Abstract
Cardiomyocytes (CMs) are nonregenerative. Self-renewable pluripotent human embryonic stem cells (hESCs) can differentiate into CMs for cell-based therapies. We recently reported that Ca2+ handling, crucial to excitation-contraction coupling of hESC-derived CMs (hESC-CMs), is functional but immature. Such immature properties as smaller cytosolic Ca2+ transient amplitudes, slower kinetics, and reduced Ca2+ content of sarcoplasmic reticulum (SR) can be attributed to the differential developmental expression profiles of specific Ca2+ handling and regulatory proteins in hESC-CMs and their adult counterparts. In particular, calsequestrin (CSQ), the most abundant, high-capacity but low-affinity, Ca2+-binding protein in the SR that is anchored to the ryanodine receptor, is robustly expressed in adult CMs but completely absent in hESC-CMs. Here we hypothesized that gene transfer of CSQ in hESC-CMs suffices to induce functional improvement of SR. Transduction of hESC-CMs by the recombinant adenovirus Ad-CMV-CSQ-IRES-GFP (Ad-CSQ) significantly increased the transient amplitude, upstroke velocity, and transient decay compared with the control Ad-CMV-GFP (Ad-GFP) and Ad-CMV-CSQΔ-IRES-GFP (Ad-CSQΔ, which mediated the expression of a nonfunctional, truncated version of CSQ) groups. Ad-CSQ increased the SR Ca2+ content but did not alter L-type Ca2+ current. Pharmacologically, untransduced wild-type, Ad-GFP-, Ad-CSQΔ-, and Ad-CSQ-transduced hESC-CMs behaved similarly. Whereas ryanodine significantly reduced the Ca2+ transient amplitude and slowed the upstroke, thapsigargin slowed the decay. Neither triadin nor junctin was affected. We conclude that CSQ expression in hESC-CMs facilitates Ca2+ handling maturation. Our results shed insights into the suitability of hESC-CMs for therapies and as certain heart disease models for drug screening.
Keywords: calcium transients, ryanodine receptor, adenovirus
human embryonic stem cells (hESCs), isolated from the inner cell mass of blastocysts, can self-renew while maintaining their pluripotency to differentiate into all cell types (33), including cardiomyocytes (CMs) (7, 16, 19, 24, 37). Therefore, hESCs may provide an unlimited ex vivo source of CMs for cell-based heart therapies. Although hESC-derived CMs (hESC-CMs) have been reported to improve cardiac function in several animal myocardial infarct models (7, 16), numerous hurdles need to be overcome before their clinical applications. For instance, we (19) and others (10, 26) have recently reported that although Ca2+ handling is functional in hESC-CMs, such Ca2+ transient properties as smaller peak amplitude, slower rise, and decay kinetics are indeed immature relative to the adult form. A number of crucial Ca2+-handling proteins are differentially expressed in hESC-, fetal, and adult CMs (19). During an action potential of adult CMs, Ca2+ entry into the cytosol through sarcolemmal L-type Ca2+ current (ICa,L or Cav1.2) channels triggers the release of Ca2+ from the intracellular Ca2+ stores (also known as sarcoplasmic reticulum, SR) via the ryanodine receptor (RyR). This process, the so-called Ca2+-induced Ca2+-release (CICR) (4), escalates the cytosolic Ca2+ concentration ([Ca2+]i) to activate the contractile apparatus for contraction. For relaxation, elevated [Ca2+]i gets pumped back into the SR by the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) and extruded by the Na+/Ca2+ exchanger (NCX) to return to the resting [Ca2+]i level. Such a rise and subsequent decay of [Ca2+]i is known as Ca2+ transient. Both the contractile force (inotropic) and frequency (chronotropic) of CMs are dependent on the amplitude and kinetic properties of Ca2+ transients. Given the central importance of CICR in cardiac excitation-contraction coupling, proper Ca2+-handling properties of hESC-CMs are therefore crucial for their successful functional integration with the recipient heart after transplantation. Indeed, abnormal Ca2+ handling, as in the case of heart failure, can even be arrhythmogenic (e.g., to cause delayed after depolarization) (4, 34).
Ca2+ homeostasis is dependent on such Ca2+-handling proteins as ICa,L channels, RyR, SERCA, and NCX. RyRs are arranged in large organized arrays (up to 200 nm in diameter with more than 100 RyRs) at the junctions between the SR and sarcolemma (i.e., t-tubules) beneath ICa,L channels. These arrays constitute a large functional Ca2+ release complex. RyRs are also coupled to other proteins at the luminal SR surface such as triadin, junctin, and calsequestrin (CSQ). As the most abundant, high-capacity but low-infinity Ca2+-binding protein in the SR, the cardiac isoform CSQ2 can store up to 20 mM Ca2+ while buffering the free SR [Ca2+] at ∼1 mM. This allows repetitive muscle contractions without rundown. Although CSQ is robustly expressed in adult CMs, we have previously shown that CSQ is completely absent in hESC-CMs (19). Indeed, the role of CSQ in the Ca2+-handling properties of hESC-CMs is completely unknown. Here we tested the hypothesis that CSQ2 plays a pivotal role in the development of Ca2+-handling properties of hESC-CMs via somatic gene transfer. We conclude that expression of the missing protein CSQ in hESC-CMs facilitates maturation in the levels of SR activity by increasing the Ca2+ load and enhancing RyR-mediated Ca2+ release. A better basic understanding will help evaluate the suitability and use of hESC-CMs for therapies and as certain heart disease models for drug screening.
