Abstract
Neuropeptide Y (NPY) conjugated with a ribosomal inactivating toxin, saporin (SAP), is a toxin that targets NPY receptor-expressing cells. Injection of NPY–SAP into the rat arcuate nucleus (Arc) and basomedial hypothalamus (BMH) destroys two populations of NPY-receptor-expressing neurons important for the control of food intake and body weight, NPY and pro-opiomelanocortin (POMC) and cocaine and amphetamine related transcript (CART) neurons, and produces profound hyperphagia and obesity. Here, we investigated the contribution of lateral hypothalamus (LHA) orexigenic peptides, orexins and melanocortin concentrating hormone (MCH), to these lesion effects. We microinjected NPY–SAP into two sites on each side of the Arc, causing a loss of NPY and POMC/CART neurons that was limited to the Arc. Lesioned rats rapidly became hyperphagic and obese. However, MCH and prepro-orexin mRNA expression were not increased in the LHA in the lesioned rats, but were decreased at some levels of the LHA or were unchanged. NPY–SAP-induced obesity therefore differs from dietary obesity and from obesity associated with leptin or leptin receptor deficiency in which MCH gene expression is increased. The Arc NPY–SAP lesion produces obesity and hyperphagia that does not require overexpression of hypothalamic neuropeptides currently considered to provide major stimulatory drive for food intake: NPY, agouti gene-related protein, MCH or orexins. The source of the seemingly unregulated stimulatory drive for feeding in these animals has not been identified, but may be associated with hindbrain or endocrine mechanisms.
Keywords: Saporin, Arcuate nucleus, MCH, Orexins, LHA, Rats
1. Introduction
The arcuate nucleus (Arc) in the basomedial hypothalamus (BMH) is crucial for control of food intake and energy expenditure. Two groups of neurons in the Arc that are importantly involved in these functions are neuropeptide Y (NPY) and agouti gene-related protein (AGRP) co-expressing neurons and the pro-opiomelanocortin (POMC) and cocaine-and amphetamine-regulated transcript (CART) co-expressing neurons [6,16,24,28]. When administered centrally, NPY and AGRP are orexigenic, whereas POMC and CART are anorexigenic. Electrolytic lesions of BMH and chemical lesions produced by monosodium glutamate or gold thioglucose, which dramatically increase food intake and body weight, have highlighted the importance of this brain region for energy homeostasis [6,13,34,36,37].
NPY–saporin, a conjugate of NPY and the ribosomal inactivating toxin, saporin (SAP), is a chemical lesioning agent that targets NPY receptor-expressing neurons [7]. Selective destruction of NPY receptor-expressing neurons is mediated by agonist-driven receptor internalization of the NPY–SAP conjugate. Once present in the soma, saporin inactivates ribosomes and destroys the affected neuron. Like most peptide-SAP conjugates, NPY–SAP is not retrogradely transported and so destroys NPY receptor-expressing cell bodies locally at the injection site, but does not destroy distant NPY neurons that terminate there [7]. The fact that NPY/AGRP and CART/POMC neurons express NPY receptors [9,25] makes both cell types targets for NPY–SAP internalization and the initial study of this targeted toxin [7] showed that Arc injections of NPY–SAP destroyed NPY/AGRP and CART/POMC neurons. These lesions virtually eliminated feeding responses to lateral ventricular injection of leptin and ghrelin, peptides whose major actions are known to be heavily dependent on those neurons. As with other lesions of the BMH, NPY–SAP lesions caused profound hyperphagia and obesity.
The neural circuitry responsible for the hyperphagia and obesity following NPY–SAP-induced lesions and other lesions of the BMH is poorly understood [6,26]. Therefore, in the present experiment, we injected NPY–SAP into the Arc and examined the effects on expression of two orexigenic peptide genes in the lateral hypothalamic area (LHA), melanin-concentrating hormone (MCH) mRNA and prepro-orexin mRNA [5,17], that are potential mediators of the NPY–SAP-induced hyperphagia and obesity. MCH [4,35,40] and orexin [10,46] cell bodies are abundant and almost exclusively located in the LHA.
Both MCH and orexins increase food intake when administered centrally [12,15,40,46] and both MCH and orexin genes are sensitive to changes in energy balance. Evidence for participation of MCH in control of energy expenditure, food intake and body weight is particularly compelling. Expression of the MCH gene is increased by fasting [40]. Mice lacking the MCH gene are lean [1], and mice in which the MCH gene is chronically over expressed are obese [32]. Expressions of MCH mRNA and peptide production are increased in genetically obese (ob/ob) mice [40,49] and diet-induced obesity [18]. Central injection of orexin-A simulates feeding and feeding is disrupted by knockout of the prepro-orexin gene, immunoneutralization of orexin-A, or pharmacological blockade of orexin A receptors. Fasting has been reported to increase orexin gene expression [11,53], an effect that is modulated by leptin. However, the relationship of orexins to obesity differs from that of MCH that prepro-orexin gene expression is decreased, not increased, in genetically obese (ob/ob and db/db) mice [53].
Arc NPY/AGRP and CART/POMC neurons may contribute to the control of LHA prepro-orexin and MCH gene expression by leptin. Leptin receptors are present on Arc NPY/AGRP and CART/POMC neurons that innervate LHA orexin and MCH neurons [5,17]. Furthermore, food deprivation-induced prepro-orexin mRNA expression can be reversed by central leptin administration [31], and chronic treatment with central leptin injection for one week has been reported to significantly decrease orexin-A concentration in the LHA region [2]. Prior intraventricular injection of leptin blocks MCH-induced stimulation of food intake [43] and leptin decreases MCH gene expression [44].
These data predict that LHA prepro-orexin or MCH gene expression would be altered by NPY–SAP induced deletion of Arc NPY/AGRP and POMC/CART neurons. Because this NPY–SAP lesion causes profound hyperphagia and obesity, we predicted that the lesion would increase the expression of MCH, which is increased in other forms of obesity. Furthermore, if this lesion is similar to genetic and dietary forms of obesity examined thus far, we would predict that obese NPY–SAP rats would have decreased or unchanged levels of prepro-orexin gene expression. Understanding the components of feeding circuitry and their interactions is crucial for development of pharmacological approaches for control of ingestion.
2. Materials and methods
2.1. Animals
Male and female Sprague Dawley rats were purchased from Simonsen Laboratories (Gilroy, CA) and housed individually in suspended wire mesh cages in an animal care facility approved by the Association for Assessment and Accreditation of Laboratory Animal Care. Rats were maintained on a 12-h light (0700–1900) and 12-h dark cycle with ad libitum access to standard pelleted rodent chow (F6 Rodent diet; Harlan Teklad, Madison, WI) and tap water. All experimental procedures were approved by Washington State University Institutional Animal Care and Use Committee, which conforms to NIH Guidelines.
