Abstract
Large-conductance, Ca2+-activated, and voltage-gated potassium channels (BK, BKCa, or Maxi-K) play an important role in electrical tuning in nonmammalian vertebrate hair cells. Systematic changes in tuning frequency along the tonotopic axis largely result from variations in BK channel kinetics, but the molecular changes underpinning these functional variations remain unknown. Auxiliary β1 have been implicated in low-frequency tuning at the cochlear apex because these subunits dramatically slow channel kinetics. Tamoxifen (Tx), a (xeno)estrogen compound known to activate BK channels through the β-subunit, was used to test for the functional presence of β1. The hypotheses were that Tx would activate the majority of BK channels in hair cells from the cochlear apex due to the presence of β1 and that the level of activation would exhibit a tonotopic gradient following the expression profile of β1. Outside-out patches of BK channels were excised from tall hair cells along the apical half of the chicken basilar papilla. In low-density patches, single-channel conductance was reduced and the averaged open probability was unaffected by Tx. In high-density patches, the amplitude of ensemble-averaged BK current was inhibited, whereas half-activation potential and activation kinetics were unaffected by Tx. In both cases, no tonotopic Tx-dependent activation of channel activity was observed. Therefore, contrary to the hypotheses, electrophysiological assessment suggests that molecular mechanisms other than auxiliary β-subunits are involved in generating a tonotopic distribution of BK channel kinetics and electric tuning in chick basilar papilla.
Keywords: calcium-activated potassium channel, KCNMA1, KCNMB1, outside-out patch, electrical tuning
large-conductance, ca2+-activated, and voltage-gated K+ channels (BK, BKCa, or Maxi-K) are expressed in a variety of cells, including vertebrate cochlear hair cells. In the cochlea, BK channels are involved in signal transduction by shaping the hair cell receptor potential and decreasing the membrane time constant (21, 45). However, the role these channels play in mammalian and nonmammalian cochlea appears different, largely due to differences in the calcium sources that modulate channel activity. In mammals, BK channels are unresponsive to calcium influx through voltage-gated calcium channels (39), whereas in nonmammals, the response of these channels is intimately associated with voltage-dependent calcium influx (16). In fish, frogs, alligators, and chicks, the interplay between BK and calcium channels contributes to an electrical resonance in the receptor potential and a mechanism for intrinsic electrical tuning to specific sound frequencies (see Ref. 15 for a review). BK channels are key components of electrical tuning due to a tonotopic distribution of channel kinetics from slow to fast along an apical-basal, or low-frequency to high-frequency, gradient (14, 15, 47). The mechanisms for systematically changing channel kinetics are unknown but may include alternative splicing of pore-forming α-subunits and coassembly with auxiliary β-subunits (25, 26, 51, 52).
Four human β-isoforms (β1–β4, KCNMB1-4) have been identified (46), and transcripts of β1 and β4 have been discovered in mammalian inner hair cells (32). In avian auditory organs, a gradient in β1-subunit expression was found using in situ hybridization, revealing high mRNA expression in apical hair cells and low mRNA expression in basal hair cells (52). In general, the incorporation of β-subunits in the channel complex slows kinetics (7, 40), which is also characteristic of BK channels from apical hair cells in the chick auditory epithelium (14). These observations led to the suggestion that β-subunits are uniquely suited to control gradients in electrical tuning (50). However, biophysical data from the chick auditory organ, or basilar papilla, argued against a dominant role for β1 in hair cells (14).
The (xeno)estrogen compound tamoxifen (Tx) activates BK channels specifically through β-subunits, including β1 (6, 10, 13). In heterologously expressed channels of known configuration, tamoxifen increased channel open probability, shifted activation curves to more negative potentials, and shortened activation time constants when the β1-subunit was present (13). These well-defined pharmacological effects provide a useful tool to test for BK channel β1-subunits in hair cells. We hypothesized that Tx would activate BK channels from the cochlear apex and that the level of activation would be tonotopically distributed. In the present study, voltage-clamp in vitro electrophysiological studies were conducted on outside-out patches excised from chick auditory hair cells. Contrary to our hypothesis, the majority of hair cell BK channels were unaffected or inhibited by Tx.
MATERIALS AND METHODS
Animal preparation.
All experimental protocols were approved by the University of Michigan Committee on Use and Care of Animals. Animal care was under the supervision of the University of Michigan's Unit for Laboratory Animal Medicine. The ages of chicks (White Leghorn) were 5 to 14 days posthatch. The chicks were anesthetized by intramuscular injection with 0.3–0.5 ml of 20 mg/ml ketamine and 5 mg/ml xylazine depending on the age of the animal. The chicks were decapitated within 10 min after the anesthetic was applied. The external auditory canal and middle ear cavity were opened, and the basilar papilla was extracted through the oval window. After a brief treatment with 0.01% protease (Type XXIV, Sigma) in artificial perilymph (154 mM NaCl, 6 mM KCl, 5 mM CaCl2, 2 mM MgCl2, and 5 mM HEPES, buffered to pH 7.4 with NaOH), the basilar papilla was pinned in a dish for further microdissection. The tegmentum vasculosum and tectorial membrane were removed, exposing the sensory epithelium. The apical half of the epithelium (∼2 mm in length) was isolated and divided into four segments, each measuring ∼0.5 mm in length. Epithelial segments were then mounted on coverslips with hair bundles facing downward exposing the basolateral surface of the hair cells. Tissue samples were observed on a fixed-stage upright microscope (BX51WI, Olympus, Japan) with a ×60, 0.9 numerical aperture water-immersion objective.
Electrophysiological recording.
