Summary
The (re)design of enzymes to catalyze “new” reactions is a topic of considerable practical and intellectual interest. Directed evolution (random mutagenesis followed by screening/selection) has been used widely to identify novel biocatalysts. However, “rational” approaches using either natural divergent evolution or computational predictions based on chemical principles have been less successful. This review summarizes recent progress in evolution- and computation-based (re)design.
Introduction
The idea of “made-to-order” enzymes both fascinates and challenges chemical biologists. The fascination is that if we understand principles of catalysis, we should be able to design “new” enzymes, using either modifications of the structures of natural proteins or, even, de novo scaffolds. The challenge is that, in contrast to natural evolution, laboratory design must be focused and rapid.
This review focuses on (re)design of members of mechanistically diverse superfamilies. Also, examples of computational (re)design of novel activities in unrelated structural scaffolds are described. Structure-based approaches for (re)design [e.g., 1–4] have been reviewed [e.g., 5,6].
Natural evolution of function: mechanistically diverse superfamilies
Mechanistically diverse superfamilies provide opportunities to decipher Nature’s strategies for evolving “new” functions in a conserved structural scaffold and to use that information to guide (re)design [7]. The members catalyze different reactions that share a catalytic strategy [8]. In the enolase superfamily (the first mechanistically diverse superfamily that was discovered [9]), an active site base abstracts the α-proton of a carboxylate substrate to generate an enediolate intermediate stabilized by coordination to an essential Mg2+; different products are formed in a second, divergent partial reaction [10,11]. Interchanging reactions catalyzed by members of mechanistically diverse superfamilies might be envisioned as “easy” exercises in (re)design: if Nature did it, why can’t we? Indeed, such speculation began as soon as the enolase superfamily was discovered [12]. Anecdotally, many attempts at interchanging activities in mechanistically diverse superfamilies have since been attempted, but few successes have been realized. Homologous enzymes that catalyze different reactions usually share ≤40% sequence identity, suggesting that optimization of a “new” function requires many substitutions. However, the introduction of a selectable level of a “new” function should be expected to occur with a minimal number of substitutions (one ?) [13].
Members of mechanistically diverse superfamilies often are promiscuous, catalyzing their physiological reaction as well as other reactions that may not be those catalyzed by homologous members of the superfamily [13]. This promiscuity provides opportunistic “starting points” for both in vivo and in vitro evolution of new functions. Indeed, the enhancement and exploitation of catalytic promiscuity has emerged as an important strategy for developing novel biocatalysts that has been reviewed extensively [5,6,14–18].
Evolution-based design: “new” functions
The most difficult challenge in (re)design is the introduction of substitutions that allow a “new” reaction, i.e., not simply enhancement of a promiscuous reaction. The idea behind evolution-based design is that the comparisons of sequences of members of mechanistically diverse superfamilies should provide sufficient information to (re)design active sites to catalyze reactions catalyzed by homologues.
Enolase Superfamily
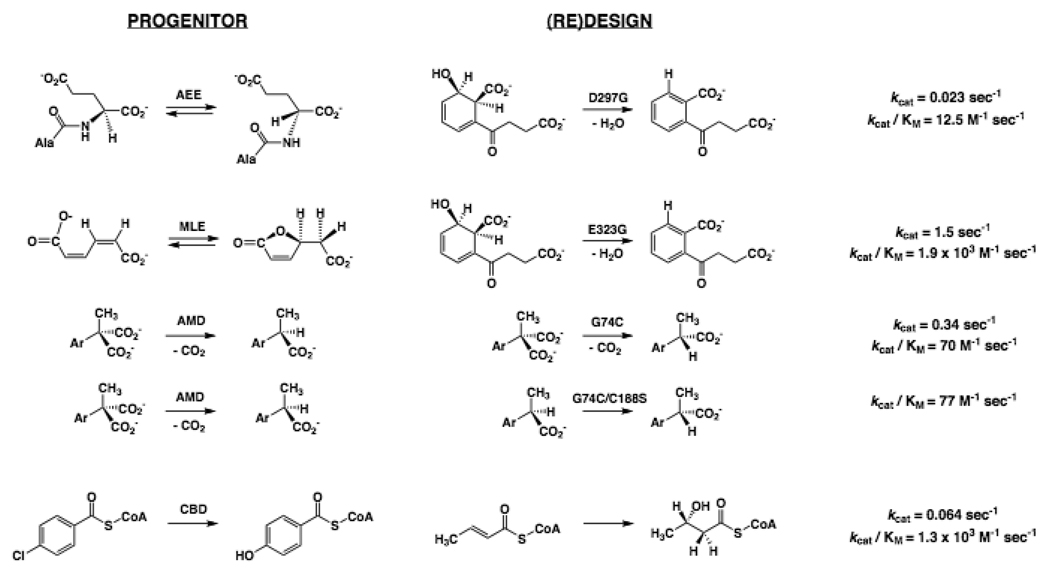
In the enolase superfamily, a single substitution permits both an L-Ala-D/L-Glu epimerase (AEE) and a muconate lactonizing enzyme (MLE) to catalyze the o-succinylbenzoate synthase (OSBS) reaction (Figure 1); neither progenitor is promiscuous for the OSBS reaction [19]. The natural enzymes that catalyze the AEE, MLE, and OSBS reactions share potential Lys acid/base catalysts on opposite faces of the active site at the ends of the second and sixth β-strands in the (β/α)7β-barrel (modified TIM-barrel) domain. Design in the case of the AEE from E. coli and directed evolution (random mutagenesis/metabolic selection) in the case of the MLE from Pseudomonas sp. P51 identified homologous single-residue substitutions that introduced OSBS activity while retaining a reduced level of the progenitor’s activity: substitution of a Gly for an Asp (AEE) or Glu (MLE) at the end of the eighth β-strand was sufficient to introduce OSBS activity. These produced remarkable increments in the rate accelerations for the OSBS reaction: 108 for the (re)designed AEE and 1010 for the MLE variant.
Figure 1.
Progenitors, (re)designed reactions, and kinetic constants for the “new” reactions obtained using evolution-based approaches.