2.2. Injection of NPY-SAP and control SAP
For stereotaxic microinjections, rats were anesthetized using 0.1 ml/100 g body weight of a ketamine-xylazine-acepromazine cocktail (5 ml ketamine HCl, 100 mg/ml, Fort Dodge Animal Health, Fort Dodge, IA; 2.5 ml xylazine, 20 mg/ml, Vedco, Inc., St. Joseph, MO; 1 ml acepromazine, 10 mg/ml, Vedco, Inc.; and 1.5 ml 0.9% saline solution). Injection parameters were modified from previous work with NPY–SAP [7]. NPY-SAP (24 ng, Advanced Targeting Systems, Carlsbad, CA) or the equivalent amount of control blank saporin (B-SAP), dissolved in 50 nl of 0.1 M phosphate buffer (PBS, pH 7.4), was injected bilaterally into two sites per side on the dorsal border of the Arc. Stereotaxic coordinates of the rostral injection sites were 2.5 caudal and + or −0.4 mm lateral to bregma and 8.3 mm ventral to dura mater. Coordinates for the caudal sites were 3.5 caudal and + or −0.4 mm lateral to bregma and 8.5 mm ventral to dura mater [38]. Injections were made with a Picospritzer (Parker; Cleveland, OH) connected to a pulled glass capillary pipette (30-µm tip diameter). Fluid movement was monitored microscopically. The solution was injected slowly over a 5-min period.
2.3. Body weight and food intake
Our previous studies have shown that NPY–SAP rats eat more than controls during the daytime, but not at night [7]. Therefore, in this experiment we measured 4-h intake of the rats’ standard pelleted food during the light phase (09:00–13:00) every 1–3 weeks after the injection.
2.4. Tissue preparation
Rats were euthanized 10–12 weeks after NPY–SAP or B-SAP injection for analysis of lesion effects and gene expression. They were anesthetized deeply with Halothane (Halocarbon Laboratories, River Edge, NJ). Transcardial perfusion was initiated just prior to cessation of the heartbeat using PBS (pH 7.4) followed by cold 4% paraformaldehyde in PBS. After perfusion, brains were removed rapidly and placed in 4% paraformaldehyde/PBS at 4 °C for overnight, then into a series of sucrose (10–25%) in PBS for a total of 20 h. Coronal hypothalamic sections (20 µm thick) were cut and collected into five sets of serial sections that were mounted onto SuperFrost Plus slides (Fisher Scientific, Los Angeles, CA). After drying, sections were stored in desiccated slide boxes at −80 °C until processed for in situ hybridization as described below.
2.5. In situ hybridization
The protocol used in the present study was modified from our previous reports [29,30]. Antisense riboprobes of rat NPY, CART, MCH, and mouse prepro-orexin were transcribed with RNA polymerase in the presence of 33P-UTP (Perkin-Elmer, Indianapolis, IN) using a MAXIscript kit (Ambion, Austin, TX), as described previously [7,29]. Unincorporated isotope was removed using spin columns (Roche, Indianapolis, IN). Probes were quantified in a scintillation counter. Before use, the probe was combined in a 1/20 ratio of Torula RNA (Sigma) and 0.1 M Tris/0.01 M EDTA (pH 8.0), and then mixed with hybridiztion buffer (containing 6.25% deionized formamide, 12.5% dextran sulfate, 0.375 M NaCl, 10 mM Tris (pH 8.0), 1.6 mM EDTA, 1.25×Denhardt’s solution, and 10 mM DTT) at a ratio of 1:3. Probe mix was heat denatured at 65 °C for 15 min. The final volume of probe mix plus hybridization buffer was 150 µl/slide (or 1.5 × 106 cpm/slide). Sections were treated in 0.1 M triethanolamine with 250 µl/ml acetic anhydride for 10 min, rinsed in 2× SSC (sodium citrate-sodium chloride buffer) for 5 min, dehydrated in graded ethanol solutions (70%, 90%, and 100%; 3 min each), and then allowed to air dry before the hybridization procedure. After adding the hybridization mix to the slides, the sections were covered with Parafilm (Pechiney Plastic Packaging, Menasha, WI) and placed in humidity chambers that were then placed into an oven set at the calculated hybridization temperature (47–50 °C) for 16–17 h. Following hybridization, the slides were washed twice in 2× SSC for 30 min each at room temperature. Slides were then incubated for 30 min at 37 °C in RNase buffer (10 mM Tris, 0.5 M NaCl, 1 mM EDTA, pH 8.0) containing RNase-A (0.02 mg/ ml) followed by incubation for 10 min at 37 °C in the buffer without RNase-A. Next, after a 5-min wash in 2× SSC at room temperature, slides were incubated in 0.1× SSC at 58–60 °C for 30 min twice, then again twice for 5 min in fresh 0.1 M SSC at room temperature. Sections were then dehydrated in graded ethanol (50%, 70%, 90%, and 100%; 3 min each) and allowed to air dry.
Then the slides were exposed to BioMax MR films (Eastman Kodak Co., Rochester, NY) for 2–7 days at –80 °C. After exposure, films were developed. The slides were then counterstained in 0.5% cresyl violet in acetate buffer, dehydrated in graded ethanol and cleared in Citrasol (VWR Scientific Products Inc.) and coverslipped with DPX (EM Sciences, Fort Washington, PA).
2.6. Quantification and analysis of gene expression
After the films were developed, the hybridization signal on the film was quantified using densitometric analysis. Briefly, hybridization signals on the films were digitized using a high-resolution scanner set at a constant setting for each experiment. The digitized images were then analyzed using Scion Image (Scion Co., Frederick, MD). The optical density (OD) of a rectangular area enclosing each hypothalamic region of interest was measured. Three sections from each rat were quantified at each 300 um interval along the coronal plane throughout the structure of interest. After subtracting out the background, the OD of a sample area, in arbitrary units of density × area, was recorded as peptide mRNA expression values. Brain tissue from both sexes was used for in situ hybridization Images from 3–5 rats per group were quantified.
2.7. Statistical analysis
All data are presented as mean ± S.E.M. Unpaired t test was used for analyzing the difference between NPY–SAP and B-SAP rats. Differences were considered significant if P ≤ 0.05.