Voltage-clamp recordings were made using an Axopatch 200B amplifier with a Digidata 1322A digitizer (Molecular Devices, Sunnyvale, CA). Data were acquired using pCLAMP 9 software (Molecular Devices). Glass electrodes were pulled from borosilicate capillaries (1B100F-4, World Precision Instruments, Sarasota, FL) to achieve an electrical resistance of 6–15 MΩ in the recording solutions. Electrodes were coated with R6101 (Dow Corning, Midland, MI) to reduce stray capacitance and filled with a high-potassium saline buffered to 1 μM free calcium concentration ([Ca2+]) (see Solutions and drugs). After seal formation (>1 GΩ) onto the basolateral part of the cell, brief suction was applied to break the membrane, and the outside-out patch was then excised. Patches were obtained from so-called tall hair cells along the superior edge of the epithelial segments, since these afferently innervated cells are enriched with BK channels clustered at synaptic active zones (18, 24, 53). If BK channels were present in the patch, a control saline with 1 μM free [Ca2+] was locally perfused onto the patch via a quartz micromanifold with a tip inner diameter of 200 μm (ALA Scientific, Westbury, NY). Salines on both sides of the patch contained equimolar potassium to maintain a reversal potential of 0 mV. Tamoxifen was locally perfused at a concentration of 1 μM, diluted from stocks into the 1 μM free [Ca2+] saline. To ensure that there was no obvious rundown during experiments, the excised patches were initially allowed to stabilize in control saline for 2 min before recording. If channel activity did not stabilize in this time period, the patches were discarded (<30% of total). When Tx was applied, recordings were again initiated after a 2-min time period to allow the drug effect to stabilize, since a previous study indicated that it takes 1 or 2 min for tamoxifen to modify BK channel activity (10). Data were sampled at 10 kHz and low-pass filtered with a 4-pole Bessel filter at a 5 kHz cutoff frequency. All recordings were made at room temperature (21–23°C). Low-density membrane patches, containing three or fewer BK channels, were recorded under steady-state conditions at multiple holding potentials for 1 min each. Data were further filtered by a low-pass Gaussian filter at 2 kHz, and baseline (leak) current was manually subtracted off-line. High-density patches with more than three channels were stepped through a series of activation voltages and ensemble averaged to determine the voltage dependence of activation. Patches were initially held at negative potentials to deactivate channels. A series of activation steps were then applied in 10-mV intervals, each followed by a deactivation step. The stimulus was repeated for a total of 30–50 presentations, and the resulting currents were averaged. Leak currents and capacitative transients were subtracted off-line.
Solutions and drugs.
Outside-out patches were exposed to symmetric potassium solutions. Both pipette and control saline contained 142 mM KCl, 0.5 mM MgCl2, 5 mM HEPES, 2 mM dibromoBAPTA, and 0.7 mM CaCl2 to give a free calcium concentration of 1 μM. Solutions were set to a pH of 7.2 with KOH. MaxChelator software (http://www.stanford.edu/∼cpatton/maxc.html) was used to estimate the amount of CaCl2 necessary to achieve the desired free [Ca2+] concentration. However, free [Ca2+] was more precisely determined using a calcium electrode (World Precision Instruments) calibrated with multiple standard solutions ranging from 0.1 to 100 μM free [Ca2+] (CALBUF-2, World Precision Instruments). The tamoxifen solution was created from a 10 mM Tx stock dissolved in 100% DMSO. The final DMSO concentration was 0.01%, a concentration that has been previously shown to have no effect on channel activity (10, 35, 37, 63). Paxilline was purchased from BIOMOL (Plymouth Meeting, PA). All other chemicals were obtained from Sigma Chemical (St. Louis, MO).
Data analysis.
In steady-state recordings from low-density patches, open probability (NPo) was estimated using Clampfit in the pCLAMP software suite. To calculate NPo, single-channel current (i) was determined from all-points amplitude histograms constructed from the steady-state current traces. In low-density patches with more than one channel, only the first opening level on the histogram was used to obtain i. NPo was calculated by dividing the mean value of current in the trace by i. The stability of NPo was determined for several patches maintained in control saline over the duration of a typical experiment (up to 6 min following the initial 2-min wait period). Individual NPo for consecutive 20-s periods was calculated and normalized to NPo from the first period. A comparison of mean normalized NPo across time confirmed that the recordings were stable during the experiments (Fig. 1E). The 95% confidence interval was determined for the normalized NPo periods in control saline (grayed region in Fig. 3). Thus, any Tx-induced NPo change enclosed within this confidence interval could be attributed to temporal variance in the recording rather than drug action. Even during drug applications, there was no indication of channel rundown or instability during our recordings, leading to the conclusion that our confidence interval is well suited for distinguishing between drug-dependent and drug-independent changes in NPo. For single-channel events, unitary currents were estimated from histograms held at multiple holding voltages ranging from −40 mV to 100 mV. Single-channel conductance was determined using the slope of a linear least-squares fit to current-voltage relationships.
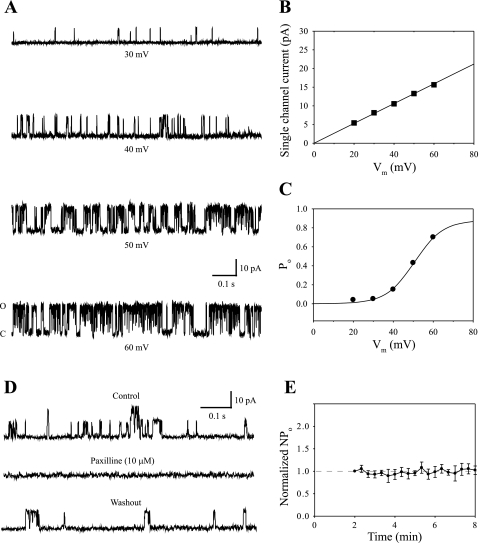
Fig. 1.
Single-channel large-conductance, Ca2+-activated potassium channel (BK) activity. A: single-channel current traces in steady-state recordings are shown at four holding voltages for an outside-out patch excised from a hair cell [1.03 mm distance from the apical end of the epithelium (DAE)] and exposed to isometric potassium. At each voltage, channel opening is upward, with C and O indicating the closed and open state of the channel, respectively. B: for the BK channel shown in A, the single-channel current level is plotted against each holding voltage. The linear regression fit indicates a single-channel conductance of 265 pS. C: for the BK channel shown in A, open probability (Po) was determined from Gaussian fits to all-points amplitude histograms and plotted against holding voltage. The resulting steady-state activation curve was fitted with a single Boltzmann function. Half-activation voltage (V1/2) was ∼50.6 mV. Vm, membrane potential. D: paxilline blocked BK currents. Single-channel current traces are shown for one outside-out patch with control saline, 10 μM paxilline, and after washing out paxilline. Membrane potential was held at 30 mV. Paxilline eliminated all voltage-dependent currents. The drug effect was partially reversible after 10-min washout. E: BK channel recordings were stable during the experiments. Channel activity was allowed to stabilize for 2 min followed by steady-state recordings for an additional 6 min. The time course of averaged open probability (NPo), normalized to the initial activity level, is shown in control saline (n = 5).