MATERIALS AND METHODS
hESC culturing and differentiation.
H1 cells (33) (WiCells, Madison, WI) were grown on irradiated mouse embryonic fibroblasts from 13.5-day embryos of CF-1 mice and propagated as previously described (19, 35, 37). The culture medium consisted of 80% Dulbecco's modified Eagle's medium, 20% knockout serum replacement, 4 ng/ml basic fibroblast growth factor (b-FGF), 1 mmol/l l-glutamine, 0.1 mmol/l β-mercaptoethanol, and 1% nonessential amino acid solution (all from Invitrogen). To induce the formation of embryoid bodies (EBs), H1 cells were detached using 1 mg/ml type IV collagenase and transferred to petri dishes containing 80% Dulbecco's modified Eagle's medium, 20% fetal bovine serum defined (HyClone, Logan, UT), 1 mmol/l l-glutamine, and 1% nonessential amino acid stock in the absence of b-FGF. The aggregates were cultured in suspension for 7 days followed by being plated on gelatin-coated (0.1%; Sigma-Aldrich, St. Louis, MO), six-well plates to form hESC-CMs.
Construction of recombinant adenoviruses.
To mediate the expression of CSQ in hESC-CMs, the human cardiac CSQ isoform 2 (sc119365, Origene technologies) was subcloned into the adenoviral shuttle vector pAdCMV-IRES-GFP (pAd-GFP) that we described previously (38) using primers containing the Bmt and SpeI restriction sites to generate pAdCMV-CSQ-IRES-GFP (pAd-CSQ). IRES (internal ribosomal entry site) enables simultaneous translation of CSQ and green fluorescent protein (GFP) with a single transcript. A truncated CSQ mutant CSQΔ was constructed in Ad-CSQ by deleting 817 bp (53 bp-869 bp) using the two EcoNI sites within the coding sequence of CSQ. A similar truncated CSQ created by introducing a stop codon after amino acid residue 71 has been shown to be nonfunctional (32). Adenoviral particles were generated by Cre-lox recombination of purified ψ5 viral DNA and shuttle vector DNA using Cre4 cells as previously described (12). The recombinant products were plaque purified, amplified, and purified again by Vivapure Adenopack Kit (Vivascience), yielding concentrations of the order of 109 plaque-forming units per milliliter.
Isolation of hESC-CMs and adenoviral gene transfer.
For isolating hESC-CMs, beating outgrowths were microsurgically dissected from hESC-derived EBs (21 to 28 days) by a glass knife, followed by incubation in collagenase II (1 mg/ml) at 37°C for 30 min. The isolated cells were incubated with Kraftbrühe solution containing (mM): 85 KCl, 30 K2HPO4, 5 MgSO4, 1 EGTA, 2 Na2-ATP, 5 pyruvic acid, 5 creatine, 20 taurine, and 20 d-glucose, at room temperature for 30 min. After the cells were plated on gelatin-coated glass coverslips for 1 h at 37°C, regular culture medium was added. After 48 h, plated hESC-CMs were transduced, incubated at 37°C, followed by the selection of GFP-positive hESC-CMs for recordings or other experiments within 24–48 h.
Real-time PCR.
Total RNA was extracted with RNeasy Mini kit (Qiagen). The amount of RNA was measured with a spectrophotometer. The purity was confirmed by the absorbance ratio at A260/280. Reverse transcription was done with the QuantiTect Reverse Transcription Kit (Qiagen). Quantitative PCR was carried out using Platinum SYBR green qPCR SuperMix-UDG (Invitrogen) and MyiQ Optical Module (Bio-Rad) according to the manufacturer's instructions. Primers for CSQ, RyR, junctin, triadin, SERCA2a, Cav1.2, and calreticulin were designed using Oligo Perfect Software (Invitrogen).
Western blot.
Proteins (20 μg) were loaded in SDS-polyacrylamide (10%) gel and separated by electrophoresis at 150 V for 2 h. After being transferred to nitrocellulose membrane, they were probed with anti-SERCA2a (ab2861, Abcam), anti-Cav1.2 (c1603, Sigma), anti-calsequestrin (ab3516, Abcam), anti-triadin (sc-33391, Santa Cruz Biotechnology), anti-junctin (sc-33367, Santa Cruz Biotechnology), or anti-calreticulin (ab22683, Abcam). Detection was performed with an ECL Plus Western blotting detection system.
Measurements of cytosolic Ca2+.
A spectrofluorometric method with Fura-2/AM as the Ca2+ indicator was used for measuring [Ca2+]i. hESC-CMs were incubated with 10 μM Fura-2/AM and 0.2% pluronic F-127 for 30 min at 37°C. Fluorescent signals obtained upon excitation at 340 nm (F340) and 380 nm (F380) were recorded from cells perfused with Tyrode solution containing (in mM) 140 NaCl, 5.0 KCl, 1.0 CaCl2, 1.0 MgCl2, 10.0 glucose, and 10 HEPES (pH 7.4) unless otherwise indicated. Data were analyzed using the Ionwizard software (Version 5, IonOptix) to generate the Ca2+ transient parameters reported in this study. The F340/F380 ratio was used to represent cytosolic [Ca2+]i. To induce cytoplasmic Ca2+ transients, CMs were stimulated by electrically pulsing from 0.1 to 0.5 Hz or by caffeine application as indicated. For electrical stimulations, Ca2+ transients were recorded and analyzed after a series of depolarizations that enabled each transient to fully decay so as to establish a steady-state SR content.
ICa,L measurements.
