Abstract
BACKGROUND
Pulmonary embolism causes significant morbidity in hospitalized patients, yet few studies have explored the impact of spiral computed tomography (CT) scanning on diagnosis and clinical outcome.
METHODS
Incidence rates of pulmonary embolism, chest and spiral CT rates, D-dimer assay, anticoagulation, and in-hospital mortality were assessed on statewide pulmonary embolism discharge data (1997–2001) from the Pennsylvania Health Care Cost Containment Council.
RESULTS
The incidence of pulmonary embolism increased from 47 to 63 per 100,000 patients from 1997 to 2001 (mean of 0.004% per year, P < .001). Mean pulmonary embolism incidence rates were higher for African American patients (0.031% per year higher than for white patients), patients aged 70 years or more (0.007% higher than for patients aged < 70 years), and female patients (0.013% higher than for male patients) (all P < .001). Concomitantly, the proportion undergoing CT (including spiral) scans increased from 23.23% to 45.18% (odds ratio = 1.30; P < .001), controlling for age, gender, race, and cancer, whereas rates for other procedures remained unchanged. By comparing 1999 and before with 2000 and after, there was a significant decrease in the 2 highest Atlas Severity of Illness categories (49.4%–37.7%) and a significant increase in the 3 lowest categories (50.6%–62.3%; P < .001). The risk of in-hospital deaths among patients with pulmonary embolism decreased in this period from 12.8% to 11.1% (P < .001).
CONCLUSION
The incidence of pulmonary embolism is increasing with the increasing use of spiral CT scans, with a lower severity of illness and lower mortality, suggesting the increase is due to earlier diagnosis.
Keywords: Hospital discharges, Pulmonary embolism, Spiral computed tomography, Venous thromboembolism
Pulmonary embolism is a major public health problem, accounting for 10% of hospital deaths,1 and the most common preventable cause of hospital death.2 With the emergence of spiral computed tomography (CT) scanning to enhance visualization of the pulmonary circulation in the last 10 to 15 years, spiral CT has emerged as a sensitive, specific, and widely available procedure. Since the 1999 publication of its emerging role in pulmonary embolism diagnosis in the emergency department,3 spiral CT has become the first-line method for evaluating patients with suspected pulmonary embolism.4 The impact of spiral CT, newer noninvasive diagnostic tools (ie, D-dimer assay),5 more effective treatment, and prophylaxis strategies (ie, low molecular weight heparin [LMWH])6 for those at risk for pulmonary embolism have not been thoroughly studied. We determined the incidence of pulmonary embolism by analyzing inpatient hospital discharge data (1997–2001) from the state of Pennsylvania by age of occurrence, gender, race, diagnostic procedures, and severity of illness. We hypothesized that the introduction of spiral CT, alone or together with the D-dimer assay and LMWH therapy, has led to earlier pulmonary embolism diagnosis and lower pulmonary embolism mortality rates.
MATERIALS AND METHODS
Discharge data for pulmonary embolism for the most recent 5-year period available were obtained from the Pennsylvania Health Care Cost Containment Council (PHC4). This independent agency collects inpatient hospital discharge data and outpatient procedure records from hospitals and ambulatory surgery centers in the state of Pennsylvania to monitor health care cost.7 Data for this analysis were limited to Pennsylvania residents hospitalized between 1997 and 2001, excluding pregnant patients and other than first hospital admission for pulmonary embolism. All patient identifiers were removed. Vital status was collected, including in-hospital deaths. The total number of Pennsylvania discharges for Pennsylvania residents and the length of stay for each patient were obtained from the PHC4 website to determine the population risk period.8 Total discharges were analyzed by year for age, race, and sex, and Mediqual Atlas Severity of Illness scores.7 The Atlas database or Cardinal Health-Atlas Research Database (formerly MedisGroup, Marlborough, Mass) collects and analyzes information from hospital admissions from more than 200 acute care hospitals in the United States to determine predictors of outcome and cost, including patient demographics, length of stay, ventilator use, intensive care stay, hospital changes, and more than 400 clinical findings, including vital signs, laboratory tests, culture results, and medical assessment.9
Clinical data were specified by diagnostic International Classification of Diseases, 9th Revision codes for pulmonary embolism, 415.11 and 415.19,10 excluding second or later admissions for pulmonary embolism; and by diagnostic procedure revenue codes, 320, including general roentgenogram; 323, arteriography; 324, chest roentgenogram; 341, nuclear medicine scan; and 352, CT scan (including spiral scan). Pennsylvania population figures were obtained from the US Census Bureau.11 Comorbid diagnoses were analyzed by the following International Classification of Diseases, 9th Revision codes: 140 to 230 for cancer/neoplasms, excluding 210 to 229, benign neoplasms, and 230 to 234 carcinoma in situ; 428, 398.91 for congestive heart failure; and 800 to 959, trauma, including 800 to 829, fractures. Spiral CT scan, D-dimer assay, and anticoagulation data, not available in the PHC4 database, were obtained on a subset of PHC4 subjects cared for at the University of Pittsburgh Medical Center (UPMC), Pittsburgh, Pennsylvania, whose records were retrieved from the UPMC Medical Archival Record System and linked to the original PHC4 dataset using an honest broker system.