In the AEE, the ability of the Asp to Gly mutant to catalyze the OSBS reaction is attributed to relaxation of a strict substrate specificity for a dipeptide substrate determined by the Asp that hydrogen bonds to the ammonium group of the substrate; presumably, the Asp prevents binding (by steric and electrostatics) of the substrate for the OSBS reaction, with these restrictions relieved by the Gly substitution. Further evidence that alteration of specificity determinants is sufficient to change the identity of the reaction was obtained by directed evolution of the AEE variant. The Y19F substitution in the 20s loop of the capping domain that sequesters the substrate from solvent produced increases in kcat and kcat/KM [20]; in subsequent rounds, additional beneficial substitutions (R24C, R24C/L277W, and R24W) within the active site cavity were identified [21]. Thus, the identity of the reaction was changed by altering a specificity-determining residue, not a catalytic residue.
As the catalytic efficiency for the OSBS reaction was increased, the efficiency of the progenitor’s AEE markedly decreased. In these experiments, the promiscuity generated by the designed substitution was rapidly “lost” as the OSBS reaction was enhanced, i.e., if this process mimics natural divergent evolution of function, the “new” activity is established at the expense of the progenitor’s activity. Tawfik has summarized other examples of directed evolution of new functions in which the progenitor’s activity is not compromised as the new activity is enhanced [17,22].
Asp/Glu racemase superfamily
A minimal number of substitutions is sufficient to interchange activities in the Cys-dependent Asp/Glu racemase superfamily that includes members catalyzing decarboxylation of (prochiral) α-aryl-α-methylmalonates to yield (R)-α–arylpropionates (AMD). In the paradigm racemases, Cys residues located on opposite faces of the active site are the acid/base catalysts, and the enolate intermediate is stabilized by hydrogen-bonding with main-chain NH groups. In the decarboxylases, loss of CO2 generates the enolate intermediate, so only a single Cys residue is present [23]. Homology-based (re)designs of the decarboxylase from Alkaligenenesis bronchiosepticus (Figure 1) generated α–arylpropionate racemase activity by introducing a Cys on the opposite face of the active site (G74C) [24]; and 2) inverted the stereospecificity of decarboxylation by removing the wild type Cys and introducing a Cys on the opposite face of the active site (G74C/C188S) [23]. The designed racemase retained decarboxylase activity but produced racemic α–arylpropionate products, as expected for symmetric protonation of the enolate intermediate. In both examples of (re)design, the kinetic constants for the “new” activities were low; however, the activity of “inverted” decarboxylase could be improved by directed evolution [25].
Enoyl-CoA hydratase superfamily
The reactions catalyzed by most members of the enoyl-CoA hydratase (crotonase) superfamily involve enolate intermediates derived from coenzyme A-thioesters, including syn-hydration of trans-2-enoyl-CoAs by crotonase and dehalogenation of 4-chlorobenzoyl-CoA by a dehalogenase (CBD) [26]. Hydration involves stepwise syn-addition of water catalyzed by two Glu residues (Glu 144 and 164 in the rat liver enzyme); dehalogenation involves displacement of chloride by Asp 145 to form an acyl-enzyme intermediate followed by hydrolysis of the aryl ester with the assistance of His 90. Installation of two Glu residues in the dehalogenase at the same positions as the catalysts in crotonase produced an unstable protein [27]. Six additional substitutions were engineered to allow proper orientation of the Glu catalysts; the octamutant catalyzed slow syn-hydration of trans-2-butenoyl-CoA (Figure 1). Although the efficiency of the (re)designed protein is less than that of the natural crotonase, this success is remarkable because the mechanisms for generating the enolate intermediates are very different (nucleophilic addition to the aromatic ring to form a Meisenheimer complex in the progenitor dehalogenase; addition of water to the conjugated enoyl ester in the designed enoyl-CoA hydratase).
Evolution-based design: enhancement of promiscuous functions
A more successful (re)design approach has been to incorporate substitutions, based on sequence and structure alignments, that enhance promiscuous activities, often, but not always, at the expense of the activity of the starting template.
N-Acetylneuraminate lyase (NAL) superfamily
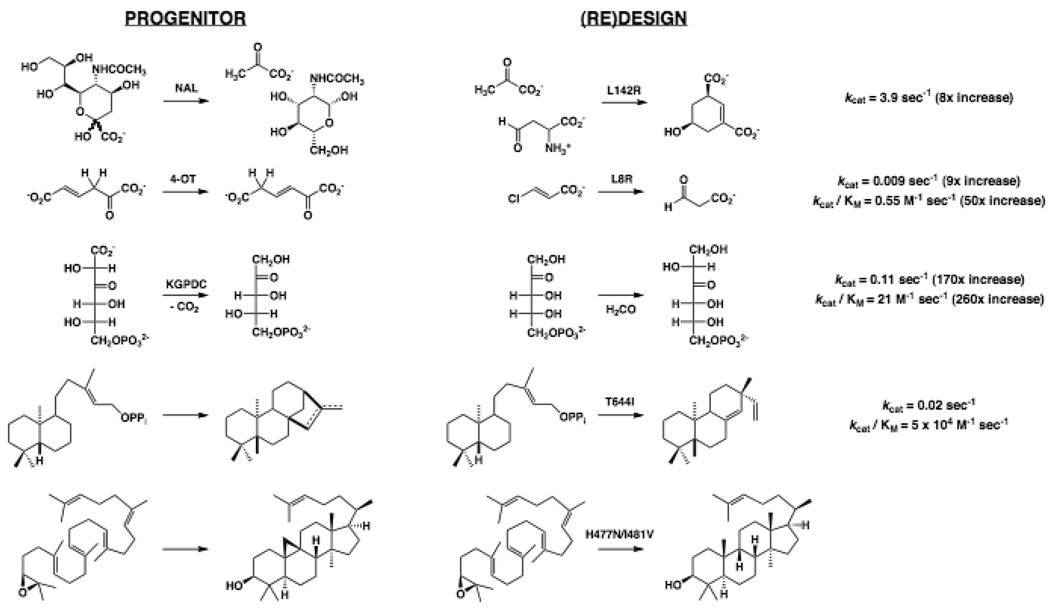
The members of the NAL superfamily catalyze reactions involving formation of a Schiff base with an α-ketoacid followed by (retro)aldol condensation, β-elimination of water, and/or vinylogous decarboxylation reaction [28,29]. The paradigm NAL catalyzes the retroaldol cleavage of N-acetylneuraminate to yield pyruvate and N-acetyl-D-mannosamine; the homologous dihydrodipicolinate synthase (DHDPS) catalyzes the condensation of pyruvate with L-aspartate-β-semialdehyde (ASA) in lysine biosynthesis. NAL is promiscuous for the DHDPS reaction, with sequence alignments identifying a Leu in NAL that is replaced by an Arg in DHDPS; this residue had been implicated in binding the aldehyde cosubstrate [30]. The Leu to Arg substitution was constructed in the NAL scaffold, with additional constructs containing substitutions for spatially proximal residues so that the Arg might be better accommodated (Figure 2). Modest enhancements in the DHDPS activity were observed, with increases in kcat and decreases in the Km for ASA. The NAL activity of the template was compromised, as the result of increases in the Km for N-acetylneuraminate.