In ensemble-averaged recordings, curve fits were accomplished using Clampfit. Steady-state currents were used to construct the conductance-voltage (G-V) curves, which were fitted with a first-order Boltzmann function:
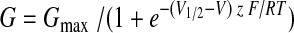 |
(1) |
where Gmax is the maximum conductance level, V1/2 is the half-activating voltage, z is the slope, F is Faraday's constant, R is the universal gas constant, and T is the absolute temperature. The effect of tamoxifen on steady-state activation was represented by the difference of V1/2 in control and drug conditions (Fig. 5):
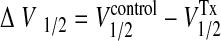 |
(2) |
In this way, positive values for ΔV1/2 reflect a Tx-dependent shift in activation toward more negative voltages, which is interpreted as an excitatory effect of the drug. Activation time constants (τ) were obtained by fitting single exponentials to the ensemble currents. The voltage dependence of activation kinetics was used to calculate the forward gating charge (Qf) using the following equation:
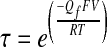 |
(3) |
which assumes a simplified two-state model for channel gating. Averaged results for various biophysical parameters are reported as means ± SE. The Pearson's product-moment correlation coefficient was used when exploring the tonotopic distribution of various channel properties. When comparing mean values, the criterion for a statistically significant difference was P < 0.05.
RESULTS
Chick hair cell BK channels in outside-out patches.
Outside-out patches were excised from the apical half of the chick sensory epithelium, specifically from tall hair cells along the superior edge of the tissue. The cell's location along the tonotopic axis of the cochlea was noted as the distance from the apical end of the epithelium (DAE). Membrane patches were excised from the basolateral surface of the hair cell, since BK channels are organized near neurotransmitter release sites at the base of the cell (18, 24, 53). Because of the tendency for BK channels to cluster into hotspots, patches often contained multiple channels. However, with high-resistance, small-diameter electrodes, it was possible to obtain low-density membrane patches with one to three channels. In this recording configuration, Tx-dependent effects on channel gating and single-channel conductance could be determined. The presence of other non-BK-like channels could be readily identified based on differences in the conductance and voltage sensitivity of single-channel events. BK channels exhibit a large single-channel conductance nearly two to ten times that of other voltage-gated cation channels (20). To be considered BK-like, channel activation had to be voltage dependent, single-channel conductance had to be above 200 pS, and transient, inactivating channels had to be absent from the recordings. These procedures allowed us to limit the possibility of contamination by other channel types. It is possible that some BK channels in long-term subconductance states were excluded from the analysis based on these procedures.
An exemplar voltage-clamp recording from a single BK channel under control conditions is shown in Fig 1, A–C. The voltage dependence of this channel was evident as more time spent in the open state when the patch was held at increasingly more positive voltages (Fig. 1A). All-points amplitude histograms were constructed from 1-min recordings at each holding voltage, facilitating calculation of both single-channel conductance and open probability. The amplitude of the single-channel current was plotted against holding voltage (Vm) and was fit by linear regression to determine single-channel conductance. For the patch shown, single-channel conductance was 265 pS (Fig. 1B). The relationship between open probability and holding voltage for this channel is also shown (Fig. 1C). The resulting activation curve was fit with a single first-order Boltzmann equation with a half-activation voltage of 50.6 mV. These results are similar to previous reports for chick hair cell BK channels (14). At all holding voltages, there were no obvious signs of ionic currents other than the BK channel current. All-points amplitude histograms at each voltage level revealed only two narrow peaks, representing the closed current level and a single open current level (data not shown). Contaminating currents from other ion channels in the patch would have resulted in additional peaks in the amplitude histograms, since all other classes of voltage-gated cation channels in chick hair cells exhibit a much lower single-channel conductance compared with BK. Thus, there was no contamination from other voltage-gated channels in this patch.
The identity of the channels was further confirmed pharmacologically. A specific BK channel antagonist paxilline, an indole alkaloid from Penicillium paxilli, blocks BK currents with extracellular concentrations of 5–10 μM (23). In the present study, 10 μM paxilline was applied extracellularly during steady-state recordings. In each case, voltage-dependent currents were eliminated after drug application (Fig. 1D, n = 5). The blockage was partially reversible after 10 to 20 min washout with control saline.
Caution was taken to ensure that the recordings of BK currents were stable during experiments. All excised patches were initially allowed to stabilize for 2 min in control saline and then again for another 2 min when Tx was introduced. Considering these wait periods and the time to make each recording, the total length of an experiment was about 6 to 8 min. Steady-state recordings were stable during this time period (Fig. 1E).
Tamoxifen modulates open probability and alters single-channel conductance.
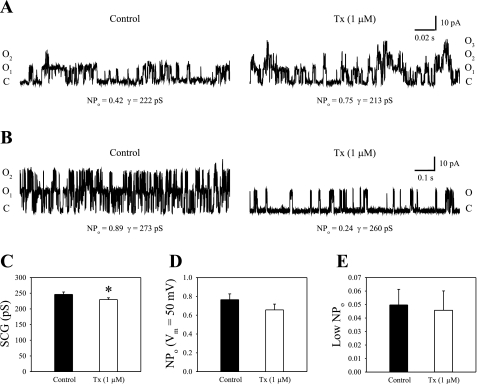
Tamoxifen was applied at a concentration of 1 μM, since this concentration can most easily distinguish between subunit composition (13). Higher concentrations of Tx can inhibit channel gating via action on the α-subunit alone, masking the activation reported to occur through the β-subunit (13). All patches were exposed to a single intracellular calcium concentration (1 μM) since Tx-dependent modulation of channel properties appears independent of calcium (10). Tamoxifen was perfused for 2 min before recording. Application of 1 μM Tx resulted in both activation (Fig. 2A) and inhibition (Fig. 2B) of the channels. In Fig. 2A, data are shown for one patch (0.10 mm, DAE) with several BK channels that were activated by Tx. In control saline, two open levels are seen in the current trace. When 1 μM Tx was perfused onto the patch, a third open level could be observed, indicating at least three channels present in this patch and an increase in channel open probability in the presence of Tx. NPo increased nearly two-fold from 0.42 in control saline to 0.75 in Tx. At the same time, single-channel conductance slightly decreased from 222 pS in control saline to 213 pS in Tx. Data from a second patch (1.40 mm, DAE) are shown in Fig. 2B. In this case, the channel was inhibited by Tx, with an overall decrease in NPo from 0.89 in control saline to 0.24 in the drug. Although the effect of Tx on NPo was opposite that in Fig. 2A, the effect on conductance was similar, decreasing from 273 pS in control saline to 260 pS in Tx.
Fig. 2.