Analysis of continuous data was by the Student t test, for example, pulmonary embolism incidence by year. True population risk was calculated as the proportion of pulmonary embolism discharges per year during the population risk period, estimated from the total Pennsylvania discharges each year and the individual length of stay. The proportion of patients undergoing CT scan by year was analyzed by multivariate logistic regression controlling for age, race, and gender, and the UPMC subset. Odds ratios were estimated for mortality by age, gender, race, and comorbidities by multiple logistic regression. In-hospital mortality was analyzed by age, gender, race, and comorbidity using logistic regression.
RESULTS
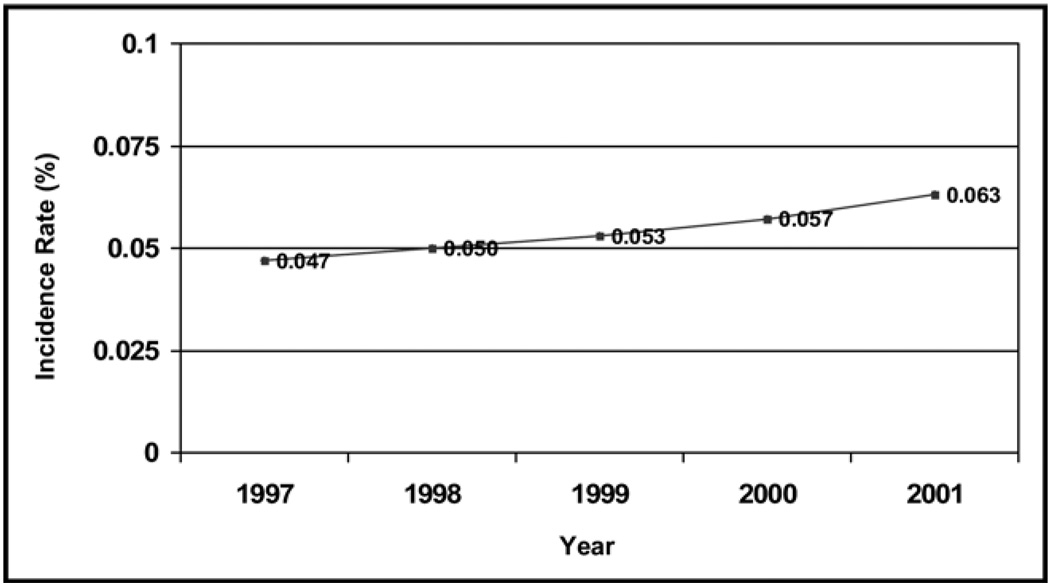
The hospital discharges for pulmonary embolism increased from 1997 to 2001 (Table 1). The incidence of pulmonary embolism, based on 37,892 Pennsylvania residents hospitalized for pulmonary embolism from 1997 to 2001, increased from a mean of 0.047% to 0.063% between 1997 and 2001, a mean increase of 0.004% per year (P < .001) (Figure 1). The true population risk, based on all discharges and length of stay during the period, increased from 0.056% in 1997 to 0.088% in 2001, a mean increase of 0.008% per year (P < .001) (Table 1). Risk factors associated with pulmonary embolism were: age more than 70 years, female gender, and African American race (mean annual increase in risk, 0.0007%; age > 70 vs ≤ 70 years; female vs male, 0.013%; African American race vs white, 0.031%; all P < .0001).
Table 1.
