Abstract
Prostate cancer remains the most common non-cutaneous malignancy among American men. Since the advent of PSA testing, most men are diagnosed with localized disease, but a proportion of men will be diagnosed with metastatic disease, many will eventually receive chemotherapy with docetaxel and prednisone. However, responses are not durable and all men will ultimately progress on this treatment. As such, continued efforts are geared towards the discovery of new agents and mechanisms of targeting prostate cancer. Angiogenesis has been shown to play an important role in tumorigenesis, proliferation and metastasis in prostate cancer. Here we discuss the major angiogenic signaling pathway involving VEGF in prostate cancer progression and the role of various promising agents that targets this pathway. This includes bevacizumab, thalidomide and its analogues, tyrosine kinase inhibitors sorafenib and AZD2171, and other inhibitors of angiogenic signaling pathways. Results of key clinical trials associated with the use of these agents and future directions are discussed herein.
Keywords: Angiogenesis, Prostate Cancer, Bevacizumab, Thalidomide, Lenalidomide, AZD2171, Sorafenib
INTRODUCTION
Prostate cancer is the most common non-cutaneous malignancy among American men [1]. While the majority of men are diagnosed in early stages, a subset will develop recurrence and eventually, metastatic disease. The cornerstone of treatment for metastatic disease includes hormonal therapy for androgen-responsive disease. Almost all respond initially but most eventually develop castration resistant prostate cancer (CRPC). The emergence of initial castration resistance leads to alternative treatment options, including anti-androgen withdrawal, secondary hormonal manipulations, and eventually chemotherapy once hormonal treatments fail. Docetaxel and prednisone is the standard for patients requiring chemotherapy. However, responses are not durable and almost all will progress. Therefore, there is a need for identification of better or alternative treatment strategies for improving current standard treatment. Angiogenesis is an important process for growth, progression, and metastasis of solid tumors [2]. Tumor vascularity as measured by microvessel density has also been shown to be a prognostic factor in a variety of solid tumors, including prostate cancer [3–6]. Multiple agents are currently under study for targeting various steps in the angiogenic cascade, with inhibitors for vascular endothelial growth factor (VEGF) being in the forefront and currently approved for colon, lung, and breast carcinoma in the metastatic setting [7, 8]. The following discussion will review the VEGF signaling pathway and the various agents currently targeting tumor angiogenesis in the field of prostate cancer.
MOLECULAR BASIS OF TUMOR ANGIOGENESIS IN PROSTATE CANCER
The concept of angiogenesis inhibition as a novel anti-cancer treatment strategy emerged with the observation that neovascularization is required in order to sustain expansile growth for solid tumors [2, 9]. One method of analyzing neovascularizaton is the measurement of endogenous proteins involved in the angiogenic cascade, including basic fibroblast growth factor (bFGF), VEGF, platelet-derived growth factor (PDGF), among others. Other methods of evaluating neovascularization include histological studies looking at microvessel density (MVD) by staining endothelial cells with antibodies directed to Factor VIII, CD34 or CD31, and determining mRNA, protein or receptor expression [2, 5, 9]. In prostate cancer, MVD has been used as a prognostic factor for determining aggressiveness and predicting metastasis [6, 10]. Although there has been discrepant results in the overall prognostic utility of MVD as a marker of prostate cancer progression [11], there is clinical evidence that targeting angiogenesis, especially in conjunction with cytotoxic chemotherapy, is a promising strategy in the treatment of prostate cancer [12–15].
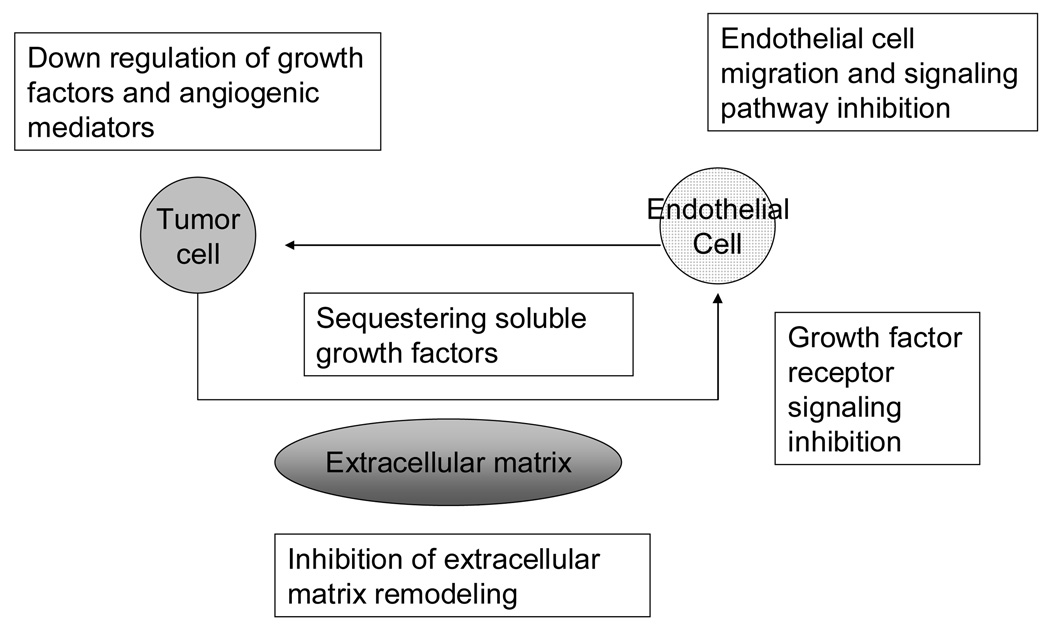
The molecular targets for angiogenesis can be inhibited in various ways, either selectively or indirectly [16]. It has been proposed that endothelial cells possess stable genomes that render them incapable of acquiring resistance to agents that would inhibit them. Thus, targeting the endothelium might be an effective way of treating cancer [17, 18]. Indeed, it has been shown that utilizing angiogenesis inhibitors resulted in normalization of tumor vasculature that allowed for better delivery of cytotoxic chemotherapy in various models [19, 20]. However, various mechanisms of resistance to anti-angiogenic therapy have been postulated [21], including cytogenetic abnormalities in tumor-associated endothelial cells which could compromise the efficacy of anti-angiogenic inhibitors [22, 23]. Various levels of inhibition can take place in the angiogenic cascade resulting in the arrest of growth or progression of vascularization [24]. This includes downregulation of tumor growth factors involved in the endothelial cell locomotion or proliferation (i.e., VEGF, bFGF), blocking of growth factor receptor signaling or endothelial cell-specific signaling pathways or inhibition of other positive regulators of angiogenesis (Figure 1).