Figure 2.
Progenitors, (re)designed reactions, and kinetic constants for enhanced promiscuous reactions obtained using evolution-based approaches.
4-Oxalocrotonate tautomerase (4-OT) superfamily
The members of 4-oxalocrotonate tautomerase (4-OT) superfamily catalyze reactions involving an enolate intermediate derived from a carboxylate substrate using a conserved N-terminal Pro as either a general acid or general base catalyst, depending on the reaction [31]. In tautomerization reactions, e.g., that catalyzed by 4-OT, the pKa of Pro 1 is 6.4, and it functions as a general base; in conjugate addition reactions, e.g., that catalyzed by trans-3-chloroacrylate dehalogenase (CaaD), the pKa of Pro 1 is 9.2, and it functions as a general acid. In 4-OT, Arg 11 stabilizes the enolate intermediate; in CaaD, the intermediate is stabilized by Arg 8 and Arg 11, although this active site also contains Glu 52 that facilitates addition of water. 4-OT is promiscuous for the CaaD reaction. Replacement of Leu 8 with Arg (L8R) in 4-OT enhances CaaD activity (Figure 2), consistent with enhanced stabilization of the enolate intermediate in hydration [32]. The L8R substitution did not significantly impact the 4-OT activity. The structures of the 4-OT and the L8R mutant reveal only small changes in active site structure.
OMPDC suprafamily
The orotidine 5’-monophosphate decarboxylase (OMPDC) suprafamily includes OMPDC (stabilization of a vinyl carbanion intermediate [33,34]) as well as 3-keto-L-gulonate 6-phosphate decarboxylase (KGPDC, decarboxylation of a β-ketoacid via an enolate intermediate [35]) and D-arabino-hex-3-ulose 6-phosphate synthase (HPS; aldol condensation via an enolate intermediate) [36]. The reaction catalyzed by OMPDC is metal ion-independent; those catalyzed by KGPDC and HPS require Mg2+ to stabilize the enolate intermediate. Interchange of the OMPDC and KGPDC/HPS reactions would be impressive examples of (re)design, but this has not been accomplished. KGPDC from E. coli and HPS from Methylomonas aminofaciens share 29% sequence identity; each is promiscuous for the other reaction. Residues conserved in KGPDCs but not in HPSs include hydrogen bond donors involved in substrate recognition and others that facilitate stereorandom protonation of the enediolate intermediate derived [37]. A tetramutant of KGPDC that incorporates consensus HPS residues increased kcat for the HPS reaction by a factor of 170; a trimutant increased kcat/KM by a factor of 260. Both displayed retained reduced KGPDC activity (Figure 2). Structures for the triple mutant complexed with the D-ribulose 5-phosphate substrate for the HPS reaction and the L-xylulose 5-phosphate product for the KGPDC reaction revealed only small changes in active site geometry relative to wild type KGPDC complexed with the same ligands [38], preventing a definitive explanation for the large enhancement of the HPS activity.
Terpene synthases/oxidosqualene cyclases
The members of the terpene synthase and oxidosqualene cyclase superfamilies involve reactive carbocation intermediates obtained from allylic (e.g., geranyl, farnesyl, or geranylgeranyl) pyrophosphates in the terpene synthases and epoxides in the oxidosqualene cyclases. In contrast to reactions that involve enolate intermediates that must be stabilized to allow kinetic competence, the carbocations are directed to products by an appropriately shaped active site cavity with functional groups to quench the product cation by proton removal or reaction with water. These enzymes often are promiscuous as the result of rearrangements, incorrect deprotonation, and/or reaction with water [39]. If the structural features that allow these “mistakes” can be identified, specific and/or “novel” synthases can be (re)designed. Several examples of (re)design in both superfamilies have been reported.
Kaurene synthase in the terpene synthase superfamily has been converted to a pimaradiene synthase by the substitution of an Ile with Thr [40] (Figure 2); the aborted cyclization is attributed to the Thr OH that assists in deprotonation of a carbocation intermediate on the reaction coordinate. Similar approaches have been used with other terpene synthases [41–43], establishing the essential role of the hydrophobic active site to direct the reactive intermediates to the “correct” products. Analogously, cycloartenol synthase in the oxidosqualene cyclase superfamily has been (re)designed as a lanosterol synthase by substitutions for two conserved active site residues [44] (Figure 2).
A similar approach for (re)designing terpene synthases is evaluation of the effect of in vitro substitutions for active site residues on product promiscuity and use of that information to combine those substitutions that favor a desired product. γ-Humulene synthase was (re)designed to provide seven cyclases that form different sesquiterpenes from farnesyl pyrophosphate [45]. This approach also allows the discovery of novel promiscuities that may be cryptic in evolved cyclases but were properties of evolutionary intermediates [46,47].