Incidence Rates of Pulmonary Embolism in Pennsylvania
| 1997 | 1998 | 1999 | 2000 | 2001 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PE cases | 5775 | 0.047% | 6143 | 0.050% | 6415 | 0.052% | 6987 | 0.057% | 7699 | 0.063% |
| Pennsylvania population |
12,227,814 | 12,245,672 | 12,263,805 | 12,282,591 | 12,287,150 | |||||
| Pennsylvania discharges |
1,754,633 | 1,718,525 | 1,745,181 | 1,789,147 | 1,812,898 | |||||
| Length of stay (d) | 10,384,079 | 9,043,073 | 8,424,381 | 8,554,508 | 8,732,616 | |||||
| Population risk | 0.056% | 0.068% | 0.076% | 0.082% | 0.088% | |||||
| Male | 2447 | 0.042% | 2639 | 0.046% | 2687 | 0.047% | 2938 | 0.049% | 3153 | 0.053% |
| 5,774,474 | 5,768,439 | 5,765,533 | 5,932,973 | 5,945,018 | ||||||
| Female | 3328 | 0.053% | 3504 | 0.056% | 3728 | 0.060% | 4049 | 0.064% | 4546 | 0.071% |
| 6,241,414 | 6,233,890 | 6,228,483 | 6,352,519 | 6,353,345 | ||||||
| White | 4692 | 0.044% | 4884 | 0.046% | 4965 | 0.047% | 5408 | 0.051% | 5995 | 0.056% |
| 10,644,566 | 10,621,032 | 10,602,898 | 10,676,627 | 10,669,055 | ||||||
| African American | 615 | 0.053% | 643 | 0.055% | 598 | 0.051% | 712 | 0.056% | 760 | 0.060% |
| 1,162,680 | 1,166,137 | 1,170,095 | 1,260,689 | 1,267,387 | ||||||
| Age ≥ 70 y | 2894 | 0.219% | 3137 | 0.223% | 3134 | 0.221% | 3333 | 0.231% | 3746 | 0.259% |
| 1,386,751 | 1,403,276 | 1,415,445 | 1,441,115 | 1,446,076 | ||||||
| Age < 70 y | 2881 | 0.027% | 3006 | 0.028% | 3281 | 0.031% | 3654 | 0.034% | 3953 | 0.036% |
| 10,629,137 | 10,599,053 | 10,578,571 | 10,844,992 | 10,857,028 | ||||||
| Associated Disorders | ||||||||||
| CHF | 1181 | 17.83% | 1256 | 17.99% | 1254 | 17.04% | 1349 | 16.81% | 1603 | 18.01% |
| Cancer | 1589 | 23.98% | 1736 | 24.87% | 1784 | 24.24% | 2013 | 25.08% | 2262 | 25.41% |
| Trauma | 562 | 8.48% | 577 | 8.27% | 610 | 8.29% | 621 | 7.74% | 734 | 8.25% |
| Atlas Severity of Illness Score |
||||||||||
| Missing | 641 | 9.68% | 757 | 10.85% | 548 | 7.44% | 484 | 6.03% | 662 | 7.44% |
| None | 96 | 1.45% | 108 | 1.55% | 117 | 1.59% | 113 | 1.41% | 203 | 2.28% |
| Minimal | 791 | 11.94% | 767 | 10.99% | 831 | 11.29% | 1543 | 19.23% | 1491 | 16.75% |
| Moderate | 2738 | 41.33% | 2840 | 40.69% | 3160 | 42.93% | 3735 | 46.54% | 4110 | 46.17% |
| Severe | 2202 | 33.24% | 2332 | 33.41% | 2534 | 34.42% | 2107 | 26.26% | 2360 | 26.51% |
| Maximal | 157 | 2.37% | 176 | 2.52% | 171 | 2.32% | 43 | 0.54% | 75 | 0.84% |
| Mortality | 759 | 11.46% | 831 | 11.91% | 785 | 10.66% | 818 | 10.19% | 819 | 9.20% |
| Diagnostic Procedures | ||||||||||
| 320 general x-rays | 5006 | 42.59% | 5043 | 39.27% | 5040 | 36.47% | 5307 | 33.88% | 5636 | 31.31% |
| 323 Arteriography | 136 | 1.16% | 190 | 1.48% | 255 | 1.85% | 247 | 1.58% | 265 | 1.47% |
| 324 chest x-rays | 3049 | 25.94% | 3361 | 26.17% | 3761 | 27.21% | 4241 | 27.07% | 4922 | 27.35% |
| 341 nuclear scans | 1968 | 16.74% | 2327 | 18.12% | 2426 | 17.55% | 2760 | 17.62% | 3011 | 16.73% |
| 352 CT scans | 1273 | 10.83% | 1589 | 12.37% | 1963 | 14.20% | 2724 | 17.39% | 3681 | 20.45% |
| Missing | 321 | 2.73% | 331 | 2.58% | 375 | 2.71% | 387 | 2.47% | 483 | 2.68% |
PE = pulmonary embolism; CHF = congestive heart failure; CT = computed tomography.
Figure 1.
Incidence of pulmonary embolism in the Pennsylvania population from 1997 to 2001.