Overall, tamoxifen (Tx) had an insignificant effect on BK channel open probability while reducing single-channel conductance. A and B: single-channel current traces are shown for two outside-out patches excised from two hair cells positioned at different locations in the epithelium [0.10 mm DAE (A) and 1.40 mm DAE (B)]. The holding voltage was 50 mV in each case. The patches were locally perfused with control saline (left) and 1 μM Tx (right). C, closed state; O1…On, open levels 1 to n, respectively. In A, tamoxifen increased BK channel open probability in the patch. In B, tamoxifen decreased BK channel gating. C: the averaged single-channel conductances (SCG) are shown in control condition and 1 μM Tx. Tamoxifen reduced single-channel conductance from 246 ± 8 pS in control to 232 ± 6 pS in 1 μM Tx (n = 19). *P < 0.05. D: NPo at 50 mV is shown in control saline and 1 μM Tx. Open probability was 0.77 ± 0.06 in the control condition and 0.66 ± 0.06 in the presence of Tx (n = 19). The difference was statistically insignificant (P > 0.05, paired Student's t-test). E: the effect of Tx was also examined while BK channels were held at low NPo. Open probability was 0.049 ± 0.012 in the control condition and 0.046 ± 0.014 in the presence of tamoxifen (P > 0.05, paired Student's t-test, n = 6). Thus, tamoxifen had no effect on BK channel gating when the activity level was initially high or low.
A total of 19 patches with three or fewer BK channels were obtained from hair cells throughout the apical half of the basilar papilla. The mean single-channel conductance was 246 ± 8 pS in control saline and 232 ± 6 pS in 1 μM Tx (∼6% reduction, P < 0.05) (Fig. 2C). This result is consistent with other studies, reporting a Tx-dependent decrease in BK single-channel conductance regardless of subunit composition (10, 11, 13) (summarized in Table 1). The heterogeneity in our data, illustrated by the differences in Fig. 2, A and B, may reflect variations in channel configuration in native cochlear tissue. On the basis of the hypothesis that BK channel β1-subunits are predominantly expressed in the apical half of chick cochlea, we expected to see Tx-mediated activation in a large proportion of channels in our data set. However, differences in average NPo in control (0.77 ± 0.06) and 1 μM Tx (0.66 ± 0.06) were insignificant (Fig. 2D, P > 0.05). A recent study has argued that the dual-regulation by Tx is dependent on the initial activity level of the channel rather than subunit composition (48). In that study, Tx inhibited BK channels when the control condition produced a high open probability. In contrast, under low open probability conditions, Tx facilitated gating. The results were the same in BK channels from normal mice and β-subunit deletion mutants. These results stand in stark contrast to numerous studies reporting a subunit-dependent action of Tx (6, 9–11, 13, 42). Nevertheless, to see whether Tx modulation was dependent on basal activation level in our study, several low-density BK patches excised from tall hair cells throughout the apical basilar papilla were also held at low voltages to examine the drug effect under low open probability conditions (NPo < 0.20). The averaged values were 0.049 ± 0.012 in the control condition and 0.046 ± 0.014 in 1 μM Tx (Fig. 2E, n = 6). Statistical tests showed that the difference was insignificant (P > 0.05). Therefore, BK channels from the apical half of the cochlea were on average unaffected by Tx, regardless of the initial channel activity, suggesting that the majority of the BK channels in these data were not regulated by auxiliary β1-subunits. However, it remained possible that, even though poorly expressed, β1-subunits may still be expressed in a gradient within the apical basilar papilla.
Table 1.
Comparison of tamoxifen-induced effects on BK channels in various cell systems
| Chick HCs | cslo α Only (13) | Myocytes of β1-Null Mice (11) | Native Smooth Muscle Cells (β Present) (10) | cslo-α + cβ1 (13) | |
|---|---|---|---|---|---|
| NPo | Insignificant at 50 mV | Insignificant at 60 mV | Insignificant at 80 mV | 3- to 4-Fold increase at 80 mV | 3- to 4-Fold increase at 60 mV |
| SCG | Reduced | Reduced | Reduced | Reduced | Reduced |
| V1/2 shift | 5.2 mV Rightward* | 3.6 mV Rightward | Insignificant | 10-15 mV Leftward | 13.8 mV Leftward |
| Gmax | Reduced | Reduced | Reduced | Reduced | Reduced |
| Activation τ (Tx/Ctrl) | 1.28* | 1.28* | NA | NA | 0.71 |
| Qf (Ctrl to Tx) | 0.44 to 0.40* | 0.49 to 0.41* | NA | NA | 0.61 to 0.46 |
BK, large-conductance, Ca2+-activated potassium channel; HC, hair cell; NPo, averaged open probability; SCG, single-channel conductance; V1/2, half-activating voltage; Gmax, maximum conductance level; τ, activation time constant; Tx, tamoxifen; Ctrl, control; Qf, forward gating charge; NA, not available. Reference numbers are shown in parentheses.
P > 0.05.
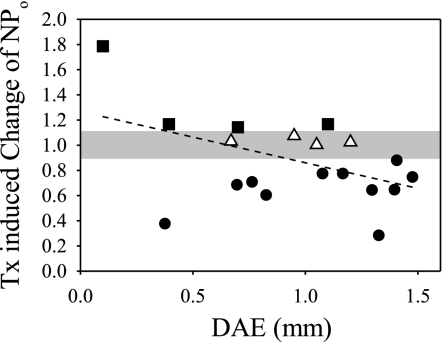
To examine this possibility, tamoxifen's effect on open probability was calculated as the ratio of NPo in Tx to NPo in control saline (NPoTx/NPocontrol) and was plotted for each patch against the distance from the apical end of the papilla (Fig. 3). Four recordings obtained from the apical basilar papilla (squares) showed increases in NPo in 1 μM Tx. In another four patches, open probability ratios were enclosed in the confidence interval associated with channel stability (grayed region, 1.00 ± 0.11, see materials and methods), suggesting that these channels were unaffected by the drug. The majority of the patches (11 of 19) were inhibited by Tx. Overall, the Tx effect on apical BK channels seemed to be inhibitory and independent of tonotopic location along the cochlea (ρ = −0.41, P = 0.68, n = 19). Even for those patches that appeared to be activated by Tx, the level of activation fell below what was expected on the basis of prior studies on BK β1 (6, 10, 11).
Fig. 3.