Evolution-based (re)design of “new” functions: grafting of loops
The previous examples of (re)design changed function with one (or a few) substitutions. The evolution of different functions in mechanistically diverse superfamilies likely were initiated by such processes, so these in vitro experiments provide information about the early stages of natural divergent evolution [13]. However, sequence alignments reveal many examples of insertions/deletions of residues, raising the issue of the importance of such processes in natural (re)design [48]. Also, sequences that support different functions (families) within mechanistically superfamilies typically share <40% sequence identity, explaining why one (or a few) point mutations in a progenitor may not produce variants that catalyze “new” reaction with large values of kcat or kcat/Km. Therefore, more aggressive in vitro mutagenesis strategies may be required to (re)design members of mechanistically diverse superfamilies.
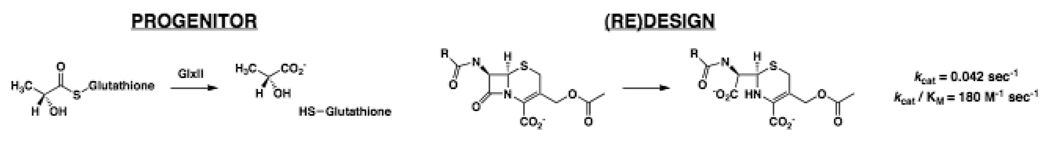
The members of a metallohydrolase superfamily catalyze reactions of carboxylate esters, sulfate esters, or phosphate esters. Glyoxalase II (D-lactoylglutathione, binuclear metal center with Fe, Mn, and/or Zn, GlxII) has been converted to a β-lactamase (binuclear Zn center) by insertions/deletions and loop swapping [49]. GlxII and IMP-1, the “target” β-lactamase, share low levels of sequence identity, different metal ligands, and loops of differing size/sequence that determine substrate specificity. After deletion of the glutathione binding domain, redesign of the metal binding site, randomization of specificity determining loops, and directed evolution, a variant was isolated that conferred antibiotic resistance and retained 59% sequence identity to the progenitor and shared 25% identity to the “target”; however, the kinetic parameters for cefotaxime are considerably less than those for IMP-1 (Figure 2). Thus, “aggressive” (re)design of a scaffold is possible. Whether this approach provides useful insights into the molecular events responsible for natural divergent evolution of novel functions is arguable, but implementing (re)design of active site loops clearly is essential for fully exploring structure-function space [48].
Computation-based (re)design of “new” functions
Evolution-based (re)design provides catalysts for natural reactions. Recently, catalysts for unnatural reactions have been obtained by two approaches: 1) alteration in the specificity-determining residues when the reaction is catalyzed by a natural enzyme, e.g., the generation of enantiospecific hydrolases from lipases using directed evolution [50–52]; and 2) de novo (computation-based) (re)design using a structurally characterized scaffold as the template for the “new” active site. The first approach is now widely used for the development of biocatalysts; the second approach represents the “frontier” for testing our understanding of the structural bases of enzymatic catalysis.
Two examples of computation-based (re)design of enzymes have been reported, both from the Baker laboratory [53]. The approach involves 1) in silico construction (using RosettaMatch) of active sites with an appropriate spatial arrangement of functional groups surrounding the presumed transition state in a scaffold that catalyzes an unrelated reaction; 2) in silico optimization of the active site geometry by changes in “outer sphere” residues; and 3) experimental confirmation by gene synthesis, protein purification, enzymatic assays, and x-ray crystallography.
Retro-aldolase
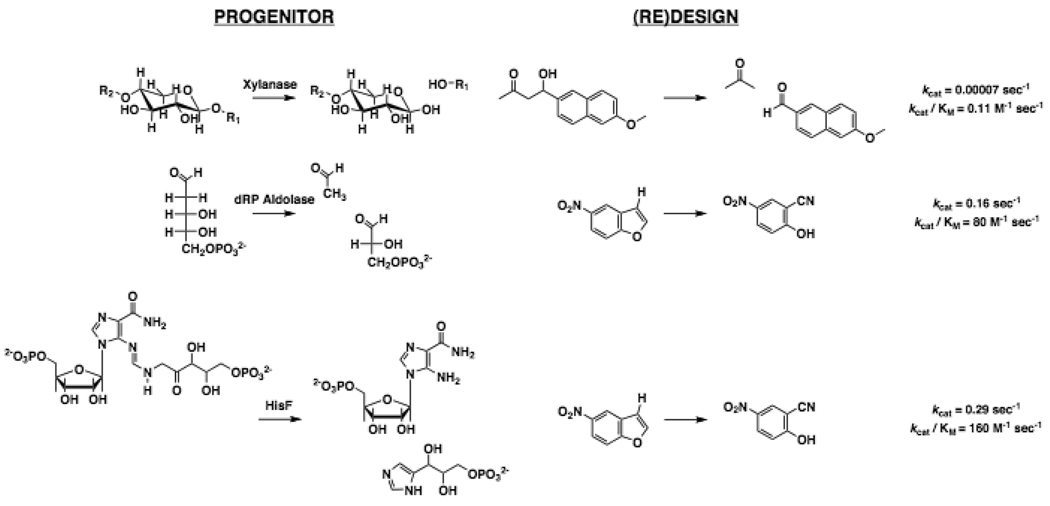
A “retro-aldolase” for cleavage of 4-hydroxy-4-(6-methoxy-2-naphthyl)-2-butanone was (re)designed using this approach; the aldehyde product is fluorescent, thereby providing a facile, sensitive assay [53]. Four variants of a Schiff base mechanism were designed: the mechanism is multi-step, requiring proton transfers to/from the covalent adduct as well as stabilization of several transition states. Seventy-one structural scaffolds, representing five different folds, were used as templates for the designed active sites; seventy-two designs were tested, based on either a jelly-roll or TIM-barrel scaffold. Twenty-eight were catalytically active: 8 using a His-Asp dyad to shuttle protons, and 20 using a water molecule, thereby mimicking the mechanism of 2-deoxy-D-ribose 5-phosphate aldolase (also a TIM-barrel scaffold) [54]. The “best” retroaldolase, based on the jelly-roll scaffold of a thermophilic β-xylanase (1M4W), provided a rate acceleration (kcat/kuncat) of 2.4 × 104 and an efficiency (kcat/Km) of 0.3 M−1 sec−1 (vs. 1 × 105 M−1 sec−1 for 2-deoxy-D-ribose 5-phosphate aldolase). Structures were determined for three retroaldolases; each showed good agreement with the predicted (re)design.