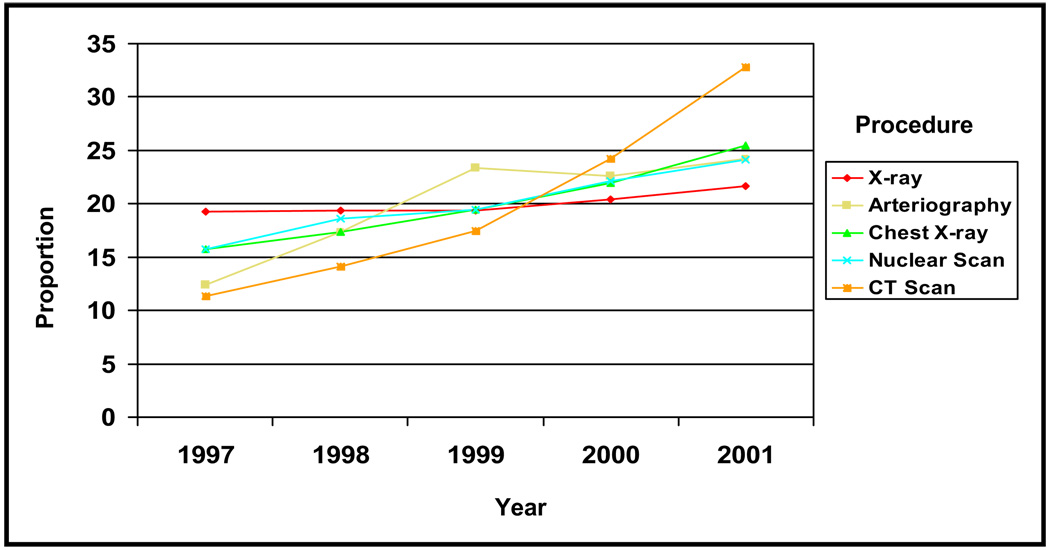
Concurrently, the proportion of patients with pulmonary embolisms undergoing CT scans, as determined by revenue codes, increased from 23.23% to 45.18% (odds ratio 1.30 per year; P <.001), after controlling for age, race, and gender (Table 1; Figure 2). No significant trends were observed for other procedures or in the proportion of patients with pulmonary embolism and congestive heart failure, cancer, or trauma (Table 1). Among the subset of PHC4 subjects cared for at UPMC, the proportion undergoing spiral CT scans increased significantly between 1997 and 2001, from 13.54% to 48.92% (P < .001) (Table 2). Spiral CT scans accounted for an increasing proportion of all CT scans performed, 30.3% in 1997 to 71.2% in 2001 (P < .001), by Cochran-Armitage test. During this same period, there also was a significant increase in enoxaparin use, 13.97% to 32.56% (P < .001), concomitant with its licensing, but not in D-dimer testing (P = .36), heparin use (P = .29), or warfarin use (P = .17). Compared with the PHC4 group as a whole, the UPMC subset was somewhat younger (60.77 vs 65.55 years; P < .0001), more likely to be male (49.60% vs 42.22%; P < .0001), and more likely to be African American (14.51% vs 11.12%; P = .0435).
Figure 2.
Proportion of patients hospitalized for pulmonary embolism in Pennsylvania who underwent diagnostic procedures between 1997 and 2001. CT = computed tomography.
Table 2.
Pennsylvania Health Care Cost Containment Council Subset University of Pittsburgh Medical Center Discharge Data: Characteristics and Procedures
| 1997 |
1998 |
1999 |
2000 |
2001 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PHC4 | MARS | PHC4 | MARS | PHC4 | MARS | PHC4 | MARS | PHC4 | MARS | |
| No. of discharges | 5575 | 163 | 6143 | 165 | 6415 | 237 | 6987 | 175 | 7699 | 209 |
| Sex | ||||||||||
| % Female | 57.35 | 48.34 | 56.93 | 49.67 | 57.60 | 51.49 | 57.93 | 50.00 | 58.83 | 51.74 |
| % Male | 42.65 | 51.66 | 43.07 | 50.33 | 42.40 | 48.51 | 42.07 | 50.00 | 41.17 | 48.26 |
| Race | ||||||||||
| % Black | 11.35 | 13.38 | 11.39 | 16.44 | 10.66 | 15.18 | 11.25 | 13.13 | 10.97 | 14.36 |
| % Hispanic | 1.57 | 0.00 | 1.69 | 0.68 | 1.55 | 0.00 | 1.22 | 0.00 | 1.32 | 0.53 |
| % Other | 0.66 | 1.41 | 0.39 | 0.68 | 0.77 | 0.52 | 0.51 | 1.25 | 0.69 | 0.00 |
| % White | 86.42 | 85.21 | 86.53 | 82.19 | 87.02 | 84.29 | 87.02 | 85.63 | 87.02 | 85.11 |
| Age (mean y) | 66.03 | 60.11 | 66.14 | 63.27 | 65.46 | 58.70 | 64.98 | 60.49 | 65.29 | 61.29 |
| By Decade | ||||||||||
| % 0–10 yr | 0.10 | 0.00 | 0.06 | 0.00 | 0.16 | 0.00 | 0.09 | 0.00 | 0.03 | 0.00 |
| % 11–20 yr | 0.54 | 0.00 | 0.58 | 0.00 | 0.74 | 2.97 | 0.78 | 0.61 | 0.56 | 0.00 |
| % 21–30 yr | 2.96 | 5.96 | 2.81 | 5.88 | 2.71 | 4.95 | 3.31 | 3.66 | 2.78 | 4.98 |
| % 31–40 yr | 5.50 | 6.62 | 5.37 | 5.88 | 6.00 | 6.44 | 5.82 | 9.15 | 6.02 | 6.47 |
| % 41–50 yr | 9.53 | 16.56 | 9.63 | 8.50 | 9.99 | 15.84 | 10.93 | 16.46 | 11.07 | 16.42 |