Fig. 1. Targets for angiogenic inhibition.
Potential targets of angiogenesis inhibition. Interactions between the tumor cell, its secreted growth factors, and extracellular matrix are all potential targets for angiogenesis inhibition. Figure adapted from Figg et. al. (Ref. 24 Figg, W. D.; Kruger, E. A.; Price, D. K.; Kim, S.; Dahut, W. D. Inhibition of angiogenesis: treatment options for patients with metastatic prostate cancer. Invest. New Drugs 2002, 20, 183–194.)
VEGF SIGNALING PATHWAY
Of the various angiogenic signaling cascade, the inhibition of VEGF is probably the best studied. The VEGF family of polypeptide growth factor, of which at least 7 members have been described, regulates receptor tyrosine kinases resulting in multiple downstream effects [25]. VEGF-A (121 amino acids) plays an important role in tumor angiogenesis. It binds two major receptor VEGF tyrosine receptor kinases, namely VEGFR-1 (Flt-1 or fms-like tyrosine kinase receptor 1) that is expressed mainly in vascular endothelial cells, and VEGFR-2 (Flk-1 in mice and KDR, kinase insert domain- containing receptor in humans), which is important in cell trafficking [26, 27].
Targeted Agents
Bevacizumab
Bevacizumab (Avastin®; Genentech, Inc., South San Francisco, CA), is a humanized monoclonal antibody IgG1 which was developed from a murine antihuman vascular endothelial growth factor (VEGF) monoclonal antibody that blocks the binding of human VEGF to its receptors. Initial preclinical studies demonstrated the ability of VEGF inhibition in preventing further tumor growth of the prostate cancer cell line DU 145 implanted in nude mice [28]. Early phase I studies demonstrated tolerability of bevacizumab although dose-response relationship was not consistently demonstrated across all tumor types [29–31].
Single agent bevacizumab has been utilized in a phase II trial with 15 CRPC patients. The dose of bevacizumab was 10 mg/kg intravenously every 2 weeks for 6 infusions that made up one cycle, followed by additional treatments for those who achieved a response or had stable disease [32]. No prostate specific antigen (PSA) decline of > 50% was noted in the 14 patients who were evaluable for response although 4 patients had PSA declines of < 50%. Of the 8 patients with measurable disease, no objective definitive responses were noted at day 70. This study demonstrates the general lack of single agent activity observed in the use of bevacizumab in several solid tumors, including prostate cancer. However, the promising results of combined VEGF inhibition with conventional cytotoxic chemotherapy in other solid tumors provided the rationale for the initiation of a trial using the combination of bevacizumab and docetaxel by the Cancer and Leukemia Group B (CALGB) [33]. CALGB 90006 was a phase II trial that used bevacizumab (15 mg/kg given on day 2 every 3 weeks) in addition to docetaxel 70 mg/m2 on day 2 as well as estramustine 280 mg three times daily on days 1 – 5. As of the last follow-up, this trial enrolled 79 patients, with 42% (14 out of 33 patients who had measurable disease) achieving a partial response (PR). A PSA decline of >50% was also observed in 79% of patients1 (unpublished data). One promising trial conducted at the National Cancer Institute (NCI), utilizing a combination of bevacizumab at 15 mg/kg every 21 days, docetaxel 75 mg/m2 every 21 days, in addition to prednisone 5 mg twice daily, and thalidomide 200mg every night, given with a thromboprophylaxis regimen, has currently accrued 60 patients2 (unpublished data). Of the 60 evaluable patients, PSA declines of ≥50% were observed in 52 pts (90%) with median ≥50% PSA-duration of 11 cycles (range: 0 – 46). Thirty-two patients with measurable disease were also evaluable with majority having partial response (n=18) or stable disease (n=11) and two complete response, making up an overall response rate of 63%. The estimated median PFS was 18.2 months and median OS of 26.7 months. This compares favorably to the initial phase III trial comparing two dosing schedules of docetaxel and prednisone with mitoxantrone and prednisone, where the median OS and PSA response for the group that received every 3 weeks of docetaxel compared to the mitoxantrone group was 18.9 months (95% confidence interval 17 – 21.2, P=0.009) and 45%, respectively [34]. The combination regimen with bevacizumab and thalidomide was also well tolerated, with a median number of treatment cycles of 12 (2–47 cycles), with the following toxicities reported: febrile neutropenia (5/60), requiring subsequent myeloid growth factor support following administration of each cycle; syncope (5/60), thrombosis (3/60), gastrointestinal perforation (2/60), grade 3 bleeding (2/60), nephrotic syndrome (1/60), and thrombosis (3/60). These adverse events were no more than what would be expected of treatment with a bevacizumab-containing regimen. Overall, the addition of bevacizumab and thalidomide to the current existing standard of care holds promise in the treatment of metastatic CRPC.
Thalidomide and its analogues
Thalidomide (Thalomid®, Celgene Corporation, Summit, NJ), was originally marketed as a sedative and antiemetic but reports of teratogenicity led to its withdrawal in the European market in the 1960’s [35, 36]. Its initial Food and Drug Administration (FDA) approved indication in the United States was for treatment of erythema nodosum leprosum. Subsequent studies elucidating the nature of thalidomide in causing embryopathy showed its ability to exert antiangiogenic activity in a rabbit cornea model [37], although demonstration of this species-specific effect requires metabolic activation [38]. The clinical efficacy of thalidomide has been demonstrated in several neoplasms, and it is currently FDA-approved for Multiple Myeloma.