Kemp “eliminase”
The report of the (re)designed retro-aldolase was followed by the report of a (re)designed “Kemp eliminase” [55], an exergonic reaction initiated by proton abstraction from carbon that has no natural counterpart. Two catalytic strategies were evaluated, one using Asp or Glu as the base and the second using a His-Asp dyad. Fifty-nine designs were experimentally tested, with 8 showing activity that utilized TIM-barrels as scaffolds (1JCL, 2-deoxy-D-ribose 5-phosphate aldolase, and 1THF, imidazoleglycerol phosphate synthase, HisF). The values of kcat/Km ranged from 6 to 160 M−1 sec−1. Experimentally determined structures confirmed the accuracies of the models.
The values of the rate acceleration and efficiency (1.5 × 104 and 12 M−1 sec−1) for one Kemp eliminase were improved by seven rounds of directed evolution, increased to 1.2 × 106 and 2.6 × 103 M−1 sec−1. The mutations were located in the active site, adjacent to the catalytic residues to fine-tune their positions relative to the substrate/transition state and/or adjust electrostatic stabilization of the transition state. Directed evolution to optimize the computation-based (re)design enhances the utility of this approach.
Conclusions
Surprisingly few successes in (re)design have been reported, although many have been attempted. And, when successful, the rate accelerations and catalytic efficiencies almost always fall far short of evolved enzymes. Why should this be the case?
Catalysis is exquisitely sensitive to active site geometry. Subsequent directed evolution provides a potential solution to this problem, as illustrated by enhancement of the evolution-based (re)design OSBS activity on the AEE scaffold [21] and, also, the computation-based (re)design of the Kemp eliminase [55].
Templates optimized for a specific function may not be appropriate for (re)design, e.g., substitutions often are destabilizing so (re)design requires that the progenitor tolerate functional substitutions [56–58]. The sequence divergence associated with different functions in homologous proteins provides essential stabilization for the active site structures that catalyze different reactions [59]. At present, the choice of progenitor is “trial and error”; consensus sequences associated with phylogenetically reconstructed (stable) ancestors may provide the necessary stabilization for robust (re)design [60]
Natural evolution of new functions likely involves concomitant optimization of dynamical properties that direct the reaction coordinate [61–64]. (Re)design of the structural features that determine dynamics in concert with those that determine mechanism represents a formidable challenge that must be overcome
As the field of enzyme (re)design moves forward, solutions to these challenges will be required to obtain catalysts that rival those obtained by natural evolution. As these problems are addressed and solved, (re)design will provide insights into the structural requirements for both natural divergent evolution of enzyme function as well as strategies for obtaining novel catalysts for a wide range of reactions.
Figure 3.
Progenitor, (re)designed reaction, and kinetic constants for a “new” reaction obtained using evolution-based grafting of loops.
Figure 4.
Progenitors, (re)designed reactions, and kinetic constants for “new” reactions obtained using computation-based approaches.
Acknowledgements
The authors thank NIH R01 GM052594 (to JAG), R01 GM060595 (to PCB), R01 GM065155 (to JAG), and P01 GM071790 (to JAG and PCB) for support of research in their laboratories.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
• • of outstanding interest
- 1.Nixon AE, Firestine SM, Salinas FG, Benkovic SJ. Rational design of a scytalone dehydratase-like enzyme using a structurally homologous protein scaffold. Proc Natl Acad Sci U S A. 1999;96:3568–3571. doi: 10.1073/pnas.96.7.3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jurgens C, Strom A, Wegener D, Hettwer S, Wilmanns M, Sterner R. Directed evolution of a (beta alpha)8-barrel enzyme to catalyze related reactions in two different metabolic pathways. Proc Natl Acad Sci U S A. 2000;97:9925–9930. doi: 10.1073/pnas.160255397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seebeck FP, Hilvert D. Conversion of a PLP-dependent racemase into an aldolase by a single active site mutation. J Am Chem Soc. 2003;125:10158–10159. doi: 10.1021/ja036707d. [DOI] [PubMed] [Google Scholar]
- 4.Hederos S, Broo KS, Jakobsson E, Kleywegt GJ, Mannervik B, Baltzer L. Incorporation of a single His residue by rational design enables thiol-ester hydrolysis by human glutathione transferase A1-1. Proc Natl Acad Sci U S A. 2004;101:13163–13167. doi: 10.1073/pnas.0403045101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woycechowsky KJ, Vamvaca K, Hilvert D. Novel enzymes through design and evolution. Adv Enzymol Relat Areas Mol Biol. 2007;75:241–294. doi: 10.1002/9780471224464.ch4. xiii. [DOI] [PubMed] [Google Scholar]
- 6.Toscano MD, Woycechowsky KJ, Hilvert D. Minimalist active-site redesign: teaching old enzymes new tricks. Angew Chem Int Ed Engl. 2007;46:3212–3236. doi: 10.1002/anie.200604205. [DOI] [PubMed] [Google Scholar]
- 7.Glasner ME, Gerlt JA, Babbitt PC. Mechanisms of Protein Evolution and Their Application to Protein Engineering. Adv. Enzymol. Relat. Area. Mol. Biol. 2007;75:193–239. doi: 10.1002/9780471224464.ch3. [DOI] [PubMed] [Google Scholar]