The effect of tamoxifen on NPo does not depend on tonotopic location. Tamoxifen-induced change of NPo (NPoTx/NPocontrol) for each patch is plotted according to the location from which that patch was taken along the basilar papilla. All patches were held at 50 mV. The confidence interval representing NPo stability in control saline is marked in gray. Data within this region were unaffected by drug exposure (▵). Data above (▪) or below (•) this interval were activated or inhibited by Tx, respectively. Overall, the effect of tamoxifen on BK channel open probability is independent of tonotopic location. Black dashed line represents a linear regression to the data.
Tamoxifen modulates steady-state activation in ensemble-averaged recordings.
Since BK channels are present in clusters at synaptic active zones, it is possible that low-density patches, like those reported above, were obtained from membrane regions outside clustered hotspots, potentially reflecting a subset of the whole cell channel population. Therefore, we conducted a separate study on Tx effects using high-density patches more generally reflecting the average properties of clustered BK channels expressed by the cell (13). Previous studies have shown that Tx facilitates BK channel activity by shifting steady-state activation to more negative potentials in channels coassembled with β1 (11, 13). In contrast, Tx inhibits channels consisting of α alone by reducing, to a moderate degree, the voltage sensitivity of channel activation (i.e., a more shallow conductance-voltage curve) (13) (Table 1). To explore the effect of Tx on a more representative channel population from the chick cochlea, ensemble-averaged recordings were obtained on outside-out patches containing multiple BK channels excised from hair cells throughout the apical half of the basilar papilla. Although patches were pulled from membrane regions enriched with BK channels, it was possible that excised patches also included other voltage-gated K+ channels (16, 47). Patches were initially held at low voltages (i.e., low BK open probability) to examine whether ionic conductances were present other than those from large-conductance BK channels. In addition, steady-state activation curves were examined for multiple voltage-sensitive components in Boltzmann equation fits, which would suggest distinct current components and potential contamination by delayed rectifier potassium channels. If non-BK conductances were present in the patch, the data were excluded from the study. Furthermore, 10 mM tetraethylammonium (TEA) was also used on several high-density patches to determine the proportion of current that could be attributed to BK. Previous studies showed that low concentrations of TEA effectively and specifically block chick hair cell BK channels when applied extracellularly (16, 47). In the present study, 10 mM TEA blocked 91 ± 5% (n = 4) of the outward potassium current. Additionally, 10 μM paxilline also effectively blocked outward potassium currents in several ensemble-averaged recordings (data not shown), further confirming that the majority of voltage-sensitive current in ensemble-averaged patches was carried by BK channels.
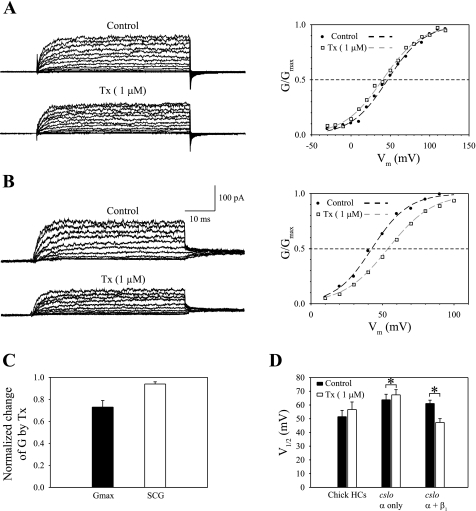
Single-channel data showed that the majority of channels were either inhibited or unaffected by Tx (Figs. 2 and 3). In Fig. 4A, ensemble-averaged currents are shown from one patch (1.14 mm, DAE) in which Tx had an excitatory effect on the voltage dependence of channel activation even while inhibiting channel conductance. Maximum conductance was reduced from 1.11 nS in control to 0.96 nS in 1 μM Tx. The half-activation potential was shifted to a more negative value (ΔV1/2 = 6.5 mV). A second example is shown in Fig. 4B (0.85 mm, DAE), where the effect of Tx was inhibition. Tamoxifen reduced Gmax from 1.44 nS in control to 0.93 nS, and activation was shifted to a more positive potential (ΔV1/2 = −11.6 mV).
Fig. 4.
Overall, tamoxifen had an insignificant effect on BK channel activation in ensemble-averaged recordings. A, left: activation currents are shown for one outside-out patch excised from a hair cell 1.14 mm DAE. The patch was perfused with control saline (top) and 1 μM Tx (bottom). The holding voltage was −20 mV. Activation steps ranged from −40 mV to 120 mV, followed by a deactivation step to −20 mV. Current traces were averaged from 30 stimulus presentations. A, right: conductance-voltage (G-V) curves were constructed from steady-state activation currents for control and drug conditions and normalized to maximum conductance level (Gmax). Normalized G-V curves were fit to a first-order Boltzmann function (dashed lines). The fit parameters are as follows: for control saline, Gmax = 1.11 nS, z = 1.08, and V1/2 = 46.7 mV; for Tx, Gmax = 0.96 nS, z = 1.03, and V1/2 = 40.2 mV. B: a second example is shown, illustrating Tx-dependent inhibition of BK channel activity. Left: activation currents are shown for an outside-out patch excised from a hair cell 0.85 mm away from the apical end. Activation steps ranged from 10 to 100 mV, followed by a deactivation step to 40 mV. Right: normalized G-V curves were fit to a first-order Boltzmann function. The fit parameters are as follows: for control saline, Gmax = 1.44 nS, z = 1.78, and V1/2 = 42.3 mV; for Tx, Gmax = 0.93 nS, z = 1.6, and V1/2 = 53.9 mV. C: the Tx-induced change of averaged maximum steady-state conductance, GmaxTx/Gmaxcontrol = 0.73 ± 0.06 (n = 19), is compared with the change of averaged single-channel conductance in the presence of Tx, SCGTx/SCGControl = 0.94 ± 0.02 (deduced from Fig. 2C). Tx-dependent reduction of Gmax cannot be fully explained by Tx reduction of SCG, as described previously. D: the averaged V1/2 was 51.5 ± 4.6 mV in control saline and 56.7 ± 5.5 mV in Tx (n = 19). The difference was statistically insignificant (P > 0.05, paired Student's t-test). Also shown are measurements of V1/2 in human embryonic kidney (HEK)-293 cells transfected with cDNA encoding chick BK channel: cslo α-subunit alone and cslo α with chick β1-subunits. In recombinant channels, the averaged V1/2 increased from 63.8 ± 4 mV to 67.4 ± 3.8 mV by 1 μM Tx if cells were transfected with α-subunits alone, but it decreased from 61.1 ± 2.4 mV to 47.3 ± 2.8 mV in cells cotransfected with α and β1 (data are based on Ref. 13). *P < 0.05. HC, hair cell.