3. Results
3.1. Body weight gain and food intake after NPY-SAP injection
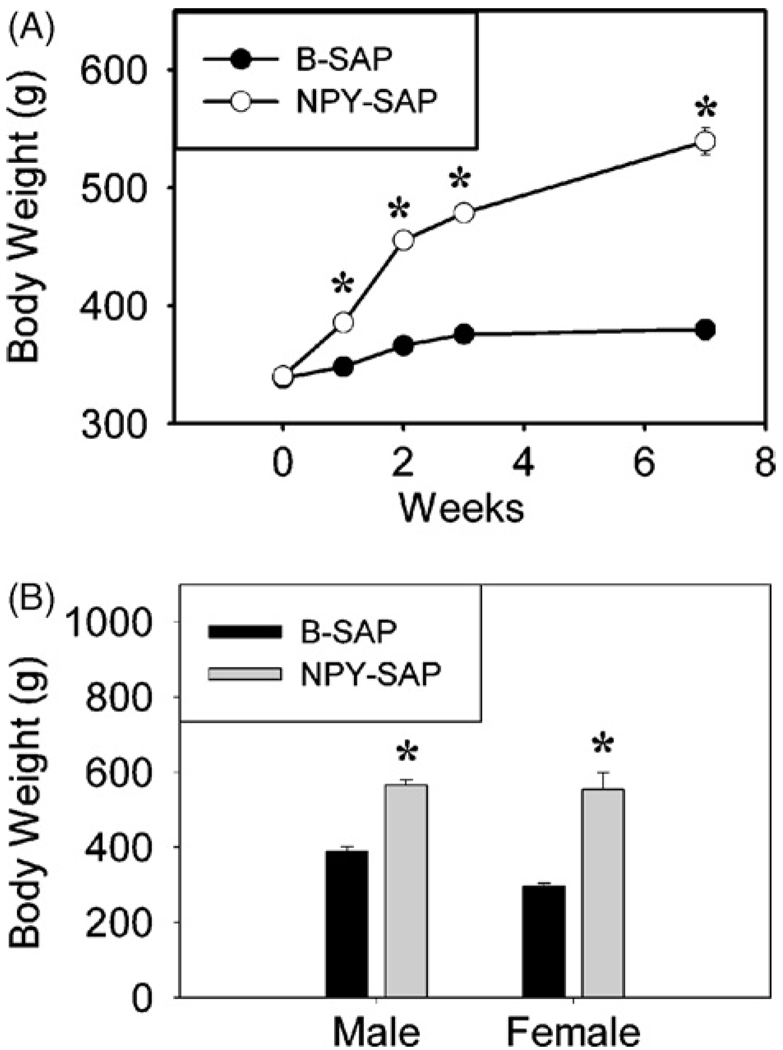
Rats with NPY-SAP injections into the BMH gained weight rapidly. Only one week after injection, the increase above B-SAP control was already statistically significant. As shown for male rats in Fig. 1A, body weight was significantly increased at all time points after the infusion (Ps < 0.001 for all cases; n = 5 for each group). Fig. 1B shows body weight for male and female rats just prior to sacrifice 10–12 weeks after NPY–SAP and B-SAP administration (P < 0.001). In male rats, body weight of B-SAP controls increased 49 g (from 338.4 ± 2.6 g to 387.8 ± 13.0 g), while body weight of NPY–SAP rats increased 226 g (from 340.4 ± 0.7 g to 566.2 ± 14.4 g) during their 10 weeks post injection survival period. Body weights female B-SAP controls increased 10 g (from 286.0 ± 7.0 g to 296.2 ± 7.8 g, while female NPY–SAP rats increased 289 g (from 265.0 ± 8.3 g to 553.9 ± 44.9 g) during their 12 week post-injection survival period. As noted previously for unconjugated saporin injections [41], effects of the control B-SAP injection did not appear to contribute to the effects we observed. Weight gain of male and female B-SAP injected rats over the experimental period was as expected for normal, uninjected rats. Normal untreated male Sprague-Dawley rats from our supplier (Simonsen Laboratories), with starting weights of approximately 350 g and on the same standard rodent maintenance diet as used in this experiment, gain approximately 50–60 g over a 10-week period. Normal females with starting weights of about 280 g gain approximately 10–20 g over a 12-week period.
Fig. 1.
Body weight gain after NPY–SAP or B-SAP infusion into the hypothalamus. A, Weekly body weight (g) changes of male rats after bilateral infusion of NPY–SAP or control blank SAP (B-SAP) into the basomedial hypothalamus, just dorsal to the Arc. B, Body weight (g) of male and female rats at the conclusion of the experiment, 10–12 weeks after NPY–SAP or B-SAP infusion into the hypothalamus. (n = 3–5 rats for each group; #P < 0.005; *P < 0.001; unpaired t-test vs. B-SAP rats).
Daytime food intake was also increased above that of controls in the NPY–SAP injected rats, as shown previously [7]. Food intake in the 4-h daytime tests in male rats was increased to 376% (6.4 ± 1.6 g vs.1.7 ± 0.9 g), 1233% (3.7 ± 1.2 g vs. 0.3 ± 0.1 g), 620% (3.1 ± 0.4 g vs. 0.5 ± 0.3 g), and 364% (4.0 ± 1.1 g vs. 1.1 ± 0.9 g) of the intake of B-SAP rats, respectively, at 10 days, 3, 5, and 8 weeks after NPY–SAP injection (Ps < 0.05).
3.2. NPY mRNA and CART mRNA expression in the hypothalamus
The expression of NPY and CART mRNAs in the hypothalamus was examined to confirm the lesion of NPY-receptor-expressing NPY and CART neurons in Arc by NPY-SAP. The expression of NPY and CART mRNAs was examined in the dorsomedial hypothalamic nucleus (DM) to establish the localization of the lesion.
