Abstract
Hydrogels that mimic the natural extracellular matrix (ECM) are used in three-dimensional cell culture, cell therapy, and tissue engineering. A semi-synthetic ECM based on cross-linked hyaluronana offers experimental control of both composition and gel stiffness. The mechanical properties of the ECM in part determine the ultimate cell phenotype. We now describe a rheological study of synthetic ECM hydrogels with storage shear moduli that span three orders of magnitude, from 11 to 3 500 Pa, a range important for engineering of soft tissues. The concentration of the chemically modified HA and the cross-linking density were the main determinants of gel stiffness. Increase in the ratio of thiol-modified gelatin reduced gel stiffness by diluting the effective concentration of the HA component.
Keywords: biomaterials, hyaluronic acid, hydrogels, rheology, tissue engineering
Introduction
Synthetic extracellular matrix (sECM) hydrogels can be used for three-dimensional cell culture, wound repair, and tissue engineering. These sECM gels provide a three-dimensional (3-D) environment, in which cells can grow, proliferate, and migrate, that simulates the in vivo environment more closely than two-dimensional (2-D) surfaces, such as tissue culture plastic.[1–3] Additional benefits come from tissue culture materials with the ability to be remodeled as cells degrade the matrix and replace it with ECM components of their own making.[4]
The material properties of these hydrogels contribute to their usefulness in various applications. In medical applications, the stiffness of the implanted material should roughly match that of the tissue it replaces.[5,6] For example, the Young’s modulus, E, of a material used to aid in bone reconstruction must be much higher than that of a gel used to prevent abdominal tissue adhesions. In addition, the shear modulus, G, of materials used as cell culture substrates, whether 2-D or 3-D, helps determine cell phenotype and stem cell fate.[7,8] When a cell attaches to a substrate with adhesion molecules on the cell membrane, such as integrins, the cytoskeleton transmits traction forces between the multiple attachment sites, which deform the culture substrate according to its stiffness. In this way, cells can feel the stiffness of their substrates and then respond by remodeling the cytoskeleton and changing the expression of adhesion molecules.[9,10] It has also been shown that matrix elasticity can be used to select cells by type[11] and to direct the differentiation of stem cells.[12] These effects can be used to manipulate the development of cells and tissues and have begun to be incorporated into computer simulation models to predict the outcome of such experiments.[13]
A wide range of methods have been used to characterize the mechanical properties of biological tissues and tissue substitutes, each suited to particular modulus ranges and variables of interest. Bulk mechanical properties of many tissues are well tabulated in the literature in certain deformation modes.[14] Ultrasonic and magnetic resonance measurements of these properties, making up the field of elastography, can even be used as diagnostic tools for medical evaluation as an alternative to palpation.[15–19] Mechanical properties have also been measured at the cellular level using a variety of methods including atomic force microscopy (AFM) and microrheology.[20–24] Unlike native tissues, the material properties of synthetic tissue substitutes can be tailored to suit particular applications, and therefore need to be characterized as well. This characterization has been performed both in bulk, using tension,[25,26] compression,[27] and shear rheology methods,[11,28] and at small scales using AFM-related techniques[29–31] and particle tracking microrheology.[32,33] In addition, when the mechanical properties of the culture substrate are known, the forces exerted by cells on the substrate can be calculated using microrheological methods[6,34].
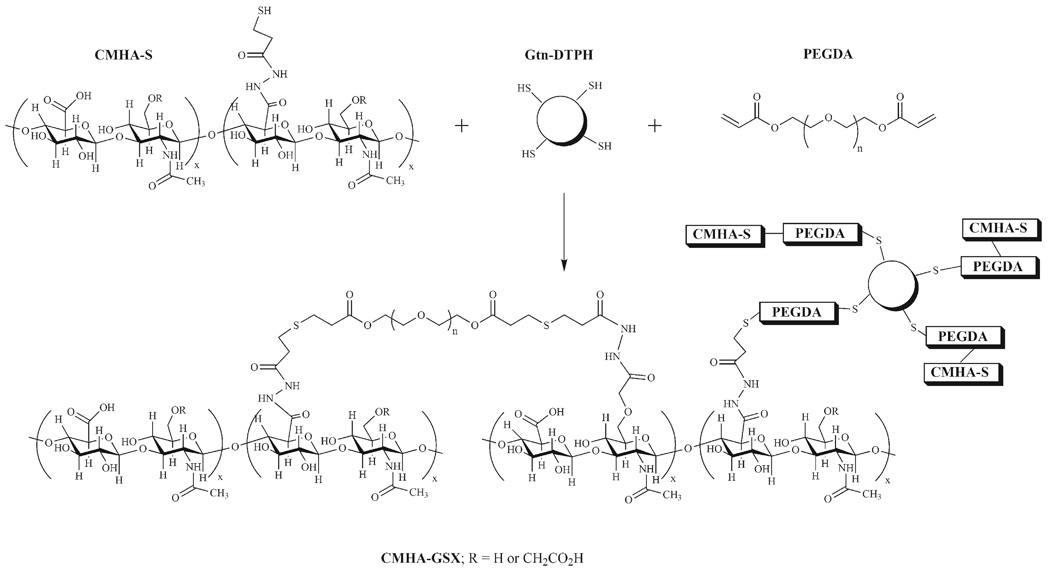
Thiol-modified hyaluronic acid (HA) and thiol-modified gelatin (Gtn) have been cross-linked into biocompatible materials and used to aid the development and repair of cells and tissues.[35] HA is a nonsulfated glycosaminoglycan present in the ECM of mammalian tissues that promotes cell motility and supports tissue morphology.[36] This thiol-modified HA [3,3′-dithio-bis-(propanoic dihydrazide) (HA-DTPH)] or thiol-modified carboxymethyl HA (CMHA-S) is cross-linked into a hydrogel using poly(ethylene glycol)-bis-acrylate (PEGDA) and used an injectable scar-free healing aid, as a 3-D cell culture material, and a tissue engineering scaffold.[37–40] CMHA-S differs chemically from HA-DTPH only by the addition of a carboxymethyl group to the primary C-6 alcohol, which then has the potential to also be modified with a DTPH functionality, thus increasing the range of thiol modification possible.[41] In studies of adhesion prevention, wound repair and 3-D cell culture, biomaterials from CMHA-S have been found to be superior to those derived from HADTPH.[41,42] The cytoadhesiveness of the material can be increased by co-cross-linking gelatin, modified with the same thiol functional group (Gtn-DTPH), into the gel to provide peptide adhesion ligands for integrins on cell surfaces. The concentration of Gtn-DTPH varies from a low of 5% for optimal healing of vocal folds, to 75% for optimal behavior of fibroblasts cultured in 3-D.[37,43] The cross-linking reaction forms a gel known as CMHA-GSX, or Extracel, and is shown in Figure 1.