ICa,L was recorded from single hESC-CMs 24–48 h after adenoviral transduction using the whole cell patch-clamp technique with an Axopatch 200B amplifier and the pClamp9.2 software (Axon Instruments, Foster City, CA) in a bath solution containing (in mM) 110 NaCl, 30 KCl, 1.8 CaCl2, 0.5 MgCl2, 5 HEPES, and 10 glucose (pH 7.4) at 37°C. Patch pipette solution contained (in mM) 110 K+ aspartate, 20 KCl, 1 MgCl2, 0.1 Na-GTP, 5 Mg-ATP, 5 Na2-phospocreatine, 1 EGTA, 10 HEPES, pH adjusted to 7.3 with KOH. To elicit ICa,L, cells were held at a −40 mV potential and pulsed from −40 mV to +60 mV with 10-mV increments for 2 s. ICa,L was defined as 5 mM nifedipine-sensitive currents.
Di-8-ANEPPS staining of T-tubule.
hESC-CMs were incubated with 10 μM Di-8-ANEPPS (Invitrogen) for 5 min at room temperature. After being wash for 10 min with PBS, the midplanes of the cell height were imaged using a confocal laser-scanning miscroscope (C1si; Nikon, Tokyo, Japan).
Statistical analysis.
All data were expressed as means ± SE. One-way ANOVA followed by Newman-Keuls multiple comparison tests or paired t-test was carried out to test for differences between the mean values within the same study. A difference of P < 0.05 was considered significant.
RESULTS
Ad-CSQ transduction of hESC-CMs upregulated CSQ but did not alter other Ca2+-handling proteins.
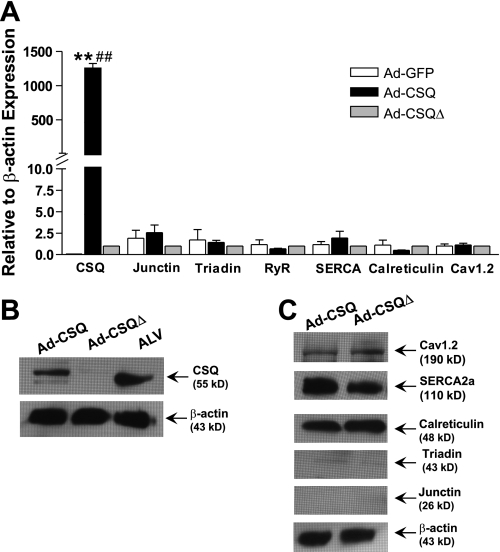
As we previously reported, CSQ is completely absent in hESC-CMs (19). In the present study, we first confirmed the efficacy of our adenoviral constructs to mediate CSQ expression in hESC-CMs. Figure 1A shows that the CSQ mRNA transcript was significantly elevated in Ad-CSQ-transduced hESC-CMs compared with the Ad-GFP-transduced control group. The increased transcript level translated into robust CSQ protein expression in Ad-CSQ-transduced hESC-CMs, although it remained less than that of human adult ventricular CMs (Fig. 1B). As anticipated, Ad-CSQΔ led to detectable increase in neither the CSQ transcript nor protein, same as Ad-GFP (P > 0.05). Transduction of hESC-CMs by Ad-GFP, Ad-CSQ, or Ad-CSQΔ had no effect on the transcript levels of other Ca2+-handling proteins such as RyR, triadin, junctin, SERCA, Cav1.2, and calreticulin (Fig. 1A). Consistently, the expression levels of the same Ca2+-handling proteins were identical in Ad-CSQ- and Ad-CSQΔ-transduced hESC-CMs (Fig. 1C).
Fig. 1.
Relative expression levels of the transcripts of various Ca2+ handling proteins probed by real-time PCR (A) and their representative protein bands from Western blot (B and C) after adenoviral transduction. Cav1.2, L-type Ca2+ channel. ALV, human adult left ventricular cardiomyocytes. Data for Ad-GFP and Ad-CSQ groups were normalized to that in Ad-CSQΔ. Values shown as means ± SE; n = 3. **P < 0.01 vs. Ad-CSQΔ; ##P < 0.01 vs. Ad-GFP.
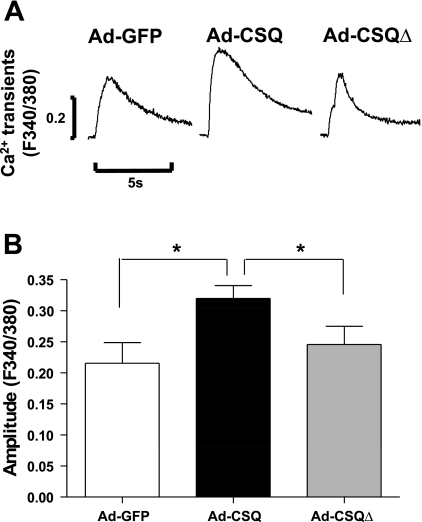
Ad-CSQ transduction increased the SR Ca2+ content of hESC-CMs.
Given the known importance of CSQ in determining the SR Ca2+ load, we next assessed the effect of CSQ expression on the Ca2+ storage capacity of SR in hESC-CMs by caffeine (10 mM) application. As anticipated from previous results (19, 26), a brief exposure to caffeine elicited robust increases of cytosolic Ca2+ in Ad-GFP-, Ad-CSQ-, and Ad-CSQΔ-transduced hESC-CMs (Fig. 2). Interestingly, the Ca2+ transient peak amplitude was significantly larger in Ad-CSQ- (n = 20) than that of Ad-GFP-transduced hESC-CMs (n = 12) by 32.7% (P < 0.05), indicating a substantial increase in SR Ca2+ content presumably due to the higher Ca2+ binding activity conferred by the expressed CSQ. The peak amplitudes of Ad-CSQΔ- and Ad-GFP-transduced hESC-CMs were not different (P > 0.05).