Thalidomide has also been studied in several neoplasms, with promising activity in some. In prostate cancer, a phase II, open-label, randomized study was completed at the NCI and compared low-dose (200 mg/day) versus high-dose (up to 1200 mg/day) thalidomide in 63 patients (50 patients in the low-dose arm, and 13 patients in the high-dose arm) [12]. The high-dose arm was terminated after 13 patients were accrued since no significant declines in PSA were observed and patients could not tolerate doses beyond 200 mg per day. Accrual of the low-dose arm was expanded to include a total of 50 patients. Observed responses were modest, with a total of 28% of all patients having a >40% decline in PSA, with 18% of patients in the low-dose arm having a ≥50% PSA decline. Although there were no objective partial response on CT scan, 2 patients on the low dose arm who had a > 50% PSA decline and stable PSA for > 9 months respectively, had improvement in bone scan lesions. PSA changes also correlated with changes or improvements in the positron emission tomography (PET) images of the lesions, suggesting responses in tumor blood flow and metabolism [39, 40]. These encouraging results with single-agent thalidomide prompted further study of this agent in combination with weekly docetaxel [14], since the superiority of the every 3-week docetaxel has not yet been demonstrated at the time of conduct of this study [34]. This phase II trial enrolled 75 chemotherapy-naïve patients with metastatic CRPC and were randomly assigned to receive either docetaxel 30 mg/m2 intravenously weekly for 3 consecutive weeks, followed by a 1-week rest period (n = 25); or docetaxel at the same dose and schedule, plus thalidomide 200 mg orally each day (n = 50). PSA working group consensus criteria combined with RECIST criteria was used to determine the proportion of patients with a PSA decline, as well as time to progression. Patients in the combination arm had a greater than 50% decline in PSA of 53% versus 37% in docetaxel-alone arm after a median follow-up of 26.4 months. Progression-free survival was also superior in the combination arm, with a median of 5.9 months versus 3.7 months in the docetaxel alone arm. Median OS was not statistically different at initial reporting but updated analysis after a median follow-up of 47 months showed a statistically significant difference in median OS for the combined group (25.9 months) versus the docetaxel-alone arm (14.7 months), P2 = .0407 [41]. The combination of thalidomide and docetaxel was well tolerated, although there was an initial observation of thromboembolic events in the combination, no further events ensued after routine prophylactic use of low-molecular weight heparin was employed. The hematologic toxicities were mild in both treatment arms, and although there were more non-hematologic toxicities that developed in the combination arm, the majority of patients did not require treatment for these symptoms.
Another study that utilized a three-drug regimen of paclitaxel, thalidomide, and estramustine had been reported3 (unpublished data). Paclitaxel was given at 100 mg/m2/week for 2 of 3 weeks; Estramustine was given at 140 mg every 8 hours, 5 days/week for 2 of 3 weeks; and thalidomide was given at doses that were escalated from 200 mg/day to 600 mg/day uninterrupted for 21 days. 18 of 25 patients (72%, CI 51 – 88%) have achieved a ≥ 50% decline in PSA sustained for more than 6 weeks, and 3 of 25 (12%) of patients have achieved a ≥ 80% decline lasting for at least 8 weeks. Improvement of bone pain was also reported. Further studies utilizing this combination includes a recently concluded phase II trial combining the two drugs with estramustine, resulting in PSA declines of ≥ 50% in 18 out of 20 patients and an additional two of 10 patients with soft-tissue lesions achieving a partial response (PR) [15]. Although these responses were encouraging, the toxicity profile of estramustine has precluded use of this specific combination from further study, and use of estramustine in general in the community [42]. The search for alternative drugs that exhibit similar or better potency, but limited toxicity, led to the development of other thalidomide analogues called the immunomodulatory drugs (iMIDs) [43]. Agents that belong to this class include lenalidomide, CPS11 and CPS49.
Lenalidomide, or CC-5013 (Revlimid®, Celgene Corporation, Summit, NJ), is a thalidomide analogue that retains the biologic properties of its parent molecule, thalidomide. Lenalidomide has been studied in a phase I trial that enrolled 45 patients with advanced solid tumors [44]. Thirty-five of these patients had prostate cancer and no significant PSA declines were seen. Another phase I trial in prostate cancer studied docetaxel at two dose levels (60 mg/m2 and 75 mg/m2) every 3 weeks, and lenalidomide at doses of 10 mg, 15 mg, 20 mg, or 25 mg, along with prednisone 5 mg twice daily4 (unpublished data). Preliminary results showed promising data with 5 patients (38.5%) of the 13 patients with measurable disease achieving a PR and 7 patients (53.9%) with stable disease. Median number of cycles were 9 (range of 2 – 15) and the maximum tolerated dose (MTD) has not been reached. Another phase I trial utilizing docetaxel every 3 weeks and lenalidomide was reported5 (unpublished data). Docetaxel was administered intravenously on day 1 of each 21-day cycle and lenalidomide orally on days 1–14. Updated analysis showed the trial enrolled thirty-three patients, 9 of whom had prostate cancer. The trial was amended by giving myeloid growth factor support after significant myelosuppression was observed. The MTD was reached at 75 mg/m2 of docetaxel once every three weeks and 25 mg of lenalidomide on days 1–14, with pegfilgrastim support given on day 2. Of the nine prostate cancer patients, stable disease was seen in 5 patients with PSA declines of 32 – 95%.
Sorafenib
Sorafenib (Nexavar®, Bayer HealthCare and Onyx Pharmaceuticals, Emeryville, CA) is a multi-tyrosine kinase inhibitor that has been shown in preclinical models to inhibit wild-type and mutant b-Raf and c-Raf kinase isoforms in vitro. Its role in angiogenesis inhibition has been defined by its proposed inhibition of various factors, including p38, c-kit, VEGFR-2 and PDGFR-β,thereby causing an anti-growth and anti-apoptotic effects by events downstream of c-Raf [45, 46]. There have been several phase II trials in prostate cancer using this agent. The first trial enrolled an initial 22 patients but had no PSA declines > 50%, however, a discordance between increasing PSA values and improvement in bone lesions by bone scintigraphy scan was observed in two patients, [47] leading to further accrual of this trial to a total of 46 patients [48]. Another phase II trial utilized sorafenib for chemo-naïve mCRPC patients with a primary endpoint of progression-free survival of at least 12 weeks using RECIST criteria or PSA-based progression if patients had no measurable disease. Published report of this trial showed a total of 55 evaluable patients, with scans obtained at baseline, every 6 weeks until 12 weeks, then every 8 weeks thereafter [49]. Results showed stable disease in 4 patients according to RECIST and 11 patients according to PSA-based criteria with 2 PSA responders. A third trial has also reported on 28 chemo-naïve patients with mCRPC [50]. The primary end point was PSA decline of ≥ 50% for at least 4 weeks with a PSA decline rate of 3.6% [95% confidence interval (CI) 0.1% to 18.3%]. However, such PSA response may not be that meaningful since data suggests that sorafenib increases PSA secretion [47]. Other trials seek to combine sorafenib with either docetaxel and prednisone or mitoxantrone and prednisone, in patients with CRPC who are progressing despite systemic chemotherapy (http://clinicaltrials.gov/show/NCT00414388).