Figure 1.
Chemical cross-linking of macromolecular thiols to form CMHA-GSX (Extracel™) hydrogel.
The shear modulus of hydrogels composed of PEGDA cross-linked HA-DTPH[28] and CMHA-S[44] has been measured rheologically. These studies have tested the effects of both cross-linker concentration and cure time, which both influence on the total cross-link density. The effect of cocross-linked Gtn-DTPH on material properties, however, has not yet been studied. Since these cell-adhesive gels are required to culture most anchored cell types, the rheological properties need to be evaluated so that the effects of substrate material properties on these cells can be measured. Thus, in this work, hydrogels with a wide range of stiffness have been synthesized from a few components in variable concentrations and characterized rheologically. The dependence of dynamic shear modulus, G*, on these composition variables will be discussed.
Experimental Part
All polymers used for casting hydrogels were obtained from Glycosan BioSystems, Inc. (Salt Lake City, UT). Correlations to Glycosan product names are: CMHA-S=Glycosil, Gtn-DTPH=Gelin-S, and PEGDA=ExtraLink. Thiolated polymer solutions were made by dissolving the materials with degassed, deionized water to prevent disulfide cross-linking.
Chemical Characterization
The molecular weights of CMHA-S and Gtn-DTPH were determined using gel permeation chromatography (GPC) and light scattering detection. The GPC setup included a Waters 515 HPLC pump, a Waters Ultrahydrogel 1000 column, a Waters 486 tunable absorbance detector, and a Wyatt miniDAWN TriStar light scattering detector. The mobile phase was 200×10−3 m sodium phosphate buffer, pH 6.7, in water/methanol 80:20 v/v with a flow rate of 0.5 mL·min−1. Retention time data were calibrated with molecular weight standards from Hyalose and light scattering data were analyzed using a measured extinction coefficient for HA, ε210 nm, of 1.87×103 mL·g−1·cm−1 and a measured differential index of refraction, dn/dc, of 0.157 mL·g−1. The molecular weight of PEGDA was not determined as it was synthesized from PEG starting material with a molecular weight 3 400 g · mol−1.
Thiol modification degree of thiol-containing polymers was measured using a modified 2-nitro-5-thiosulfobenzoate (NTSB) colorimetric assay.[45] An NTSB stock solution was prepared by dissolving 300 mg 5,5′-dithiobis-2-nitrobenzoic acid (DTNB, Ellman’s reagent) in 30 mL of 1 m Na2SO3, adjusting the pH to 7.5, and bubbling oxygen through the solution in a 38 °C water bath until the color changed from a bright orange to a pale yellow, indicating cleavage of the disulfide bond to form NTSB. This stock solution was stored at −30 °C until needed. The NTSB assay solution was prepared by diluting 1 mL stock solution with 100 mL buffer at pH 9.5 containing 100×10−3 m Na2SO3 and 5×10−3 m EDTA. Thiolated polymers were dissolved at 1 mg · mL−1 water, of which 100 µL was added to 3 mL NTSB assay solution. This assay mixture was then stored in the dark to prevent photodegradation of the colorimetric species, 2-nitro-5-thiobenzoate (NTB), for 20 min before absorbance at 412 nm was measured.
Hydrogel Casting
Hydrogels were cast in a variety of compositions according to Table 1 and 2 where the quantities of CMHA-S, Gtn-DTPH, and PEGDA were varied, within a similar range of compositions as is often used for cell culture. Four hydrogels were cast using PureCol™ purified rat tail Type I collagen (INAMED Biomaterials, Fremont, CA, molecular weight 300 kDa) instead of Gtn-DTPH. Three other hydrogels were cast using no PEGDA cross-linker, but instead the solutions were vigorously mixed with ambient air for 15 s to accelerate disulfide bond formation via oxidation. For each composition, three or more samples were cast in 60 mm diameter tissue culture dishes (actual surface area 21 cm2) with 4.2 mL of gel in each dish to make gels approximately 2 mm thick. To create a level surface with uniform thickness for rheology, the stacking ridges were removed from all tissue culture dishes using either a razor blade or a powered grinder before the gels were cast. The gels were allowed to cure covered on a level surface for at least 30 min at room temperature (21 ± 1 °C) before the dishes were sealed with parafilm and placed inside a larger container with a thin layer of water at the bottom to maintain humidity and prevent dehydration of the gels. The gels were cured this way at room temperature for 24 h before rheological characterization. The gels that were cross-linked via disulfide bonds, without PEGDA, were allowed to cure for 48 h. These time points were selected to allow the majority of cross-linking to occur, which more accurately represents the steady-state material properties.
Table 1.
Hydrogel compositions for rheology, with numbers corresponding to figures. HA=CMHA-S, Gtn=Gtn-DTPH, XL=PEGDA cross-linker.
