, migration, permeability, tube formation, and sprouting, as has been summarized in excellent reviews (Brindle et al., 2006; Eklund and Olsen, 2006). However, studies on the ability of Ang1 to stimulate proliferation of cultured endothelial cells are controversial, ranging from no effect (Davis et al., 1996; Witzenbichler et al., 1998; Fujikawa et al., 1999) to mild effects (Koblizek et al., 1998; Teichert-Kuliszewska et al., 2001) to substantial effects (Kanda et al., 2005). The in vivo studies with Ang1 and COMP-Ang1 in newborns (Cho et al., 2005; Thurston et al., 2005; Kim et al., 2007) suggest that vessel enlargement was accompanied by endothelial cell proliferation, establishing that Tie-2 can stimulate proliferation of endothelial cells in vivo. Like them, we found that Tie-2–dependent proliferation was independent from sprout formation and occurred within endothelial cord structures.
Kanda et al. (2005) have shown that blocking Erk can partially inhibit Ang1-stimulated endothelial cell proliferation of cultured endothelial cells. This is in good agreement with our finding that treatment with anti–VE-PTP antibodies feeds into the Tie-2–signaling pathway leading to Erk activation. Importantly, the fact that the Erk1/2 inhibitors PD98059 and U0126 blocked the anti–VE-PTP effect on endothelial cell proliferation and on enlargement of vascular structures in the allantois indicates that Tie-2 triggers endothelial proliferation in the allantois via Erk1/2 and that VE-PTP counteracts this pathway. Collectively, these results indicate that anti–VE-PTP antibodies induce enlargement of vascular structures in the allantois by stimulating endothelial cell proliferation via the Tie-2, Erk1/2 pathway.
It is intriguing that VE-PTP molecules associated with Tie-2 were selectively sensitive to anti–VE-PTP antibody-triggered dissociation and endocytosis, whereas VE-cadherin–associated VE-PTP molecules were not sensitive for this effect. Likewise, anti–VE-PTP antibodies selectively affected tyrosine phosphorylation of Tie-2, but not the phosphorylation pattern of the components of the VE-cadherin complex in endothelial adherens junctions. We assume that VE-PTP complexed with VE-cadherin may not be accessible for antibodies, possibly masked within VE-cadherin clusters at cell contacts. Indeed, incubation of living, intact endothelial cells or allantois explants with anti–VE-PTP antibodies did not allow to stain endothelial cell contacts, whereas fixing and permeabilizing the specimens rendered extracellular epitopes of VE-PTP accessible to antibody staining (Fig. 3 and Videos 5–7).
In conclusion, our results establish VE-PTP as an essential negative regulator of Tie-2, which controls Tie-2–driven endothelial cell proliferation, which in turn affects blood vessel remodeling during embryonic development and determines blood vessel size during perinatal growth. In light of the publications analyzing the function of the Tie-receptor system in tumor angiogenesis (Shim et al., 2007) it will be interesting to test a potential role of VE-PTP in this pathological process. Furthermore, because VE-PTP is an endothelial-specific transmembrane protein and antibodies against its extracellular part affect endothelial cell proliferation and angiogenesis in vivo, VE-PTP is generally an easily accessible, interesting novel target for pro- or anti-angiogenic therapeutic interventions.
Materials and methods
Mice
Tie-2–deficient mice were provided by Daniel Dumont (Sunnybrook and Women’s Research Institute, Toronto, Canada; Dumont et al., 1994). For timed matings, mice were mated for 3 h.