| % 51–60 yr | 12.69 | 14.57 | 12.69 | 14.38 | 13.89 | 18.32 | 13.84 | 14.63 | 14.35 | 13.93 |
| % 61–70 yr | 21.08 | 18.54 | 20.66 | 18.30 | 20.32 | 19.31 | 19.74 | 17.68 | 19.25 | 19.40 |
| % 71–80 yr | 29.33 | 27.15 | 29.72 | 33.99 | 27.81 | 22.28 | 27.72 | 23.78 | 27.94 | 24.38 |
| % 81–90 yr | 15.67 | 9.27 | 16.34 | 11.76 | 15.83 | 7.43 | 15.64 | 11.59 | 15.47 | 13.93 |
| % 91–100 yr | 2.60 | 1.32 | 2.13 | 1.31 | 2.56 | 2.48 | 2.10 | 2.44 | 2.51 | 0.50 |
| % 100 yr | 0.00 | 0.00 | 0.02 | 0.00 | 0.00 | 0.00 | 0.03 | 0.00 | 0.01 | 0.00 |
| Associated Disorders | ||||||||||
| % Cancer | 17.89 | 21.47 | 18.65 | 23.03 | 18 | 15.61 | 19.34 | 16 | 19.19 | 15.31 |
| % CHF | 16.66 | 17.79 | 16.89 | 18.18 | 15.84 | 15.61 | 15.61 | 15.43 | 16.49 | 13.40 |
| % Trauma | 6.78 | 10.43 | 6.53 | 6.06 | 6.49 | 8.86 | 6.22 | 13.14 | 6.48 | 13.88 |
| Diagnostic Procedures | ||||||||||
| % Spiral CT | – | 13.54 | – | 17.84 | – | 14.39 | – | 24.15 | – | 48.92 |
| % Spiral CT of all CT scans |
– | 30.33 | – | 34.7 | – | 25.3 | – | 35.2 | – | 71.2 |
| % D-dimer assay | – | 6.15 | – | 4.02 | – | 7.28 | – | 2.60 | – | 6.02 |
| Anticoagulation | ||||||||||
| % Enoxaparin | – | 13.97 | – | 15.52 | – | 28.74 | – | 21.35 | – | 32.56 |
| % Heparin | – | 93.85 | – | 91.95 | – | 85.82 | – | 90.63 | – | 89.77 |
| % Warfarin | – | 70.39 | – | 71.26 | – | 73.18 | – | 72.40 | – | 78.14 |
PHC4 = Pennsylvania Health Care Cost Containment Council; MARS = Medical Archival Record System; CHF = congestive heart failure; CT = computed tomography.
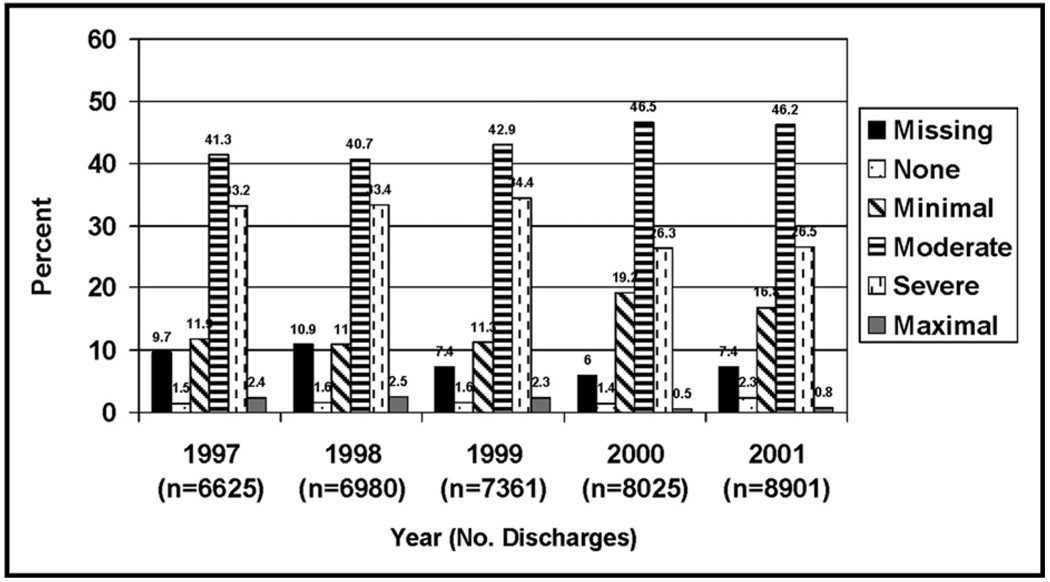
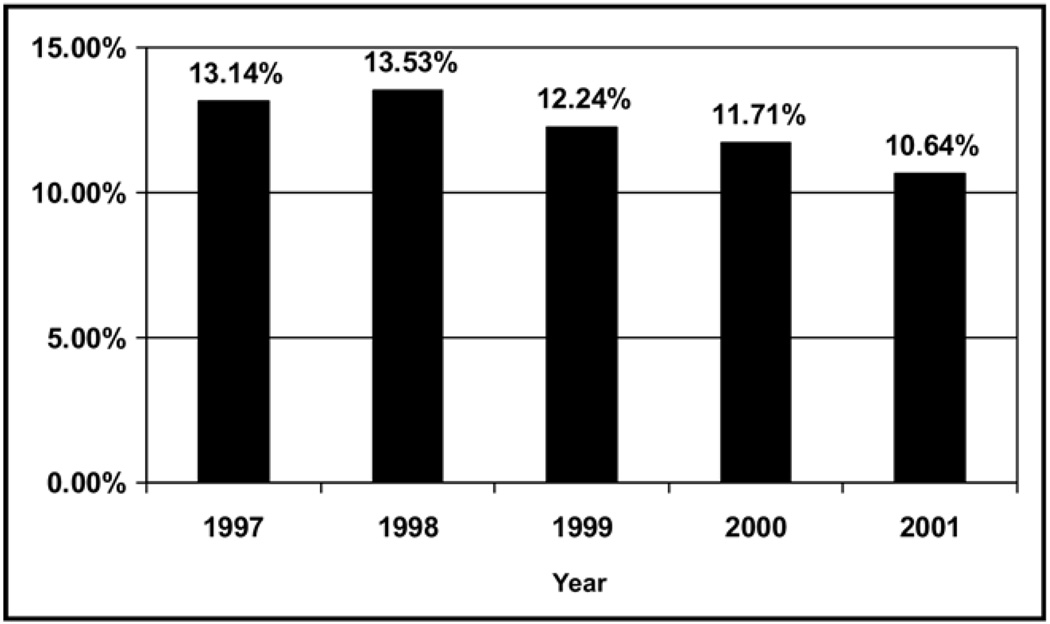
By comparing 1999 and before with 2000 and after, the 2 highest Atlas Severity of Illness scores decreased from 49.4% to 37.7%, and the 3 lowest scores increased from 50.6% to 62.3% (P < .0001) (Figure 3). This trend persisted after controlling for age, race, and gender. The proportion of in-hospital deaths among patients with pulmonary embolism decreased from 12.8% in 1999 and before to 11.1% in 2000 and after (P < .0001) (Figure 4). In logistic regression, significant predictors for mortality included cancer, congestive heart failure, age more than 70 years, and African American race (all P < .05), but not spiral CT (P = .055). Within the smaller UPMC subset, D-dimer testing and treatment with enoxaparin or warfarin also were predictors of mortality (all P < .05), but not the use of spiral CT scans (P = .055).
Figure 3.
Atlas Severity of Illness scores by year for patients hospitalized with pulmonary embolism in Pennsylvania.
Figure 4.
Mortality rates by year for patients hospitalized with pulmonary embolism in Pennsylvania.
DISCUSSION
