Abstract
Stem cells use both transcriptional and epigenetic mechanisms to control gene expression and regulate tissue development and homeostasis. In this issue, Gu et al. (Gu, B., P. Sun, Y. Yuan, R.C. Moraes, A. Li, A. Teng, A. Agrawal, C. Rhéaume, V. Bilanchone, J.M. Veltmaat, et al. 2009. J. Cell Biol. 185:811–826) reveal an important link between these two mechanisms in mammary epithelial stem cells by showing that transcriptional activation of β-catenin downstream of Wnt signaling can be regulated epigenetically through a chromatin remodeling factor, Pygo2.
The recent characterization of stem cells in mammary epithelium has opened the door for understanding how mammary epithelial stem cells are regulated during tissue development, homeostasis, and tumorigenesis (Shackleton et al., 2006; Stingl et al., 2006). Fully functional mammary gland formation occurs in three stages: embryonic development, puberty, and pregnancy. During embryogenesis, cells of the multipotent ventral ectoderm condense to form placodes that migrate into the dense mammary mesenchyme to form a rudimentary ductal tree. In response to hormonal changes during puberty, the ductal tree matures into a complex, branched network of growing ducts that terminate into terminal end buds (TEBs), each containing stem cells with the capacity to form either ductal or luminal alveolar cell types. Pregnancy activates rapid growth and proliferation of TEB stem cell populations to form expansive ductal networks and to differentiate into secretory alveoli, which produce milk that is secreted into the ductal lumens during lactation. At the end of lactation, ductal and alveoli structures regress, and stem cells of the TEB await another round of growth and differentiation during subsequent pregnancies. It is thought that stem cells in the mammary epithelium are involved in tumor development (Molyneux et al., 2007).
Multiple stages of mammary development and tumorigenesis are regulated by Wnt/β-catenin signaling (Boras-Granic and Wysolmerski, 2008). Wnt ligands signal by increasing cytoplasmic pools of β-catenin. In unstimulated cells, β-catenin is phosphorylated and targeted to ubiquitin-mediated degradation through its interaction with the multiprotein axin complex containing adenomatous polyposis coli protein and GSK3β. When secreted Wnt proteins bind to Frizzled receptors, formation of the axin complex is inhibited, leading to cytoplasmic accumulation and nuclear translocation of β-catenin. In coordination with Tcf (T cell factor)/lymphoid enhancer factor (Lef) DNA–binding proteins, β-catenin regulates the transcription of several gene targets and is crucial for mammary development. Deletion of Lef1 (van Genderen et al., 1994) or overexpression of Dkk1, a soluble inhibitor of Wnt ligands, halts mammary gland development (Andl et al., 2002; Chu et al., 2004). In addition, Wnts can promote tumorigenesis in mammary epithelium (Nusse and Varmus, 1982) and can increase mammary stem cell number four- to sixfold (Shackleton et al., 2006).
Pygopus (Pygo) genes were first identified in Drosophila melanogaster as one mechanism that controls Wnt/β-catenin signaling. In Drosophila, Pygo proteins control β-catenin–Lef–Tcf transcriptional complexes through regulation of either β-catenin nuclear translocation or by binding to β-catenin via the adapter protein BCL9 (Belenkaya et al., 2002; Kramps et al., 2002; Parker et al., 2002; Thompson, 2004). Pygo proteins also contain a plant homeo domain (PHD; Fiedler et al., 2008) that binds to methylated residues on lysine 4 of histone H3 (H3K4me), an epigenetic mark linked to active transcription (Santos-Rosa et al., 2002). However, the biological relevance of Pygo’s binding to methylated histone residues or how these interactions function to regulate Wnt signaling was unclear.
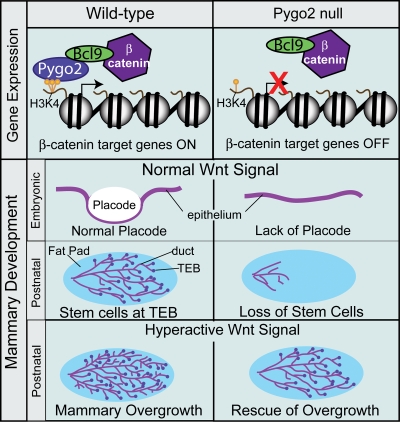
Now, Gu et al. (see p. 811 of this issue) make the first link between Pygo expression and regulation of Wnt signaling in a mammalian tissue. Two pygo genes exist in mammals, mPygo1 and mPygo2, with the latter having a broader expression pattern (Li et al., 2004). Examination of the phenotype of Pygo2 deletion in mammary epithelium revealed reduced formation of mammary placodes during embryogenesis caused by decreased progenitor proliferation. In epithelial-specific Pygo2-null mice, postnatal mammary development that does progress to form ductal networks exhibits reduced ductal elongation and a lack of TEB structures (Fig. 1). Analysis of the number and function of mammary stem cells (Lin−CD24+/CD29High) in these mutant ductal structures revealed reductions in both stem cell number and the ability to form mammary structures in transplantation assays, thus confirming a role for Pygo2 in mammary stem cell self-renewal. Furthermore, Wnt signaling is reduced in Pygo2-null mammary epithelium. Thus, Pygo2 controls proliferation and self-renewal of embryonic mammary epithelial progenitors and postnatal stem cells through the promotion of Wnt signaling (Fig. 1).