Reagents and antibodies
The following reagents and antibodies were used: gelatin (Sigma-Aldrich), mowiol (Sigma-Aldrich), fibronectin (Sigma-Aldrich), Hoechst (Invitrogen), PD 98059 (Calbiochem), COMP-angiopoietin-1 (201–314; Qbiogene), angiopoietin-1 (923-AN; R&D Systems), angiopoietin-2 (623-AN; R&D Systems), mAb 109.1 against VE-PTP (Baumer et al., 2006), pAb VE-PTP-C against VE-PTP (crossreactive to the human homologue hVE-PTP; Nawroth et al., 2002), pAb PTP 1–8 against the extracellular fibronectin type III-like domains 1–8 of VE-PTP, pAb D17 against the extracellular membrane proximal fibronectin type III-like domain of VE-PTP, mAb 3G1 against Tie-2 (Koblizek et al., 1997), mAb against human Tie-2 (Millipore), pAb against Tie-1 (Santa Cruz Biotechnology, Inc.), pAb against phospho-Tie-2-Tyr992, pAb against phospho-Erk1/2-Thr202/Tyr204 pAb and mAb against Erk1/2, pAb against phospho-Akt-Ser473, pAb against Akt (Cell Signaling Technology), mAb 11D4.1 and pAb C5 against murine VE-cadherin (Gotsch et al., 1997), pAb against human VE-cadherin (Santa Cruz Biotechnology, Inc.), mAb 1G8 against mouse ESAM (Nasdala et al., 2002), pAb against human ESAM (Nottebaum et al., 2008), mAb against PECAM-1 1G5.1 and 5D2.6 (Wegmann et al., 2006), mAb against plakoglobin (BD Biosciences), mAb 4G10 against phosphotyrosine (Millipore), and mAb and pAb against phospho-Histone H3-Ser10 (Millipore). Secondary antibodies were purchased from Dianova; Alexa 488–, Alexa 633–, and Alexa 568–coupled antibodies from Invitrogen; and DyLight680 and DyLight800-coupled antibodies from Thermo Fisher Scientific.
Cell culture
The following cells were propagated as described: bEnd.3 and bEnd.5 (Reiss et al., 1998), Tie2+/+ and Tie2−/− mouse endothelioma cells (Jones et al., 2003), and HUVEC (Baumeister et al., 2005). Polyoma middle T immortalization of embryonic endothelial cells was performed as described previously (Reiss and Kiefer, 2004), starting from E9.5 mouse embryos. For stimulation with angiopoietins and antibody treatment, bEnd.5 cells were grown to confluence in DMEM, 10% FCS, starved with MCDB 131 medium (Invitrogen) containing 1% BSA overnight, and stimulated with 200 ng/ml recombinant COMP-angiopoietin-1 (COMP-Ang1) or 600 ng/ml Ang1 and Ang2, respectively, in MCDB 131 medium (with 1% BSA) for indicated time periods. Cells were treated with 50 µg/ml mAb 109.1 (anti-VE-TP), 1G8.1 (anti-ESAM), pAb PTP 1–8 (anti-VE-PTP), or preimmune serum in starvation medium for 30–90 min. HUVECs were grown to confluence in Medium 199 (Invitrogen), 20% FCS, starved with Medium 199 containing 2% FCS for 6 h and stimulated with angiopoietins as described above for bEnd.5 cells.
Constructs and adenoviral transfection
Flag VE-PTP (lacking the first 16 FNIII-like repeats, but containing the extracellular most membrane-proximal 17th domain) and Flag VE-PTP C/S (Fachinger et al., 1999) with a phosphatase-dead mutant (cysteine in the active center replaced by seine) were cloned into pENTR 2B (Invitrogen). Using the LR Recombination Reaction (Gateway Technology; Invitrogen), the pAd-DEST vector was created and used for lipofection of 293A cells. The viral lysate was prepared by lysis of the transfected 293A cells. HUVECs were transduced with adFlag-VE-PTP or adFlag-VE-PTPC/S for 18 h, washed, and starved for 4 h in Medium199, 2% FCS. Subsequently, cells were treated with antibodies against the 17th extracellular domain of VE-PTP for indicated time periods.