| ID | Sample Name | HA |
Gtn |
XL |
G' |
std |
|---|---|---|---|---|---|---|
| % | % | % | Pa | % | ||
| 2a | 0.8% HA | 0.8 | 0 | 0.4 | 340 | 7 |
| 2b | 99:1 HA/Gtn | 0.792 | 0.008 | 0.4 | 330 | 10 |
| 2c | 95:5 HA/Gtn | 0.76 | 0.04 | 0.4 | 280 | 8 |
| 2d | 83:17 HA/Gtn | 0.65 | 0.13 | 0.44 | 160 | 8 |
| 2e | 70:30 HA/Gtn | 0.56 | 0.24 | 0.4 | 120 | 21 |
| 2f | 50:50 HA/Gtn | 0.4 | 0.4 | 0.4 | 83 | 12 |
| 2g | 20:80 HA/Gtn | 0.16 | 0.64 | 0.4 | 23 | 24 |
| 2h | 99:1 HA/PureCola) | 0.792 | 0.008 | 0.4 | 240 | 16 |
| 2i | 95:5 HA/PureCol | 0.76 | 0.04 | 0.4 | 270 | 10 |
| 2j | 85:15 HA/PureCol | 0.68 | 0.12 | 0.4 | 290 | 7 |
| 2k | 70:30 HA/PureCol | 0.56 | 0.24 | 0.4 | 270 | 39 |
| 3a | 1.6% HA | 1.6 | 0 | 0.8 | 700 | 35 |
| 3b | 50:50 HA/Gtn | 0.8 | 0.8 | 0.8 | 800 | 15 |
| 3c | 20:80 HA/Gtn | 0.32 | 1.28 | 0.8 | 270 | 9 |
| 3d | 1.6% Gtn | 0 | 1.6 | 0.8 | 78 | 7 |
| 4a | 0.8% HA+Gtn, 0.1%XL | 0.4 | 0.4 | 0.1 | 14.5 | 4 |
| 4b | 0.8% HA+Gtn, 0.16% XL | 0.4 | 0.4 | 0.16 | 23 | 10 |
| 4c | 0.8% HA+Gtn, 0.2% XL | 0.4 | 0.4 | 0.2 | 37 | 5 |
| 4d | 0.8% HA+Gtn, 0.3% XL | 0.4 | 0.4 | 0.3 | 72 | 7 |
| 4e | 0.8% HA+Gtn, 0.8% XL | 0.4 | 0.4 | 0.8 | 280 | 15 |
| 4f | 0.8% HA+Gtn, 1.6% XL | 0.4 | 0.4 | 1.6 | 510 | 19 |
| 4g | 1.6% HA+gtn, 0.2 XL | 0.8 | 0.8 | 0.2 | 180 | 8 |
| 4h | 1.6% HA+Gtn, 0.4% XL | 0.8 | 0.8 | 0.4 | 370 | 7 |
| 4i | 1.6% HA+Gtn, 0.6% XL | 0.8 | 0.8 | 0.6 | 520 | 8 |
| 4j | 0.8% HA, 0.8% XL | 0.8 | 0 | 0.8 | 710 | 9 |
| 4k | 0.8% HA, 1.2% XL | 0.8 | 0 | 1.2 | 1100 | 11 |
| 4l | 0.8% HA, 1.6% XL | 0.8 | 0 | 1.6 | 1300 | 11 |
Concentrations of PureCol™ are shown in the % Gtn column.
Table 2.
Hydrogel compositions not shown in figures. HA=CMHA-S, Gtn=Gtn-DTPH, XL=PEGDA cross-linker.
| ID | Sample name | HA |
Gtn |
XL |
G' |
std |
|---|---|---|---|---|---|---|
| % | % | % | Pa | % | ||
| a | 20:80 HA/Gtn, 0.32% XL | 0.32 | 1.28 | 0.32 | 28 | 14 |
| b | 1.6% HA, 1.6% XL | 1.6 | 0 | 1.6 | 3500 | 12 |
| c | 0.6% HA+Gtn, 0.8% XL | 0.3 | 0.3 | 0.8 | 11 | 59 |
| d | 0.4% HA+Gtn, 0.8% XL | 0.2 | 0.2 | 0.8 | 21 | 34 |
| e | 2% HA+Gtn, no XL | 1 | 1 | 0 | 100 | 12 |
| f | 2% HA, no XL | 2 | 0 | 0 | 80 | 19 |
| g | 1% HA, 0.45% XL | 1 | 0 | 0.45 | 500 | 13 |
Rheology
Oscillatory shear measurements of the elastic modulus, G', and the viscous modulus, G", were obtained at room temperature using a constant stress rheometer (AR550 from TA Instruments, New Castle, DE). When these measurements were performed on the pregel solutions using the customary parallel plate steel fixtures, it proved difficult to control evaporation at the sample/air interface. Therefore, the alternate procedure of Ghosh et al.[28] was adopted to circumvent this problem. Gels of thickness 1–3 mm were first cast (see previous section) in a covered plastic tissue culture plate of diameter 60 or 100 mm. When negligible difference was observed between samples of these different geometries, further sample compositions were run using gels cast at 3 mm thick and 60 mm diameter. After curing the gel for 24 h, the entire tissue culture plate was affixed to the lower rheometer stage using double stick tape, and the upper rheometer fixture, a stainless steel disc (diameter 40 mm) was lowered until it made contact with the top surface of the sample. The value of G' was then measured at oscilllation frequency 1 Hz in a stress sweep test from 0.6 to 20 Pa. This stress sweep was performed in order to determine the limit of the linear viscoelastic regime, and also to detect the possible presence of “slip” at the interface between the sample and plastic or steel. In almost all cases, the measured phase angle between the stress and the strain was less than two degrees, and the measured G' values were independent of the imposed stress, results that imply the absence of slip. As a further check on interfacial adhesion, measurements were repeated with the sample under a slight degree of compression between the upper steel fixture and the lower plastic plate, and no change in G' results was observed. Furthermore, for a given gel composition, measured G' values were independent of sample thickness in the range of 1–3 mm.
Data Analysis
All G' and G" values were corrected by dividing by the apparent gap and multiplying by the true thickness of the gel as determined by the difference between the apparent gap and the thickness of the dish with adhesive. Values were averaged over the region that G' was relatively constant with respect to the gap distance. Error bars in all figures represent the sample standard deviation for the values averaged.
Results
Chemical Characterization
Multi-angle laser light scattering molecular weight determination for CMHA-S was performed, resulting in a weight-average molecular weight, M̅w of 68 kDa with a polydispersity index of 1.94. The retention time data were not valid as the retention time of much of the peak was outside the range of the calibration curve, with the lowest standard at approximately 30 kDa, thus invalidating the results. Nevertheless, the portion of the peak that was within the calibration curve was analyzed and showed to be 47 kDa. When Gtn-DTPH was analyzed similarly, the retention time was also outside the range of the calibration curve and, as determined by light scattering, was 13 kDa.
A modified Ellman’s assay determined the thiol modification degree to be 38% of acid groups on the CMHA to be converted to DTPH groups, corresponding to 0.89 mmol thiol per gram of CMHA-S. The sample standard deviation for this measurement was 2% conversion, or 0.04 mmol thiol per gram of CMHA-S. The same assay determined a thiol content of 0.57 mmol thiol per gram of Gtn-DTPH, with sample standard deviation of 0.02 mmol thiol per gram of Gtn-DTPH.