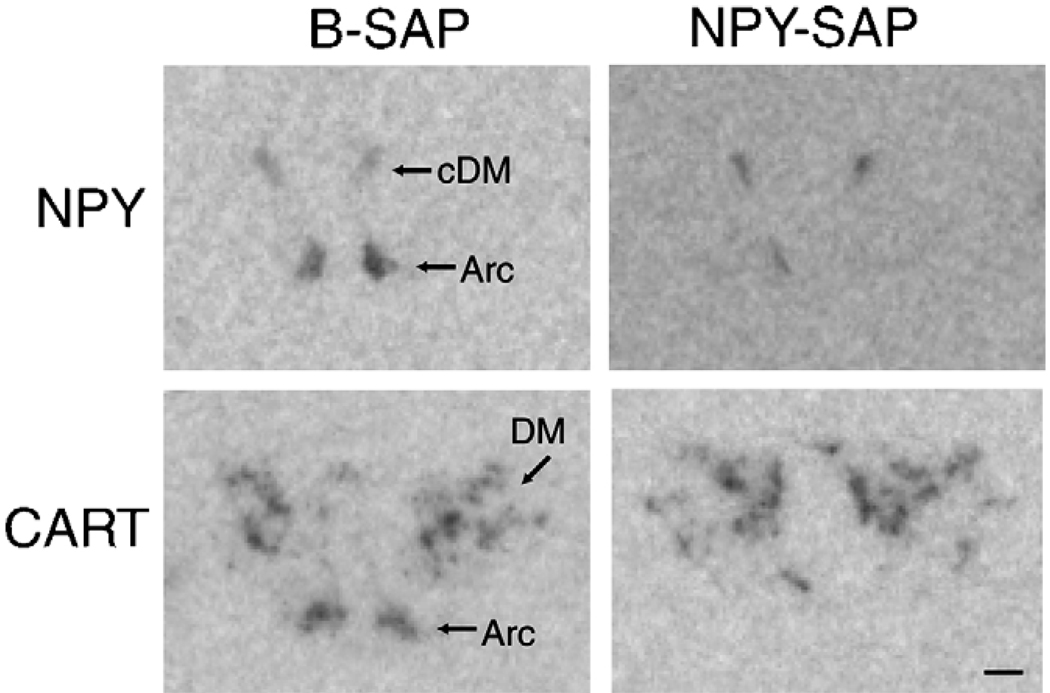
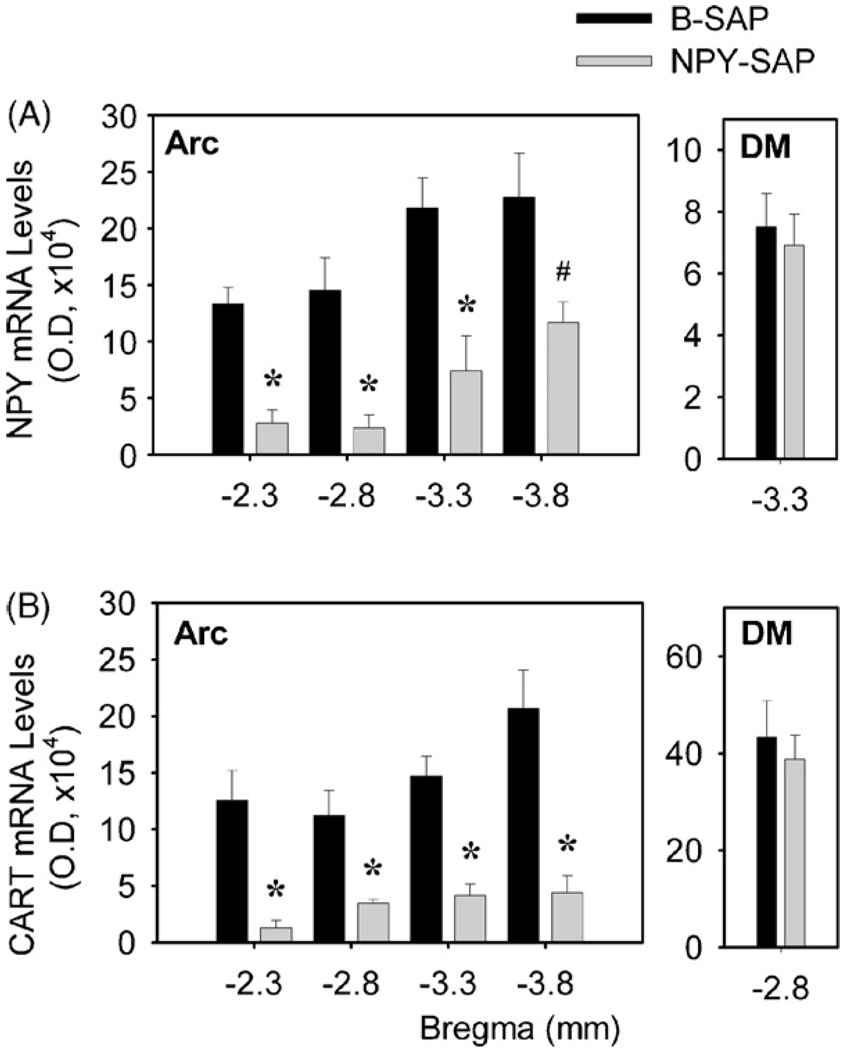
In control B-SAP rats (Fig. 2), NPY mRNA expression was found in Arc between 2.3 to 3.8 mm caudal to bregma and in the compact part of DM at 3.3 mm caudal to bregma. NPY mRNA expression (Fig. 3A) was dramatically decreased in Arc in NPY–SAP rats, at all levels measured (P < 0.05 for all comparisons; n = 5). In contrast, NPY mRNA expression in the compact part of DM did not differ between NPY–SAP and B-SAP rats (P > 0.7). Quantitative data showed that overall NPY mRNA levels in Arc and DM in NPY–SAP rats were 25.3 and 92.0% of the control B-SAP rats, respectively.
Fig. 2.
Representative images showing expression of NPY and CART mRNA in coronal sections from hypothalamus (3.3 and 2.8 mm caudal to bregma, respectively). NPY–SAP dramatically decreased expression of both mRNAs in the Arc, but not in the DM, indicating that the lesion was localized in the Arc (see quantitative data in Fig. 3). Calibration bar = 0.5 mm.
Fig. 3.
Expression of hypothalamic NPY mRNA and CART mRNA, as determined by quantification of optical density. Panel (A) shows NPY mRNA expression in the Arc (left) and the compact part of DM (right) at different coronal levels. Distance (mm) caudal to bregma is indicated on the X-axis. Panel (B) shows optical density of CART mRNA expression in the Arc (left) and DM (right) at different levels caudal to bregma. #P < 0.05; *P < 0.01 (n = 5 for each group; unpaired t test; vs. B-SAP-infused rats).
In B-SAP rats (Fig. 2), CART mRNA expression was observed in Arc between 2.3 and 3.8 mm caudal to bregma and also in DM at these same levels. As shown in Fig. 3B, the expression levels of CART mRNA was dramatically decreased at all levels of the Arc by NPY–SAP, compared to B-SAP (P < 0.01 for all comparisons; n = 5). However, there were no significant differences between groups in the DM (P > 0.6). Quantitative data showed that overall CART mRNA levels in Arc and DM in NPY–SAP rats were 23.2 and 96.1% of the control rats, respectively. The decrease in NPY and CART expression in the Arc, but not in the DM, of NPY–SAP injected rats indicates that the NPY–SAP was effective in producing a lesion and that the lesion was highly localized within the Arc.