Fig. 2.
Ad-CSQ transduction increased the caffeine-induced Ca2+ transient peak amplitude. n = 12, 20, and 14 for Ad-GFP, Ad-CSQ, and Ad-CSQΔ, respectively. Values shown as means ± SE; *P < 0.05 vs. Ad-CSQ.
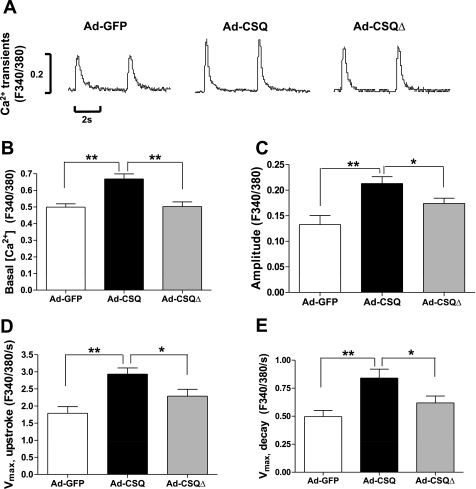
Ad-CSQ transduction enhanced magnitude and kinetics of Ca2+ transients.
To investigate whether CSQ expression in hESC-CMs facilitates the development of their Ca2+-handling properties, electrically induced Ca2+ transients of Ad-GFP, Ad-CSQ, and Ad-CSQΔ-transduced hESC-CMs were characterized and compared. Consistent with a larger SR load, Fig. 3 shows that Ad-CSQ-transduced hESC-CMs (n = 29) indeed generated larger Ca2+ transients with higher upstroke and decay velocity relative to the Ad-GFP-transduced control group (n = 12, P < 0.05). When the same electrically stimulated Ca2+ transient parameters were assessed, however, Ad-CSQΔ-transduced hESC-CMs were not different from the Ad-GFP control (P > 0.05). Previously we reported that hESC-CMs have lower cytosolic Ca2+ than human fetal and adult CMs (19). Indeed, basal cytosolic Ca2+ was elevated in Ad-CSQ-transduced (n = 20; P < 0.05) but not Ad-CSQΔ-transduced (n = 9; P > 0.05) compared with Ad-GFP-transduced hESC-CMs (n = 11). Taken collectively, the above results indicate that CSQ expression in hESC-CMs enhanced their SR Ca2+ release and development of mature Ca2+ homeostasis.
Fig. 3.
Electrically induced Ca2+ transients. A: representative tracings; bar graphs summarizing basal Ca2+ (B), amplitude (C), maximum upstroke velocity (Vmax,upstroke) (D), and maximum decay velocity (Vmax,decay) (E) of transients. Values shown as means ± SE; n = 12, 29, and 15 for Ad-GFP, Ad-CSQ, and Ad-CSQΔ, respectively. *P < 0.05, **P < 0.01 vs. Ad-CSQ.
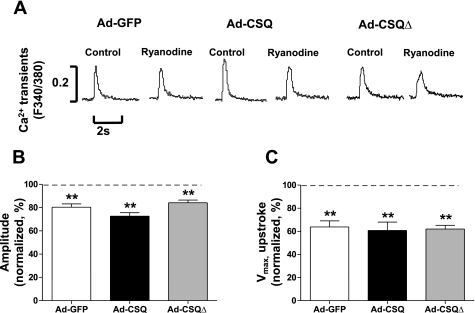
Pharmacological responses of Ad-CSQ-transduced hESC-CMs in Ca2+ transients.
When anchored to the RyR complex via triadin and junctin, CSQ is known to modulate RyR activity (2). To investigate the effect of CSQ expression on RyR activity of hESC-CMs, we studied and compared the responses of electrically induced Ca2+ transients of Ad-GFP-, Ad-CSQ-, and Ad-CSQΔ-transduced cells to ryanodine and thapsigargin, known inhibitors of RyR and SERCA, respectively. Similar to our previous finding (19), Fig. 4 shows that application of 10 μM ryanodine to Ad-GFP-transduced hESC-CMs significantly (P < 0.05) decreased the electrically evoked Ca2+ transient amplitude and slowed the upstroke velocity to 80.4 ± 2.8% and 63.8 ± 5.2% (n = 5) of the drug-free values, respectively. The inhibitory effects of ryanodine on the peak amplitude and upstroke velocity in Ad-CSQ (72.7 ± 3.0% and 60.8 ± 7.2%, respectively; n = 8)- and Ad-CSQΔ (84.2 ± 2.3% and 62.0 ± 3.2%, respectively; n = 5)-transduced hESC-CMs were identical to the Ad-GFP control (P > 0.05).
Fig. 4.
Effects of ryanodine on electrically induced Ca2+ transients. A: representative tracings; amplitude (B)and maximum Vmax,upstroke (C) after ryanodine application normalized to values recorded under control ryanodine-free conditions (dashed line i.e., 100%). n = 5, 8, and 5 for Ad-GFP, Ad-CSQ, and Ad-CSQΔ, respectively. **P < 0.01 vs. dashed line.