Rheology
Multiple compositions were cast using different concentrations of CMHA-S, Gtn-DTPH, PureCol™, and PEGDA. These gels were then characterized rheologically to measure G' and G". For most gels, the dynamic shear modulus, G*, where G* = G' + iG" and |G*| = [(G')2 + (G")2]1/2, was dominated by G' because values for G" were minimal. While stress sweeps were conducted over the range of 0.6–20 Pa, the results were mostly consistent over the stress sweep, therefore all figures and tables shown represent modulus values as measured at a constant stress of τ=6 Pa. Table 1 shows composition and modulus information for data included in the figures, while Table 2 shows the same information for sample data not included in any figures shown.
Constant Total Concentration
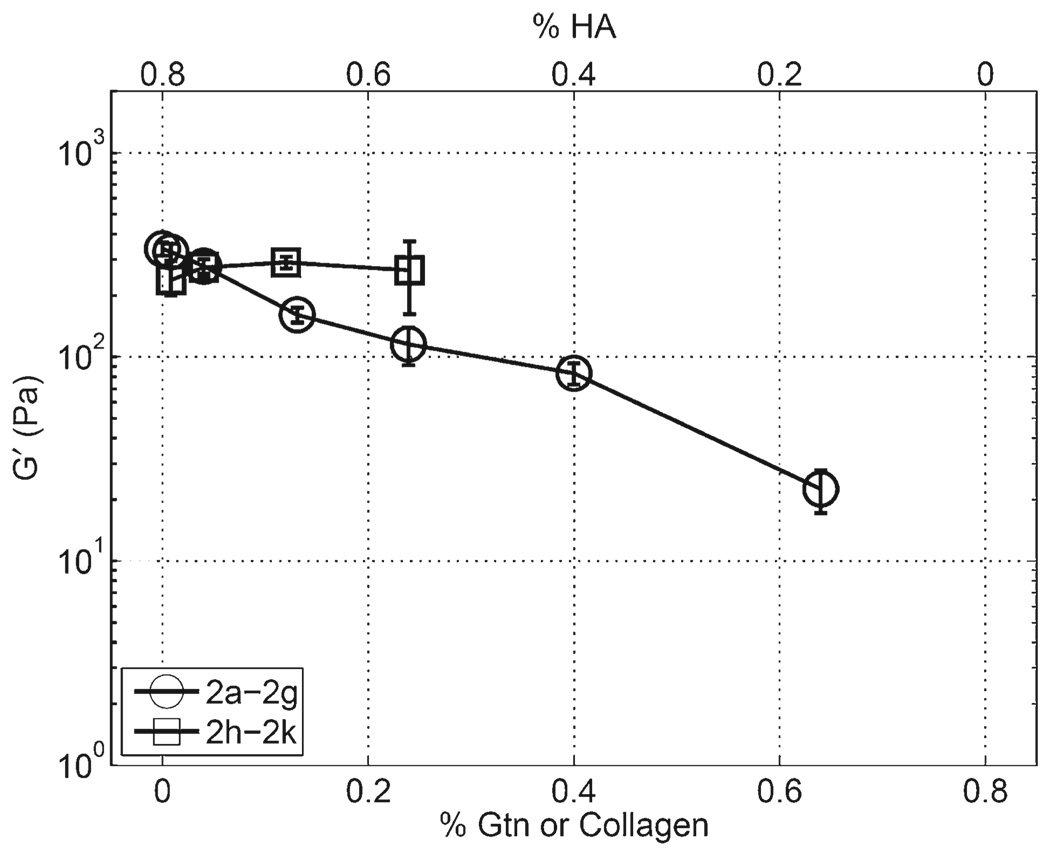
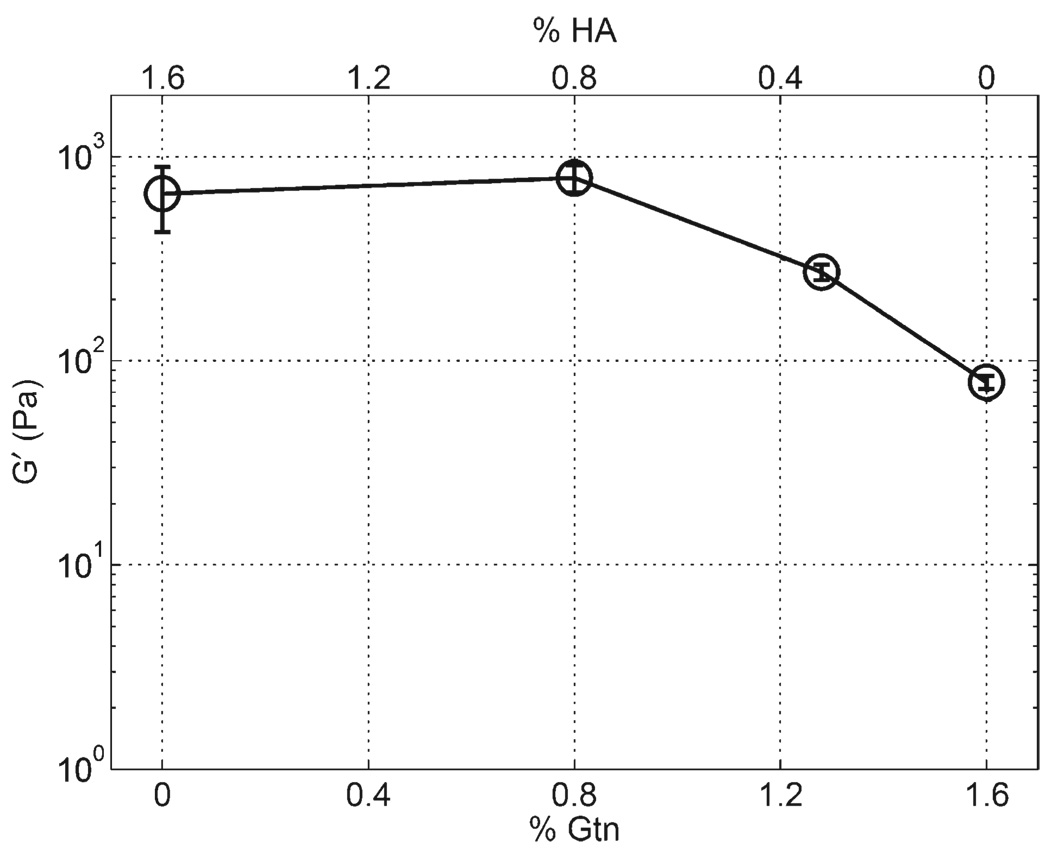
For gels where the total mass concentration of polymer and the concentration of cross-linker were held constant at 0.8 and 0.4% w/v, respectively, and the ratio of CMHA-S to Gtn-DTPH was varied, G' decreased with a decrease in CMHA-S to Gtn-DTPH ratio, as shown in Figure 2. Importantly, this trend is not present, however, when PureCol™ is substituted for Gtn-DTPH, where G' was observed to remain relatively constant. The same trend was found for a similar experiment using 1.6% total polymer concentration and 0.8% cross-linker as shown in Figure 3. Sample 3a in this set, containing 1.6% CMHA-S and no Gtn-DTPH, did not show any region where G' was independent of the gap distance, so the value reported is the average for all gap distances measured. For this sample, G' increased exponentially with a decrease in the gap distance (data not shown), causing the sample standard deviation to be relatively high.