Figure 1.
Pygo2 regulates Wnt signaling during mammary gland development. Pygo2 promotes trimethylation (yellow) of lysine 4 of histone H3 (H3K4) and synergistically binds these residues and BCL9–β-catenin complexes to activate β-catenin target genes in mammary epithelium. Loss of Pygo2 in mice results in embryonic defects of mammary progenitor cells and placode (white circle) formation. In addition, loss of Pygo2 results in postnatal defects in mammary ductal networks caused by the lack of stem cells in TEBs (purple circles). Pygo2-null mammary glands rescue mammary overgrowth phenotypes when Wnt signaling is hyperactivated.
How does Pygo2 regulate Wnt signaling and progenitor proliferation in the mammary gland? Using biochemistry, cell biology, and mouse genetics, Gu et al. (2009) demonstrate that Pygo2 promotes Wnt signaling in mammary epithelium through its synergistic interactions with di- and trimethylated histones and the recruitment of β-catenin–BCL9 complexes to promoters of β-catenin target genes. Pygo2 expression in mammary epithelium is required for Lef1 expression and Wnt signaling reporter activation similar to its role in Drosophila and Xenopus laevis. Furthermore, loss of Pygo2 can suppress overgrowth in mammary glands expressing activating mutations of β-catenin (Fig. 1). The regulation of mammary cell proliferation requires Pygo2’s ability to interact with trimethylated K4 residues of histone H3 and its interaction with BCL9–β-catenin complexes at β-catenin target genes. Together, these data support the key role of Pygo2 as a link between activated chromatin and the recruitment of β-catenin to its target genes.
The regulation of developmental processes by proteins that bind to and activate H3 K4 methylation has been described for other PHD-containing proteins, including WDR5. Interestingly, WDR5 also regulates Xenopus development and murine osteoblast formation (Wysocka et al., 2005; Gori et al., 2006) and can have specificity by regulating Wnt signaling (Zhu et al., 2008). Additional chromatin regulators have been shown to link epigenetic and transcriptional control of gene expression, as shown for MLL1 at Hox genes (Guenther et al., 2005). However, the regulatory mechanisms that link these epigenetic regulatory factors to transcription are not clear. Future insights into how proteins that recognize activating or repressive epigenetic histone marks through their recruitment of specific transcription factors will further extend our knowledge of the important link between epigenetic and transcriptional regulation of gene expression.
Beyond its function in regulating gene expression, Pygo’s role in the proliferation of mammary progenitors reveals several interesting aspects of mammary biology. First, common mechanisms that regulate embryonic mammary progenitors and adult mammary stem cells have not been identified. The data presented by Gu et al. (2009) suggest that Pygo2 regulates proliferation of both embryonic mammary progenitors and postnatal mammary stem cells of the TEB, revealing the first mechanism of shared regulation by these pools of mammary progenitor populations. Furthermore, the control of proliferation by Pygo2 suggests that blocking Pygo2 may serve as a potential therapeutic target given its striking ability to block mammary overgrowth with Wnt hyperactivation (Fig. 1). Future studies identifying molecules or proteins that regulate the activity or the interactions of Pygo2 may serve as potent mammary tumor inhibitors.
Acknowledgments
I thank Drs. Weimin Zhong and Matthew Rodeheffer for comments on this manuscript.
The Horsley laboratory is funded by grants from the National Institutes of Health (4R00AR054775) and the Connecticut Department of Public Health (09SCAYALE30).