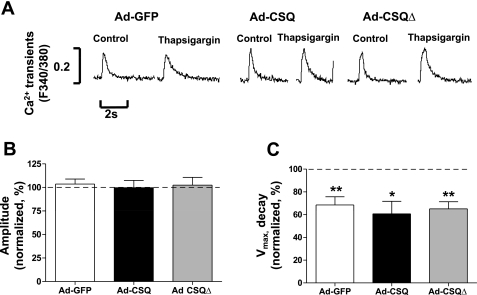
Consistent with the notion that SERCA mediates Ca2+ reuptake for SR reload in hESC-CMs, 0.5 μM thapsigargin significantly decreased the decay velocity of Ad-GFP-, Ad-CSQ-, and Ad-CSQΔ-transduced hESC-CMs to 68.5 ± 7.2% (n = 6), 60.7 ± 11% (n = 7), and 65.2 ± 6.2% (n = 6), respectively (Fig. 5). However, the transient amplitudes were not affected by thapsigargin (103.6 ± 5.3%, n = 7; 99.7 ± 7.9%, n = 6; and 102.4 ± 8.4%, n = 5, respectively; P > 0.05). These responses of all three groups of hESC-CMs were not different from each other (P > 0.05).
Fig. 5.
Effects of thapsigargin on electrically induced Ca2+ transients. A: representative tracings; amplitude (B) and Vmax,decay (C) normalized to values recorded under control thapsigargin-free conditions (dashed line, i.e., 100%); n = 6, 7, and 6 for Ad-GFP, Ad-CSQ, and Ad-CSQΔ, respectively. *P < 0.05, **P < 0.01 vs. dashed line.
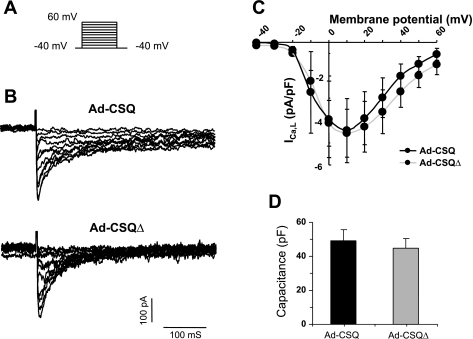
CSQ expression in hESC-CMs did not alter ICa,L and capacitance.
Since ICa,L mediates the entry of Ca2+ and is known to express in even early hESC-CMs (24), its activity if increased by Ad-CSQ transduction can potentially augment CICR and SR Ca2+ release, thereby leading to our observations presented above. To test this possibility, we examined the current-voltage relationships of ICa,L recorded from Ad-CSQ- and Ad-CSQΔ-transduced hESC-CMs. Figure 6, A–C, shows that the magnitudes of peak ICa,L of the two experimental groups were not different across the entire voltage range investigated (P > 0.05), consistent with the finding that ICa,L is expressed in early hESC-CMs and not substantially changed during maturation (25). Therefore, CSQ expression did not alter ICa,L. It is known that cardiac overexpression of CSQ leads to hypertrophy with increased heart mass and cell size (14, 27). Indeed, such a transgenic approach has been used as a mouse model of hypertrophy (9, 39). Therefore, we also measured the membrane capacitance to assess cell hypertrophy after CSQ expression. However, no changes could be detected (P > 0.05, Fig. 6D).
Fig. 6.
Voltage-clamp protocol (A) used to investigate the current-voltage (I-V) curve of L-type Ca current (ICa,L, C) in Ad-CSQ and Ad-CSQΔ groups. B: typical tracings of ICa,L; n = 4 for Ad-CSQ and 3 for Ad-CSQΔ. D: membrane capacitance; n = 13 for Ad-CSQ and 10 for Ad-CSQΔ.
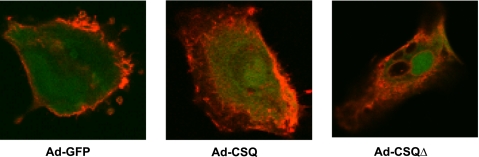
CSQ expression did not induce the formation of t-tubules.
The membrane-selective lipophilic dye Di-8-ANEPPS was used to detect the presence of t-tubules, invaginations of the surface membrane of CMs. Regardless of CSQ expression, only the periphery, but not the cellular midplane, of hESC-CMs was positively stained (Fig. 7). This was different from the organized pattern of staining found in adult CMs (18, 20), indicating that CSQ expression did not induce the formation of t-tubules not otherwise expressed in hESC-CMs (17).
Fig. 7.
Typical t-tubule imaging (×60) from Ad-GFP, Ad-CSQ, and Ad-CSQΔ groups. Green, green fluorescent protein (GFP) expression from the successful transduction with either Ad-GFP, Ad-CSQ, or Ad-CSQΔ; Red, di-8-ANEPPS staining of the lipid membrane.
DISCUSSION
In the present study, we evaluated the consequences of CSQ expression in various functional and structural aspects of hESC-CMs. The major findings were that somatic gene transfer of CSQ that is otherwise absent in hESC-CMs renders their Ca2+-handling properties more mature, as reflected by an increased SR load, an elevated basal cytosolic Ca2+, augmented Ca2+-transient amplitude, and accelerated kinetics. Although our observations with hESC-CMs share a number of similarities with previous studies of CSQ in isolated adult CMs (23, 24) and transgenic models (14, 27), several differences were also noticeable. As further elaborated and discussed below, such similarities and differences can be attributed to their common identities as CMs and different developmental stages, respectively.