Figure 2.
CMHA-S to Gtn-DTPH (2a–2g) and CMHA-S to Pure-Col™(2h–2k) ratio sweeps with total polymer concentration fixed at 0.8% w/v cross-linked with 0.4% w/v PEGDA.
Figure 3.
Rheology for CMHA-S to Gtn-DTPH ratio sweep with total polymer concentration fixed at 1.6% w/v cross-linked with 0.8% w/v PEGDA, showing samples 3a–3d.
Constant Component Concentration
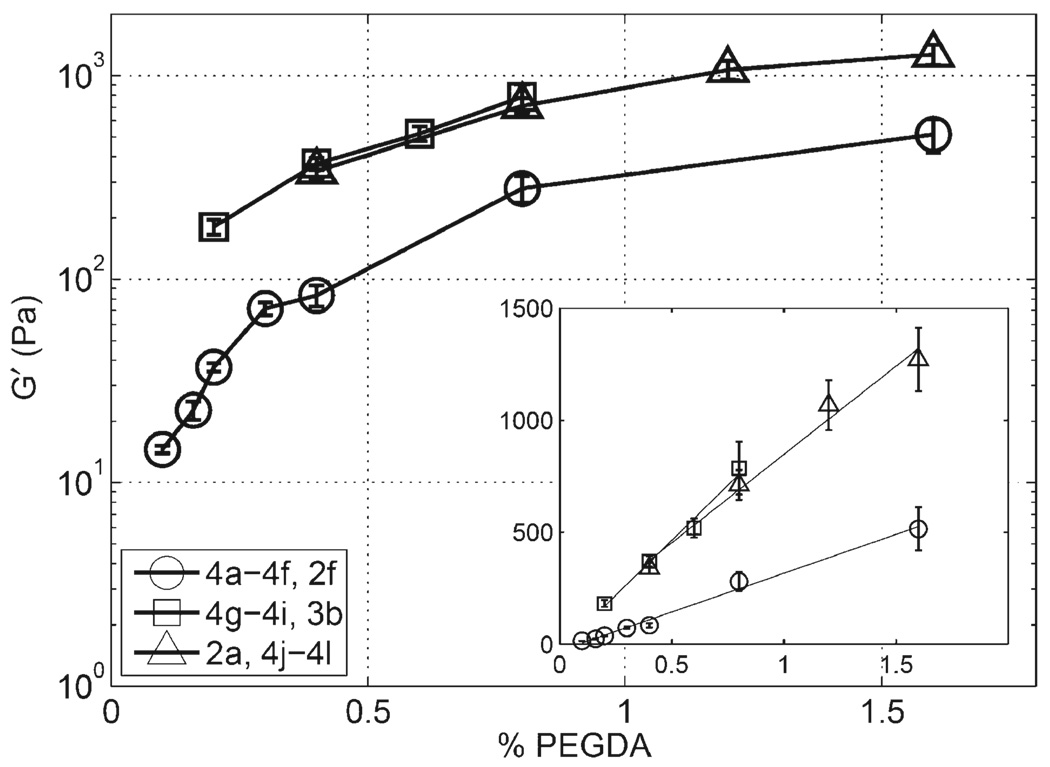
For gels where the concentrations of CMHA-S and Gtn-DTPH were held constant at either 0.4 or 0.8% w/v, or when 0.8% CMHA-S was used alone, the modulus increased approximately linearly with increase in PEGDA concentration, as shown in Figure 4. Linear regression of these data shows a slope of 350 Pa/% PEGDA for gels containing 0.4% CMHA-S and Gtn-DTPH with a correlation coefficient, r2, of 0.9910, 980 Pa/% PEGDA and r2 0.9857 for gels containing 0.8% of each thiolated polymer, and 790 Pa/% PEGDA and r2 0.9843 for gels containing only 0.8% CMHA-S, although this last slope is likely skewed by the sample 4l because the available thiols on the CMHA-S become the theoretical limiting reagent for PEGDA concentrations above 1.2% for this concentration of CMHA-S. Neglecting sample 4l from the regression, the slope becomes 910 Pa/% PEGDA with r2 equal to 0.9999. Samples with the same concentration of CMHA-S with and without Gtn-DTPH, comparing 4h with 2a and 3b with 4j, were noted to have very similar values.
Figure 4.
PEGDA concentration sweep for gels composed of 0.4% w/v CMHA-S & Gtn-DTPH (4a–4d, 2f, 4e–4f), 0.8% w/v CMHA-S and Gtn-DTPH (4g–4i, 3b), and 0.8% CMHA-S (2a, 4j–4l). Inset shows the same data on a linear scale with linear regression lines with the following slopes and r2 values in the same order: 350 Pa/% XL, 0.991; 980 Pa/% XL, 0.986; 790 Pa/% XL, 0.984.
Other Compostions
Seven material compositions not represented in the figures showing composition trends are reported in Table 2. Sample b, having the highest CMHA-S and PEGDA concentrations of any sample, showed the highest value for G', at 3.5 kPa, of all gels tested. Samples c and d, with low concentrations of both CMHA-S and Gtn-DTPH, showed low G' values that were exceeded by their corresponding G" values, indicating they had not formed true gels. Gels e and f were cured 48 h without PEGDA and showed very low G' values compared to similar polymer concentrations cross-linked with PEGDA. A third gel related to e and f, composed of 2% Gtn-DTPH with no PEGDA cross-linker, was cast but formed a heterogeneous material with separate gel and fluid layers, so the rheological results are omitted.