3.3. MCH and prepro-orexin expression in the LHA
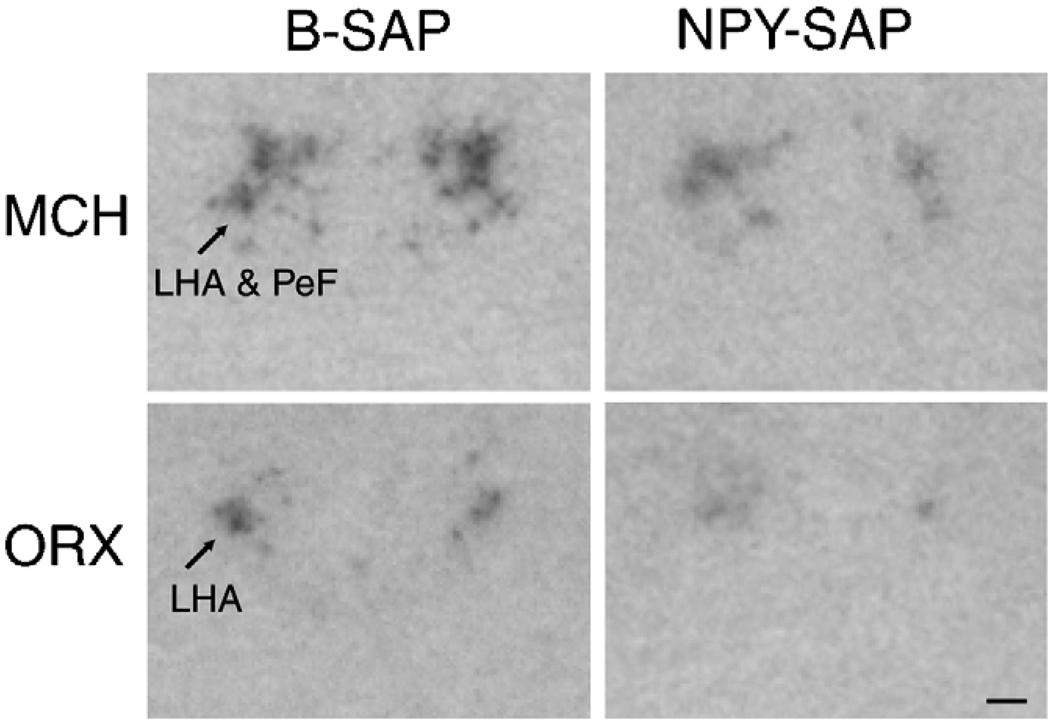
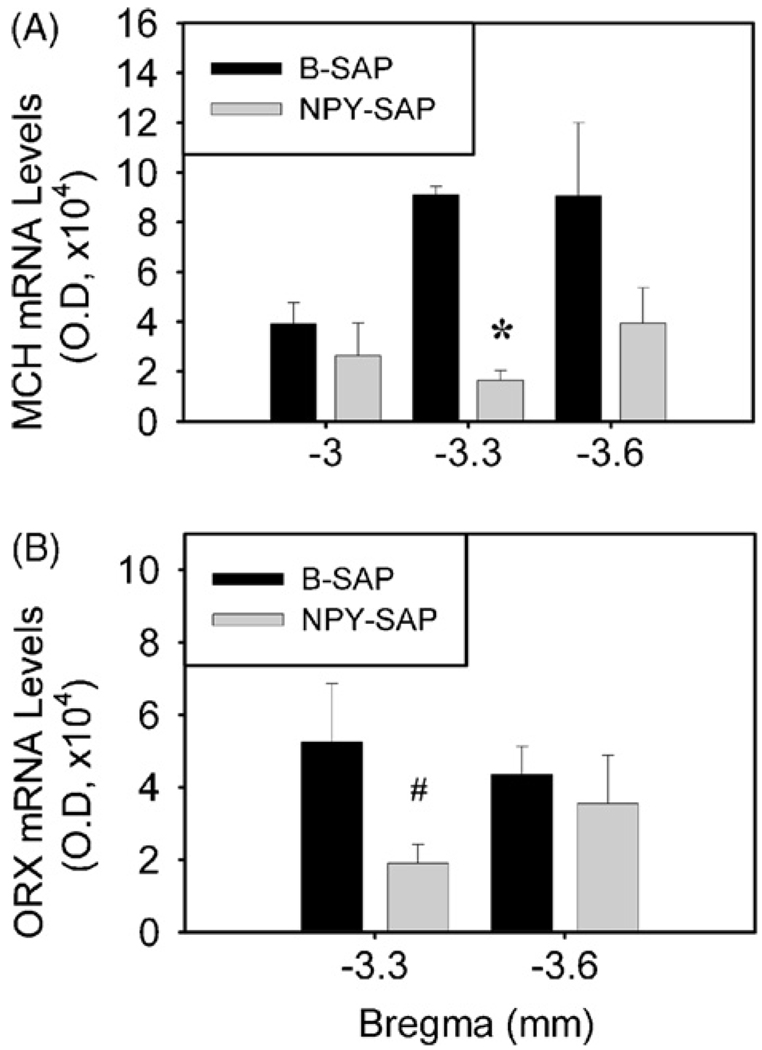
In B-SAP controls, MCH mRNA expression was present exclusively in LHA and nearby perifornical nucleus (PeF) between 3.0 and 3.6 mm caudal to bregma, while prepro-orexin mRNA was found exclusively in the LHA between 3.3 and 3.6 mm caudaltobregma (Fig. 4). In NPY–SAP injected rats, MCH mRNA expression in LHA/PeF did not differ from controls at 3.0 or 3.6 mm caudal to bregma (P > 0.1), but was significantly decreased at 3.3 mm caudal to bregma (P < 0.01). The overall expression of MCH mRNA in LHA/PeF in NPY–SAP rats was 41.4% of the control.
Fig. 4.
Representative images showing expression of MCH and ORX mRNAs in coronal sections from hypothalamus (3.3 and 2.8 mm caudal to bregma, respectively). NPY–SAP slightly decreased expression of both mRNAs in the LHA (see quantitative data in Fig. 5). Calibration bar = 0.5 mm.
Prepro-orexin mRNA expression did not differ between groups at 3.6 mm caudal to bregma (P > 0.6), but was significantly decreased in the NPY–SAP group at 3.3 mm caudal to bregma (P = 0.05). The overall prepro-orexin mRNA expression in LHA was only 56.7% of control in NPY–SAP rats.
4. Discussion
Injection of NPY–SAP into the Arc dramatically reduced expression of NPY and CART mRNAs at the injection site, as described previously [7], due to destruction of NPY-receptor-expressing neurons (including NPY and CART neurons) in the vicinity of the injection site. As reported previously [7], lesioned rats gained weight rapidly and became obese. Male NPY–SAP rats gained 4.6 times more body weight than male SAP controls during the 10 weeks post injection period. Female NPY–SAP rats gained 29 times more body weight than female SAP controls during 12 post injection weeks. Consistent with our previous report [7], rats injected with NPY–SAP were hyperphagic, consuming 3.6–12.3 fold more food than controls during 4-h daytime tests. Although additional metabolic and pair feeding experiments may reveal a lesion-induced decrease in metabolic rate, clearly hyperphagia was a major contributor to the obesity induced by the Arc NPY–SAP lesion.
Despite the hyperphagia and obesity induced by the Arc NPY–SAP injections, neither the MCH nor the prepro-orexin gene was upregulated in these animals. mRNA expression for both genes was either unchanged or reduced in obese NPY–SAP injected rats. In the case of prepro-orexin, the failure of the NPY–SAP lesion to increase prepro-orexin gene expression is consistent with the fact that expression of this gene is not increased in genetically obese mice (ob/ob and db/db) or rats (fa/fa Zucker rats) [8,50,53]. However, in the case of MCH, our results are surprising because MCH has been reported to be upregulated in both genetic and dietary-induced forms of obesity [18,40,49] and because decreased gene expression is associated with a lean phenotype [1,48]. It therefore appears from these data that NPY–SAP induced hyperphagia and obesity do not require upregulation of either MCH or prepro-orexin genes, or the presence of NPY/AGRP co-expressing neurons, which are lesioned by the NPY–SAP injection.