- 8.Gerlt JA, Babbitt PC. DIVERGENT EVOLUTION OF ENZYMATIC FUNCTION: Mechanistically Diverse Superfamilies and Functionally Distinct Suprafamilies. Annu Rev Biochem. 2001;70:209–246. doi: 10.1146/annurev.biochem.70.1.209. [DOI] [PubMed] [Google Scholar]
- 9.Neidhart DJ, Kenyon GL, Gerlt JA, Petsko GA. Mandelate racemase and muconate lactonizing enzyme are mechanistically distinct and structurally homologous. Nature. 1990;347:692–694. doi: 10.1038/347692a0. [DOI] [PubMed] [Google Scholar]
- 10.Babbitt PC, Gerlt JA. Understanding enzyme superfamilies. Chemistry as the fundamental determinant in the evolution of new catalytic activities. J Biol Chem. 1997;272:30591–30594. doi: 10.1074/jbc.272.49.30591. [DOI] [PubMed] [Google Scholar]
- 11.Gerlt JA, Babbitt PC, Rayment I. Divergent evolution in the enolase superfamily: the interplay of mechanism and specificity. Arch Biochem Biophys. 2005;433:59–70. doi: 10.1016/j.abb.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman M. On the road to mandelate … racemase. Science. 1991;251:31–32. doi: 10.1126/science.1986411. [DOI] [PubMed] [Google Scholar]
- 13.O'Brien PJ, Herschlag D. Catalytic promiscuity and the evolution of new enzymatic activities. Chem Biol. 1999;6:R91–R105. doi: 10.1016/S1074-5521(99)80033-7. [DOI] [PubMed] [Google Scholar]
- 14.Bornscheuer UT, Kazlauskas RJ. Catalytic promiscuity in biocatalysis: using old enzymes to form new bonds and follow new pathways. Angew Chem Int Ed Engl. 2004;43:6032–6040. doi: 10.1002/anie.200460416. [DOI] [PubMed] [Google Scholar]
- 15.Kazlauskas RJ. Enhancing catalytic promiscuity for biocatalysis. Curr Opin Chem Biol. 2005;9:195–201. doi: 10.1016/j.cbpa.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Aharoni A, Gaidukov L, Khersonsky O, Mc QGS, Roodveldt C, Tawfik DS. The 'evolvability' of promiscuous protein functions. Nat Genet. 2005;37:73–76. doi: 10.1038/ng1482. [DOI] [PubMed] [Google Scholar]
- 17.Khersonsky O, Roodveldt C, Tawfik DS. Enzyme promiscuity: evolutionary and mechanistic aspects. Curr Opin Chem Biol. 2006;10:498–508. doi: 10.1016/j.cbpa.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Hult K, Berglund P. Enzyme promiscuity: mechanism and applications. Trends Biotechnol. 2007;25:231–238. doi: 10.1016/j.tibtech.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 19. Schmidt DM, Mundorff EC, Dojka M, Bermudez E, Ness JE, Govindarajan S, Babbitt PC, Minshull J, Gerlt JA. Evolutionary potential of (beta/alpha)8-barrels: functional promiscuity produced by single substitutions in the enolase superfamily. Biochemistry. 2003;42:8387–8393. doi: 10.1021/bi034769a. The introduction of a "new" function in a mechanistically diverse superfamily Ienolase) can be accomplished with a single substitution of a specificity determininng residue that allows the substrate for the "new" reaction to bind productively vis a vis the "hard-wired" functional groups. This in vitro (re)design study has important implications for the early steps in natural divergent evolution of function.
- 20.Vick JE, Schmidt DM, Gerlt JA. Evolutionary potential of (beta/alpha)8-barrels: in vitro enhancement of a "new" reaction in the enolase superfamily. Biochemistry. 2005;44:11722–11729. doi: 10.1021/bi050963g. [DOI] [PubMed] [Google Scholar]
- 21. Vick JE, Gerlt JA. Evolutionary potential of (beta/alpha)8-barrels: stepwise evolution of a "new" reaction in the enolase superfamily. Biochemistry. 2007;46:14589–14597. doi: 10.1021/bi7019063. This study and that reported in the previous reference demonstrate that directed evolution and selection improve rational (re)design by fine-tuning active site structure.
- 22.Gould SM, Tawfik DS. Directed evolution of the promiscuous esterase activity of carbonic anhydrase II. Biochemistry. 2005;44:5444–5452. doi: 10.1021/bi0475471. [DOI] [PubMed] [Google Scholar]
- 23. Ijima Y, Matoishi K, Terao Y, Doi N, Yanagawa H, Ohta H. Inversion of enantioselectivity of asymmetric biocatalytic decarboxylation by site-directed mutagenesis based on the reaction mechanism. Chem Commun (Camb) 2005:877–879. doi: 10.1039/b416398b. As demonstrated in this and the following reference, "new" reactions can be (re)designed by rational addition/deletion of acid/base catalysts.
- 24.Terao Y, Miyamoto K, Ohta H. Introduction of single mutation changes arylmalonate decarboxylase to racemase. Chem Commun (Camb) 2006:3600–3602. doi: 10.1039/b607211a. [DOI] [PubMed] [Google Scholar]
- 25.Terao Y, Miyamoto K, Ohta H. Improvement of the activity of arylmalonate decarboxylase by random mutagenesis. Appl Microbiol Biotechnol. 2006;73:647–653. doi: 10.1007/s00253-006-0518-z. [DOI] [PubMed] [Google Scholar]
- 26.Hamed RB, Batchelar ET, Clifton IJ, Schofield CJ. Mechanisms and structures of crotonase superfamily enzymes--how nature controls enolate and oxyanion reactivity. Cell Mol Life Sci. 2008;65:2507–2527. doi: 10.1007/s00018-008-8082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xiang H, Luo L, Taylor KL, Dunaway-Mariano D. Interchange of catalytic activity within the 2-enoyl-coenzyme A hydratase/isomerase superfamily based on a common active site template. Biochemistry. 1999;38:7638–7652. doi: 10.1021/bi9901432. The first example of (re)designed guided by natural divergent evolution within a mechanistically diverse superfamily (enoyl-CoA hydratase), although additional substitutions were required to accommodate the (re)designed acid/base catalysts.