Discussion
The purpose of this study was to explore the dependence of mechanical properties on the composition of sECM hydrogels containing HA, gelatin, and PEGDA. As this dependence is understood, hydrogel composition can be tuned to produce gels with the desired material properties. These gels can then be used to study the effects of substrate stiffness on cultured cells, to measure the mechanical forces exerted by cells, to select cells on the basis of type, and to direct the phenotype of cells, including stem cells.
Factors affecting hydrogel mechanical properties include, but are not limited to: polymer molecular weight, concentration, cross-link density, chain entanglement, cross-linker length, and osmotic pressure. The primary variables evaluated in this study are concentration, crosslink density, and molecular weight.
The molecular weight of polymer chains has an important effect on the shear modulus of HA-based hydrogels. Ghosh et al.[28] used HA-DTPH with 158 kDa with 42% of HA disaccharide units modified with thiol-containing DTPH groups, while the molecular weight of the CMHA-S used in this study was measured as 68 kDa and 38% thiol substitution. One hydrogel, sample g, was formulated to correspond to the sample referred to as 4:1 in the work by Ghosh. Sample g had a G' of 500 Pa while Ghosh 4:1 had a G' of about 1 kPa. Clearly, molecular weight could be used to modulate mechanical properties, since a higher molecular weight would result in more chain entanglement and hence a stiffer material, but with much less flexibility for cell and tissue researchers than concentration and cross-link density, which can be adjusted at the time the gels are cast, rather than requiring a separate synthesis.
Figure 2 illustrates the dependence on polymer concentration, where the stiffness decreases dramatically with decrease in CMHA-S concentration in series 2a–2g. In this case, the increasing concentration of Gtn-DTPH is insufficient to prevent the reduction in stiffness due to the lowered concentration of CMHA-S. Series 3a–3d in Figure 3 shows the same decrease in shear modulus as a result of the dilution of CMHA-S as it is replaced by the same mass of Gtn-DTPH. Series 2h–2k, however, does not show the same reduction in stiffness due to the higher molecular weight of collagen, despite its inability to co-cross-link with CMHA-S. The experimental variable between the Gtn-DTPH and collagen samples was the effect of cross-linking ability versus high molecular weight of two commercially available materials that can both be used to increase the cytoadhesiveness of hydrogels. It appears that when Gtn-DTPH is added, while keeping the total polymer concentration constant, the rule of mixtures applies, where the stiffness of the mixture material is equal to the weighted average of the component materials. Since Gtn-DTPH has such low modulus relative to that of CMHA-S, the modulus decreases as it would if there were no Gtn-DTPH present, and the CMHA-S were merely being diluted. The stiffness of gels containing only Gtn-DTPH could not be measured in this context because the resulting gels were not homogeneous. These effects are not noticed, however, in the comparison between samples 3a and 3b, which are not significantly different, presumably due to the high variance for sample 3a caused by the fact that the readings were not constant as a function of gap distance. The principle of dilution is further supported by the data shown in Figure 4, in which series 4g–4i, 3b and series 2a, 4j–4l have overlapping data points, where the concentrations of CMHA-S and PEGDA are identical in the two materials, and one material contains 0.8% w/v Gtn-DTPH in addition. In this case, the added Gtn-DTPH contributes nothing to the bulk mechanical properties of the gel, although it is still an important component needed to allow cellular attachment, spreading, and migration.[46] These results indicate that while material stiffness can be held constant by entangling high molecular weight collagen (PureCol M̅W 300 kDa) within CMHA-S hydrogels using a weight concentration replacement technique, the alternative use of Gtn-DTPH (M̅W 13 kDa) must be used in addition to the desired concentration of CMHA-S. While Gtn-DTPH has the ability to cross-link in the presence of cells, unmodified gelatin is cross-linked with noncytocompatible reagents such as glutaraldehyde. The effects of a step increase in total concentration of CMHA-S can be seen in Figure 4 where the relatively constant gap between curves on a logarithmic scale represents a constant proportionality between them over the entire range of cross-link density. Linear regression of the data in Figure 4 shows good linear dependence of shear modulus, G, on cross-linker concentration with a constant thiolated polymer concentration, which is in agreement with rubber elasticity theory where an ideal rubber should follow G=sveRT, where R is the gas constant, T the temperature, s a constant that depends on the polymer physics model used, and ve is the volumetric network chain density, which is a function of cross-link density.[47] There are several reasons, however, why these hydrogels cannot be considered ideal rubbers, especially the fact that the modulus still increased between samples 4k and 4l, when the thiol/acrylate ratio of sample 4k was nearly 1:1. It is clear from this example that the cross-linking reaction does not consume all of the limiting reagent under these conditions, and therefore ve cannot be explicitly derived from hydrogel composition.
Cross-link density, as predominantly determined by the concentration of PEGDA cross-linker,[28] is likely to be the most frequently varied parameter when adjusting the material properties of this class of hydrogels for use in cell culture and tissue engineering. Adjusting the cross-linker concentration provides the widest range in shear modulus without changing the concentration of CMHA-S, which would likely lead to differences in other properties of the hydrogel, such as density, porosity, and degradability. The concentration of PEGDA is also varied more easily as it is much more soluble in water than CMHA-S, which is impractical to use in concentrations over 3% w/v. The major limitation of the ability to increase G by increase in cross-link density is the number of available thiols, or substitution degree, of CMHA-S. For comparisons with other tissues and cell culture materials, it is important to note that the relationship between Young’s modulus, E, and shear modulus, G, is E=2G(1+v). Furthermore, when a material can be assumed to be incompressible, its Poisson’s ratio, v, approaches 0.5 and this relationship approaches E=3G. This assumption for HA hydrogels is supported by a research showing that n for polyacrylamide hydrogels is nearly 0.5,[48] and because these gels are typically used under very low strain. This relationship between E and G then provides a useful tool for comparing mechanical properties of substrates and tissues that have been determined using other methods of measurement.