The findings of this study demonstrate that the incidence of pulmonary embolism diagnosis is increasing with the increasing use of CT scans, and specifically with the increasing use of spiral CT scans. Previous studies have been limited by small sample size and relatively homogeneous populations without wide applicability to the general population, such as the Worcester Deep Venous Thrombosis Study and the Olmstead County study.2,12 Because PCH4 reporting is mandatory, and virtually all patients with pulmonary embolism are hospitalized, the PCH4 database overcomes such limitations, capturing most, if not all, such diagnoses.
This study for the first time demonstrates an increase in population-based incidence rates of pulmonary embolism since the advent of spiral CT, consistent with the community hospital study showing a 1 per 1000 increase in pulmonary embolism admissions with spiral CT.13 The observed increase in pulmonary embolism we observed was accompanied by a 3.6-fold increase in spiral CT use and a 2.3-fold increase in enoxaparin use, during the period from 1997 to 2001, but not by any increase in D-dimer assays.
Those diagnosed with pulmonary embolism later in the time period (ie, 2000 and after) were “less sick” than those diagnosed earlier, as evidenced by the decrease in the 2 highest and an increase in the 3 lowest Atlas Severity of Illness scores. The in-hospital mortality rates also decreased from 1999 and before, compared with 2000 and after. We suspect that wider use of spiral CT during this period may have led to earlier pulmonary embolism diagnosis than with conventional methods, resulting in lower acuity of illness. This has significant public health implications, because earlier scanning may lead to better health outcomes.
The higher incidence of pulmonary embolism with advancing age and African American race is similar to that in previous studies,2,14,15 but the higher incidence in female patients was unexpected and in contrast with past reports.2,12 Although the gender difference was not large, it may have been related to the size of our database and may reflect the fact that PHC4 does not collect data from smaller federal hospitals, including Veterans Administration hospitals. Despite this, there has been no decrease in the proportion of female admissions in the Veterans Administration system database, either nationally (4.36% in fiscal year 2005 and 4.47% in fiscal year 2006) or locally in Pennsylvania (3.72% in fiscal year 2005 and 3.77% in fiscal year 2006), nor do these figures exceed the estimated 5% of veterans who are female (Michael Fine, MD, personal communication, June 2007). Given the widespread use of hormonal agents and other gender-specific risks in women, however, our findings suggest important areas for further research.
Decreasing pulmonary embolism mortality in the UPMC sample could be predicted by LMWH and warfarin use, and by D-dimer testing (P < .05) in logistic regression analysis, but only LMWH use significantly increased during this period (1997–2002). Although decreasing pulmonary embolism mortality may also be related to earlier diagnosis, spiral CT scan was not a significant predictor of decreasing mortality, perhaps related to the smaller subset for whom these data were available.