Detection of VE-PTP endocytosis by immunofluorescence staining
For detection of endocytosed VE-PTP, bEnd.3 endothelioma cells were seeded on fibronectin-coated glass chamber slides and grown to confluence, starved with MCDB 131 medium containing 1% BSA overnight, and treated with 50 µg/ml pAb PTP 1–8 (anti-VE-PTP), mAb 109.1 (anti-VE-PTP), or control antibodies in starvation medium for indicated time periods. Subsequently, cells were washed extensively, fixed with paraformaldehyde, permeabilized, and blocked with 3% BSA/PBS. Endocytosed antibodies were detected with Alexa 568–coupled secondary antibodies. For controls cells were stained with secondary antibodies without permeabilization. Cells were immunostained with anti–Tie-2, anti–VE-cadherin, anti-ESAM, and anti-EEA1 antibodies for 30 min at room temperature. Subsequently, primary antibodies were detected with Alexa 488–, Alexa 568– and Alexa 633–coupled antibodies for 30 min at room temperature. Cell nuclei were counterstained with 10 µg/ml Hoechst in 3% BSA/PBS for 2 min and examined with a fluorescence microscope (Axioskop; Carl Zeiss, Inc.) in conjunction with a digital camera (RT KE/SE Spot; Diagnostic Instruments, Inc.) or using a confocal laser-scan microscope (Carl Zeiss, Inc.).
Immunoprecipitation and immunoblotting
For coimmunoprecipitations (coIPs), cells were lysed in lysis buffer (20 mM imidazole, pH 6.8, 100 mM NaCl, 2 mM CaCl2, 1% Triton X-100, 0.04% NaN3, and 1x Complete EDTA-free protease inhibitor cocktail [Roche]). For detection of phosphotyrosine after immunoprecipitation, cells were lysed in lysis buffer containing 20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 2 mM CaCl2, 1 mM Na3VO4, 1% Triton X-100, 0.04% NaN3, and 1x Complete EDTA-free. At no time were intact cells exposed to vanadate or peroxyvanadate. Lysates were centrifuged at 4°C for 30 min at 20,000 g, and aliquots were set aside for direct blot analysis; for IP aliquots were incubated for 2 h at 4°C with protein A– or G–Sepharose loaded with the respective antibodies. Immunocomplexes were washed five times with lysis buffer and analyzed by SDS-PAGE. Total cell lysates (3 × 105 cells/lane) or immunoprecipitated material was separated by electrophoresis on 6% (IP) or 8% (direct immunoblots of cell lysates) SDS-PAGE and transferred to nitrocellulose (Whatman) by wet blotting. Blots were analyzed as described previously (Nawroth et al., 2002) or with fluorescent dye-coupled secondary antibodies and Starion fluorescence Image Analyzing system (Fujifilm). For detection of phosphotyrosine, milk powder in the blocking buffer was replaced by 2% BSA and 200 µM Na3VO4 was added.
RNA interference
For RNA interference of mouse and human VE-PTP expression the following siRNAs were used: VE-PTP-34: 5′-CCUCACUGAGGGUAACAGU-3′ (targeting mVE-PTP) and siPTP-82: 5′-GACAGUAUGAGGUGGAAGU-3′ (targeting hVE-PTP; Ambion). For negative controls an siRNA was used that does not target any known mammalian gene (5′-UUCUCCGAACGUGUCACGU-3′; QIAGEN 1022076). Routinely 106 bEnd.5 cells or HUVECs were transfected with 3 or 4 µg of siRNA, respectively, using nucleofection (Amaxa Biosystems) according to the manufacturer’s instructions. For detection of tyrosine phosphorylation, single nucleofection reactions of bEnd.5 cells and HUVECs were scaled up fivefold and threefold, respectively.