AZD2171
AZD2171 (Cediranib®, Astra Zeneca, Wilmington, DE) is an oral, highly potent, indoleether quinazoline ATP-competitive small molecule that inhibits proliferation via inhibition of all VEGF receptors [51, 52]. A phase I study has been conducted in 83 patients with advanced solid tumors, with the primary objective of determining the maximum tolerated dose (MTD). The most common dose-limiting toxicity (DLT) was hypertension (n=7) occurring at doses of 20 mg and higher [53]. A separate phase I dose-escalation study was performed in prostate cancer [54]. Doses of 1, 2.5, 5, 10, 15, 20, and 30 mg were administered to 26 patients. DLTs occurred at the 30 mg dose and the MTD was defined as 20 mg. Although safety, tolerability and pharmacokinetics were the main objectives of the study, an objective response was observed in one patient and 4 patients were observed to have PSA reductions after drug discontinuation. There is currently an ongoing phase II trial using this agent at the NCI that has enrolled 18 of a planned 35 patients at a dose no higher than 20 mg daily in patients with metastatic CRPC who have progressed after docetaxel, with encouraging responses6 (unpublished data). Two out of 11 evaluable patients had PR (personal communication). Decreases in lymph node metastases as well as in lung, liver and bone lesions have been noted although discordance has been observed between the imaging and the PSA responses. The safety and early activity profile makes AZD2171 a promising candidate for future 2nd line therapy post-docetaxel failure.
Other agents targeting angiogenic signaling
A promising VEGF antagonist that is a fusion protein combining the Fc portion of human IgG1 with the principal extracellular ligand-binding domains of the human VEGF receptors (VEGFR) 1 and 2 is called VEGF-Trap. A phase III trial has been launched using aflibercept with the primary objective of demonstrating an improvement of overall survival in patients receiving docetaxel and prednisone (http://clinicaltrials.gov/ct2/show/NCT00519285). Secondary endpoints include PSA and pain response, time to occurrence of skeletal related events and progression free survival, as well as safety and pharmacokinetics.
Apart from directly targeting VEGF or its receptors, other strategies for targeting angiogenesis includes inhibition of other VEGF-stimulated tyrosine phosphorylation (using selective inhibitors of the Flk-1/KDR tyrosine kinase receptors), or inhibition or down-regulation of key cellular regulatory proteins, including Matrix Metalloproteinase (MMP-2) and endostatin.
SU5416 (Semaxinib®, Pharmacia, San Francisco, CA) is a small, lipophilic, highly protein-bound synthetic tyrosine kinase inhibitor (TKI) that inhibits VEGFR-2 (VEGF receptor-2; Flk-1/KDR) autophosphorylation [55, 56]. SU5416 has been shown to inhibit tumor vascularization and growth inhibition in vivo. Early phase I studies showed an MTD of 145 mg/m2 via twice weekly intravenous infusions, but clotting abnormalities were observed [57]. SU11248 (Sutent®, Pfizer Inc., New York, NY) exhibited more pharmacologic stability and efficacy than SU5416 [58], and exhibited potent anti-angiogenic properties pre-clinically [59, 60]. It is currently being investigated in a phase I/II trial for patients with chemotherapy-naïve metastatic CRPC in combination with docetaxel and prednisone with primary outcome measures which include the pharmacokinetic characterization, safety, tolerability, and anti-tumor activity of this combination (http://clinicaltrials.gov/show/NCT00137436).
Alteration of calcium-sensitive signal transduction pathway is another potential strategy for inhibiting cellular signaling that likely affects proliferation. Carboxyamido-triazole (CAI) is an inhibitor of signal transduction via non-voltage gated calcium channels with anti-angiogenic effects as a result of inhibition of downstream phosphorylation events involving phospholipase Cγ and inositol phosphate [61, 62]. CAI has been studied pre-clinically with prostate cell lines and was found to inhibit proliferation [63]. It has been studied in a phase I trial with a MTD of 300 mg/m2/day and DLT of cerebellar ataxia and confusion occurring at 350 mg/m2/day [64]. However, in a pharmacokinetic-based phase II study in metastatic CRPC, CAI was not associated with any clinical responses although a decrease in serum VEGF was observed [65].
Endostatin is a 20 kDa C-terminal fragment of collagen XVIII, of which the isolated murine form demonstrated potent antiangiogenic and antitumor activity in vitro and in vivo [66, 67]. Although the exact mechanism for antiangiogenesis is not fully elucidated, a possible mechanism is through MMP-2 inhibition [68]. As MMPs are overexpressed and related to progression of prostate cancer [69], targeting this pathway could be a viable option for anticancer therapy, perhaps in the earlier stages [70, 71]. Other agents that have been studied in prostate cancer though not with much success includes a metabolite of estradiol, named 2-Methoxyestradiol (2ME), which has been studied in a phase I trial in advanced malignancies [72], with the trial prematurely closing because of the extremely low plasma concentrations achieved relative to the doses administered without discernible effects on microvessel density, and another agent called TNP-470, which is an anti-angiogenic synthetic analog of fumagillin and has been studied in prostate cancer. In a phase I study, the MTD was found to be 70.88 mg/m2 of body surface area, with neuropsychiatric symptoms as the DLT [73].
CONCLUSIONS
Targeting angiogenesis is an emerging and exciting field of cancer research. Although much has been learned regarding the tumor biology and molecular pharmacology of agents targeting this pathway, there are still major road blocks in the identification of appropriate individuals who may benefit more from these agents. Perhaps the identification of better tumor markers will help in the assessment of patients. It is also likely that combination of anti-angiogenic agents with conventional cytotoxic therapy will yield better clinical responses compared to either agent alone. The challenge remains as to how to best combine these agents and how to measure the responses seen with these treatments.