One possible explanation for the lack of upregulation of MCH and prepro-orexin genes in our experiment is that the NPY–SAP injected into the Arc may have diffused into the LHA region in quantities sufficient to lesion MCH and orexin neurons. Because they express NPY receptors [14,21,27], these neurons are potential targets of NPY–SAP. However, this seems unlikely due to the evidence that the NPY–SAP lesions were highly localized in the Arc. Small injection volumes (50 nl/site) were used. In the DM, which lies dorsal to the Arc and along the tract of the injector pipette, there was no evidence of significant loss of NPY or POMC/CART gene expression, although NPY receptor genes are strongly expressed in DM NPY neurons [22]. Even if LHA neurons were lesioned by NPY–SAP, we could still conclude that increased prepro-orexin and MCH gene expression are not required for the hyperphagic and obesifying effects of the lesion.
It is also possible that gene overexpression was suppressed in the NPY–SAP lesioned rats by direct effects of leptin on orexin and MCH neurons. Both cell types express leptin receptors and both genes are down regulated by leptin. However, while this might explain the effects of the lesion on orexin and MCH gene expression, it does not explain the underlying mechanisms of obesity and hyperphagia.
NPY–SAP injection destroyed NPY/AGRP neurons, which innervate and provide a major source for activation of MCH and orexin neurons [5,17]. However, it is unlikely that loss of NPY or AGRP is the cause of the NPY–SAP-induced hyperphagia and obesity, since these peptides are orexigenic and their loss would be likely to decrease rather than increase food intake. NPY knockout mice and NPY/AGRP double knockout mice do not become obese, but exhibit normal body weight and food intake [19,39]. Furthermore, loss of the NPY/AGRP-expressing neurons themselves, and thus deletion of all neuroactive substances expressed by these same neurons, is not likely to be the cause of obesity in our experiment. Selective targeted ablation of NPY/AGRP neurons has been shown to produce a lean, hypophagic phenotype [3], and selective deletion of these neurons in adult animals causes anorexia and rapid starvation [23,33,51].
Alternatively, obesity and hyperphagia associated with the NPY–SAP lesion seems more likely to be a consequence of POMC neuron lesion. Both mice with mutation of the POMC gene [54] and those with selective ablation of POMC neurons [52] become obese. Loss of POMC neurons may result in disinhibition of feeding circuitry due to loss of the projections from CART/POMC neurons. However, the failure of NPY–SAP lesion to upregulate the orexin and MCH genes in our experiment indicates that these neurons were probably not disinhibited. Thus, if disinhibition is a cause of hyperphagia and obesity in NPY–SAP lesioned rats, this effect is not dependent on orexin or MCH neurons.
An interesting possibility is that hindbrain controls of appetite may have contributed to the hyperphagia and obesity in the NPY–SAP lesioned rats. Previously we reported that Arc NPY–SAP injections virtually eliminated feeding responses to lateral ventricular injection of leptin and ghrelin, peptides whose major actions are known to be heavily dependent on NPY/AGRP and CART/POMC neurons. However, glucoprivic and lipoprivic stimulation of feeding and glucagon-like peptide-1 and cholecystokinin-induced suppression of feeding, responses not heavily reliant on NPY/AGRP and CART/POMC neurons, were not impaired. Thus, important controls of feeding emanating from the hindbrain survive this lesion and may be adequate to cause hyperphagia and obesity in the absence of modulating influences from hypothalamic circuitry. It is also possible that in the aftermath of the NPY–SAP lesion, the hindbrain input to the hypothalamus is re-wired in a way that leads to dysregulation. For example, hypothalamic neurons are innervated by hindbrain NPY/ catecholamine co-expressing neurons, as well as by NPY neurons with cell bodies in the Arc [20,45,47]. Our previous study showed that NPY-immunoreactive fibers and terminals in hypothalamic paraventricular nucleus (PVN) were significantly reduced 10 days after NPY–SAP injection into Arc [7]. However, several weeks later, NPY-positive fibers both in Arc and PVN recovered to the levels seen in controls [7], suggesting post-lesion growth of new terminal processes. Since NPY neurons in the Arc had been destroyed, we concluded that hindbrain NPY neurons were a likely source of new hypothalamic terminals [41,42]. In this type of situation, surviving NPY-responsive neurons in the hypothalamus may be re- innervated after the lesion by different NPY neurons controlled by different signals, thus resulting in dysregulation of appetite. Additional studies will be required to shed light on the effects of Arc NPY–SAP lesions on feeding circuits.
Our results show that lesion of NPY receptor-expressing neurons in the Arc causes hyperphagia that occurs despite profound reduction of Arc neurons containing NPY and AGRP, two major orexigenic peptides. In addition, the hyperphagia and obesity are not associated with a compensatory increase in expression of the genes for the lateral hypothalamic orexigenic peptides, MCH and orexin. The source of the stimulatory drive for feeding in rats with Arc NPY–SAP lesions remains unknown.
Fig. 5.
Expression of MCH and ORX mRNAs in the LHA, as determined by quantitative analysis of optical density. Panel (A) shows MCH mRNA expression at 3, 3.3 and 3.6 mm caudal to bregma. Panel (B) shows ORX mRNA expression at 3.3 and 3.6 mm caudal to bregma. #P < 0.05; *P < 0.01 (n = 3–5 rats for each group; unpaired t test; vs. B-SAP-infused rats).
Acknowledgments
This work was supported by Public Health Service Grant DK 40498 and NS 4552004 to S.R.