References
- Andl T., Reddy S., Gaddapara T., Millar S. 2002. WNT signals are required for the initiation of hair follicle development.Dev. Cell. 2:643–653 [DOI] [PubMed] [Google Scholar]
- Belenkaya T.Y., Han C., Standley H.J., Lin X., Houston D.W., Heasman J. 2002. pygopus Encodes a nuclear protein essential for wingless/Wnt signaling.Development. 129:4089–4101 [DOI] [PubMed] [Google Scholar]
- Boras-Granic K., Wysolmerski J.J. 2008. Wnt signaling in breast organogenesis.Organogenesis. 4:116–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu E.Y., Hens J., Andl T., Kairo A., Yamaguchi T.P., Brisken C., Glick A., Wysolmerski J.J., Millar S.E. 2004. Canonical WNT signaling promotes mammary placode development and is essential for initiation of mammary gland morphogenesis.Development. 131:4819–4829 [DOI] [PubMed] [Google Scholar]
- Fiedler M., Sanchez-Barrena M.J., Nekrasov M., Mieszczanek J., Rybin V., Muller J., Evans P., Bienz M. 2008. Decoding of methylated histone H3 tail by the Pygo-BCL9 Wnt signaling complex.Mol. Cell. 30:507–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gori F., Friedman L.G., Demay M.B. 2006. Wdr5, a WD-40 protein, regulates osteoblast differentiation during embryonic bone development.Dev. Biol. 295:498–506 [DOI] [PubMed] [Google Scholar]
- Gu B., Sun P., Yuan Y., Moraes R.C., Li A., Teng A., Agrawal A., Rhéaume C., Bilanchone V., Veltmaat J.M., et al. 2009. Pygo2 expands mammary progenitor cells by facilitating histone H3 K4 methylation.J. Cell Biol. 185:811–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther M.G., Jenner R.G., Chevalier B., Nakamura T., Croce C.M., Canaani E., Young R.A. 2005. Global and Hox-specific roles for the MLL1 methyltransferase.Proc. Natl. Acad. Sci. USA. 102:8603–8608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramps T., Peter O., Brunner E., Nellen D., Froesch B., Chatterjee S., Murone M., Züllig S., Basler K. 2002. Wnt/wingless signaling requires BCL9/legless-mediated recruitment of pygopus to the nuclear beta-catenin-TCF complex.Cell. 109:47–60 [DOI] [PubMed] [Google Scholar]
- Li B., Mackay D., Ma J., Dai X. 2004. Cloning and developmental expression of mouse pygopus 2, a putative Wnt signaling component.Genomics. 84:398–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneux G., Regan J., Smalley M.J. 2007. Mammary stem cells and breast cancer.Cell. Mol. Life Sci. 64:3248–3260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R., Varmus H. 1982. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome.Cell. 31:99–109 [DOI] [PubMed] [Google Scholar]
- Parker D.S., Jemison J., Cadigan K.M. 2002. Pygopus, a nuclear PHD-finger protein required for Wingless signaling in Drosophila.Development. 129:2565–2576 [DOI] [PubMed] [Google Scholar]
- Santos-Rosa H., Schneider R., Bannister A.J., Sherriff J., Bernstein B.E., Emre N.C., Schreiber S.L., Mellor J., Kouzarides T. 2002. Active genes are tri-methylated at K4 of histone H3.Nature. 419:407–411 [DOI] [PubMed] [Google Scholar]
- Shackleton M., Vaillant F., Simpson K.J., Stingl J., Smyth G.K., Asselin-Labat M.L., Wu L., Lindeman G.J., Visvader J.E. 2006. Generation of a functional mammary gland from a single stem cell.Nature. 439:84–88 [DOI] [PubMed] [Google Scholar]
- Stingl J., Eirew P., Ricketson I., Shackleton M., Vaillant F., Choi D., Li H.I., Eaves C.J. 2006. Purification and unique properties of mammary epithelial stem cells.Nature. 439:993–997 [DOI] [PubMed] [Google Scholar]
- Thompson B.J. 2004. A complex of Armadillo, Legless, and Pygopus coactivates dTCF to activate wingless target genes.Curr. Biol. 14:458–466 [DOI] [PubMed] [Google Scholar]
- van Genderen C., Okamura R., Fariñas I., Quo R., Parslow T., Bruhn L., Grosschedl R. 1994. Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice.Genes Dev. 8:2691–2703 [DOI] [PubMed] [Google Scholar]
- Wysocka J., Swigut T., Milne T.A., Dou Y., Zhang X., Burlingame A.L., Roeder R.G., Brivanlou A.H., Allis C.D. 2005. WDR5 associates with histone H3 methylated at K4 and is essential for H3 K4 methylation and vertebrate development.Cell. 121:859–872 [DOI] [PubMed] [Google Scholar]
- Zhu E.D., Demay M.B., Gori F. 2008. Wdr5 is essential for osteoblast differentiation.J. Biol. Chem. 283:7361–7367 [DOI] [PubMed] [Google Scholar]