Allantois explant cultures and immunolabeling
Allantoides were dissected from E8.0 to E8.5 C57BL/6 wild-type or Tie-2–deficient embryos, cultured for 22 h before fixation, in the presence of 50 µg/ml mAb 109.1 (anti–VE-TP), 1G8.1 (anti-ESAM), pAb PTP 1–8 (anti–VE-PTP), pAb VE-19 (anti-ESAM), or preimmune serum, or left untreated for 12–22 h. For stimulation with growth factors, allantoides were cultured in the presence of 600 ng/ml COMP-Ang1 or Ang1. For inhibiting Erk1/2 activation, allantoides were pretreated with 50 µM/L PD 98059 or 10 µM U0126 for 30 min and subsequently incubated with antibodies in the presence of 50 µM/L PD 98059 or 10 µM U0126 for 12 h. Explants were fixed as described previously (Drake and Fleming, 2000), immunolabeled (Baumer et al., 2006), and examined with a fluorescence microscope (Axioskop; Carl Zeiss, Inc.) in conjunction with a digital camera (RT KE/SE Spot; Diagnostic Instruments, Inc.). For identifying proliferating endothelial cells, allantoides were fixed and immunolabeled with anti–PECAM-1 and anti–phospho-Histone 3 antibodies as described previously (Baumer et al., 2006). Fluorescence signal was detected using a confocal laser-scan microscope (Carl Zeiss, Inc.). Optical sections were collected along the z-axis and 3D images were reconstructed using LSM Image Examiner software (Video 4). Phospho-Histone 3 positive endothelial cells were counted in four volumes (257 × 257 × 24 µm) set randomly per allantois.
Antibody treatment of 1–2-wk-old mice
For treatment of young mice (P7), pups from litters of C57BL/6 mice were injected i.p. with 50 µg mAb 109.1 (anti–VE-TP), 1G8.1 (anti-ESAM), pAb PTP 1–8 (anti–VE-PTP), or preimmune serum for controls daily for a period of 7 or 4 d. Tongues from mouse pups were harvested and fixed in paraformaldehyde (4% PFA for 3 h); lungs from the same mice were removed and rapidly frozen, and tyrosine phosphorylation of Tie-2 was analyzed as described previously (Thurston et al., 2005). Longitudinal vibratome sections (100 µm) of tongues and trachea whole mounts were fixed for another 30 min, permeabilized with 0.5% Triton X-100, and stained with mAbs against PECAM-1 and pAbs against phospho-Histone 3, followed by detection with Alexa 488– and Alexa 568–coupled secondary antibodies and mounted in mowiol (Sigma-Aldrich). Confocal fluorescence images were collected using a confocal laser-scan microscope (Carl Zeiss, Inc.). Measurements of vessel diameter were performed on three tongues per group on four representative regions per tongue. Counting of phospho-Histone 3 positive endothelial cells was performed in three tongues per group on nine representative regions per tongue.
Live imaging of allantois explants
For live imaging, allantois explants were dissected from E8.0 to E8.5 VE-cadherin-EGFP expressing embryos, cultured on 0.5% gelatin-coated µ-slides (Ibidi) in a live imaging chamber (Ibidi), which guaranteed 85% humidity, 5% CO2, and 37°C. After cultivation for 6–12 h, the EGFP fluorescence signal was detected using a confocal laser-scan microscope (Carl Zeiss, Inc.). Optical sections of the cultured explants were collected along the z-axis and collapsed into a single focal plane for each indicated time point. After stopping confocal analysis, allantoides were fixed and immunolabeled with anti–PECAM-1 and phospho-Histone 3 antibodies as described previously (Baumer et al., 2006).
Microscopy
All images were acquired using a fluorescence microscope (Axioskop; Carl Zeiss, Inc.) with 10×/NA 0.5, 20×/NA 0.75, 40×/NA 0.75, or 100×/NA 1.3 objectives (Carl Zeiss, Inc.) in conjunction with a digital camera (RT KE/SE Spot; Diagnostic Instruments, Inc.). Alternatively, a confocal laser-scan microscope (510 META; Carl Zeiss, Inc.) was used with 20×/NA 0.8, 40×/NA 1.2, or 63×/NA 1.4 objectives (Carl Zeiss, Inc.).