REFERENCES
- 1.Alon T, Friedman JM. Late-onset leanness in mice with targeted ablation of melanin concentrating hormone neurons. J Neurosci. 2006;26:389–397. doi: 10.1523/JNEUROSCI.1203-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck B, Richy S. Hypothalamic hypocretin/orexin and neuropeptide Y: divergent interaction with energy depletion and leptin. Biochem Biophys Res Commun. 1999;258:119–122. doi: 10.1006/bbrc.1999.0605. [DOI] [PubMed] [Google Scholar]
- 3.Bewick GA, Gardiner JV, Dhillo WS, Kent AS, White NE, Webster Z, et al. Post-embryonic ablation of AgRP neurons in mice leads to a lean, hypophagic phenotype. FASEB J. 2005;19:1680–1682. doi: 10.1096/fj.04-3434fje. [DOI] [PubMed] [Google Scholar]
- 4.Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, et al. The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J Comp Neurol. 1992;319:218–245. doi: 10.1002/cne.903190204. [DOI] [PubMed] [Google Scholar]
- 5.Broberger C, De Lecea L, Sutcliffe JG, Hökfelt T. Hypocretin/orexin- and melanin-concentrating hormone-expressing cells form distinct populations in the rodent lateral hypothalamus: relationship to the neuropeptide Y and agouti gene-related protein systems. J Comp Neurol. 1998;402:460–474. [PubMed] [Google Scholar]
- 6.Broberger C, Johansen J, Johansson C, Schalling M, Hökfelt T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc Natl Acad Sci U S A. 1998;95:15043–15048. doi: 10.1073/pnas.95.25.15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bugarith K, Dinh TT, Li AJ, Speth RC, Ritter S. Basomedial hypothalamic injections of neuropeptide Y conjugated to saporin selectively disrupt hypothalamic controls of food intake. Endocrinology. 2005;146:1179–1191. doi: 10.1210/en.2004-1166. [DOI] [PubMed] [Google Scholar]
- 8.Cai XJ, Lister CA, Buckingham RE, Pickavance L, Wilding J, Arch JR, et al. Down-regulation of orexin gene expression by severe obesity in the rats: studies in Zucker fatty and zucker diabetic fatty rats and effects of rosiglitazone. Brain Res Mol Brain Res. 2000;77:131–137. doi: 10.1016/s0169-328x(00)00041-3. [DOI] [PubMed] [Google Scholar]
- 9.Cone RD, Cowley MA, Butler AA, Fan W, Marks DL, Low MJ. The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int J Obes Relat Metab Disord. 2001;25 Suppl. 5:S63–S67. doi: 10.1038/sj.ijo.0801913. [DOI] [PubMed] [Google Scholar]
- 10.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diano S, Horvath B, Urbanski HF, Sotonyi P, Horvath TL. Fasting activates the nonhuman primate hypocretin (orexin) system and its postsynaptic targets. Endocrinology. 2003;144:3774–3778. doi: 10.1210/en.2003-0274. [DOI] [PubMed] [Google Scholar]
- 12.Dube MG, Kalra SP, Kalra PS. Food intake elicited by central administration of orexins/hypocretins: identification of hypothalamic sites of action. Brain Res. 1999;842:473–477. doi: 10.1016/s0006-8993(99)01824-7. [DOI] [PubMed] [Google Scholar]
- 13.Dube MG, Xu B, Kalra PS, Sninsky CA, Kalra SP. Disruption in neuropeptide Y and leptin signaling in obese ventromedial hypothalamic-lesioned rats. Brain Res. 1999;816:38–46. doi: 10.1016/s0006-8993(98)00985-8. [DOI] [PubMed] [Google Scholar]
- 14.Dumont Y, Jacques D, Bouchard P, Quirion R. Species differences in the expression and distribution of the neuropeptide Y Y1, Y2, Y4, and Y5 receptors in rodents, guinea pig, and primates brains. J Comp Neurol. 1998;402:372–384. [PubMed] [Google Scholar]
- 15.Edwards CM, Abusnana S, Sunter D, Murphy KG, Ghatei MA, Bloom SR. The effect of the orexins on food intake: comparison with neuropeptide Y, melanin-concentrating hormone and galanin. J Endocrinol. 1999;160:R7–R12. doi: 10.1677/joe.0.160r007. [DOI] [PubMed] [Google Scholar]
- 16.Elias CF, Lee C, Kelly J, Aschkenasi C, Ahima RS, Couceyro PR, et al. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron. 1998;21:1375–1385. doi: 10.1016/s0896-6273(00)80656-x. [DOI] [PubMed] [Google Scholar]
- 17.Elias CF, Saper CB, Maratos-Flier E, Tritos NA, Lee C, Kelly J, et al. Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J Comp Neurol. 1998;402:442–459. [PubMed] [Google Scholar]
- 18.Elliott JC, Harrold JA, Brodin P, Enquist K, Bäckman A, Byström M, et al. Increases in melanin-concentrating hormone and MCH receptor levels in the hypothalamus of dietary-obese rats. Brain Res Mol Brain Res. 2004;128:150–159. doi: 10.1016/j.molbrainres.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Erickson JC, Clegg KE, Palmiter RD. Sensitivity to leptin and susceptibility to seizures of mice lacking neuropeptide Y. Nature. 1996;381:415–421. doi: 10.1038/381415a0. [DOI] [PubMed] [Google Scholar]
- 20.Everitt BJ, Hokfelt T, Terenius L, Tatemoto K, Mutt V, Goldstein M. Differential co-existence of neuropeptide Y (NPY)-like immunoreactivity with catecholamines in the central nervous system of the rat. Neuroscience. 1984;11:443–462. doi: 10.1016/0306-4522(84)90036-8. [DOI] [PubMed] [Google Scholar]
- 21.Gehlert DR, Gackenheimer SL. Differential distribution of neuropeptide Y Y1 and Y2 receptors in rat and guinea-pig brains. Neuroscience. 1997;76:215–224. doi: 10.1016/s0306-4522(96)00340-5. [DOI] [PubMed] [Google Scholar]
- 22.Glavas MM, Grayson BE, Kirigiti MA, Cowley MA, Smith MS, Grove KI. Comparison of neurotransmitter receptor expression in NPY neurons of the arcuate and dorsomedial hypothalamus during development. Program No. 300.2; 2007 Neuroscience Meeting Planner; San Diego, CA: Society for Neuroscience; 2007. [Google Scholar]
- 23.Gropp E, Shanabrough M, Borok E, Xu AW, Janoschek R, Buch T, et al. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat Neurosci. 2005;8:1289–1291. doi: 10.1038/nn1548. [DOI] [PubMed] [Google Scholar]
- 24.Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci. 1998;1:271–272. doi: 10.1038/1082. [DOI] [PubMed] [Google Scholar]
- 25.Hökfelt T, Broberger C, Zhang X, Diez M, Kopp J, Xu Z, et al. Neuropeptide Y: some viewpoints on a multifaceted peptide in the normal and diseased nervous system. Brain Res Brain Res Rev. 1998;26:154–166. doi: 10.1016/s0165-0173(97)00052-0. [DOI] [PubMed] [Google Scholar]
- 26.Homma A, Li HP, Hayashi K, Kawano Y, Kawano H. Differential response of arcuate proopiomelanocortin- and neuropeptide Y-containing neurons to the lesion produced by gold thioglucose administration. J Comp Neurol. 2006;499:120–131. doi: 10.1002/cne.21097. [DOI] [PubMed] [Google Scholar]