ACKNOWLEDGEMENTS
This project has been supported by the Intramural Research Program of the National Cancer Institute, Center for Cancer Research, National Institutes of Health. The content of this publication does not reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
ABBREVIATIONS
- CRPC
Castration Resistant Prostate Cancer
- VEGF
Vascular Endothelial Growth Factor
- bFGF
basic Fibroblast Growth Factor
- PDGF
platelet-derived growth factor
- MVD
microvessel density
- CALGB
Cancer and Leukemia Group B
- FDA
Food and Drug Administration
- PSA
Prostate specific antigen
- PFS
Progression-free survival
- OS
Overall Survival
- PR
Partial Response
- iMIDs
immunomodulatory drugs
- RECIST
Response Evaluation Criteria for Solid Tumors
- MTD
Maximum tolerated dose
- DLT
dose-limiting toxicity
- NCI
National Cancer Institute
- MMP
Matrix Metalloproteinase
- CAI
Carboxyamido-triazole
- 2ME
2-Methoxyestradiol
Footnotes
Picus,J. Docetaxel/bevacizumab (Avastin) in prostate cancer. Cancer Invest. 2004, Supp 1, pp 60, Abstract # 46.
Ning, Y. M.; Gulley, J.; Arlen, P.; Latham, L.; Srinavasan, R.; Wright, J.; Parnes, H.; Figg, W. D.; Dahut, W. L. A phase II trial of thalidomide, bevacizumab, and docetaxel in patients (pts) with metastatic castration-refractory prostate cancer (CRPC). ASCO Genitourinary Cancers Symposium, San Francisco, CA 2008, Abstract # 164.
Daliani, D.; Dieringer, C. N.; Papandreou, L.; Pagliaro, L.; Tannir, N.; Davies, K.; Logothetis, C. J. A tolerance and efficacy study of thalidomide, paclitaxel, and estramustine for patients with chemotherapy refractory androgen independent prostate cancer. Cancer Invest. 2004, 22, 62, Abstract # 48.
Moss, R.; Mohile, S.; Shelton, G.; Melia, J.; Petrylak, D. P. A phase I open-label study using lenalidomide and docetaxel in androgen-independent prostate cancer (AIPC). Prostate Cancer Symposium 2007, Abstract # 89.
Sanborn, S. L.; Cooney, M.; Gibbons, J.; Brell, J.; Savvides, P.; Krishnamurthi, S.; Bokar, J.; Horvath, N.; Ness, A.; Remick, S. Phase I trial of daily lenalidomide and docetaxel given every three weeks in patients with advanced solid tumors. J Clin. Oncol. (Meeting Abstracts) 2007, 25, Abstract # 3570.
Karakunnel, J. J.; Gulley, J.; Arlen, P.; Mulquin, M.; Wright, J.; Turkby, I. B.; Choyke, P.; Ahlers, C. M.; Figg, W.; Dahut, W. Response evaluation by dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) in a phase II study of cediranib in docetaxel-resistant, castrate resistant prostate cancer (CRPC). 2008 ASCO GU Symposium Proceedings 2008, Abstract # 189.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J. Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 3.Weidner N, Folkman J. Tumoral vascularity as a prognostic factor in cancer. Important Adv. Oncol. 1996:167–190. [PubMed] [Google Scholar]
- 4.Weidner N, Folkman J, Pozza F, Bevilacqua P, Allred EN, Moore DH, Meli S, Gasparini G. Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast arcinoma. J. Natl. Cancer Inst. 1992;84:1875–1887. doi: 10.1093/jnci/84.24.1875. [DOI] [PubMed] [Google Scholar]
- 5.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N. Engl. J. Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 6.Weidner N, Carroll PR, Flax J, Blumenfeld W, Folkman J. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am. J. Pathol. 1993;143:401–409. [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen MH, Gootenberg J, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab plus FOLFOX4 as second-line treatment of colorectal cancer. Oncologist. 2007;12:356–361. doi: 10.1634/theoncologist.12-3-356. [DOI] [PubMed] [Google Scholar]
- 8.Cohen MH, Gootenberg J, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab (Avastin) plus Carboplatin and Paclitaxel as first-line treatment of advanced/metastatic recurrent nonsquamous non-small cell lung cancer. Oncologist. 2007;12:713–718. doi: 10.1634/theoncologist.12-6-713. [DOI] [PubMed] [Google Scholar]
- 9.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 10.Strohmeyer D, Rossing C, Strauss F, Bauerfeind A, Kaufmann O, Loening S. Tumor angiogenesis is associated with progression after radical prostatectomy in pT2/pT3 prostate cancer. Prostate. 2000;42:26–33. doi: 10.1002/(sici)1097-0045(20000101)42:1<26::aid-pros4>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 11.Gettman MT, Pacelli A, Slezak J, Bergstralh EJ, Blute M, Zincke H, Bostwick DG. Role of microvessel density in predicting recurrence in pathologic Stage T3 prostatic adenocarcinoma. Urology. 1999;54:479–485. doi: 10.1016/s0090-4295(99)00202-2. [DOI] [PubMed] [Google Scholar]
- 12.Figg WD, Dahut W, Duray P, Hamilton M, Tompkins A, Steinberg SM, Jones E, Premkumar A, Linehan WM, Floeter MK, Chen CC, Dixon S, Kohler DR, Kruger EA, Gubish E, Pluda JM, Reed E. A randomized phase II trial of thalidomide, an angiogenesis inhibitor, in patients with androgen-independent prostate cancer. Clin. Cancer Res. 2001;7:1888–1893. [PubMed] [Google Scholar]
- 13.Figg WD, Arlen P, Gulley J, Fernandez P, Noone M, Fedenko K, Hamilton M, Parker C, Kruger EA, Pluda J, Dahut WL. A randomized phase II trial of docetaxel (taxotere) plus thalidomide in androgen-independent prostate cancer. Semin. Oncol. 2001;28:62–66. doi: 10.1016/s0093-7754(01)90157-5. [DOI] [PubMed] [Google Scholar]