We recognize certain additional limitations to our dataset. First, because this analysis excludes Pennsylvania residents hospitalized in other states, non-Pennsylvania residents, and those managed as outpatients, the incidence of pulmonary embolism in our population may have been underestimated. The overwhelming majority of patients with pulmonary embolism are treated in the inpatient setting.16,17 The only “outpatient” pulmonary embolism treatment study of more than 40 subjects was not purely an outpatient study because it included those hospitalized for up to 3 days.18 Thus, the criteria for safe outpatient anticoagulation in patients with newly diagnosed pulmonary embolism are not yet established and an important area of ongoing research.19,20 The incidence rates we found were similar to or greater than those of previously published population-based studies,11,15 suggesting it is unlikely that a significant proportion of patients are missing because of these factors. Moreover, outpatient pulmonary embolism cases should have little to no effect on the change in incidence rates during a 5-year period.
A second limitation was the use of revenue codes for CT scans, because there are no specific codes for spiral scans. However, a subset analysis of spiral CT scan data on the UPMC discharges supports this conclusion, as do the findings of Prologo and colleagues,21 who found a significant increase in spiral CT in patients with suspected pulmonary embolism at their academic medical center between 1997 and 1998 and 2002 and 2003. Moreover, this is corroborated by the lower incidence of pulmonary embolism in clinical studies before the use of spiral scans,2 as well as the high sensitivity and specificity of spiral CT scans for pulmonary embolism.22,23 These data justify our assumption that the increased use of CT scans in our 5-year study was the result of an increase in spiral procedures evaluating pulmonary embolism.
It is possible, although not proven, that the increasing incidence of pulmonary embolism corresponds to the diagnosis of smaller, possibly very small, pulmonary emboli (eg, isolated subsegmental pulmonary emboli), the significance of which is not established.24 If so, then diagnosing an increasing number of smaller, possibly irrelevant, pulmonary emboli could be deleterious if these were treated with anticoagulant therapy with its inherent bleeding risk. Because neither bleeding symptoms nor anticoagulation use is available in the PHC4 database, it is not possible to determine this risk, although should significant bleeding have occurred and led to a decrease in hemoglobin or blood pressure, the severity of illness scores would be unlikely to have improved.
CONCLUSIONS
The increasing incidence of pulmonary embolism in the state of Pennsylvania seems to be related to increasing pulmonary embolism diagnosis concomitant with the introduction of spiral CT, leading to earlier diagnosis at lower severity of illness. Although it is possible there are biologic factors causing an actual increase in the disease, we found no data to support this. Pulmonary embolism is still a major cause of morbidity and mortality in hospitalized patients,1 and our findings suggest that earlier diagnosis and earlier intervention might improve health outcomes. Further studies are needed to answer this important question. Because many hospitals now mandate the use of prophylactic anticoagulation in at-risk hospitalized patients, this is an ideal time to initiate prospective studies to help determine to what extent this approach reduces disease burden.
CLINICAL SIGNIFICANCE.
Pulmonary embolism causes significant morbidity in hospitalized patients.
The impact of spiral CT scans on the diagnosis of pulmonary embolism has not been well characterized.
This study of Pennsylvania hospital discharge data shows the incidence of pulmonary embolism is increasing with the use of spiral CT scans.
This increase is associated with lower severity of illness and lower mortality, suggesting the increase is due to earlier diagnosis.
ACKNOWLEDGMENTS
We acknowledge Diane Comer, from the Center for Healthcare Research at the University of Pittsburgh, for the efforts in management and analysis of the large volume of data. We acknowledge the expertise, dedication, and work of PHC4 staff, including Jayne Jones, epidemiologist; Debra Heikes, Judith Good, Jill Wiest, and Rachel Anspach, data analysts; and Joseph Martin, Director, Data Requests and Press Secretary Public Relations. We thank Melissa Saul, Director Clinical Research Office, UPMC, who served as honest broker for UPMC database, and Marc P. Volavka, Executive Director, PHC4, who served as honest broker for the PHC4 database.
This work was sponsored by a grant from the Jewish Healthcare Foundation, Pittsburgh, Pennsylvania. The sponsor had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the article.