- 28.Lawrence MC, Barbosa JA, Smith BJ, Hall NE, Pilling PA, Ooi HC, Marcuccio SM. Structure and mechanism of a sub-family of enzymes related to N-acetylneuraminate lyase. J Mol Biol. 1997;266:381–399. doi: 10.1006/jmbi.1996.0769. [DOI] [PubMed] [Google Scholar]
- 29.Choi KH, Lai V, Foster CE, Morris AJ, Tolan DR, Allen KN. New superfamily members identified for Schiff-base enzymes based on verification of catalytically essential residues. Biochemistry. 2006;45:8546–8555. doi: 10.1021/bi060239d. [DOI] [PubMed] [Google Scholar]
- 30.Joerger AC, Mayer S, Fersht AR. Mimicking natural evolution in vitro: an N-acetylneuraminate lyase mutant with an increased dihydrodipicolinate synthase activity. Proc Natl Acad Sci U S A. 2003;100:5694–5699. doi: 10.1073/pnas.0531477100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poelarends GJ, Veetil VP, Whitman CP. The chemical versatility of the beta-alpha-beta fold: Catalytic promiscuity and divergent evolution in the tautomerase superfamily. Cell Mol Life Sci. 2008 doi: 10.1007/s00018-008-8285-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Poelarends GJ, Almrud JJ, Serrano H, Darty JE, Johnson WH, Jr, Hackert ML, Whitman CP. Evolution of enzymatic activity in the tautomerase superfamily: mechanistic and structural consequences of the L8R mutation in 4-oxalocrotonate tautomerase. Biochemistry. 2006;45:7700–7708. doi: 10.1021/bi0600603. Modest changes in promiscuity were accomplished by rational (re)design, although the structure of the mutant enzyme revealed only small changes in active site structure. Presumably, very small changes in structure are responsible for large changes in kinetic constants.
- 33.Toth K, Amyes TL, Wood BM, Chan K, Gerlt JA, Richard JP. Product deuterium isotope effect for orotidine 5'-monophosphate decarboxylase: evidence for the existence of a short-lived carbanion intermediate. J Am Chem Soc. 2007;129:12946–12947. doi: 10.1021/ja076222f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amyes TL, Wood BM, Chan K, Gerlt JA, Richard JP. Formation and stability of a vinyl carbanion at the active site of orotidine 5'-monophosphate decarboxylase: pKa of the C-6 proton of enzyme-bound UMP. J Am Chem Soc. 2008;130:1574–1575. doi: 10.1021/ja710384t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yew WS, Wise EL, Rayment I, Gerlt JA. Evolution of enzymatic activities in the orotidine 5'-monophosphate decarboxylase suprafamily: mechanistic evidence for a proton relay system in the active site of 3-keto-L-gulonate 6-phosphate decarboxylase. Biochemistry. 2004;43:6427–6437. doi: 10.1021/bi049741t. [DOI] [PubMed] [Google Scholar]
- 36.Wise E, Yew WS, Babbitt PC, Gerlt JA, Rayment I. Homologous (beta/alpha)8-barrel enzymes that catalyze unrelated reactions: orotidine 5'-monophosphate decarboxylase and 3-keto-L-gulonate 6-phosphate decarboxylase. Biochemistry. 2002;41:3861–3869. doi: 10.1021/bi012174e. [DOI] [PubMed] [Google Scholar]
- 37. Yew WS, Akana J, Wise EL, Rayment I, Gerlt JA. Evolution of enzymatic activities in the orotidine 5'-monophosphate decarboxylase suprafamily: enhancing the promiscuous D-arabino-hex-3-ulose 6-phosphate synthase reaction catalyzed by 3-keto-L-gulonate 6-phosphate decarboxylase. Biochemistry. 2005;44:1807–1815. doi: 10.1021/bi047815v. This and the following reference describe sequence-based changes in active site structure to enhance a promiscuous reaction. Again, only very small changes in active site structure were observed in a (re)engineered mutant, so the structural bases for the changes in promiscuity could not be identified.
- 38.Wise EL, Yew WS, Akana J, Gerlt JA, Rayment I. Evolution of enzymatic activities in the orotidine 5'-monophosphate decarboxylase suprafamily: structural basis for catalytic promiscuity in wild-type and designed mutants of 3-keto-L-gulonate 6-phosphate decarboxylase. Biochemistry. 2005;44:1816–1823. doi: 10.1021/bi0478143. [DOI] [PubMed] [Google Scholar]
- 39.Lodeiro S, Xiong Q, Wilson WK, Kolesnikova MD, Onak CS, Matsuda SP. An oxidosqualene cyclase makes numerous products by diverse mechanisms: a challenge to prevailing concepts of triterpene biosynthesis. J Am Chem Soc. 2007;129:11213–11222. doi: 10.1021/ja073133u. [DOI] [PubMed] [Google Scholar]
- 40.Xu M, Wilderman PR, Peters RJ. Following evolution's lead to a single residue switch for diterpene synthase product outcome. Proc Natl Acad Sci U S A. 2007;104:7397–7401. doi: 10.1073/pnas.0611454104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilderman PR, Peters RJ. A single residue switch converts abietadiene synthase into a pimaradiene specific cyclase. J Am Chem Soc. 2007;129:15736–15737. doi: 10.1021/ja074977g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Morrone D, Xu M, Fulton DB, Determan MK, Peters RJ. Increasing complexity of a diterpene synthase reaction with a single residue switch. J Am Chem Soc. 2008;130:5400–5401. doi: 10.1021/ja710524w. A sequence-based change in products in the terpene synthase superfamily with a single substitution that presumably changes the shape/polity of the active site cavity that directs reactive carbocation intermediates to product(s).
- 43.Kampranis SC, Ioannidis D, Purvis A, Mahrez W, Ninga E, Katerelos NA, Anssour S, Dunwell JM, Degenhardt J, Makris AM, et al. Rational conversion of substrate and product specificity in a Salvia monoterpene synthase: structural insights into the evolution of terpene synthase function. Plant Cell. 2007;19:1994–2005. doi: 10.1105/tpc.106.047779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lodeiro S, Schulz-Gasch T, Matsuda SP. Enzyme redesign: two mutations cooperate to convert cycloartenol synthase into an accurate lanosterol synthase. J Am Chem Soc. 2005;127:14132–14133. doi: 10.1021/ja053791j. A sequence-based change in products in the oxidosqualene cyclase superfamily with a single substitution that presumably changes the shape/polity of the active site cavity that directs reactive carbocation intermediates to product(s).