- 14.Dahut WL, Gulley JL, Arlen PM, Liu Y, Fedenko KM, Steinberg SM, Wright JJ, Parnes H, Chen CC, Jones E, Parker CE, Linehan WM, Figg WD. Randomized phase II trial of docetaxel plus thalidomide in androgen-independent prostate cancer. J. Clin. Oncol. 2004;22:2532–2539. doi: 10.1200/JCO.2004.05.074. [DOI] [PubMed] [Google Scholar]
- 15.Figg WD, Li H, Sissung T, Retter A, Wu S, Gulley JL, Arlen P, Wright JJ, Parnes H, Fedenko K, Latham L, Steinberg SM, Jones E, Chen C, Dahut W. Pre-clinical and clinical evaluation of estramustine, docetaxel and thalidomide combination in androgen-independent prostate cancer. BJU Int. 2007;99:1047–1055. doi: 10.1111/j.1464-410X.2007.06763.x. [DOI] [PubMed] [Google Scholar]
- 16.Folkman J, Browder T, Palmblad J. Angiogenesis research: guidelines for translation to clinical application. Thromb. Haemost. 2001;86:23–33. [PubMed] [Google Scholar]
- 17.Kerbel RS. Inhibition of tumor angiogenesis as a strategy to circumvent acquired resistance to anti-cancer therapeutic agents. Bioessays. 1991;13:31–36. doi: 10.1002/bies.950130106. [DOI] [PubMed] [Google Scholar]
- 18.Boehm T, Folkman J, Browder T, O'Reilly MS. Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature. 1997;390:404–407. doi: 10.1038/37126. [DOI] [PubMed] [Google Scholar]
- 19.Gerber HP, Ferrara N. Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res. 2005;65:671–680. [PubMed] [Google Scholar]
- 20.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 21.Kerbel RS. Tumor angiogenesis. N. Engl. J. Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hida K, Hida Y, Amin DN, Flint AF, Panigrahy D, Morton CC, Klagsbrun M. Tumor-associated endothelial cells with cytogenetic abnormalities. Cancer Res. 2004;64:8249–8255. doi: 10.1158/0008-5472.CAN-04-1567. [DOI] [PubMed] [Google Scholar]
- 23.Hida K, Klagsbrun M. A new perspective on tumor endothelial cells: unexpected chromosome and centrosome abnormalities. Cancer Res. 2005;65:2507–2510. doi: 10.1158/0008-5472.CAN-05-0002. [DOI] [PubMed] [Google Scholar]
- 24.Figg WD, Kruger EA, Price DK, Kim S, Dahut WD. Inhibition of angiogenesis: treatment options for patients with metastatic prostate cancer. Invest. New Drugs. 2002;20:183–194. doi: 10.1023/a:1015626410273. [DOI] [PubMed] [Google Scholar]
- 25.Epstein RJ. VEGF signaling inhibitors: More pro-apoptotic than anti-angiogenic. Cancer Metastasis Rev. 2007;26:443–452. doi: 10.1007/s10555-007-9071-1. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Q, Egashira K, Hiasa K, Ishibashi M, Inoue S, Ohtani K, Tan C, Shibuya M, Takeshita A, Sunagawa K. Essential role of vascular endothelial growth factor and Flt-1 signals in neointimal formation after periadventitial injury. Arterioscler. Thromb. Vasc. Biol. 2004;24:2284–2289. doi: 10.1161/01.ATV.0000147161.42956.80. [DOI] [PubMed] [Google Scholar]
- 27.Zhong H, Bowen JP. Molecular design and clinical development of VEGFR kinase inhibitors. Curr. Top. Med. Chem. 2007;7:1379–1393. doi: 10.2174/156802607781696855. [DOI] [PubMed] [Google Scholar]
- 28.Borgstrom P, Bourdon MA, Hillan KJ, Sriramarao P, Ferrara N. Neutralizing anti-vascular endothelial growth factor antibody completely inhibits angiogenesis and growth of human prostate carcinoma micro tumors in vivo. Prostate. 1998;35:1–10. doi: 10.1002/(sici)1097-0045(19980401)35:1<1::aid-pros1>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 29.Gordon MS, Margolin K, Talpaz M, Sledge GW, Jr, Holmgren E, Benjamin R, Stalter S, Shak S, Adelman D. Phase I safety and pharmacokinetic study of recombinant human anti-vascular endothelial growth factor in patients with advanced cancer. J. Clin. Oncol. 2001;19:843–850. doi: 10.1200/JCO.2001.19.3.843. [DOI] [PubMed] [Google Scholar]
- 30.Margolin K, Gordon MS, Holmgren E, Gaudreault J, Novotny W, Fyfe G, Adelman D, Stalter S, Breed J. Phase Ib trial of intravenous recombinant humanized monoclonal antibody to vascular endothelial growth factor in combination with chemotherapy in patients with advanced cancer: pharmacologic and long-term safety data. J. Clin. Oncol. 2001;19:851–856. doi: 10.1200/JCO.2001.19.3.851. [DOI] [PubMed] [Google Scholar]
- 31.Panares RL, Garcia AA. Bevacizumab in the management of solid tumors. Expert Rev. Anticancer Ther. 2007;7:433–445. doi: 10.1586/14737140.7.4.433. [DOI] [PubMed] [Google Scholar]
- 32.Reese DM, Fratesi P, Corry M, Novotny W, Holmgren E, Small E. A Phase II Trial of Humanized Anti-Vascular Endothelial Growth Factor Antibody for the Treatment of Androgen-Independent Prostate Cancer. Prostate. 2001;3:65–70. [Google Scholar]
- 33.Ryan CJ, Lin AM, Small EJ. Angiogenesis inhibition plus chemotherapy for metastatic hormone refractory prostate cancer: history and rationale. Urol. Oncol. 2006;24:250–253. doi: 10.1016/j.urolonc.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 34.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Theodore C, James ND, Turesson I, Rosenthal MA, Eisenberger MA. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 35.Kelsey FO. Thalidomide update: regulatory aspects. Teratology. 1988;38:221–226. doi: 10.1002/tera.1420380305. [DOI] [PubMed] [Google Scholar]
- 36.Mellin GW, Katzenstein M. The saga of thalidomide. Neuropathy to embryopathy, with case reports of congenital anomalies. N. Engl. J. Med. 1962;267:1184–1192. doi: 10.1056/NEJM196212062672305. [DOI] [PubMed] [Google Scholar]