Tamoxifen-induced conductance change is summarized in Fig. 4C for 19 high-density patches. In 1 μM Tx, the averaged reduction in Gmax was greater than the averaged reduction in single-channel conductance (Fig. 2C). This is comparable to the effect of 1 μM Tx on Gmax and single-channel conductance in human embryonic kidney-293 cells heterologously expressing chick BK subunits (13). The results suggest that in native tissue, as in the expression system, tamoxifen further decreased the maximum open probability of the channel in addition to a reduction in single-channel conductance.
The overall effect of 1 μM Tx on BK channel activity was estimated by comparing the mean V1/2 in Tx to that in control saline. If β1-subunits are predominantly expressed in the apical half of the cochlea, tamoxifen should significantly shift V1/2 to more negative voltages, similar to the effect on recombinant channels coexpressed with α- and β1-subunits (13). However, in chick hair cells, the averaged V1/2 was 51.5 ± 4.6 mV in control and 56.7 ± 5.5 mV in 1 μM Tx. A two-tailed paired Student's t-test revealed that the difference in these values was statistically insignificant (P > 0.05) (Fig. 4D). The behavior of hair cell BK channels was compared with results from recombinant channels (Fig. 4D). The averaged values from chick hair cells were slightly lower than recombinant channels consisting of α-subunits alone. This increased sensitivity in the native channels has been detailed previously (14). Nevertheless, the difference in the mean response of hair cell BK between control and drug conditions is more similar to that of recombinant channels with α-subunits alone rather than those incorporating chick β1.
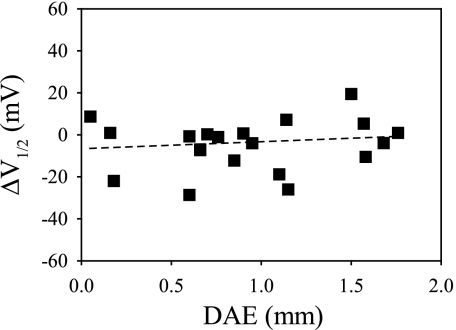
The tamoxifen-induced shift of V1/2 (ΔV1/2) was also plotted according to tonotopic location along the basilar papilla (Fig. 5). In a total of 19 ensemble-averaged recordings, only one patch, excised 1.50 mm away from the apical end, exhibited a large leftward shift in voltage activation (ΔV1/2 = 19.4 mV), indicating Tx-induced activation of channel activity and suggesting the presence of BK channel β1-subunits in this patch. However, similar to results for low-density BK patches, tamoxifen's effect on half-activation was independent of target cell location (ρ = 4.17, P = 0.09).
Fig. 5.
The effect of tamoxifen on V1/2 does not depend on tonotopic location. The tamoxifen-induced shift of half-activation potential for each patch is plotted according to its tonotopic location. Positive values indicate activation by Tx. Similar to the effect of tamoxifen on NPo in steady-state recordings (Fig. 3), the distribution of ΔV1/2 did not show an apical-to-basal pattern, arguing against the notion of a tonotopic gradient of β-subunits in hair cell BK channels. Black dashed line represents a linear regression to the data.
Tamoxifen had little effect on channel kinetics in ensemble-averaged recordings.
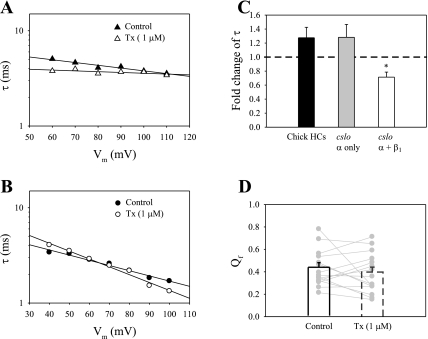
In recombinant channels, tamoxifen's action on open probability is reflected in changes to channel rate constants and macroscopic kinetics depending on the presence or absence of β1-subunits in the channel complex (10, 13). For example, in one study, 1 μM Tx decreased activation time constants in channels with β1 but unaffected those consisting of α-subunits alone (13). In the present study, activation rate (τ) was determined by fitting single exponentials to activating currents from ensemble-averaged recordings. Examples are shown in Fig. 6, A and B, for the current traces shown in Fig. 4, A and B, respectively. In one patch (Fig. 6A), activation time constants decreased at most voltages in Tx compared with control saline, suggesting a decrease in the closed-state dwell time when Tx was present. On the basis of previous results with recombinant channels, the drug-induced effects on kinetics, as well as on steady-state G-V curves (Fig. 4A), are consistent with the suggestion that channels in this patch included auxiliary β1-subunits. Notably, this patch was excised from the most apically positioned hair cell in our data set. In contrast, activation time constants for another patch (Fig. 6B, from current traces in Fig. 4B) were largely unaffected by Tx, although there was a small increase in the estimated forward gating charge, Qf (i.e., from 0.54 in control condition to 0.59 in 1 μM Tx). This result, as well as the steady-state inhibition in Fig. 4B, suggests that auxiliary β1-subunits were absent from this patch. Similar data were obtained for 15 high-density patches distributed throughout the apical half of the chick sensory epithelium. On average, the activation time constant at 70 mV changed 1.28 ± 0.15-fold in 1 μM Tx compared with control saline (Fig. 6C). However, a Student's t-test on paired samples indicated that any difference in mean activation rates between control and Tx treatments was statistically insignificant (P > 0.05). Data from cloned BK channels are plotted alongside the hair cell data (Fig. 6C; data based on Ref. 13). The native hair cell channels responded to Tx-like recombinant channels consisting of α-subunits alone, but unlike the channels incorporating β1-subunits.
Fig. 6.
The effects of tamoxifen on activation rates appeared similar to those in HEK-293 cells transfected with α-subunit alone. A and B: for the ensemble-averaged recordings shown in Fig. 4, A and B, activation time-constants (τ) were estimated from single-exponential fits to activation currents in control saline (▴ and •) and 1 μM Tx (▵ and ○). Straight lines represent curve fits to Eq. 3, where Qf is forward gating charge, F is Faraday's constant, R is the universal gas constant, and T is absolute temperature. C: activation time constants in 1 μM Tx were calculated as fold changes compared with those in control saline (n = 15). Data were analyzed for a constant holding potential of 70 mV. In addition, similar measurements are shown for patches from HEK-293 cells transfected with cslo α-subunit only or cotransfected with cslo α- and β-subunits (data are based on Ref. 13). The fold changes of τ were 1.28 ± 0.15 in hair cell BK channels, 1.28 ± 0.18 in transfected BK channels with α-subunit alone, and 0.71 ± 0.07 in BK channels cotransfected with α + β1-subunit. Tamoxifen decreased τ only in recombinant BK channels incorporating β1 (*P < 0.05). D: Qf in control saline and Tx is shown in pairs for each patch (gray dots connected by lines, n = 15). The averaged value in control saline was 0.44 ± 0.04 and 0.40 ± 0.04 in 1 μM Tx. The differences in the means between these conditions was statistically insignificant (P > 0.05).