- 27.Kopp J, Xu ZQ, Zhang X, Pedrazzini T, Herzog H, Kresse A, et al. Expression of the neuropeptide Y Y1 receptor in the CNS of rat and of wild-type and Y1 receptor knock-out mice. Focus on immunohistochemical localization. Neuroscience. 2002;111:443–532. doi: 10.1016/s0306-4522(01)00463-8. [DOI] [PubMed] [Google Scholar]
- 28.Kristensen P, Judge ME, Thim L, Ribel U, Christjansen KN, Wulff BS, et al. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature. 1998;393:72–76. doi: 10.1038/29993. [DOI] [PubMed] [Google Scholar]
- 29.Li AJ, Ritter S. Glucoprivation increases expression of neuropeptide Y mRNA in hindbrain neurons that innervate the hypothalamus. Eur J Neurosci. 2004;19:2147–2154. doi: 10.1111/j.1460-9568.2004.03287.x. [DOI] [PubMed] [Google Scholar]
- 30.Li AJ, Wang Q, Ritter S. Differential responsiveness of dopamine-beta-hydroxylase gene expression to glucoprivation in different catecholamine cell groups. Endocrinology. 2006;147:3428–3434. doi: 10.1210/en.2006-0235. [DOI] [PubMed] [Google Scholar]
- 31.López M, Seoane L, García MC, Lago F, Casanueva FF, Señarís R, et al. Leptin regulation of prepro-orexin and orexin receptor mRNA levels in the hypothalamus. Biochem Biophys Res Commun. 2000;269:41–45. doi: 10.1006/bbrc.2000.2245. [DOI] [PubMed] [Google Scholar]
- 32.Ludwig DS, Tritos NA, Mastaitis JW, Kulkarni R, Kokkotou E, Elmquist J, et al. Melanin-concentrating hormone overexpression in transgenic mice leads to obesity and insulin resistance. J Clin Invest. 2001;107:379–386. doi: 10.1172/JCI10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- 34.Marshall NB, Barrnett RJ, Mayer J. Hypothalamic lesions in goldthioglucose injected mice. Proc Soc Exp Biol Med. 1955;90:240–244. doi: 10.3181/00379727-90-21995. [DOI] [PubMed] [Google Scholar]
- 35.Naito N, Kawazoe I, Nakai Y, Kawauchi H. Melanin-concentrating hormone-like immunoreactive material in the rat hypothalamus; characterization and subcellular localization. Cell Tissue Res. 1988;253:291–295. doi: 10.1007/BF00222284. [DOI] [PubMed] [Google Scholar]
- 36.Olney JW, Adamo NJ, Ratner A. Monosodium glutamate effects. Science. 1971;172:294. doi: 10.1126/science.172.3980.294. [DOI] [PubMed] [Google Scholar]
- 37.Olney JW. Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science. 1969;164:719–721. doi: 10.1126/science.164.3880.719. [DOI] [PubMed] [Google Scholar]
- 38.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3rd ed. San Diego: Academic Press; 1997. [DOI] [PubMed] [Google Scholar]
- 39.Qian S, Chen H, Weingarth D, Trumbauer ME, Novi DE, Guan X, et al. Neither agouti-related protein nor neuropeptide Y is critically required for the regulation of energy homeostasis in mice. Mol Cell Biol. 2002;22:5027–5035. doi: 10.1128/MCB.22.14.5027-5035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, et al. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380:243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- 41.Ritter S, Bugarith K, Dinh TT. Immunotoxic destruction of distinct catecholaminergic subgroups produces selective impairment of glucoregulatory responses and neuronal activation. J Comp Neurol. 2001;432:197–216. doi: 10.1002/cne.1097. [DOI] [PubMed] [Google Scholar]
- 42.Ritter S, Dinh TT, Zhang Y. Localization of hindbrain glucoreceptive sites controlling food intake and blood glucose. Brain Res. 2000;856:37–47. doi: 10.1016/s0006-8993(99)02327-6. [DOI] [PubMed] [Google Scholar]
- 43.Sahu A. Evidence suggesting that galanin (GAL), melanin-concentrating hormone (MCH), neurotensin (NT), proopiomelanocortin (POMC) and neuropeptide Y (NPY) are targets of leptin signaling in the hypothalamus. Endocrinology. 1998;139:795–798. doi: 10.1210/endo.139.2.5909. [DOI] [PubMed] [Google Scholar]
- 44.Sahu A. Leptin decreases food intake induced by melanin-concentrating hormone (MCH), galanin (GAL) and neuropeptide Y (NPY) in the rat. Endocrinology. 1998;139:4739–4742. doi: 10.1210/endo.139.11.6432. [DOI] [PubMed] [Google Scholar]
- 45.Sahu A, Kalra SP, Crowley WR, Kalra PS. Evidence that NPY-containing neurons in the brainstem project into selected hypothalamic nuclei: implication in feeding behavior. Brain Res. 1988;457:376–378. doi: 10.1016/0006-8993(88)90710-x. [DOI] [PubMed] [Google Scholar]
- 46.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 47.Sawchenko PE, Swanson LW, Grzanna R, Howe PR, Bloom SR, Polak JM. Colocalization of neuropeptide Y immunoreactivity in brainstem catecholaminergic neurons that project to the paraventricular nucleus of the hypothalamus. J Comp Neurol. 1985;241:138–153. doi: 10.1002/cne.902410203. [DOI] [PubMed] [Google Scholar]
- 48.Shimada M, Tritos NA, Lowell BB, Flier JS, Maratos-Flier E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature. 1998;396:670–674. doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- 49.Stricker-Krongrad A, Dimitrov T, Beck B. Central and peripheral dysregulation of melanin-concentrating hormone in obese Zucker rats. Brain Res Mol Brain Res. 2001;92:43–48. doi: 10.1016/s0169-328x(01)00130-9. [DOI] [PubMed] [Google Scholar]
- 50.Stricker-Krongrad A, Richy S, Beck B. Orexins/hypocretins in the ob/ob mouse: hypothalamic gene expression, peptide content and metabolic effects. Regul Pept. 2002;104:11–20. doi: 10.1016/s0167-0115(01)00344-5. [DOI] [PubMed] [Google Scholar]
- 51.Wu Q, Howell MP, Cowley MA, Palmiter RD. Starvation after AgRP neuron ablation is independent of melanocortin signalling. Proc Natl Acad Sci U S A. 2008;105:2687–2692. doi: 10.1073/pnas.0712062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu AW, Kaelin CB, Morton GJ, Ogimoto K, Stanhope K, Graham J, et al. Effects of hypothalamic neurodegeneration on energy balance. PLoS Biol. 2005;3:e415. doi: 10.1371/journal.pbio.0030415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamamoto Y, Ueta Y, Date Y, Nakazato M, Hara Y, Serino R, et al. Down regulation of the prepro-orexin gene expression in genetically obese mice. Brain Res Mol Brain Res. 1999;65:14–22. doi: 10.1016/s0169-328x(98)00320-9. [DOI] [PubMed] [Google Scholar]
- 54.Yaswen L, Diehl N, Brennan MB, Hochgeschwender U. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat Med. 1999;5:1066–1070. doi: 10.1038/12506. [DOI] [PubMed] [Google Scholar]