Conclusion
Design principles should recreate the interwoven set of biochemical and mechanical cues in the cellular microenvironment. Recently, the importance of using the simplest applicable material to facilitate manufacture and regulatory approval has been emphasized.[1,49] In other words, the end-user’s requirements are key design criteria for materials for cell therapy.[49] End-user parameters that can now be met by sECMs include: (i) experimentally variable composition, (ii) experimentally variable compliance, (iii) controllable biodegradability, (iv) physical forms, (v) batch-to-batch consistency, (vi) cross-linkable under physiological conditions, and (vii) transparent. These sECMs are modular, which allows researchers to add soluble factors, attachment peptides, matricellular proteins, cross-linkers, and macromomers as desired.[43]
The storage shear moduli of HA hydrogels in this study range from 11 Pa to 3.5 kPa. While this represents the low range of stiffness for tissues and cell-culture substrates, as summarized in Table 3,[12,19,48] it does cover an important compliance range in soft tissue engineering. Tissue engineering applications requiring higher stiffness, such as bone regeneration, have typically used lyophilized hydrogel sponges,[50] which provide a much stiffer scaffold at the cellular scale, but a quantitative study of this stiffness has yet to be performed. Using the results of this study, cell researchers and tissue engineers can tailor the mechanical properties of CMHA-S-based hydrogels to the needs of their experiments, thus facilitating tuning the mechanics of materials to better suit the biology of cells and tissues.[9]
Table 3.
Approximate shear moduli for tissues and culture substrates.
| Material |
G |
|---|---|
| Pa | |
| Brain, nerves | 102–103 |
| Liver, fat, relaxed muscle, breast gland tissue | 103–104 |
| Dermis, connective tissue, contracted muscle | 105–106 |
| Eepidermis, cartilage | 107–108 |
| Polyacrylamide gels | 103–104 |
| Bone, polystyrene | 108–1010 |
| Glass | 1011 |
Acknowledgements
Major funding was provided by the Centers of Excellence Program of the State of Utah. Partial support for rheometry work was provided by NIH grant 5R21EB4947. The authors (M. A. and J. J. M.) acknowledge the Donors of the Petroleum Research Fund (PRF45968AC9). We thank Dr. J. A. Scott, Glycosan BioSystems (Salt Lake City) for constructive advice and commercial products for evaluation.
Contributor Information
Janssen L. Vanderhooft, Department of Bioengineering, University of Utah, 419 Wakara Way, Suite 205, Salt Lake City, Utah 84108-1257, USA
Mataz Alcoutlabi, Department of Materials Science and Engineering, University of Utah, 122 South Central Campus Drive, Room 304, Salt Lake City, Utah 84108-1257, USA.
Jules J. Magda, Department of Materials Science and Engineering, University of Utah, 122 South Central Campus Drive, Room 304, Salt Lake City, Utah 84108-1257, USA Department of Chemical Engineering, University of Utah, 50 South Central Campus Drive, Room 3290, Salt Lake City, Utah 84108-1257, USA.
Glenn D. Prestwich, Department of Medicinal Chemistry, University of Utah, 419 Wakara Way, Suite 205, Salt Lake City, Utah 84108-1257, USA; Center for Therapeutic Biomaterials, University of Utah, 419 Wakara Way, Suite 205, Salt Lake City, Utah 84108-1257, USA.
References
- 1.Prestwich GD. J. Cell. Biochem. 2007;101:1370. doi: 10.1002/jcb.21386. [DOI] [PubMed] [Google Scholar]
- 2.Panorchan P, Lee JSH, Kole TP, Tseng Y, Wirtz D. Biophys. J. 2006;91:3499. doi: 10.1529/biophysj.106.084988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pedersen J, Swartz M. Ann. Biomed. Eng. 2005;33:1469. doi: 10.1007/s10439-005-8159-4. [DOI] [PubMed] [Google Scholar]
- 4.Uchida T, Ikeda S, Oura H, Tada M, Nakano T, Fukuda T, Matsuda T, Negoro M, Arai F. J. Biotechnol. 2008;133:213. doi: 10.1016/j.jbiotec.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 5.Engler AJ, Griffin MA, Sen S, Bonnemann CG, Sweeney HL, Discher DE. J. Cell Biol. 2004;166:877. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghosh K, Pan Z, Guan E, Ge S, Liu Y, Nakamura T, Ren X-D, Rafailovich MH, Clark RAF. Biomaterials. 2007;28:671. doi: 10.1016/j.biomaterials.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rehfeldt F, Engler A, Eckhardt A, Ahmed F, Discher D. Adv. Drug Delivery Rev. 2007;59:1329. doi: 10.1016/j.addr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang YL, Pelham RJJ. Methods Enzymol. 1998;298:489. doi: 10.1016/s0076-6879(98)98041-7. [DOI] [PubMed] [Google Scholar]
- 9.Discher DE, Janmey P, Wang Y-L. Science. 2005;310:1139. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 10.Pelham RJJ, Wang YL. Biol. Bull. 1998;194:348. doi: 10.2307/1543109. [DOI] [PubMed] [Google Scholar]
- 11.Georges PC, Miller WJ, Meaney DF, Sawyer ES, Janmey PA. Biophys. J. 2006;90:3012. doi: 10.1529/biophysj.105.073114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engler AJ, Sen S, Sweeney HL, Discher DE. Cell. 2006;126:677. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 13.Byrne DP, Lacroix D, Planell JA, Kelly DJ, Prendergast PJ. Biomaterials. 2007;28:5544. doi: 10.1016/j.biomaterials.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Duck FA. “Physical Properties of Tissue: A Comprehensive Reference Book”. London: Academic Press; 1990. [Google Scholar]
- 15.Greenleaf JF, Fatemi M, Insana M. Annu. Rev. Biomed. Eng. 2003;5:57. doi: 10.1146/annurev.bioeng.5.040202.121623. [DOI] [PubMed] [Google Scholar]