Statistical analysis
The endothelial cord diameter measurements in allantois explants were made by drawing a straight line from edge to edge of the vessel (diameter) at a point in the vessel located mid-distance between adjacent branches. The detailed method is illustrated by a drawing in LaRue et al. (2003). Western blots were analyzed by measurements of pixel intensity using Multi Gauche software (Fujifilm). P-values were calculated by using two-way Student’s t or Mann-Whitney U tests. Error bars indicate standard deviation of the mean.
Online supplemental material
Figure S1 shows that polyclonal and monoclonal antibodies against VE-PTP trigger vessel enlargement in allantois explants and do not affect junctional proteins. Figure S2 demonstrates that endocytosed VE-PTP colocalizes first with early endosomes and is subsequently degraded, whereas junctional structures stay intact. Figure S3 shows that endogenously expressed Tie-2 and VE-PTP associate in endothelial cells. Figure S4 shows allantois explants treated with anti–VE-PTP antibodies in the presence of the Erk1/2 inhibitor U0126. Figure S5 demonstrates that Tie-2 is no longer cell surface distributed as shown in Fig. 3 A, but located at cell–cell contacts upon stimulation with COMP-Ang1. The same monoclonal antibody against Tie-2 was used as in Fig. 3 A. Videos 1–3 show allantois explants expressing VE-cadherin-GFP imaged for 10–14 h in the presence of polyclonal antibodies against VE-PTP (Video 2), preimmune antibodies (Video 1), or 600 ng/ml COMP-Ang1 in an Ibidi live-imaging chamber (Video 3). Video 4 shows an animation of a 3D reconstruction of an allantois cut-out. Allantois was treated with control antibodies and subsequently stained for PECAM-1 and phospho-Histone 3. Videos 5 and 6 show that antibodies against VE-PTP trigger endocytosis of VE-PTP in endothelial cells of allantois explant cultures (Video 5) in comparison to preimmune serum (Video 6). Video 7 demonstrates that VE-PTP is localized to the cell surface and cell–cell contacts in endothelial cells of untreated allantois explant cultures. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200811159/DC1.
Acknowledgments
We thank Ralf Adams for critically reading the manuscript.
This work was supported by the Deutsche Forschungsgemeinschaft (SFB629) and by the Max-Planck-Society.
Footnotes
Abbreviations used in this paper: ESAM, endothelial cell selective adhesion molecule; VE-PTP, vascular endothelial protein tyrosine phosphatase.
References
- Baumeister U., Funke R., Ebnet K., Vorschmitt H., Koch S., Vestweber D. 2005. Association of Csk to VE-cadherin and inhibition of cell proliferation.EMBO J. 24:1686–1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumer S., Keller L., Holtmann A., Funke R., August B., Gamp A., Wolburg H., Wolburg-Buchholz K., Deutsch U., Vestweber D. 2006. Vascular endothelial cell specific phospho-tyrosine phosphatase (VE-PTP) activity is required for blood vessel development.Blood. 107:4754–4762 [DOI] [PubMed] [Google Scholar]
- Brindle N.P., Saharinen P., Alitalo K. 2006. Signaling and functions of angiopoietin-1 in vascular protection.Circ. Res. 98:1014–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho C.H., Kim K.E., Byun J., Jang H.S., Kim D.K., Baluk P., Baffert F., Lee G.M., Mochizuki N., Kim J., et al. 2005. Long-term and sustained COMP-Ang1 induces long-lasting vascular enlargement and enhanced blood flow.Circ. Res. 97:86–94 [DOI] [PubMed] [Google Scholar]