- 45. Yoshikuni Y, Ferrin TE, Keasling JD. Designed divergent evolution of enzyme function. Nature. 2006;440:1078–1082. doi: 10.1038/nature04607. This report describes rational combination of active site substitutions to produce novel terpene synthases.
- 46. Greenhagen BT, O'Maille PE, Noel JP, Chappell J. Identifying and manipulating structural determinates linking catalytic specificities in terpene synthases. Proc Natl Acad Sci U S A. 2006;103:9826–9831. doi: 10.1073/pnas.0601605103. This report also describes rational combination of active site substitutions to produce novel terpene synthases.
- 47.O'Maille PE, Malone A, Dellas N, Andes Hess B, Jr, Smentek L, Sheehan I, Greenhagen BT, Chappell J, Manning G, Noel JP. Quantitative exploration of the catalytic landscape separating divergent plant sesquiterpene synthases. Nat Chem Biol. 2008;4:617–623. doi: 10.1038/nchembio.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tawfik DS. Biochemistry. Loop grafting and the origins of enzyme species. Science. 2006;311:475–476. doi: 10.1126/science.1123883. [DOI] [PubMed] [Google Scholar]
- 49. Park HS, Nam SH, Lee JK, Yoon CN, Mannervik B, Benkovic SJ, Kim HS. Design and evolution of new catalytic activity with an existing protein scaffold. Science. 2006;311:535–538. doi: 10.1126/science.1118953. Homologous members of mechanistically diverse superfamilies often differ in the lengths and sequences of active site loops. These can be (re)designed, although in this study very significant structural alterations were required.
- 50.Reetz MT. Controlling the enantioselectivity of enzymes by directed evolution: practical and theoretical ramifications. Proc Natl Acad Sci U S A. 2004;101:5716–5722. doi: 10.1073/pnas.0306866101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reetz MT, Wang LW, Bocola M. Directed evolution of enantioselective enzymes: iterative cycles of CASTing for probing protein-sequence space. Angew Chem Int Ed Engl. 2006;45:1236–1241. doi: 10.1002/anie.200502746. [DOI] [PubMed] [Google Scholar]
- 52.Reetz MT, Kahakeaw D, Lohmer R. Addressing the numbers problem in directed evolution. Chembiochem. 2008;9:1797–1804. doi: 10.1002/cbic.200800298. [DOI] [PubMed] [Google Scholar]
- 53. Jiang L, Althoff EA, Clemente FR, Doyle L, Rothlisberger D, Zanghellini A, Gallaher JL, Betker JL, Tanaka F, Barbas CF, 3rd, et al. De novo computational design of retro-aldol enzymes. Science. 2008;319:1387–1391. doi: 10.1126/science.1152692. The first example of computation-directed (re)design of a novel retroaldolase activity in a scaffold that catalyzes an unrelated reaction. The (re)design retroaldolase is inefficient, but this study establishes that an active site can be rationally designed to stabilize intermediates/transition states in a complex reaction.
- 54.Heine A, DeSantis G, Luz JG, Mitchell M, Wong CH, Wilson IA. Observation of covalent intermediates in an enzyme mechanism at atomic resolution. Science. 2001;294:369–374. doi: 10.1126/science.1063601. [DOI] [PubMed] [Google Scholar]
- 55. Rothlisberger D, Khersonsky O, Wollacott AM, Jiang L, DeChancie J, Betker J, Gallaher JL, Althoff EA, Zanghellini A, Dym O, et al. Kemp elimination catalysts by computational enzyme design. Nature. 2008;453:190–195. doi: 10.1038/nature06879. The first example of (re)design of an active site to catalyze an unnatural reaction. The low levels of activity in the original design were enhanced by several rounds of directed evolution. This computation-directed evolution approach likely will be the strategy of choice for (re)design of "new" functions in scaffolds that catalyze different reactions.
- 56.Bloom JD, Labthavikul ST, Otey CR, Arnold FH. Protein stability promotes evolvability. Proc Natl Acad Sci U S A. 2006;103:5869–5874. doi: 10.1073/pnas.0510098103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Camps M, Herman A, Loh E, Loeb LA. Genetic constraints on protein evolution. Crit Rev Biochem Mol Biol. 2007;42:313–326. doi: 10.1080/10409230701597642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tokuriki N, Stricher F, Serrano L, Tawfik DS. How protein stability and new functions trade off. PLoS Comput Biol. 2008;4:e1000002. doi: 10.1371/journal.pcbi.1000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nagatani RA, Gonzalez A, Shoichet BK, Brinen LS, Babbitt PC. Stability for function trade-offs in the enolase superfamily "catalytic module". Biochemistry. 2007;46:6688–6695. doi: 10.1021/bi700507d. [DOI] [PubMed] [Google Scholar]
- 60.Ortlund EA, Bridgham JT, Redinbo MR, Thornton JW. Crystal structure of an ancient protein: evolution by conformational epistasis. Science. 2007;317:1544–1548. doi: 10.1126/science.1142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boehr DD, McElheny D, Dyson HJ, Wright PE. The dynamic energy landscape of dihydrofolate reductase catalysis. Science. 2006;313:1638–1642. doi: 10.1126/science.1130258. [DOI] [PubMed] [Google Scholar]
- 62.Benkovic SJ, Hammes GG, Hammes-Schiffer S. Free-energy landscape of enzyme catalysis. Biochemistry. 2008;47:3317–3321. doi: 10.1021/bi800049z. [DOI] [PubMed] [Google Scholar]
- 63.Meyer MP, Tomchick DR, Klinman JP. Enzyme structure and dynamics affect hydrogen tunneling: the impact of a remote side chain (I553) in soybean lipoxygenase-1. Proc Natl Acad Sci U S A. 2008;105:1146–1151. doi: 10.1073/pnas.0710643105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luo M, Li L, Schramm VL. Remote mutations alter transition-state structure of human purine nucleoside phosphorylase. Biochemistry. 2008;47:2565–2576. doi: 10.1021/bi702133x. [DOI] [PubMed] [Google Scholar]