CSQ2 coordinates the rates of SR Ca2+ release and load by modulating RyR activities. Indeed, the SR Ca2+ content is also known to affect the amount of Ca2+ released via CICR. For a given ICa,L trigger, a high SR Ca2+ load enhances the open probability of RyRs while directly providing more Ca2+ available for release. This is achieved via direct Ca2+ binding to the channel protein or by acting on the CSQ-RyR complex (8, 11). By contrast, ICa,L can no longer cause CICR when the SR Ca2+ content is sufficiently low. Mechanistically, CSQ senses the levels of luminal Ca2+ and affects RyRs via triadin and junctin. When SR Ca2+ declines (such as that during Ca2+ release), the increased level of Ca2+-free CSQ deactivates RyRs by binding via triadin and junctin. Alternatively, SR Ca2+ reload (e.g., upon relaxation when CICR terminates) relieves the CSQ2-mediated inhibition of RyRs. Thus, not only does CSQ buffer to increase the SR capacity to hold luminal Ca2+, but it also augments the sensitivity of RyR to changes in Ca2+ (1). Interestingly, in the absence of triadin and junctin, CSQ has been reported to activate purified RyRs reconstituted in lipid bilayer (3, 13). Since ICa,L was not altered by Ad-CSQ transduction, the larger electrically induced Ca2+ transients observed in hESC-CMs therefore did not result from a simple increase of Ca2+ influx via ICa,L. Instead, the SR load was increased as demonstrated by our caffeine experiment. Also, the accelerated rise time of electrically induced Ca2+ release was most consistent with an enhanced RyR activity. CSQ-induced inhibition of RyR was not observed probably due to the absence of triadin and junctin (at both the transcript and protein levels) in Ad-CSQ-transduced hESC-CMs. Rather, the elevated basal cytosolic Ca2+ of Ad-CSQ-transduced hESC-CMs might contribute to the faster Ca2+ transient decay due to a larger Ca2+ gradient between cytosol and SR. Alternatively, it can also be attributed to an increased NCX activitiy. Further experiments will be needed to test these possibilities.
Of note, CSQ is already expressed in adult CMs but completely absent in hESC-CMs as we already reported (19). Previous studies of adult CMs and transgenic animals obtain valuable information about the biology of CSQ by altering its expression in isolated cells or the native heart to above or below the physiological level. Our experiments, however, attempted to express the missing protein in hESC-CMs to facilitate the maturation process by rendering their Ca2+-handling properties adult-like, thereby shedding insights into its developmental and functional roles. For instance, adenovirus-mediated overexpression of CSQ2 in adult rabbit ventricular CMs in vitro has been previously reported to increase the SR Ca2+ content while reducing the excitation-contractin coupling gain (23). Although the Ca2+ load is increased in transgenic mice that overexpress CSQ, however, these animals develop hypertrophy and heart failure with impaired SR Ca2+ release and decreased Ca2+ spark frequency (14, 27, 36). By contrast, CSQ2 deletion causes a striking increase in the SR volume as a compensatory response to maintain an operable SR Ca2+ storage (15). As a result, premature spontaneous SR Ca2+ release and subsequently, arrhythmias occur due to the relieved inhibition of RyRs by CSQ (15, 31). Therefore, whereas the experimental approaches are complementary in their overall goal to obtain a better understanding of CSQ in cardiac physiology, ours differs by design. In addition, species-specific properties likely contribute to the observations in this and previous studies. Despite the various experimental outcomes, it is apparent from the collective data sets that increased CSQ2 expression generally leads to an increased SR load that is crucial for CMs maturation.
In mature ventricular CMs, CICR is optimized by the presence of t-tubules, invaginations in the sarcolemmal membrane that concentrate Cav1.2 and bring them spatially closer to RyRs (5, 6). Fast and synchronous activation of RyR translates into a larger Ca2+ transient amplitude, recruitment of more actin-myosin cross-bridge cycling, and subsequent generation of greater contractile force (6, 20). Recently, our laboratory (17) and Satin et al. (26) independently reported that such a uniform, spatially and temporally synchronized Ca2+ propagation wavefront is not observed in hESC-CMs. Although CSQ expression in hESC-CMs increased the transient peak amplitude and hastened the kinetics, t-tubule biogenesis could not be induced, consistent with the lack of CSQ-induced cellular hypertrophy as gauged by membrane capacitance. This observation again differs from the increased heart mass and cell size reported for transgenic mice that overexpress cardiac CSQ (14, 27). With reduced contractility and Ca2+ transient amplitudes, these animals have indeed been employed as a model of hypertrophy (9, 39).
Terminally differentiated CMs can undergo certain compensatory changes as a result of changes in the environment, the expression or activity of certain specific gene products. For instance, the presence of thapsigargin or small interfering RNA (siRNA)-mediated genetic suppression of SERCA2 in CMs has been reported to cause remodeling of the Ca2+ signaling mechanisms (30). In general, these responses of adult CMs can be considered as adaptation for maintenance or homeostasis because their protein synthesis is largely determined by protein turnover rather than active synthesis (28–30). By contrast, hESC-CMs are immature cells that are robustly undergoing developmental changes with active protein synthesis. As such, it has been postulated that hESC-CMs are more functionally plastic. However, Cav1.2 and an array of Ca2+-handling proteins such as junctin, triadin, RyR, SERCA, and calreticulin other than CSQ were not altered in our experiments. Furthermore, neither cellular hypertrophy nor t-tubule biogenesis was observed after Ad-CSQ transduction. Developmentally, immature CMs are known to express significant levels of calreticulin; calreticulin decreases after birth due to posttranscriptional modification and is subsequently replaced by CSQ during SR maturation (21, 22, 31). Our results show that Ad-CSQ transduction also did not affect calreticulin. Taken together, our results indicate that CSQ expression in hESC-CMs did not induce significant compensatory response to augment or counterbalance its own gene product activity. As such, our observed functional changes can be primarily attributed to CSQ.
Of note, although signs of maturation were observed, however, it is clear that the adult level has not been reached. Indeed, factors other than CSQ such as the environments and other gene products are also likely involved in the maturation of hESC-CMs. For instance, the lack of or insufficient expression of other CSQ-related proteins (e.g., junctin and triadin) might have prevented further maturation. If so, future strategies to facilitate maturation can involve the expression of specific regulatory proteins simultaneously with CSQ. Furthermore, adenovirus-mediated gene transfer is only transient. As such, it might contribute to the lack of changes of other Ca-handling proteins that are required for global maturation. Further investigations (e.g., lentiviral gene transfer for persistent genetic modification) will be needed to shed additional insights into cardiac differentiation and maturation.
In conclusion, expression of CSQ that is absent in hESC-CMs facilitates the maturation of their Ca2+-handling properties by increasing the SR load, Ca2+ transient amplitude, and kinetics. The results represent a first step to understand their development and maturation and may ultimately lead to effective approaches to derive adultlike stem cell-derived CMs with improved efficacy.
GRANTS
This work was supported by grants from the National Heart, Lung, and Blood Institute (R01 HL-72857 to R. A. Li), the Stem Cell Program of the University of California, Davis School of Medicine (to R. A. Li), the Shriners Hospital Fellowship (to J. Liu and D. K. Lieu), and the California Institute for Regenerative Medicine (to J. D. Fu and R. A. Li), and CC Wang Stem Cell Fund (to R. A. Li and H. F. Tse).
REFERENCES
- 1.Beard NA, Casarotto MG, Wei L, Varsanyi M, Laver DR, Dulhunty AF. Regulation of ryanodine receptors by calsequestrin: effect of high luminal Ca2+ and phosphorylation. Biophys J 88: 3444–3454, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beard NA, Laver DR, Dulhunty AF. Calsequestrin and the calcium release channel of skeletal and cardiac muscle. Progr Biophy Mol Biol 85: 33–69, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Beard NA, Sakowska MM, Dulhunty AF, Laver DR. Calsequestrin is an inhibitor of skeletal muscle ryanodine receptor calcium release channels. Biophys J 82: 310–320, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bers DM Cardiac excitation-contraction coupling. Nature 415: 198–205, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Brette F, Orchard C. Resurgence of cardiac t-tubule research. Physiology 22: 167–173, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Brette F, Orchard C. T-tubule function in mammalian cardiac myocytes. Circ Res 92: 1182–1192, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Caspi O, Huber I, Kehat I, Habib M, Arbel G, Gepstein A, Yankelson L, Aronson D, Beyar R, Gepstein L. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. J Am Coll Cardiol 50: 1884–1893, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Ching LL, Williams AJ, Sitsapesan R. Evidence for Ca(2+) activation and inactivation sites on the luminal side of the cardiac ryanodine receptor complex. Circ Res 87: 201–206, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Cho MC, Rapacciuolo A, Koch WJ, Kobayashi Y, Jones LR, Rockman HA. Defective beta-adrenergic receptor signaling precedes the development of dilated cardiomyopathy in transgenic mice with calsequestrin overexpression. J Biol Chem 274: 22251–22256, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Dolnikov K, Shilkrut M, Zeevi-Levin N, Gerecht-Nir S, Amit M, Danon A, Itskovitz-Eldor J, Binah O. Functional properties of human embryonic stem cell-derived cardiomyocytes: intracellular Ca2+ handling and the role of sarcoplasmic reticulum in the contraction. Stem Cells 24: 236–245, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Gyorke I, Gyorke S. Regulation of the cardiac ryanodine receptor channel by luminal Ca2+ involves luminal Ca2+ sensing sites. Biophys J 75: 2801–2810, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardy S, Kitamura M, Harris-Stansil T, Dai Y, Phipps ML. Construction of adenovirus vectors through Cre-lox recombination. J Virol 71: 1842–1849, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herzog A, Szegedi C, Jona I, Herberg FW, Varsanyi M. Surface plasmon resonance studies prove the interaction of skeletal muscle sarcoplasmic reticular Ca(2+) release channel/ryanodine receptor with calsequestrin. FEBS Lett 472: 73–77, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Jones LR, Suzuki YJ, Wang W, Kobayashi YM, Ramesh V, Franzini-Armstrong C, Cleemann L, Morad M. Regulation of Ca2+ signaling in transgenic mouse cardiac myocytes overexpressing calsequestrin. J Clin Invest 101: 1385–1393, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knollmann BC, Chopra N, Hlaing T, Akin B, Yang T, Ettensohn K, Knollmann BE, Horton KD, Weissman NJ, Holinstat I, Zhang W, Roden DM, Jones LR, Franzini-Armstrong C, Pfeifer K. Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J Clin Invest 116: 2510–2520, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O'Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nature Biotech 25: 1015–1024, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Lieu DK, Liu J, Siu CW, McNerney GP, Tse HF, Abu-Khalil A, Huser TR, Li RA. Absence of transverse tubules contributes to non-uniform Ca2+ wavefronts in mouse and human embryonic stem cell-derived cardiomyocytes. Stem Cells Dev, 2009. [DOI] [PMC free article] [PubMed]
- 18.Lipp P, Huser J, Pott L, Niggli E. Spatially non-uniform Ca2+ signals induced by the reduction of transverse tubules in citrate-loaded guinea-pig ventricular myocytes in culture. J Physiol 497: 589–597, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu J, Fu JD, Siu CW, Li RA. Functional sarcoplasmic reticulum for calcium handling of human embryonic stem cell-derived cardiomyocytes: insights for driven maturation. Stem Cells 25: 3038–3044, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Louch WE, Bito V, Heinzel FR, Macianskiene R, Vanhaecke J, Flameng W, Mubagwa K, Sipido KR. Reduced synchrony of Ca2+ release with loss of T-tubules-a comparison to Ca2+ release in human failing cardiomyocytes. Cardiovasc Res 62: 63–73, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Lynch JM, Chilibeck K, Qui Y, Michalak M. Assembling pieces of the cardiac puzzle; calreticulin and calcium-dependent pathways in cardiac development, health, and disease. Trends Cardiovasc Med 16: 65–69, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Mesaeli N, Nakamura K, Zvaritch E, Dickie P, Dziak E, Krause KH, Opas M, MacLennan DH, Michalak M. Calreticulin is essential for cardiac development. J Cell Biol 144: 857–868, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller SL, Currie S, Loughrey CM, Kettlewell S, Seidler T, Reynolds DF, Hasenfuss G, Smith GL. Effects of calsequestrin over-expression on excitation-contraction coupling in isolated rabbit cardiomyocytes. Cardiovasc Res 67: 667–677, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Mummery C, Ward-van Oostwaard D, Doevendans P, Spijker R, van den Brink S, Hassink R, van der Heyden M, Opthof T, Pera M, de la Riviere AB, Passier R, Tertoolen L. Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells. Circulation 107: 2733–2740, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Sartiani L, Bettiol E, Stillitano F, Mugelli A, Cerbai E, Jaconi ME. Developmental changes in cardiomyocytes differentiated from human embryonic stem cells: a molecular and electrophysiological approach. Stem Cells 25: 1136–1144, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Satin J, Itzhaki I, Rapoport S, Schroder EA, Izu L, Arbel G, Beyar R, Balke CW, Schiller J, Gepstein L. Calcium handling in human embryonic stem cell derived cardiomyocytes. Stem Cells, 2008. [DOI] [PubMed]
- 27.Sato Y, Ferguson DG, Sako H, Dorn GW, 2nd Kadambi VJ, Yatani A, Hoit BD, Walsh RA, Kranias EG. Cardiac-specific overexpression of mouse cardiac calsequestrin is associated with depressed cardiovascular function and hypertrophy in transgenic mice. J Biol Chem 273: 28470–28477, 1998. [DOI] [PubMed] [Google Scholar]
- 28.Seguchi M, Harding JA, Jarmakani JM. Developmental change in the function of sarcoplasmic reticulum. J Mol Cell Cardiol 18: 189–195, 1986. [DOI] [PubMed] [Google Scholar]
- 29.Seguchi M, Jarmakani JM, George BL, Harding JA. Effect of Ca2+ antagonists on mechanical function in the neonatal heart. Pediatr Res 20: 838–842, 1986. [DOI] [PubMed] [Google Scholar]
- 30.Seth M, Sumbilla C, Mullen SP, Lewis D, Klein MG, Hussain A, Soboloff J, Gill DL, Inesi G. Sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA) gene silencing and remodeling of the Ca2+ signaling mechanism in cardiac myocytes. Proc Natl Acad Sci USA 101: 16683–16688, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song L, Alcalai R, Arad M, Wolf CM, Toka O, Conner DA, Berul CI, Eldar M, Seidman CE, Seidman JG. Calsequestrin 2 (CASQ2) mutations increase expression of calreticulin and ryanodine receptors, causing catecholaminergic polymorphic ventricular tachycardia. J Clin Invest 117: 1814–1823, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terentyev D, Viatchenko-Karpinski S, Gyorke I, Volpe P, Williams SC, Gyorke S. Calsequestrin determines the functional size and stability of cardiac intracellular calcium stores: Mechanism for hereditary arrhythmia. Proc Natl Acad Sci USA 100: 11759–11764, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science 282: 1145–1147, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Tomaselli GF, Zipes DP. What causes sudden death in heart failure? Circ Res 95: 754–763, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Wang K, Xue T, Tsang SY, Van Huizen R, Wong CW, Lai KW, Ye Z, Cheng L, Au KW, Zhang J, Li GR, Lau CP, Tse HF, Li RA. Electrophysiological properties of pluripotent human and mouse embryonic stem cells. Stem Cells 23: 1526–1534, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Wang W, Cleemann L, Jones LR, Morad M. Modulation of focal and global Ca2+ release in calsequestrin-overexpressing mouse cardiomyocytes. J Physiol 524: 399–414, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xue T, Cho HC, Akar FG, Tsang SY, Jones SP, Marban E, Tomaselli GF, Li RA. Functional integration of electrically active cardiac derivatives from genetically engineered human embryonic stem cells with quiescent recipient ventricular cardiomyocytes: insights into the development of cell-based pacemakers. Circulation 111: 11–20, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Xue T, Siu CW, Lieu DK, Lau CP, Tse HF, Li RA. Mechanistic role of I(f) revealed by induction of ventricular automaticity by somatic gene transfer of gating-engineered pacemaker (HCN) channels. Circulation 115: 1839–1850, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang A, Sonin D, Jones L, Barry WH, Liang BT. A beneficial role of cardiac P2X4 receptors in heart failure: rescue of the calsequestrin overexpression model of cardiomyopathy. Am J Physiol Heart Circ Physiol 287: H1096–H1103, 2004. [DOI] [PubMed] [Google Scholar]