- Davis S., Aldrich T.H., Jones P.F., Acheson A., Compton D.L., Jain V., Ryan T.E., Bruno J., Radziejewski C., Maisonpierre P.C., Yancopoulos G.D. 1996. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning.Cell. 87:1161–1169 [DOI] [PubMed] [Google Scholar]
- Dominguez M.G., Hughes V.C., Pan L., Simmons M., Daly C., Anderson K., Noguera-Troise I., Murphy A.J., Valenzuela D.M., Davis S., et al. 2007. Vascular endothelial tyrosine phosphatase (VE-PTP)-null mice undergo vasculogenesis but die embryonically because of defects in angiogenesis.Proc. Natl. Acad. Sci. USA. 104:3243–3248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake C.J., Fleming P.A. 2000. Vasculogenesis in the day 6.5 to 9.5 mouse embryo.Blood. 95:1671–1679 [PubMed] [Google Scholar]
- Dumont D.J., Gradwohl G., Fong G.H., Puri M.C., Gertsenstein M., Auerbach A., Breitman M.L. 1994. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo.Genes Dev. 8:1897–1909 [DOI] [PubMed] [Google Scholar]
- Eklund L., Olsen B.R. 2006. Tie receptors and their angiopoietin ligands are context-dependent regulators of vascular remodeling.Exp. Cell Res. 312:630–641 [DOI] [PubMed] [Google Scholar]
- Fachinger G., Deutsch U., Risau W. 1999. Functional interaction of vascular endothelial-protein-tyrosine phosphatase with the angiopoietin receptor Tie-2.Oncogene. 18:5948–5953 [DOI] [PubMed] [Google Scholar]
- Fujikawa K., de Aos Scherpenseel I., Jain S.K., Presman E., Christensen R.A., Varticovski L. 1999. Role of PI 3-kinase in angiopoietin-1-mediated migration and attachment-dependent survival of endothelial cells.Exp. Cell Res. 253:663–672 [DOI] [PubMed] [Google Scholar]
- Gale N.W., Thurston G., Hackett S.F., Renard R., Wang Q., McClain J., Martin C.B., Witte C., Witte M.H., Jackson D., et al. 2002. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1.Dev. Cell. 3:411–423 [DOI] [PubMed] [Google Scholar]
- Gotsch U., Borges E., Bosse R., Böggemeyer E., Simon M., Mossmann H., Vestweber D. 1997. VE-cadherin antibody accelerates neutrophil recruitment in vivo.J. Cell Sci. 110:583–588 [DOI] [PubMed] [Google Scholar]
- Jones N., Chen S.H., Sturk C., Master Z., Tran J., Kerbel R.S., Dumont D.J. 2003. A unique autophosphorylation site on Tie2/Tek mediates Dok-R phosphotyrosine binding domain binding and function.Mol. Cell. Biol. 23:2658–2668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda S., Miyata Y., Mochizuki Y., Matsuyama T., Kanetake H. 2005. Angiopoietin 1 is mitogenic for cultured endothelial cells.Cancer Res. 65:6820–6827 [DOI] [PubMed] [Google Scholar]
- Kim K.E., Cho C.H., Kim H.Z., Baluk P., McDonald D.M., Koh G.Y. 2007. In vivo actions of angiopoietins on quiescent and remodeling blood and lymphatic vessels in mouse airways and skin.Arterioscler. Thromb. Vasc. Biol. 27:564–570 [DOI] [PubMed] [Google Scholar]
- Koblizek T.I., Runting A.S., Stacker S.A., Wilks A.F., Risau W., Deutsch U. 1997. Tie2 receptor expression and phosphorylation in cultured cells and mouse tissues.Eur. J. Biochem. 244:774–779 [DOI] [PubMed] [Google Scholar]