- 37.D'Amato RJ, Loughnan MS, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc. Natl. Acad. Sci. U S A. 1994;91:4082–4085. doi: 10.1073/pnas.91.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bauer KS, Dixon SC, Figg WD. Inhibition of angiogenesis by thalidomide requires metabolic activation, which is species-dependent. Biochem. Pharmacol. 1998;55:1827–1834. doi: 10.1016/s0006-2952(98)00046-x. [DOI] [PubMed] [Google Scholar]
- 39.Kurdziel K, Bacharach S, Carrasquillo J, Huebsch S, Whatley M, Sellers D, Steinberg S, Libutti S, Pluda J, Reed E, Dahut W, Figg W. Using PET 18F-FDG, 11CO, and 15O-water for Monitoring Prostate Cancer During a Phase II Anti-angiogenic Drug Trial with Thalidomide. Clin. Positron Imaging. 2000;3:144. doi: 10.1016/s1095-0397(00)00056-x. 8:45–9:00. [DOI] [PubMed] [Google Scholar]
- 40.Kurdziel KA, Figg WD, Carrasquillo JA, Huebsch S, Whatley M, Sellers D, Libutti SK, Pluda JM, Dahut W, Reed E, Bacharach SL. Using positron emission tomography 2-deoxy-2-[18F]fluoro-D-glucose, 11CO, and 15O-water for monitoring androgen independent prostate cancer. Mol. Imaging Biol. 2003;5:86–93. doi: 10.1016/s1536-1632(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 41.Figg WD, Retter A, Steinberg SM, Dahut WL. In Reply. J. Clin. Oncol. 2005;23 2113-a-2114. [Google Scholar]
- 42.Gulley J, Dahut WL. Chemotherapy for prostate cancer: finally an advance! Am. J. Ther. 2004;11:288–294. doi: 10.1097/01.mjt.0000133582.68709.e3. [DOI] [PubMed] [Google Scholar]
- 43.Aragon-Ching JB, Li H, Gardner ER, Figg WD. Thalidomide Analogues as Anticancer Drugs. Rec. Patents Anticancer Drug Discov. 2007;2:167–174. doi: 10.2174/157489207780832478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tohnya TM, Ng SS, Dahut WL, Wright JJ, Arlen PM, Gulley JL, Parker C, Zeldis J, Figg WD. A phase I study of oral CC-5013 (lenalidomide, Revlimid), a thalidomide derivative, in patients with refractory metastatic cancer. Clin. Prostate Cancer. 2004;2:241–243. doi: 10.3816/cgc.2004.n.006. [DOI] [PubMed] [Google Scholar]
- 45.Wilhelm S, Chien DS. BAY 43–9006: preclinical data. Curr. Pharm. Des. 2002;8:2255–2257. doi: 10.2174/1381612023393026. [DOI] [PubMed] [Google Scholar]
- 46.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, Cao Y, Shujath J, Gawlak S, Eveleigh D, Rowley B, Liu L, Adnane L, Lynch M, Auclair D, Taylor I, Gedrich R, Voznesensky A, Riedl B, Post LE, Bollag G, Trail PA. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 47.Dahut WL, Scripture C, Posadas E, Jain L, Gulley JL, Arlen PM, Wright JJ, Yu Y, Cao L, Steinberg SM, Aragon-Ching JB, Venitz J, Jones E, Chen CC, Figg WD. A phase II clinical trial of sorafenib in androgen-independent prostate cancer. Clin. Cancer Res. 2008;14:209–214. doi: 10.1158/1078-0432.CCR-07-1355. [DOI] [PubMed] [Google Scholar]
- 48.Aragon-Ching JB, Jain L, Gulley JL, Arlen PM, Wright JJ, Steinberg SM, Draper D, Venitz J, Jones E, Chen CC, Figg WD, Dahut WL. Final analysis of a phase II trial using sorafenib for metastatic castration-resistant prostate cancer. BJU Int. 2009 doi: 10.1111/j.1464-410X.2008.08327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steinbild S, Mross K, Frost A, Morant R, Gillessen S, Dittrich C, Strumberg D, Hochhaus A, Hanauske AR, Edler L, Burkholder I, Scheulen M. A clinical phase II study with sorafenib in patients with progressive hormone-refractory prostate cancer: a study of the CESAR Central European Society for Anticancer Drug Research-EWIV. Br. J. Cancer. 2007;97:1480–1485. doi: 10.1038/sj.bjc.6604064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chi KN, Ellard SL, Hotte SJ, Czaykowski P, Moore M, Ruether JD, Schell AJ, Taylor S, Hansen C, Gauthier I, Walsh W, Seymour L. A phase II study of sorafenib in patients with chemo-naive castration-resistant prostate cancer. Ann. Oncol. 2008;19:746–751. doi: 10.1093/annonc/mdm554. [DOI] [PubMed] [Google Scholar]
- 51.Takeda M, Arao T, Yokote H, Komatsu T, Yanagihara K, Sasaki H, Yamada Y, Tamura T, Fukuoka K, Kimura H, Saijo N, Nishio K. AZD2171 shows potent antitumor activity against gastric cancer over-expressing fibroblast growth factor receptor 2/keratinocyte growth factor receptor. Clin. Cancer Res. 2007;13:3051–3057. doi: 10.1158/1078-0432.CCR-06-2743. [DOI] [PubMed] [Google Scholar]
- 52.Wedge SR, Kendrew J, Hennequin LF, Valentine PJ, Barry ST, Brave SR, Smith NR, James NH, Dukes M, Curwen JO, Chester R, Jackson JA, Boffey SJ, Kilburn LL, Barnett S, Richmond GH, Wadsworth PF, Walker M, Bigley AL, Taylor ST, Cooper L, Beck S, Jurgensmeier JM, Ogilvie DJ. AZD2171: a highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res. 2005;65:4389–4400. doi: 10.1158/0008-5472.CAN-04-4409. [DOI] [PubMed] [Google Scholar]
- 53.Drevs J, Siegert P, Medinger M, Mross K, Strecker R, Zirrgiebel U, Harder J, Blum H, Robertson J, Jurgensmeier JM, Puchalski TA, Young H, Saunders O, Unger C. Phase I clinical study of AZD2171, an oral vascular endothelial growth factor signaling inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2007;25:3045–3054. doi: 10.1200/JCO.2006.07.2066. [DOI] [PubMed] [Google Scholar]
- 54.Ryan CJ, Stadler WM, Roth B, Hutcheon D, Conry S, Puchalski T, Morris C, Small EJ. Phase I dose escalation and pharmacokinetic study of AZD2171, an inhibitor of the vascular endothelial growth factor receptor tyrosine kinase, in patients with hormone refractory prostate cancer (HRPC) Invest. New Drugs. 2007;25:445–451. doi: 10.1007/s10637-007-9050-y. [DOI] [PubMed] [Google Scholar]