Differences in activation rates at a single voltage level, as described above, could be obscured by changes in the voltage dependence of channel kinetics. To investigate this possibility, the forward gating charge, Qf, was estimated from the voltage dependence of activation kinetics using Eq. 3. The previous results on recombinant channels showed that Qf was reduced in the presence of Tx regardless of subunit composition, although differences in the means were only significant for channels incorporating β1 (13). The average Qf for chick hair cell BK channels was more similar to recombinant α-only channels in two ways (Table 1). First, the mean value in control saline was more similar to α-only channels (Fig. 6D). Second, the reduction due to Tx mirrored the effect on α-only channels, which was relatively small and statistically insignificant (P > 0.05).
DISCUSSION
Hair cell excitability is governed by a number of distinct ion channel types, several of which localize to specific domains within the cell membrane (18, 19, 27, 30, 34, 43, 61, 62). The dominant conductance in afferently innervated cochlear hair cells arises from BK channels, but there appear to be several distinct differences between channel function and localization in mammals vs. nonmammals. In mammalian cochlear hair cells, these channels are clustered near the apical pole of the cell (39, 57) and are relatively independent of calcium influx through voltage-gated calcium channels (2, 39). In contrast to other vertebrates, mammalian hair cells do not exhibit a resonant membrane potential, but the large-conductance and fast kinetics of BK channels in these cells still contribute to the temporal processing of sound by altering the hair cell membrane time constant (45). In nonmammalian vertebrates, BK channels are colocalized with voltage-gated calcium channels (18, 24, 53) and activated by depolarization-induced calcium influx (16). Functionally, these channels are key regulators in an electrical tuning mechanism, which presumably plays a critical role in frequency discrimination in these animals (15).
The mechanisms governing a systematic change in tuning and BK channel behavior along the chick basilar papilla remain unclear. Several primary articles and reviews have offered one possibility, namely, that β-subunits regulate low-frequency tuning in avians and turtles (15, 21, 25, 26, 46, 50, 51). The prevailing model concerning regulation by β1 relies on an apical-to-basal gradient of expression with high expression at the apex descending to little detectable expression by the midpoint of the basilar papilla (50). Direct support for this hypothesis rests with a single in situ hybridization study showing that β1 mRNA were more highly expressed in apical hair cells of the quail basilar papilla (52).
Functional studies, on the other hand, have often downplayed the potential influence of BK β1 on hair cell excitability. Mouse knockout models deficient in BK channel β-subunits exhibit normal auditory function (49, 54) and normal BK channel biophysics (58), challenging the notion that β-subunits are functionally involved in hearing in mammals even though gene transcripts have been found in the mammalian cochlea (32). Genetic knockouts are unavailable for nonmammalian vertebrates, making it necessary to use other approaches to test for a role for β1 in electrical tuning in nonmammals. In general, the kinetics of BK channels from chick hair cells are significantly faster than those of recombinant channels that include β1 (13, 14). Moreover, while deactivation kinetics were distributed in a tonotopic gradient along the avian basilar papilla, activation kinetics were more uniform (14), which argues against β1 as the controlling mechanism since these subunits should result in slower activation as well as deactivation. In this study, we sought to augment these results using a pharmacological tool that modulates BK channels in a subunit-dependent manner.
Pharmacological compounds have been used to modulate BK channels by interacting through auxiliary β-subunits (3–5, 8, 11, 29, 31, 38, 60). Several studies have reported β-dependent activation by estrogen-like compounds [i.e., 17-β-estradiol and the (xeno)estrogen tamoxifen], lithocholate (3–5), and dehydrosoyasaponin-I (17, 40, 44). Our study focused on tamoxifen, since it is the most widely characterized compound in this class of BK activators and it is the only one of this kind that has been validated using chick BK homologues (13). Modulation by Tx extends to channels with β1-, β2-, or β4-subunits and includes recombinant channels from chick and mouse sequences as well as a variety of native tissues (1, 6, 9, 10, 13, 29). One recent study has questioned the ability of Tx to distinguish between channel configurations, suggesting instead that Tx exerts a dual effect depending on basal channel activity independent of accessory subunits (48). In that paper, Tx was applied to smooth muscle BK channels from wild-type and β1 knockout mice. At low open probability, Tx increased activity regardless of genotype, leading to the conclusion that Tx could not be used to test for incorporation of β1. In contrast, the ability for Tx to distinguish between channel configuration has been repeatedly shown in native tissue (6, 10, 11, 42), as well as heterologous expression systems (13). Normalized conductance-voltage curves shift to the left when Tx is applied to channels incorporating β1. In other words, Tx-dependent activation could be observed throughout the voltage activation range of the channel. In the present study, Tx exhibited similar effects on channel activity regardless of basal activity level (Fig. 2, D and E). The discrepancy between Perez's (48) and other reports, including the present study, is unclear but may simply reflect additional changes in the β1-knockout mouse.
In the present study, tamoxifen was applied to BK channels from the apical half of the chick basilar papilla. Analyses were conducted essentially as described previously for heterologously expressed chick BK isoforms (13), providing us with a means to directly compare results from native tissue with those from known channel configurations. On the basis of the hypothesis that β1-subunits are more highly expressed in apical hair cells, we expected the majority of patches pulled from apical tall hair cells to show increased BK channel activity in the presence of Tx. However, in steady-state recordings from low-density patches, Tx had little effect on mean open probability (NPo, Fig. 2D). In ensemble-averaged recordings from high-density patches, Tx had an insignificant effect on the average voltage-dependence of channel activation (Fig. 4D) or kinetics (Fig. 6, C and D). As summarized in Table 1, compared with other native or recombinant BK channels, the results may indicate that BK channels in chick hair cells generally consist of α-subunits alone, not auxiliary β1-subunits.