- 16.Harrigan TP, Konofagou EE. J. Biomech. 2004;37:1215. doi: 10.1016/j.jbiomech.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 17.Kruse S, Rose G, Glaser K, Manduca A, Felmlee J, Jack CR, Jr, Ehman R. Neuroimage. 2008;39:231. doi: 10.1016/j.neuroimage.2007.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ophir J, Alam SK, Garra B, Kallel F, Konofagou E, Krouskop T, Varghese T. Proc. Inst. Mech. Eng. 1999;213:203. doi: 10.1243/0954411991534933. [DOI] [PubMed] [Google Scholar]
- 19.Sarvazyan AP, Rudenko OV, Swanson SD, Fowlkes JB, Emelianov SY. Ultrasound Med. Biol. 1998;24:1419. doi: 10.1016/s0301-5629(98)00110-0. [DOI] [PubMed] [Google Scholar]
- 20.Crocker JC, Hoffman BD. Multiple-Particle Tracking and Two-Point Microrheology in Cells. In: Wang YL, Discher DE, editors. Methods in Cell Biology. Vol. 83. Amsterdam: Academic Press; 2007. p. 141. [DOI] [PubMed] [Google Scholar]
- 21.Dangaria JH, Butler PJ. Am. J. Physiol. Cell Physiol. 2007;293:C1568. doi: 10.1152/ajpcell.00193.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kasza KE, Rowat AC, Liu J, Angelini TE, Brangwynne CP, Koenderink GH, Weitz DA. Curr. Opin. Cell Biol. 2007;19:101. doi: 10.1016/j.ceb.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Tseng Y, Kole TP, Wirtz D. Biophys. J. 2002;83:3162. doi: 10.1016/S0006-3495(02)75319-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weihs D, Mason TG, Teitell MA. Biophys. J. 2006;91:4296. doi: 10.1529/biophysj.106.081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linnes MP, Ratner BD, Giachelli CM. Biomaterials. 2007;28:5298. doi: 10.1016/j.biomaterials.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopes MA, Silva RF, Monteiro FJ, Santos JD. Biomaterials. 2000;21:749. doi: 10.1016/s0142-9612(99)00248-3. [DOI] [PubMed] [Google Scholar]
- 27.Shor L, Guceri S, Wen X, Gandhi M, Sun W. Biomaterials. 2007;28:5291. doi: 10.1016/j.biomaterials.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 28.Ghosh K, Shu XZ, Mou R, Lombardi J, Prestwich GD, Rafailovich MH, Clark RAF. Biomacromolecules. 2005;6:2857. doi: 10.1021/bm050361c. [DOI] [PubMed] [Google Scholar]
- 29.Engler AJ, Rehfeldt F, Sen S, Discher DE. Methods Cell Biol. 2007;83:521. doi: 10.1016/S0091-679X(07)83022-6. [DOI] [PubMed] [Google Scholar]
- 30.Frey MT, Engler A, Discher DE, Lee J, Wang Y-L. Methods Cell Biol. 2007;83:47. doi: 10.1016/S0091-679X(07)83003-2. [DOI] [PubMed] [Google Scholar]
- 31.Richert L, Engler AJ, Discher DE, Picart C. Biomacromolecules. 2004;5:1908. doi: 10.1021/bm0498023. [DOI] [PubMed] [Google Scholar]
- 32.Dasgupta BR, Weitz DA. Phys. Rev. E. Stat. Nonlin. Soft Matter Phys. 2005;71:021504. doi: 10.1103/PhysRevE.71.021504. [DOI] [PubMed] [Google Scholar]
- 33.Valentine MT, Perlman ZE, Gardel ML, Shin JH, Matsudaira P, Mitchison TJ, Weitz DA. Biophys. J. 2004;86:4004. doi: 10.1529/biophysj.103.037812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pelham RJJ, Wang Yl. Mol. Biol. Cell. 1999;10:935. doi: 10.1091/mbc.10.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shu XZ, Prestwich GD. Therapeutic Biomaterials from Chemically Modified Hyaluronan. In: Garg HG, Hales CA, editors. Chemistry and Biology of Hyaluronan. Amsterdam: Elsevier Press; 2004. p. 475. [Google Scholar]
- 36.Knudson CB, Knudson W. Semin. Cell Dev. Biol. 2001;12:69. doi: 10.1006/scdb.2000.0243. [DOI] [PubMed] [Google Scholar]
- 37.Duflo S, Thibeault SL, Li W, Shu XZ, Prestwich GD. Tissue Eng. 2006;12:3201. doi: 10.1089/ten.2006.12.3201. [DOI] [PubMed] [Google Scholar]
- 38.Prestwich GD, Shu XZ, Liu Y, Cai S, Walsh JF, Hughes CW, Ahmad S, Kirker KR, Yu B, Orlandi RR, Park AH, Thibeault SL, Duflo S, Smith ME. Adv. Exp. Med. Biol. 2006;585:125. doi: 10.1007/978-0-387-34133-0_9. [DOI] [PubMed] [Google Scholar]
- 39.Shu XZ, Liu Y, Luo Y, Roberts MC, Prestwich GD. Biomacromolecules. 2002;3:1304. doi: 10.1021/bm025603c. [DOI] [PubMed] [Google Scholar]
- 40.Shu XZ, Liu Y, Palumbo S, Luo Y, Prestwich GD. Biomaterials. 2004;25:1339. doi: 10.1016/j.biomaterials.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 41.Prestwich GD. Acc. Chem. Res. 2008;41:130. doi: 10.1021/ar7000827. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y, Shu XZ, Prestwich GD. Fertil. Steril. 2007;87:940. doi: 10.1016/j.fertnstert.2006.07.1532. [DOI] [PubMed] [Google Scholar]
- 43.Serban MA, Prestwich GD. Methods. 2008;45:93. doi: 10.1016/j.ymeth.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hansen JK, Thibeault SL, Walsh JF, Shu XZ, Prestwich GD. Ann. Otol. Rhinol. Laryngol. 2005;114:662. doi: 10.1177/000348940511400902. [DOI] [PubMed] [Google Scholar]
- 45.Thannhauser TW, Konishi Y, Scheraga HA. Methods Enzymol. 1987;143:115. doi: 10.1016/0076-6879(87)43020-6. [DOI] [PubMed] [Google Scholar]
- 46.Shu XZ, Liu Y, Palumbo F, Prestwich GD. Biomaterials. 2003;24:3825. doi: 10.1016/s0142-9612(03)00267-9. [DOI] [PubMed] [Google Scholar]
- 47.Flory PJ. Principles of Polymer Chemistry. Ithaca, New York: Cornell University Press; 1953. [Google Scholar]
- 48.Boudou T, Ohayon J, Picart C, Tracqui P. Biorheology. 2006;43:721. [PubMed] [Google Scholar]
- 49.Prestwich GD. Organogenesis. 2008;4:42. doi: 10.4161/org.6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Y, Ahmad S, Shu XZ, Sanders RK, Kopesec SA, Prestwich GD. J. Orthop. Res. 2006;24:1454. doi: 10.1002/jor.20148. [DOI] [PubMed] [Google Scholar]