- 55.Fong TA, Shawver LK, Sun L, Tang C, App H, Powell TJ, Kim YH, Schreck R, Wang X, Risau W, Ullrich A, Hirth KP, McMahon G. SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res. 1999;59:99–106. [PubMed] [Google Scholar]
- 56.Mendel DB, Laird AD, Smolich BD, Blake RA, Liang C, Hannah AL, Shaheen RM, Ellis LM, Weitman S, Shawver LK, Cherrington JM. Development of SU5416, a selective small molecule inhibitor of VEGF receptor tyrosine kinase activity, as an anti-angiogenesis agent. Anticancer Drug Des. 2000;15:29–41. [PubMed] [Google Scholar]
- 57.Lockhart AC, Cropp GF, Berlin JD, Donnelly E, Schumaker RD, Schaaf LJ, Hande KR, Fleischer AC, Hannah AL, Rothenberg ML. Phase I/pilot study of SU5416 (semaxinib) in combination with irinotecan/bolus 5-FU/LV (IFL) in patients with metastatic colorectal cancer. Am. J. Clin. Oncol. 2006;29:109–115. doi: 10.1097/01.coc.0000199882.53545.ac. [DOI] [PubMed] [Google Scholar]
- 58.Chow LQ, Eckhardt SG. Sunitinib: from rational design to clinical efficacy. J. Clin. Oncol. 2007;25:884–896. doi: 10.1200/JCO.2006.06.3602. [DOI] [PubMed] [Google Scholar]
- 59.Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, Schreck RE, Abrams TJ, Ngai TJ, Lee LB, Murray LJ, Carver J, Chan E, Moss KG, Haznedar JO, Sukbuntherng J, Blake RA, Sun L, Tang C, Miller T, Shirazian S, McMahon G, Cherrington JM. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin. Cancer Res. 2003;9:327–337. [PubMed] [Google Scholar]
- 60.Christensen JG. A preclinical review of sunitinib, a multitargeted receptor tyrosine kinase inhibitor with anti-angiogenic and antitumour activities. Ann. Oncol. 2007;18 Suppl 10:x3–x10. doi: 10.1093/annonc/mdm408. [DOI] [PubMed] [Google Scholar]
- 61.Hupe DJ, Behrens ND, Boltz R. Anti-proliferative activity of L-651,582 correlates with calcium-mediated regulation of nucleotide metabolism at phosphoribosyl pyrophosphate synthetase. J. Cell Physiol. 1990;144:457–466. doi: 10.1002/jcp.1041440313. [DOI] [PubMed] [Google Scholar]
- 62.Hupe DJ, Boltz R, Cohen CJ, Felix J, Ham E, Miller D, Soderman D, Van Skiver D. The inhibition of receptor-mediated and voltage-dependent calcium entry by the antiproliferative L-651,582. J. Biol. Chem. 1991;266:10136–10142. [PubMed] [Google Scholar]
- 63.Wasilenko WJ, Palad AJ, Somers KD, Blackmore PF, Kohn EC, Rhim JS, Wright GL, Jr, Schellhammer PF. Effects of the calcium influx inhibitor carboxyamido-triazole on the proliferation and invasiveness of human prostate tumor cell lines. Int. J. Cancer. 1996;68:259–264. doi: 10.1002/(SICI)1097-0215(19961009)68:2<259::AID-IJC20>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 64.Kohn EC, Figg WD, Sarosy GA, Bauer KS, Davis PA, Soltis MJ, Thompkins A, Liotta LA, Reed E. Phase I trial of micronized formulation carboxyamidotriazole in patients with refractory solid tumors: pharmacokinetics, clinical outcome, and comparison of formulations. J. Clin. Oncol. 1997;15:1985–1993. doi: 10.1200/JCO.1997.15.5.1985. [DOI] [PubMed] [Google Scholar]
- 65.Bauer KS, Figg WD, Hamilton JM, Jones EC, Premkumar A, Steinberg SM, Dyer V, Linehan WM, Pluda JM, Reed E. A pharmacokinetically guided Phase II study of carboxyamido-triazole in androgen-independent prostate cancer. Clin. Cancer Res. 1999;5:2324–2329. [PubMed] [Google Scholar]
- 66.O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 67.Oh SP, Kamagata Y, Muragaki Y, Timmons S, Ooshima A, Olsen BR. Isolation and sequencing of cDNAs for proteins with multiple domains of Gly-Xaa-Yaa repeats identify a distinct family of collagenous proteins. Proc. Natl. Acad. Sci. U S A. 1994;91:4229–4233. doi: 10.1073/pnas.91.10.4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim YM, Jang JW, Lee OH, Yeon J, Choi EY, Kim KW, Lee ST, Kwon YG. Endostatin inhibits endothelial and tumor cellular invasion by blocking the activation and catalytic activity of matrix metalloproteinase. Cancer Res. 2000;60:5410–5413. [PubMed] [Google Scholar]
- 69.Madigan MC, Kingsley EA, Cozzi PJ, Delprado WJ, Russell PJ, Li Y. The role of extracellular matrix metalloproteinase inducer protein in prostate cancer progression. Cancer Immunol. Immunother. 2008;57:1367–1379. doi: 10.1007/s00262-008-0473-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abdollahi A, Lipson KE, Sckell A, Zieher H, Klenke F, Poerschke D, Roth A, Han X, Krix M, Bischof M, Hahnfeldt P, Grone HJ, Debus J, Hlatky L, Huber PE. Combined therapy with direct and indirect angiogenesis inhibition results in enhanced antiangiogenic and antitumor effects. Cancer Res. 2003;63:8890–8898. [PubMed] [Google Scholar]
- 71.Isayeva T, Chanda D, Kallman L, Eltoum IE, Ponnazhagan S. Effects of sustained antiangiogenic therapy in multistage prostate cancer in TRAMP model. Cancer Res. 2007;67:5789–5797. doi: 10.1158/0008-5472.CAN-06-3637. [DOI] [PubMed] [Google Scholar]
- 72.Dahut WL, Lakhani NJ, Gulley JL, Arlen PM, Kohn EC, Kotz H, McNally D, Parr A, Nguyen D, Yang SX, Steinberg SM, Venitz J, Sparreboom A, Figg WD. Phase I clinical trial of oral 2-methoxyestradiol, an antiangiogenic and apoptotic agent, in patients with solid tumors. Cancer Biol. Ther. 2006;5:22–27. doi: 10.4161/cbt.5.1.2349. [DOI] [PubMed] [Google Scholar]
- 73.Logothetis CJ, Wu KK, Finn LD, Daliani D, Figg W, Ghaddar H, Gutterman JU. Phase I trial of the angiogenesis inhibitor TNP-470 for progressive androgen-independent prostate cancer. Clin. Cancer Res. 2001;7:1198–1203. [PubMed] [Google Scholar]