- Koblizek T.I., Weiss C., Yancopoulos G.D., Deutsch U., Risau W. 1998. Angiopoietin-1 induces sprouting angiogenesis in vitro.Curr. Biol. 8:529–532 [DOI] [PubMed] [Google Scholar]
- LaRue A.C., Mironov V.A., Argraves W.S., Czirók A., Fleming P.A., Drake C.J. 2003. Patterning of embryonic blood vessels.Dev. Dyn. 228:21–29 [DOI] [PubMed] [Google Scholar]
- Maisonpierre P.C., Suri C., Jones P.F., Bartunkova S., Wiegand S.J., Radziejewski C., Compton D., Mc J., Clain T.H., Aldrich N., et al. 1997. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis.Science. 277:55–60 [DOI] [PubMed] [Google Scholar]
- Murray B.W., Padrique E.S., Pinko C., McTigue M.A. 2001. Mechanistic effects of autophosphorylation on receptor tyrosine kinase catalysis: enzymatic characterization of Tie2 and phospho-Tie2.Biochemistry. 40:10243–10253 [DOI] [PubMed] [Google Scholar]
- Nasdala I., Wolburg-Buchholz K., Wolburg H., Kuhn A., Ebnet K., Brachtendorf G., Samulowitz U., Kuster B., Engelhardt B., Vestweber D., Butz S. 2002. A transmembrane tight junction protein selectively expressed on endothelial cells and platelets.J. Biol. Chem. 277:16294–16303 [DOI] [PubMed] [Google Scholar]
- Nawroth R., Poell G., Ranft A., Samulowitz U., Fachinger G., Golding M., Shima D.T., Deutsch U., Vestweber D. 2002. VE-PTP and VE-cadherin ectodomains interact to facilitate regulation of phosphorylation and cell contacts.EMBO J. 21:4885–4895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottebaum A.F., Cagna G., Winderlich M., Gamp A.C., Linnepe R., Polaschegg C., Filippova K., Lyck R., Engelhardt B., Kamenyeva O., et al. 2008. VE-PTP maintains the endothelial barrier via plakoglobin and becomes dissociated from VE-cadherin by leukocytes and by VEGF.J. Exp. Med. 205:2929–2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss Y., Kiefer F. 2004. Immortalization of endothelial cells. In Methods in Endothelial Cell Biology. Augustin H.G., editor Springer, Berlin, Heidelberg, New York: 63–72 [Google Scholar]
- Reiss Y., Hoch G., Deutsch U., Engelhardt B. 1998. T cell interaction with ICAM-1-deficient endothelium in vitro: essential role for ICAM-1 and ICAM-2 in transendothelial migration of T cells.Eur. J. Immunol. 28:3086–3099 [DOI] [PubMed] [Google Scholar]
- Rodriguez F., Vacaru A., Overvoorde J., den Hertog J. 2008. The receptor protein-tyrosine phosphatase, Dep1, acts in arterial/venous cell fate decisions in zebrafish development.Dev. Biol. 324:122–130 [DOI] [PubMed] [Google Scholar]
- Saharinen P., Kerkela K., Ekman N., Marron M., Brindle N., Lee G.M., Augustin H., Koh G.Y., Alitalo K. 2005. Multiple angiopoietin recombinant proteins activate the Tie1 receptor tyrosine kinase and promote its interaction with Tie2.J. Cell Biol. 169:239–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saharinen P., Eklund L., Miettinen J., Wirkkala R., Anisimov A., Winderlich M., Nottebaum A., Vestweber D., Deutsch U., Koh G.Y., et al. 2008. Angiopoietins assemble distinct Tie2 signalling complexes in endothelial cell-cell and cell-matrix contacts.Nat. Cell Biol. 10:527–537 [DOI] [PubMed] [Google Scholar]
- Sato T.N., Tozawa Y., Deutsch U., Wolburg-Buchholz K., Fujiwara Y., Gendron-Maguire M., Gridley T., Wolburg H., Risau W., Qin Y. 1995. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation.Nature. 376:70–74 [DOI] [PubMed] [Google Scholar]