References
- 1.Lindblad B, Eriksson A, Bergqvist D. Autopsy-verified pulmonary embolism in a surgical department: analysis of the period from 1951 to 1988. Br J Surg. 1991;78:849–852. doi: 10.1002/bjs.1800780725. [DOI] [PubMed] [Google Scholar]
- 2.Anderson FA, Jr, Wheeler HB, Goldberg RJ, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med. 1991;151:933–938. [PubMed] [Google Scholar]
- 3.Holbert JM, Costello P, Federle MP. Role of spiral computed tomography in the diagnosis of pulmonary embolism in the emergency department. Ann Emerg Med. 1999;33:520–528. doi: 10.1016/s0196-0644(99)70339-2. [DOI] [PubMed] [Google Scholar]
- 4.British Thoracic Society Standards of Care Committee Pulmonary Embolism Guideline Development Group. British Thoracic Society guidelines for the management of suspected acute pulmonary embolism. Thorax. 2003;58:470–483. doi: 10.1136/thorax.58.6.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and d-dimer. Ann Intern Med. 2001;135:98–107. doi: 10.7326/0003-4819-135-2-200107170-00010. [DOI] [PubMed] [Google Scholar]
- 6.Lee AY, Levine MN, Baker RI, et al. Randomized comparison of low-molecular-weight heparin versus oral anticoagulation for the prevention of thromboembolism in patients with cancer. N Engl J Med. 2003;349:146–153. doi: 10.1056/NEJMoa025313. [DOI] [PubMed] [Google Scholar]
- 7. [Accessed August 1, 2005];About the council. Available at: http://www.phc4.0rg/council/mission.htm.
- 8. [Accessed November 9, 2007];Pennsylvania Health Care Cost Containment Council, County Profiles, Hospital Discharge Data. Available at: http:www.phc4.0rg/countyprofiles/CountProfileResults.aspx; http:www.phc4.0rg/reports/fin1998report_volumeone.pdf.
- 9.Rello J, Ollendorf DA, Oster G, et al. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest. 2002;122:2115–2121. doi: 10.1378/chest.122.6.2115. [DOI] [PubMed] [Google Scholar]
- 10.International Classification of Diseases, Ninth Revision, Clinical Modification. Chicago: American Medical Association Press; 2004. [Google Scholar]
- 11. [Accessed February 8, 2005];National and state population estimates. Available at: http://www.census.gov/popest/estimates.php.
- 12.Silverstein MD, Heit JA, Mohr DN, et al. Trends in the incidence of deep venous thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998;158:585–593. doi: 10.1001/archinte.158.6.585. [DOI] [PubMed] [Google Scholar]
- 13.Ost D, Khanna D, Shah R, et al. Impact of spiral computed tomography on the diagnosis of pulmonary embolism in a community hospital setting. Respiration. 2004;71:444–447. doi: 10.1159/000080628. [DOI] [PubMed] [Google Scholar]
- 14.Stein PD, Hull RD, Kayali F, et al. Venous thromboembolism according to age. Arch Intern Med. 2004;164:2260–2265. doi: 10.1001/archinte.164.20.2260. [DOI] [PubMed] [Google Scholar]
- 15.White RH, Zhou H, Romano PS. Incidence of idiopathic deep venous thrombosis and secondary thromboembolism among ethnic groups in California. Ann Intern Med. 1998;128:737–740. doi: 10.7326/0003-4819-128-9-199805010-00006. [DOI] [PubMed] [Google Scholar]
- 16.Aujesky D, Obrosky DS, Stone RA, et al. A prediction rule to identify low-risk patients with pulmonary embolism. Arch Int Med. 2006;166:169–175. doi: 10.1001/archinte.166.2.169. [DOI] [PubMed] [Google Scholar]
- 17.Almahameed A, Carman TL. Outpatient management of stable acute pulmonary embolism: proposed accelerated pathway for risk stratification. Am J Med. 2007;120(10 Suppl 2):S18–S25. doi: 10.1016/j.amjmed.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 18.Kovacs MJ, Anderson D, Morrow B, et al. Outpatient treatment of pulmonary embolism with dalteparin. Thromb Haemost. 2000;83:209–211. [PubMed] [Google Scholar]
- 19.Ramzi DW, Leeper KV. DVT and pulmonary embolism: part II. Treatment and prevention. Am Fam Physician. 2004;59:2841–2848. [PubMed] [Google Scholar]
- 20.Jimenez D, Yusen RD, Otero R, et al. Prognostic models for selecting patients with acute pulmonary embolism for initial outpatient therapy. Chest. 2007;132:7–8. doi: 10.1378/chest.06-2921. [DOI] [PubMed] [Google Scholar]
- 21.Prologo JD, Gilkeson RC, Diaz M, Asaad J. CT pulmonary angiography: a comparative analysis of the utilization patterns in emergency department and hospitalized patients between 1998 and 2003. Am J Roentgenol. 2004;183:1093–1106. doi: 10.2214/ajr.183.4.1831093. [DOI] [PubMed] [Google Scholar]
- 22.Kim KKI, Muller NL, Mayo JR. Clinically suspected pulmonary embolism: utility of spiral CT. Radiology. 1999;210:693–697. doi: 10.1148/radiology.210.3.r99mr01693. [DOI] [PubMed] [Google Scholar]
- 23.Stein PD, Fowler SE, Goodman LR, et al. Multidetector computed tomograph for acute pulmonary embolism. N Engl J Med. 2006;354:2317–2327. doi: 10.1056/NEJMoa052367. [DOI] [PubMed] [Google Scholar]
- 24.Le Gal G, Righini M, Parent F, et al. Diagnosis and management of subsegmental pulmonary embolism. J Thromb Haemost. 2006;4:724–731. doi: 10.1111/j.1538-7836.2006.01819.x. [DOI] [PubMed] [Google Scholar]