When activation data were plotted against tonotopic location of the patch, the data appeared to exhibit a high degree of variance (Figs. 3 and 5), even though the standard error of the individual means was relatively small (i.e., ratio of NPo in Tx vs. control was 0.85 ± 0.08, and the shift in V1/2 due to Tx was −4.94 ± 2.70 mV). For example, data in Fig. 3 could be divided into three groups, channels that were activated, inhibited, or unaffected by Tx, by defining an exclusion boundary based on stability recordings. Data falling within this region (i.e., gray bar in Fig. 3) were deemed unaffected by Tx (4 of 19 patches). By this criterion, 4 patches from the apical region of the papilla were activated by Tx whereas 11 of 19 patches were inhibited by Tx. Similarly, ensemble-averaged recordings showed few instances of Tx-related activation, with the majority of patches being inhibited or unaffected by Tx. Overall, our results suggest that molecular mechanisms other than auxiliary β-subunits are the primary determinants of electric tuning in the chick basilar papilla.
How, then, do we reconcile observations of BK β1 mRNA with the lack of a clear biophysical role for these subunits in hair cell electrophysiology? One possibility is that we systematically excluded channels with β1 by focusing attention on basal membrane regions (i.e., the synaptic pole of the hair cell). However, extrasynaptic channels are less likely to be colocalized with voltage-gated calcium channels (55) and, thus, less likely to contribute to electrical tuning. Alternatively, β1-subunits could play a role in mitochondrial BK channels (12), providing a rationale for the detection of mRNA transcripts without robust plasma membrane expression. Finally, β1-subunits may be involved in other functional roles, such as facilitating α-subunit trafficking to the plasma membrane (28). In this latter case, additional mechanisms would need to be invoked to allow the physical assembly of α and β1 without enabling the normal biophysical modulation of gating (i.e., slower kinetics and activation by Tx).
The potential molecular variations within the population of chick hair cell BK channels are enormous (see Ref. 15 for a review). These variations likely contribute to the heterogeneity in the data reported here, but splice variation, accessory proteins, and posttranslational modifications may also regulate or interfere with the interaction between Tx and BK. Relatively few of the possible channel configurations have been tested biophysically and even less have been characterized pharmacologically. In addition, one need not focus entirely on the subunits themselves, since estrogen can modify membrane composition (59) and BK properties can be modulated by local membrane content (56). Accordingly, variations in membrane sterols and phospholipids may regulate the effect of Tx. Therefore, a greater understanding of the in situ composition of chick cochlear BK channels, and their local lipid environment, is required to unravel the molecular diversity that underlies electrical tuning. Direct molecular evidence, whether from immunocytochemical studies or genetic manipulations, will be necessary before ruling out a functional role for β1.
The inhibitory effects of Tx on hair cell BK channels could have a large impact on hair cell excitability if this compound, and presumably other estrogen-like compounds, were present in the cochlear duct. An interaction between gender, estrogen deficiency, and hearing acuity is frequently acknowledged, even if still unresolved (22). In addition to the effects on hair cell BK channels described here for the first time, estrogen and anti-estrogens modulate cochlear blood flow (33), potassium secretion by stria vascularis (36), and cochlear response to acoustic trauma (41). Acute effects of estradiol and tamoxifen on various ion channel types suggests that regulation by these compounds is complex and in some cases estrogen-receptor independent. Further work is required to fully explore the role that sex steroids play in the function and dysfunction of the normal and pathological ear.
GRANTS
This work was supported by grants from the National Institute of Deafness and Other Communication Disorders (DC-007432 to R. K. Duncan and core grant P30-DC-005188).
Acknowledgments
The authors thank Dr. Mark Crumling for helpful comments on earlier drafts of this manuscript.
REFERENCES
- 1.Behrens R, Nolting A, Reimann F, Schwarz M, Waldschutz R, Pongs O. hKCNMB3 and hKCNMB4, cloning and characterization of two members of the large-conductance calcium-activated potassium channel beta subunit family. FEBS Lett 474: 99–106, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Beurg M, Hafidi A, Skinner LJ, Ruel J, Nouvian R, Henaff M, Puel JL, Aran JM, Dulon D. Ryanodine receptors and BK channels act as a presynaptic depressor of neurotransmission in cochlear inner hair cells. Eur J Neurosci 22: 1109–1119, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Bukiya AN, Liu J, Toro L, Dopico AM. Beta1 (KCNMB1) subunits mediate lithocholate activation of large-conductance Ca2+-activated K+ channels and dilation in small, resistance-size arteries. Mol Pharmacol 72: 359–369, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Bukiya AN, McMillan J, Parrill AL, Dopico AM. Structural determinants of monohydroxylated bile acids to activate beta1 subunit-containing BK channels. J Lipid Res 49: 2441–2451, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bukiya AN, Vaithianathan T, Toro L, Dopico AM. The second transmembrane domain of the large conductance, voltage- and calcium-gated potassium channel beta(1) subunit is a lithocholate sensor. FEBS Lett 582: 673–678, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coiret G, Borowiec AS, Mariot P, Ouadid-Ahidouch H, Matifat F. The antiestrogen tamoxifen activates BK channels and stimulates proliferation of MCF-7 breast cancer cells. Mol Pharmacol 71: 843–851, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Cox DH, Aldrich RW. Role of the beta1 subunit in large-conductance Ca(2+)-activated K(+) channel gating energetics. Mechanisms of enhanced Ca(2+) sensitivity. J Gen Physiol 116: 411–432, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dick GM The pure anti-oestrogen ICI 182,780 (Faslodex) activates large conductance Ca(2+)-activated K(+) channels in smooth muscle. Br J Pharmacol 136: 961–964, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dick GM, Hunter AC, Sanders KM. Ethylbromide tamoxifen, a membrane-impermeant antiestrogen, activates smooth muscle calcium-activated large-conductance potassium channels from the extracellular side. Mol Pharmacol 61: 1105–1113, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Dick GM, Rossow CF, Smirnov S, Horowitz B, Sanders KM. Tamoxifen activates smooth muscle BK channels through the regulatory beta 1 subunit. J Biol Chem 276: 34594–34599, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Dick GM, Sanders KM. (Xeno)estrogen sensitivity of smooth muscle BK channels conferred by the regulatory beta1 subunit: a study of beta1 knockout mice. J Biol Chem 276: 44835–44840, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Douglas RM, Lai JC, Bian S, Cummins L, Moczydlowski E, Haddad GG. The calcium-sensitive large-conductance potassium channel (BK/MAXI K) is present in the inner mitochondrial membrane of rat brain. Neuroscience 139: 1249–1261, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Duncan RK Tamoxifen alters gating of the BK alpha subunit and mediates enhanced interactions with the avian beta subunit. