Abstract
Recently, genetic studies have implicated KIAA0319 in developmental dyslexia, the most common of the childhood learning disorders. The first functional data indicated that the KIAA0319 protein is expressed on the plasma membrane and may be involved in neuronal migration. Further analysis of the subcellular distribution of the overexpressed protein in mammalian cells indicates that KIAA0319 can colocalize with the early endosomal marker early endosome antigen 1 (EEA1) in large intracellular vesicles, suggesting that it is endocytosed. Antibody internalization assays with full-length KIAA0319 and deletion constructs confirmed that KIAA0319 is internalized and showed the importance of the cytoplasmic juxtamembranal region in this process. The present study has identified the medium subunit (μ2) of adaptor protein 2 (AP-2) as a binding partner of KIAA0319 in a yeast two-hybrid screen. Using Rab5 mutants or depletion of the μ-subunit of AP-2 or clathrin heavy chain by RNA interference, we demonstrate that KIAA0319 follows a clathrin-mediated endocytic pathway. We also identify tyrosine-995 of KIAA0319 as a critical amino acid required for the interaction with AP-2 and subsequent internalization. These results suggest the surface expression of KIAA0319 is regulated by endocytosis, supporting the idea that the internalization and recycling of the protein may be involved in fine tuning its role in neuronal migration.
Keywords: Rab5, adaptor protein-2, trafficking
developmental dyslexia (DD) is one of the most prevalent neurobehavioral disorders among school-age children, with a frequency between 5% and 10% (18). Dyslexic individuals have difficulties with learning to read despite intellectual ability and appropriate educational opportunities. To date, nine dyslexia susceptibility loci (DYX1–9) have been reported (28), and several genes have been proposed as candidates: DYX1C1, ROBO1, DCDC2, and KIAA0319 (10, 15, 18). All these genes participate in brain development processes such as neuronal migration and axonal guidance, and abnormalities in brain development have been reported in dyslexia (13). More recently two other genes (MRPL19 and C2ORF3) have been proposed (2) but with little functional information available.
The locus DYX2, on chromosome 6p22, is of particular interest because it has been consistently reported in several independent studies, and not one, but two, candidate DD susceptibility genes (DCDC2 and KIAA0319) have been identified (18). We have previously described a risk haplotype in this locus associated with dyslexia (11) and showed that this haplotype is associated with a reduction of KIAA0319 gene expression (19). Interference with rat Kiaa0319 expression in utero disrupts neuronal migration in the developing cerebral cortex and causes a marked change in the normal morphology of migrating neurons (19). This protein is predicted to have a motif at NH2-terminus with seven cysteine (MANSC), five polycystic kidney disease (PKD) domains and a transmembrane domain (26). We have recently shown that KIAA0319 is a highly glycosylated, dimeric, type I plasma membrane protein (27). The precise function of this protein and its involvement in neuronal migration are still unclear. The presence of PKD domains suggests a possible role in cell adhesion or cell-cell interactions (19), although the detection of a secreted, nonmembranal, minor isoform could also indicate a role in signaling (27).
Plasma membrane proteins, upon reaching the cell surface, are tightly regulated to determine whether they stay at the cell surface or are internalized in response to specific signals (14). Many proteins are internalized, including proteins involved in cell adhesion such as cadherins and integrins, and their intracellular trafficking is thought to regulate their function (6, 30). Most cell-surface receptors and integral membrane proteins are internalized by clathrin-mediated endocytosis, although several alternative endocytic pathways operate at the plasma membrane of mammalian cells (3, 9, 29). Thus clathrin-mediated endocytosis is the best characterized route of protein internalization. Clathrin is not only involved in endocytosis of plasma membrane proteins but also in the selective transport of cargo molecules between membrane-bound intracellular compartments (25). Many proteins involved in the formation of the clathrin-coated pits, the binding of cargo proteins, and the regulation of the pathway are known. One of the key regulators of the early endocytosis traffic is Rab5 (8, 22, 23). Rab proteins constitute the largest family of monomeric small GTPases, and their regulatory function lies in their ability to cycle between an active, GTP-bound, and an inactive, GDP-bound, state (31). Rab proteins function in the tethering/docking of vesicles to their target compartment, leading to membrane fusion, and thus these proteins regulate vesicular transport in endocytosis and exocytosis (22). Rab5 is localized to the early endosome where it regulates clathrin-coated, vesicle-mediated transport from the plasma membrane to the early endosomes as well as homotypic early endosome fusion (31). Sorting of cargo into clathrin-coated pits requires adaptors that recognize either directly or indirectly signals present within the cytoplasmic domain of the cargo (3). Although several adaptors have been identified, adaptor protein 2 (AP-2) is the main adaptor functioning at the plasma membrane of mammalian cells (17, 20). AP-2 is a heterotetramer composed of two large subunits (α and β2), one medium subunit (μ2), and a small subunit (σ2) (17).
Among the sorting signals present in the cargo proteins, the “tyrosine-based” and the “dileucine-based” motifs are the most characterized (5). The YXXΦ motif (where X is any amino acid and Φ a hydrophobic amino acid) is the main tyrosine-based signal, widely involved in protein sorting at the plasma membrane and at several intracellular compartments and is recognized by the μ-subunit of AP-2 and other AP complexes. NPXY, the other tyrosine-based signal, is only involved in internalization from the plasma membrane and, although it has been reported to bind AP-2 and clathrin, it is recognized by alternative adaptor proteins (3, 5, 29). The dileucine-based sorting signals also include two motifs, in the form of [DE]XXXL[LI] and DXXLL consensus sequences, although only [DE]XXXL[LI] is involved in internalization of plasma membrane proteins (5).
We recently started to characterize the protein KIAA0319 to gain further insight into its role in DD. Analysis of the trafficking of KIAA0319 is necessary to understand the function of this protein. Herein we show that, after reaching the plasma membrane, KIAA0319 is internalized back into the cell. This endocytosis follows a clathrin-mediated pathway that involves the participation of Rab5 and a direct interaction with AP-2. Furthermore, we identify the sorting signal in the cytoplasmic domain of KIAA0319 necessary for its interaction with AP-2 and, therefore, for the internalization process.
MATERIALS AND METHODS
DNA constructs.
Standard PCR and molecular cloning techniques were used to generate expression plasmids. Primer sequences and cloning conditions are available upon request. Mammalian expression plasmids pcD4KAm and pcD4Kmd20-21, encoding COOH-terminal mycHIS-tagged KIAA0319 full-length (KA) and 984-1072 deletion protein (Kd20-21), respectively, have been previously described (27). New constructs used here are pcD4Kmd20-21a and pcD4Kmd21b, encoding the COOH-terminal mycHIS-tagged KIAA0319 deletion proteins Kd20-21a (deletion of exon 20 and part of exon 21, encoding amino acids 984-1023) and Kd21b (deletion of part of exon 21, encoding amino acids 1031-1072), respectively. Plasmid pcDKAV, encoding the COOH-terminal V5-tagged KIAA0319 full-length protein (KAv), was obtained by subcloning an NdeI-BstBI fragment from pcD4KAm into pcDNA3.2/V5/GW/D-TOPO (Invitrogen). The cytoplasmic domain of KIAA0319 (KACt, amino acids 980-1072) was cloned into the yeast expression vector pAS2DD (12) to obtain pAS2_KACt. KACt and fragments f1, f2, and f3 encoding amino acids 980-1013, 1008-1044, and 1039-1072 of KIAA0319, respectively, were cloned into pGEX-6P-3 (Amersham Biosciences) for bacterial overexpression as GST-fusion proteins. Yeast control plasmids are pLAM5′-1, encoding a GAL4 DNA-Binding Domain/human Lamin C hybrid, and pGADT7-T, encoding a fusion of the SV40 large T antigen and the GAL4 Activating Domain (Clontech). Tyrosine-to-alanine change at position 995 (Y995A) of KIAA0319 was performed by site-directed mutagenesis in plasmids pcDKAV, pAS2_KACt, and pGEX-KACt, with the GeneTailor Site-Directed Mutagenesis System (Invitrogen), according to the manufacturer's protocol. All plasmids have been checked by sequencing. Constructs expressing the green fluorescent protein (GFP)-tagged constitutively active (Q79L) and dominant negative (S34N) Rab5 mutants (21) were kindly provided by Dr. Knoll.
Reagents.
Primary antibodies were the following: mouse monoclonal 9E10 anti-myc; rabbit anti-V5 (Sigma); mouse anti-early endosome antigen 1 (EEA1) (Transduction Laboratories), used as an early endosome marker; rabbit polyclonal anti-KIAA0319 R2 (27); mouse anti-AP50 (Transduction Laboratories), against the μ2-subunit of AP-2, mouse anti-clathrin heavy chain (Transduction Laboratories for Western blot, Affinity Bioreagents for IF) and mouse anti-actin (Sigma). Secondary antibodies were Alexa-Fluor (488 or 594) goat anti-mouse or anti-rabbit (Molecular Probes) for immunofluorescence and goat anti-mouse IgG-HRP (Bio-Rad). Alexa-Fluor 594-conjugated Transferrin from human serum was from Molecular Probes.
Cell culture and transfection.
HEK293T and HeLa cells were grown in DMEM (Sigma), supplemented with 10% (vol/vol) fetal bovine serum, 2 mM l-glutamine, and 100 U/ml penicillin-streptomycin, at 37°C with 5% CO2. Transfections of HEK293T and HeLa cells were performed using GeneJuice (Novagen) and ExGen 500 in vitro Transfection Reagent (Fermentas), respectively, according to the manufacturers' protocols.
Immunofluorescence and internalization assays.
Normal immunofluorescence (IF) experiments were performed as previously described (27). For internalization assays, transiently transfected HEK293T cells were washed four times with prewarmed serum-free DMEM 24–30 h after transfection and incubated in DMEM + 0.5% BSA for 30 min at 37°C. This medium was substituted by 0.25 ml of cold DMEM medium containing R2 antiserum (1:200), and cells were incubated for 30 min at 4°C. Medium was removed; cells were washed three times with cold serum-free DMEM and incubated in 0.5 ml of this medium for 1 h at 37°C. Medium was then removed and cells were washed with PBS, fixed with paraformaldehyde for 30 min, and processed for IF, except that R2 antiserum was omitted in the incubation with primary antibodies. For small interfering RNA (siRNA)-treated HeLa cells (see RNA interference), internalization assays were performed as follows: medium was removed and replaced by 0.25 ml of complete medium containing 0.5 μl of R2 antiserum; cells were incubated 10 min at 37°C, then washed twice with PBS, and incubated for 20 min at 37°C with complete medium followed by 10 min with complete medium plus Alexa-Fluor 594-transferrin. Medium was then removed and cells were washed three times with PBS, fixed with paraformaldehyde, incubated directly with anti-rabbit secondary antibody, and mounted onto microscope slides. All preparations were examined using a Zeiss LSM510 MetaHEAD Confocal Imaging System.
Yeast two-hybrid assay.
Two-hybrid screen was performed using the cytoplasmic domain of KIAA0319 cloned in fusion with GAL-BD as bait (pAS2_KACt). All experiments were performed according to the manufacturer's instruction except the X-Gal assay. The overlay X-Gal assay was performed directly on the selective medium plates by pouring on colonies a mixture of 0.25 M Na2HPO4, pH 7.5, 0.5% agar, 0.1% SDS, and 0.04% X-Gal prewarmed at 50°C. Plates were then incubated, overlay-up, at 30°C. The bait was first transformed in the yeast strain Y190 and grown on media lacking tryptophan. These transformed yeasts were then used to screen by mating the Matchmaker human brain cDNA library pretransformed in the yeast strain Y187 (Clontech). Diploids were grown either on plates lacking tryptophan, leucine, and histidine and supplemented with 20 mM 3-amino-1,2,4-triazole or on plates lacking tryptophan and leucine. Prey plasmids from positive clones were extracted and sequenced. Prey and bait plasmids were then cotransformed in Y190 for reconfirmation of interaction.
In vitro binding assay.
GST fusion proteins were overexpressed in Escherichia coli BL21 (Rosetta cells from Novagen). A single colony was grown overnight in 20 ml of Luria Bertani medium with ampicillin. The bacteria were diluted 1:10 in the same medium and cultured until OD600 was 0.6-0.8. Protein expression was induced by addition of 0.2 mM isopropyl-β-thiogalactoside for 4 h at 30°C. Cells were harvested at 3,000 rpm for 20 min at 4°C, resuspended in 10 ml of cold PBS, and sonicated. The sonicated suspension was incubated for 30 min at 4°C with Triton X-100 to a final concentration of 1% before being centrifuged at 3,000 rpm for 20 min at 4°C. The sonicated supernatant (500 μl) was then incubated with 25 μl of glutathione-Sepharose 4B beads (GE Healthcare) for 4 h at 4°C with rotation. Beads were washed twice with TNE buffer (1% NP-40, 10 mM Tris·HCl, pH 7.5, 150 mM NaCl, and 1 mM EDTA), once with TNE-NaCl buffer (1% NP-40, 10 mM Tris·HCl, pH 7.5, 600 mM NaCl,and 1 mM EDTA) and twice with TNE buffer. The washed beads were incubated overnight at 4°C with rotation with one-sixth of TNE buffer lysate obtained from HeLa cells grown to confluency on a T175 flask. Beads were then washed five times with TNE buffer or TNE-NaCl buffer (fourth wash) and resuspended in 70 μl of SDS sample buffer to be processed by immunoblotting as previously described (27).
RNA interference.
AP-2 and clathrin depletion experiments were performed using siRNA duplexes (Ambion) targeting the μ2-subunit of AP-2 (GGUGUUUGAACCGAAGCUGtt) or clathrin heavy chain (GGGUGCCAGAUUAUCAAUUtt). Scrambled siRNA used as negative control was from Qiagen (AllStars negative control siRNA). HeLa cells were seeded onto 24-well plates at a density of 3 × 104 cells/well in media without antibiotics and transfected with 100 nM duplex siRNA per well using oligofectamine (Invitrogen). The following day the medium was replaced by fresh medium with antibiotics, and cells were transfected with pcD4KAm. Approximately 24 h after transfection, cells were transferred both onto coverslips and onto a well of a six-well plate and, 24 h later, processed for internalization assays and for protein expression, respectively.
RESULTS
Plasma membrane KIAA0319 is internalized into early endosomes.
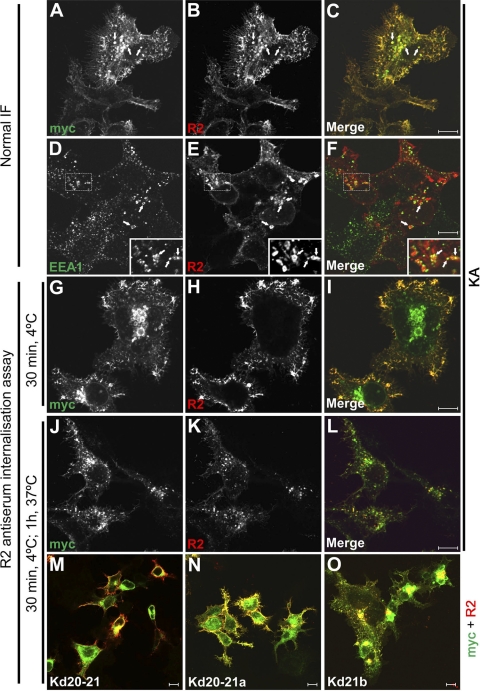
We have previously shown that full-length KIAA0319 localizes to the plasma membrane, although it is also detected in the trans-Golgi network and the endoplasmic reticulum, probably due to the overexpression of the protein (27). We observe that this protein very often localizes to intracellular vesicles, usually at a juxtanuclear position (Fig. 1, A–C). These vesicles can be very large in size in transiently transfected cells, which may be a reflection of the overexpression of the protein. Several subcellular markers were used in IF experiments, and colocalization was detected in some vesicles with the early endosomal marker EEA1 (Fig. 1, D–F). This result suggests that these vesicles could be early endosomes originated by the internalization of KIAA0319 from the plasma membrane. To test this hypothesis we performed antibody-internalization assays. For this purpose we used R2 antiserum, which recognizes an antigen in the PKD2 domain of the KIAA0319 protein. Cells were incubated with this antiserum for 30 min at 4°C; under these conditions endocytosis is inhibited and the antibodies bind to the protein located at the plasma membrane, where the antigen is accessible, but not the protein in internal compartments (Fig. 1, G–I). Subsequent incubation at 37°C allows the endocytic process to resume, and the uptake of the antibody indicates internalization of KIAA0319. Under these conditions, the R2 signal is detected in vesicles (Fig. 1, J–L) similar to those detected by normal IF (Fig. 1, A–C), indicating that KIAA0319 is endocytosed from the plasma membrane. We confirmed that this internalization process is not an artifact triggered by the binding of the polyclonal antibody itself by performing internalization assays using the Fab fragments derived from the affinity-purified-specific antibodies (supplementary Fig. 2).
Fig. 1.
KIAA0319 is internalized into early endosomes. Transiently transfected HEK293T cells expressing mycHIS-tagged full-length KIAA0319 KA (A–L) or several COOH-terminal-deletion proteins (M–O) were processed for normal IF or for internalization assays as described in materials and methods. KIAA0319 proteins were detected with polyclonal R2 antiserum and/or monoclonal anti-myc 9E10 antibody. KA is detected in vesicles (A–C), some of which show colocalization with the endosomal marker anti-EEA1 (D–F) (arrows). For the internalization assays, cells were loaded at 4°C with R2 antiserum, followed by incubation at 37°C to allow endocytosis to proceed (G–L). Antibody bound at 4°C stays at the plasma membrane (G–I) but is internalized after incubation at 37°C (J–L). Similar internalization assays performed for deletion proteins Kd20-21 (M), Kd20-21a (N), and Kd21b (O) (lacking residues 984-1072, 984-1023, and 1031-1072, respectively) show that only Kd21b is internalized. This indicates that the juxtamembranal region of the cytoplasmic domain contains a signal for endocytosis (see supplementary Fig. 1 for more details). 10-μm bars are shown in the merged panels.
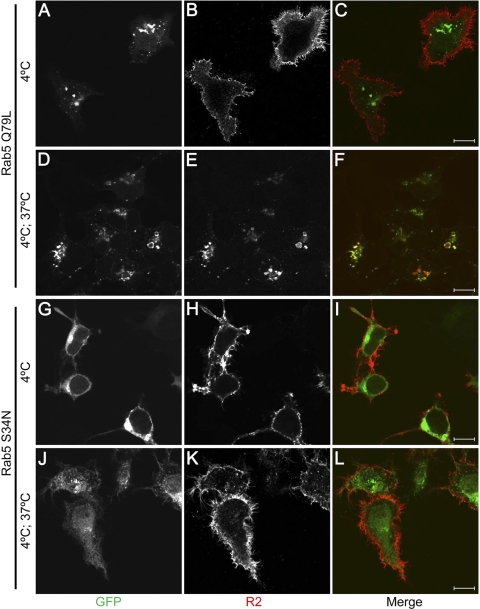
Fig. 2.
Effect of Rab5 mutants on KIAA0319 internalization. HEK293T cells coexpressing mycHIS-tagged full-length KIAA0319 and either the constitutively active Rab5 Q79L mutant (A–F) or the dominant negative Rab5 S34N mutant (G–L) were subjected to R2 antiserum internalization assays. Rab5 mutants are detected by their GFP tag (A, D, G, J). As shown in Fig. 1, R2 antiserum bound at 4°C stays at the plasma membrane (B, H). In Rab5 Q79L transfected cells (D), KIAA0319 is internalized (E) and colocalizes with the enlarged Rab5-positive endosomes (F) after incubation at 37°C. In cells overexpressing Rab5 S34N (J), the R2 antiserum stays at the plasma membrane after the incubation at 37°C (K), and no colocalization with the Rab5 mutant is detected (L), suggesting that the endocytosis of KIAA0319 is inhibited. 10-μm bars are shown in the merge panels.
The cytoplasmic juxtamembranal region of KIAA0319 is needed for internalization.
The KIAA0319 COOH-terminus (KACt, residues 980 to 1072, mostly encoded by exons 20 and 21), constitutes the cytoplasmic domain of the protein. Although it is not needed to localize to the plasma membrane (27), it might contain important signals for endocytosis. To investigate this we performed antibody internalization assays with several deletion constructs of KIAA0319. Cells expressing the Kd20-21 deletion protein, lacking most of the cytoplasmic domain, do not internalize R2 antibodies (Fig. 1M), suggesting this region of the protein is needed for endocytosis. We further analyzed the location of the signal for endocytosis by using two other constructs encoding KIAA0319-deletion proteins Kd20-21a and Kd21b, lacking residues 984 to 1023 and 1031 to 1072, respectively. Internalization assays were performed as described. Vesicles with internalized Kd21b could be observed (Fig. 1O), as seen with the full-length protein (Fig. 1L), but not with Kd20-21a (Fig. 1N), which presented a similar pattern to that of the full cytoplasmic-domain-deleted protein Kd20-21 (Fig. 1M). These internalization assays, together with normal IF staining, are shown more in detail in supplementary Fig. 1. These results suggest that the signal needed for KIAA0319 endocytosis resides in the region 984-1023 of the protein, mostly encoded by exon 20.
KIAA0319 internalization is Rab5 dependent.
We further examined the internalization of KIAA0319 by investigating the effect of Rab5 mutants. In the early endocytic pathway, Rab5 regulates clathrin-coated, vesicle-mediated transport from the plasma membrane to the early endosomes as well as homotypic early endosome fusion (7, 31). Two Rab5 mutants Q79L (“constitutively active”) and S34N (“dominant negative”) with opposing biochemical properties have been described (24): Rab5 Q79L has reduced GTPase activity and is a potent stimulator of homotypic fusion between early endosomes, whereas Rab5 S34N has preferential affinity for GDP and causes fragmentation of endosomes, inhibition of endosome fusion, and inhibition of transferrin endocytosis. R2 antiserum internalization assays were performed in cells cotransfected with KIAA0319 and one of the Rab5 mutant constructs. R2 antiserum is detected at the plasma membrane after incubation at 4°C both in cells expressing the Q79L mutant (Fig. 2, A–C) and in cells expressing the S34N mutant (Fig. 2, G–I). After incubation at 37°C, internalized KIAA0319 colocalizes with Rab5 Q79L in enlarged vesicles of transfected cells (Fig. 2, D–F). However, in cells expressing Rab5 S34N, KIAA0319 internalization is inhibited (Fig. 2, J–L). These results suggest that KIAA0319 most probably enters the early endosomal system following a clathrin-dependent endocytic pathway.
KIAA0319 interacts with the μ-subunit of AP-2.
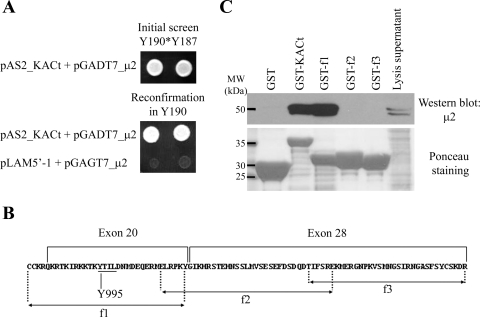
In parallel with the experiments described above, and as part of the characterization of KIAA0319, we performed a Yeast Two Hybrid (Y2H) screen to identify proteins interacting with its cytoplasmic domain (KACt). The bait KACt was first tested for transcriptional activation. As expected, neither growth on media lacking histidine nor activation of lacZ gene was observed for the Y190 strain pretransformed with the bait KACt (Y190[KACt]) (data not shown). After Y190[KACt] was mated with the pretransformed library, five independent clones encoding μ2, the μ-subunit of the AP-2 complex (accession number NM_004068) were identified from 5.5 × 107 diploids. Three of these clones contained a prey vector encoding amino acids 66-435 (codon 436 being the stop codon), one clone encoded amino acids 2-435, and the last clone amino acids 87-435 of the μ2-subunit of AP-2. We retested this protein interaction by cotransformation of Y190 with one of the purified prey plasmids encoding μ2 (pGADT7_μ2, insert encoding amino acids 66-435) and either the bait vector pAS2_KACt or the control vector pLAM5′-1. Only colonies expressing KACt and the μ2-subunit could grow on selective media lacking histidine (Fig. 3A) and activate the lacZ gene (data not shown), confirming an interaction between KACt and μ2.
Fig. 3.
KIAA0319 interacts with the μ-subunit of adaptor-2 (AP-2). A: yeast two-hybrid (Y2H) analysis. Results of initial library screening by mating (top) and reconfirmation assay (bottom). Yeast Y190 was cotransformed with the prey plasmid encoding μ2 (pGADT7_μ2) and the bait plasmid (pAS2_KACt) or a negative control plasmid (pLAM5′-1) and grown on selective media lacking tryptophan, leucine, and histidine and supplemented with 20 mM 3-amino-1,2,4-triazole (3-AT). Only yeast containing plasmids encoding KACt and μ2 could activate the reporter gene his and grow on selective media (assays shown in duplicate). B: sequence of the cytoplasmic domain of KIAA0319 (KACt) indicating exons, fragments f1, f2, and f3, and position of tyrosine 995 (see text and Fig. 4). C: GST-pulldown assays. HeLa cell protein lysates were incubated with GST or GST-fusion proteins (full-length KACt or overlapping fragments f1-f2-f3) immobilized on glutathione sepharose beads. Eluates were analyzed by Western blot using anti-AP50 antibody, against the μ2-subunit of AP-2. A Ponceau staining of the membrane indicates the expression level of GST and GST-fusion proteins used in the experiment. Only KACt and f1 GST-fusion proteins are able to pull down the μ2 subunit of AP-2.
To provide further evidence of this interaction, we performed GST pull-down experiments. After immobilization on sepharose beads, GST or the fusion protein GST-KACt were incubated with HeLa cell protein extracts, beads were washed, and bound proteins were eluted and separated by SDS-PAGE followed by Western blot analysis to detect μ2. To ascertain the region of KIAA0319 involved in the interaction with μ2, we performed the same experiment using three GST fusions containing overlapping fragments of KACt, f1, f2, or f3 (residues 980-1013, 1008–1044, and 1039-1072, respectively, of the KIAA0319 protein) (Fig. 3B). Specific binding could only be detected with the KACt and the f1 GST-fusion proteins (Fig. 3C). Ponceau S staining confirms the presence of the GST fusion proteins in all samples. These results corroborate the Y2H data showing an interaction between KIAA0319 and the μ2 subunit of AP-2. Furthermore, in agreement with the antibody-internalization results obtained with the KIAA0319-deletion constructs (Fig. 1, M–O), the interaction domain is mapped to the region 980-1013 of full-length KIAA0319.
KIAA0319 undergoes clathrin-mediated endocytosis.
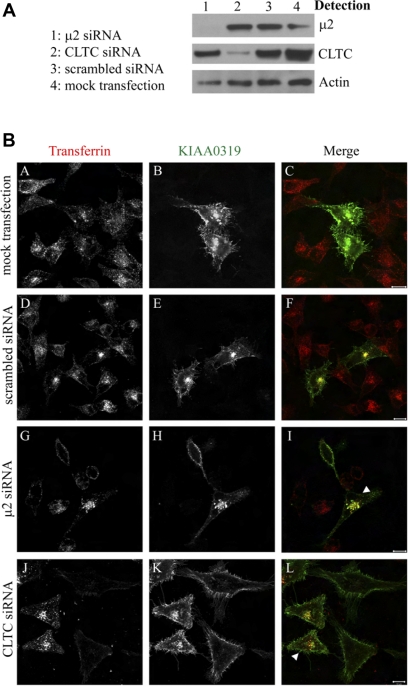
AP-2 is a key component for the clathrin-dependent endocytosis of transmembranal proteins, and the results described above strongly support that KIAA0319 follows this endocytic pathway. To further analyze KIAA0319s dependence on the AP-2 complex and clathrin for internalization, we carried out RNAi to deplete the μ2-subunit or clathrin heavy chain (CLTC) in HeLa cells overexpressing KIAA0319. Western blot analysis revealed >90% depletion of μ2 or CLTC (Fig. 4A). Because the antibodies that we tried against the μ2-subunit do not work in immunofluorescence, we used the transferrin receptor as a control. Transferrin receptor internalization is a very well-known marker for clathrin-dependent endocytosis, and knockdown of μ2 or CLTC blocks the internalization of the transferrin receptor (which also interacts with the μ2-subunit) (3). Depleted cells can be identified by the absence of labeled transferrin uptake. In these depleted cells KIAA0319 internalization is also prevented, as shown by the accumulation of the R2 antibody at the cell surface after performing the internalization assay (Fig. 4B, G–L). Nontargeting scrambled siRNA or mock transfection had no effect on the endocytosis of KIAA0319 (Fig. 4B, A–F). The same experiments as those described in Fig. 4, but detecting clathrin instead of transferrin, are presented in supplementary Fig. 3 and show the same results. Thus preventing the functioning of AP-2 or clathrin by depleting μ2 or CLTC causes retention of KIAA0319 on the cell surface where it is blocked from internalizing. These results confirm that KIAA0319 follows the clathrin-mediated endocytic pathway and that this internalization is dependent on AP-2.
Fig. 4.
KIAA0319 undergoes clathrin-mediated endocytosis. HeLa cells were treated with transfection reagent alone (mock), with 100 nM scrambled small interfering RNA (siRNA) or with 100 nM siRNA directed against either μ2 or cathrin heavy chain (CLTC). After siRNA treatment, cells were transfected with a full-length KIAA0319 construct containing a V5 tag. A: cell lysates were prepared 72 h later and Western blotted using antibodies directed against μ2, CLTC, or actin. RNAi is specific for μ2 and CTLC and does not influence actin levels. B: R2 internalization assays in siRNA-treated cells. KIAA0319 protein at the plasma membrane was labeled with anti-KIAA0319 R2 antiserum, and after a period of internalization cells were fixed and processed to detect internalized R2 (B, E, H, K). Cells silenced for μ2 or CLTC can be identified by the absence of transferrin-594 uptake (A, D, G, J). In mock transfected and scrambled control cells, R2-labeled KIAA0319 is observed intracellularly, where it colocalizes with transferrin (A–F). In cells depleted of μ2 or CLTC, R2-labeled KIAA0319 is observed only on the cell surface (H, K). The arrowheads indicate some nondepleted cells that can act as an internal negative control. 10-μm bars are shown in the merged panels.
Tyrosine-995 is needed for the endocytosis of KIAA0319.
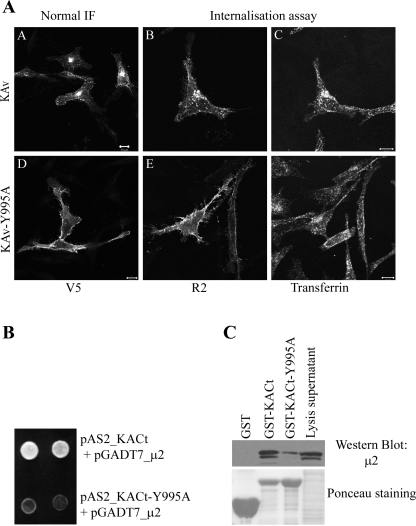
The cytoplasmic domain of KIAA0319 contains residues YTIL at position 995-998, matching the consensus sequence for the YXXΦ AP-2-binding motif (Fig. 3B). It is located in fragment f1, which we show to be necessary for interaction with AP-2. To investigate whether this motif does indeed mediate the interaction between KACt and AP-2, we performed site-directed mutagenesis to change tyrosine-995 into alanine (Y995A).
We assessed the effect of Y995A mutation on the subcellular localization and internalization of KIAA0319, either by normal IF or by anti-KIAA0319 R2 antiserum internalization assays. HeLa cells were transfected with either KIAA0319 wild-type (KAv) or mutated KIAA0319 (KAv-Y995A), both tagged with a COOH-terminal V5 tag. As previously demonstrated in the HEK293T cell line (Fig. 1), KIAA0319 wild type is observed intracellularly with some plasma membrane staining apparent (Fig. 5 A,A) and as expected, the R2 antibody is internalized (Fig. 5A,B). KAv-Y995A on the other hand is detected mainly at the cell surface by normal IF (Fig. 5A,D). Furthermore, the internalization of the R2 antibody in KAv-Y995A expressing cells is inhibited (Fig. 5A,E), suggesting that the cell surface expression of KAv-Y995A is due to a defect in endocytosis. Transferrin internalization occurs normally in both KAv- and KAv-Y995A-expressing cells (Fig. 5A, C and F) showing clathrin-mediated endocytosis is unaffected in both cases. These results indicate that tyrosine-995 is necessary for the endocytosis of KIAA0319.
Fig. 5.
Tyrosine-995 is needed for the endocytosis of KIAA0319. A: effect of Y995A mutation on the subcellular localization of KIAA0319. HeLa cells were transfected with constructs expressing either wild-type (KAv) (A–C) or mutant (KAv-Y995A) (D–F) V5-tagged full-length KIAA0319 protein. Proteins were detected with anti-V5 antibody (A, D) or by R2 antiserum internalization assays (B, E). KAv is observed on the cell surface and in internal structures, whereas KAv-Y995A is found only at the cell surface and R2 antibodies are not internalized. Internalization of transferrin-594 is unaffected by the expression of either construct demonstrating clathrin-dependent endocytosis occurs normally in both cases (C, F). b: effect of Y995A mutation on the interaction between KIAA0319 COOH-terminus and the μ2-subunit by Y2H analysis. Y190 yeast cells were cotransformed with either the bait vectors pAS2_KACt (wild-type bait) or pAS2_KACt-Y995A (mutated bait) and the prey plasmid encoding μ2 (pGADT7_μ2). Only yeast transformed with wild-type KACt could activate the reporter gene his and grow on media lacking histidine (assays shown in duplicate). c: effect of Y995A mutation on the interaction between KIAA0319 and the μ2-subunit by GST-pulldown analysis. Experiments performed as described in Fig. 3. Binding of μ2 was highly reduced with mutated KACt fragment (KACt-Y995A) compared with wild-type fragment. Ponceau staining indicates amounts of fusion protein used for the GST pulldown. Scale bars: 10 μm.
To confirm that the mutation Y995A in KIAA0319 prevents its interaction with AP-2, we performed Y2H and GST-pulldown experiments. The Y190 yeast strain was cotransformed with a prey plasmid expressing μ2 (pGADT7_μ2) and either the bait plasmid pAS2_KACt or the mutated bait plasmid pAS2_KACt-Y995A. Yeast containing the wild-type bait plasmid could grow on media lacking histidine (Fig. 5B) and activate the reporter gene lacZ (not shown), whereas yeast containing the mutated bait plasmid failed to grow and to activate lacZ under the same conditions. Further data confirming the importance of this residue were obtained from GST-pulldown experiments (Fig. 5C), with GST-KACt-Y995A showing a highly reduced binding to AP-2 compared with the result obtained with the wild-type GST-KACt. The reduction of binding is not due to lower expression of the mutated fusion protein GST-KACt (see Ponceau staining, Fig. 5C) but to the substitution of tyrosine-995 to alanine. These results indicate that the binding of AP-2 to the COOH-terminus of KIAA0319 involves the tyrosine-based motif in position 995-998 of the KIAA0319 protein.
DISCUSSION
Neuronal migration is one of the key processes leading to the formation of the cerebral cortex and thus, during the development of the brain, neurons are required to tightly regulate cell-cell adhesion and migration. Recent data on KIAA0319 suggest that it is required for appropriate cell adhesion between migrating neurons and the glial fibers in the developing neocortex (19). We have recently shown that KIAA0319 is a single transmembrane protein detected at the plasma membrane (27). This subcellular localization is necessary for the protein to be directly involved in cell adhesion/neuronal migration. However, most proteins reaching the plasma membrane are not permanent residents but instead traffic between different subcellular compartments. The observation of vesicles positive for both KIAA0319 and an early endosome marker indicated that KIAA0319 could be internalized after reaching the plasma membrane. However, neither the traffic mechanisms of KIAA0319 nor the motifs in its sequence driving this traffic were known. We have characterized such mechanisms and motifs and show here that KIAA0319 is internalized via a clathrin-dependent pathway through direct interaction with the μ-subunit of the adaptor protein AP-2. This interaction is mediated by a YXXΦ-type internalization signal in the cytoplasmic domain of KIAA0319.
Using deletion constructs, we observe that the juxtamembranal cytoplasmic region, encoded by exon 20, is necessary for the internalization of specific antibodies bound to KIAA0319 at the plasma membrane. The internalization of the protein, overexpressed in mammalian cells, appears to be a constitutive process as it is observed in normal culture conditions. For the characterization of the endocytic mechanism driving the internalization of this protein, we took advantage of the availability of Rab5 mutants. Rab5 controls critical steps for the endocytosis as it regulates clathrin-mediated transport from the plasma membrane to early endosomes as well as homotypic early endosome fusion (31). Rab5 has been reported to participate at the very early stages of the endocytic pathway as it is required for vesicle formation in vitro (16). Different Rab5 mutants have been described. The Q79L mutant is constitutively active because of a strongly decreased intrinsic GTPase activity. Overexpression of this mutant leads to a dramatic change in cell morphology, with the appearance of large early endocytic structures. The S34N is a dominant negative mutant, with a preferential affinity for GDP. Its overexpression inhibits transferrin receptor internalization, which follows the classical clathrin-mediated endocytosis pathway (24). The KIAA0319 protein, when coexpressed with these Rab5 mutants, colocalizes with the Q79L mutant in large vesicles, while its internalization is prevented in cells expressing Rab5-S34N; this is a very similar localization pattern as those reported for transferrin in the same conditions and suggests that KIAA0319 follows the same clathrin-mediated endocytic pathway.
As described before, there are two main sorting signals for proteins following clathrin-mediated endocytosis: dileucine- and tyrosine-based motifs. No sequence in the cytoplasmic COOH-terminus of KIAA0319 (KACt) fits the dileucine-based consensus signals. Regarding the tyrosine-based signals, out of the three tyrosine residues (Y995, Y1013, and Y1067) in KACt, none of them fits the NPXY sequence and only Y995, with the 995-998 region YTIL, matches the consensus for a YXXΦ motif. The presence of a sequence conforming to a sorting motif is not necessarily predictive of sorting function because these signals must be present in an appropriate context to be active (4). However, the observation that the deletion protein KAd20-21a, where the YTIL sequence is missing, could not be internalized from the plasma membrane suggests that such a sequence is likely to be the internalization signal for KIAA0319. We have shown that this is indeed the situation by identifying a direct interaction of KIAA0319 with the μ-subunit of the adaptor protein AP-2 (μ2). This interaction was found following an independent approach as a result of a parallel experiment where we used KACt as bait in a Y2H screen to identify interacting proteins. We identified several clones of μ2, encoding three different protein fragments, the smallest starting at residue 87. The NH2-terminus of μ2 (1/3 of the protein) is involved in interaction with the β-subunit of the AP complex, and the COOH-terminus (2/3 of the protein) is involved in binding to YXXΦ motifs (1). Thus all the clones detected in the Y2H screen contain the COOH-terminal region of the protein and would be able to interact with proteins containing a YXXΦ sequence. The use of fragments f1, f2, and f3 of KACt as well as the Y995A KACt mutant in Y2H experiments further confirmed that the YTIL sequence is responsible for the in vitro interaction of KIAA0319 with μ2. We confirmed that this was also the case in vivo. Depletion of μ2 or CLTC, as seen similarly in cells transfected with the Rab5 dominant-negative mutant, blocked the endocytosis of KIAA0319, indicating that the pool of KIAA0319 localized on the plasma membrane is indeed internalized via the clathrin pathway using AP-2 as an adaptor. The full-length KIAA0319-Y995A mutant protein, however, could not be internalized, confirming the importance of tyrosine 995 in the binding to AP-2 and endocytosis. This is true not only in the commonly used cell lines HEK293T or HeLa, used above, but also in neuronal cell lines such as Kelly (supplementary Fig. 4), which could be regarded as a better model to study the function of the neuron-expressed KIAA0319 protein.
Clathrin-mediated endocytosis represents the major route by which hormones, receptors, and signaling factors are internalized. Endocytosed proteins reach the early endosomes, which play an important role in their sorting and regulation. From early endosomes, two alternative routes are available: the recycling back to the cell surface or to the trans-Golgi network, or the transport through late endosomes for degradation in the lysosome. Both routes will influence the surface expression of the endocytosed proteins. The sorting signal present in the cytoplasmic domains of these proteins could also be important for the choice of the postearly endosome route followed by the protein. Thus YXXΦ motifs are involved in targeting transmembrane proteins to lysosomes and lysosome-related organelles (5), and both position within the cytoplasmic domain and actual amino acid sequence are important. Accordingly, it would be expected that KIAA0319 follows a recycling pathway after endocytosis instead of degradation. We are currently investigating this hypothesis to establish the specific steps that KIAA0319 follows after its internalization.
As it has been mentioned before, the recycling pathway of cell adhesion proteins such as cadherins is thought to have an important role in the regulation of their adhesive function (6). Understanding the trafficking pathway of the KIAA0319 protein is important in the context of its involvement in neuronal migration as it would probably allow us to gain new insight in the mechanisms used to regulate this process. We have reported the initial steps to reach this objective, and the data presented here will be a valuable base to complete this task and advance in the functional characterization of this protein.
GRANTS
This work was supported by funding from the Wellcome Trust.
Supplementary Material
Acknowledgments
The authors thank Dr. Kazuhiro Kobayashi for help in some molecular biology steps, Dr. Brian J. Knoll for kindly providing the Rab5 constructs, and the Molecular Cytogenetics and Microscopy Core group for its technical support.
REFERENCES
- 1.Aguilar RC, Ohno H, Roche KW, Bonifacino JS. Functional domain mapping of the clathrin-associated adaptor medium chains mu1 and mu2. J Biol Chem 272: 27160–27166, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Anthoni H, Zucchelli M, Matsson H, Muller-Myhsok B, Fransson I, Schumacher J, Massinen S, Onkamo P, Warnke A, Griesemann H, Hoffmann P, Nopola-Hemmi J, Lyytinen H, Schulte-Korne G, Kere J, Nothen MM, and Peyrard-Janvid M. A locus on 2p12 containing the co-regulated MRPL19 and C2ORF3 genes is associated to dyslexia. Hum Mol Genet 16: 667–677, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Benmerah A, Lamaze C. Clathrin-coated pits: vive la difference? Traffic 8: 970–982, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Bonifacino JS, Dell'Angelica EC. Molecular bases for the recognition of tyrosine-based sorting signals. J Cell Biol 145: 923–926, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem 72: 395–447, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Bryant DM, Stow JL. The ins and outs of E-cadherin trafficking. Trends Cell Biol 14: 427–434, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, Zerial M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell 70: 715–728, 1992. [DOI] [PubMed] [Google Scholar]
- 8.Cavalli V, Corti M, Gruenberg J. Endocytosis and signaling cascades: a close encounter. FEBS Lett 498: 190–196, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature 422: 37–44, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Fisher SE, Francks C. Genes, cognition and dyslexia: learning to read the genome. Trends Cogn Sci 10: 250–257, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Francks C, Paracchini S, Smith SD, Richardson AJ, Scerri TS, Cardon LR, Marlow AJ, MacPhie IL, Walter J, Pennington BF, Fisher SE, Olson RK, DeFries JC, Stein JF, Monaco AP. A 77-kilobase region of chromosome 6p22.2 is associated with dyslexia in families from the United Kingdom and from the United States. Am J Hum Genet 75: 1046–1058, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fromont-Racine M, Rain JC, Legrain P. Toward a functional analysis of the yeast genome through exhaustive two-hybrid screens. Nat Genet 16: 277–282, 1997. [DOI] [PubMed] [Google Scholar]
- 13.Galaburda AM, LoTurco J, Ramus F, Fitch RH, Rosen GD. From genes to behavior in developmental dyslexia. Nat Neurosci 9: 1213–1217, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Gong Q, Huntsman C, Ma D. Clathrin-independent internalization and recycling. J Cell Mol Med 12: 126–144, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGrath LM, Smith SD, Pennington BF. Breakthroughs in the search for dyslexia candidate genes. Trends Mol Med 12: 333–341, 2006. [DOI] [PubMed] [Google Scholar]
- 16.McLauchlan H, Newell J, Morrice N, Osborne A, West M, Smythe E. A novel role for Rab5-GDI in ligand sequestration into clathrin-coated pits. Curr Biol 8: 34–45, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Nakatsu F, Ohno H. Adaptor protein complexes as the key regulators of protein sorting in the post-Golgi network. Cell Struct Funct 28: 419–429, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Paracchini S, Scerri T, Monaco AP. The genetic lexicon of dyslexia. Annu Rev Genomics Hum Genet 8: 57–79, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Paracchini S, Thomas A, Castro S, Lai C, Paramasivam M, Wang Y, Keating BJ, Taylor JM, Hacking DF, Scerri T, Francks C, Richardson AJ, Wade-Martins R, Stein JF, Knight JC, Copp AJ, Loturco J, Monaco AP. The chromosome 6p22 haplotype associated with dyslexia reduces the expression of KIAA0319, a novel gene involved in neuronal migration. Hum Mol Genet 15: 1659–1666, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Robinson MS Adaptable adaptors for coated vesicles. Trends Cell Biol 14: 167–174, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Rosenfeld JL, Moore RH, Zimmer KP, Alpizar-Foster E, Dai W, Zarka MN, Knoll BJ. Lysosome proteins are redistributed during expression of a GTP-hydrolysis-defective rab5a. J Cell Sci 114: 4499–4508, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Somsel Rodman J and Wandinger-Ness A. Rab GTPases coordinate endocytosis. J Cell Sci 113: 183–192, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Stein MP, Dong J and Wandinger-Ness A. Rab proteins and endocytic trafficking: potential targets for therapeutic intervention. Adv Drug Deliv Rev 55: 1421–1437, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Stenmark H, Parton RG, Steele-Mortimer O, Lutcke A, Gruenberg J, Zerial M. Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J 13: 1287–1296, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Traub LM Common principles in clathrin-mediated sorting at the Golgi and the plasma membrane. Biochim Biophys Acta 1744: 415–437, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Velayos-Baeza A, Toma C, da Roza S, Paracchini S, Monaco AP. Alternative splicing in the dyslexia-associated gene KIAA0319. Mamm Genome 18: 627–634, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Velayos-Baeza A, Toma C, Paracchini S, Monaco AP. The dyslexia-associated gene KIAA0319 encodes highly N- and O-glycosylated plasma membrane and secreted isoforms. Hum Mol Genet 17: 859–871, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Williams J, O'Donovan MC. The genetics of developmental dyslexia. Eur J Hum Genet 14: 681–689, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Wolfe BL, Trejo J. Clathrin-dependent mechanisms of G protein-coupled receptor endocytosis. Traffic 8: 462–470, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Yap AS, Crampton MS, Hardin J. Making and breaking contacts: the cellular biology of cadherin regulation. Curr Opin Cell Biol 19: 508–514, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol 2: 107–117, 2001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.