- Shewchuk L.M., Hassell A.M., Ellis B., Holmes W.D., Davis R., Horne E.L., Kadwell S.H., McKee D.D., Moore J.T. 2000. Structure of the Tie2 RTK domain: self-inhibition by the nucleotide binding loop, activation loop, and C-terminal tail.Structure. 8:1105–1113 [DOI] [PubMed] [Google Scholar]
- Shim W.S., Ho I.A., Wong P.E. 2007. Angiopoietin: a TIE(d) balance in tumor angiogenesis.Mol. Cancer Res. 5:655–665 [DOI] [PubMed] [Google Scholar]
- Suri C., Jones P.F., Patan S., Bartunkova S., Maisonpierre P.C., Davis S., Sato T.N., Yancopoulos G.D. 1996. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis.Cell. 87:1171–1180 [DOI] [PubMed] [Google Scholar]
- Takahashi T., Takahashi K., St John P.L., Fleming P.A., Tomemori T., Watanabe T., Abrahamson D.R., Drake C.J., Shirasawa T., Daniel T.O. 2003. A mutant receptor tyrosine phosphatase, CD148, causes defects in vascular development.Mol. Cell. Biol. 23:1817–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichert-Kuliszewska K., Maisonpierre P.C., Jones N., Campbell A.I., Master Z., Bendeck M.P., Alitalo K., Dumont D.J., Yancopoulos G.D., Stewart D.J. 2001. Biological action of angiopoietin-2 in a fibrin matrix model of angiogenesis is associated with activation of Tie2.Cardiovasc. Res. 49:659–670 [DOI] [PubMed] [Google Scholar]
- Thurston G., Wang Q., Baffert F., Rudge J., Papadopoulos N., Jean-Guillaume D., Wiegand S., Yancopoulos G.D., McDonald D.M. 2005. Angiopoietin 1 causes vessel enlargement, without angiogenic sprouting, during a critical developmental period.Development. 132:3317–3326 [DOI] [PubMed] [Google Scholar]
- Trapasso F., Drusco A., Costinean S., Alder H., Aqeilan R.I., Iuliano R., Gaudio E., Raso C., Zanesi N., Croce C.M., Fusco A. 2006. Genetic ablation of Ptprj, a mouse cancer susceptibility gene, results in normal growth and development and does not predispose to spontaneous tumorigenesis.DNA Cell Biol. 25:376–382 [DOI] [PubMed] [Google Scholar]
- Vikkula M., Boon L.M., Carraway K.L., Calvert J.T., Diamonti A.J., Goumnerov B., Pasyk K.A., Marchuk D.A., Warman M.L., Cantley L.C., et al. 1996. Vascular dysmorphogenesis caused by an activating mutation in the receptor tyrosine kinase TIE2.Cell. 87:1181–1190 [DOI] [PubMed] [Google Scholar]
- Wegmann F., Petri J., Khandoga A.G., Moser C., Khandoga A., Volkery S., Li H., Nasdala I., Brandau O., Fässler R., et al. 2006. ESAM supports neutrophil extravasation, activation of Rho and VEGF-induced vascular permeability.J. Exp. Med. 203:1671–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzenbichler B., Maisonpierre P.C., Jones P., Yancopoulos G.D., Isner J.M. 1998. Chemotactic properties of angiopoietin-1 and -2, ligands for the endothelial-specific receptor tyrosine kinase Tie2.J. Biol. Chem. 273:18514–18521 [DOI] [PubMed] [Google Scholar]
- Yuan H.T., Venkatesha S., Chan B., Deutsch U., Mammoto T., Sukhatme V.P., Woolf A.S., Karumanchi S.A. 2007. Activation of the orphan endothelial receptor Tie1 modifies Tie2-mediated intracellular signaling and cell survival.FASEB J. 21:3171–3183 [DOI] [PubMed] [Google Scholar]
- Zhu J.W., Brdicka T., Katsumoto T.R., Lin J., Weiss A. 2008. Structurally distinct phosphatases CD45 and CD148 both regulate B cell and macrophage immunoreceptor signaling.Immunity. 28:183–196 [DOI] [PMC free article] [PubMed] [Google Scholar]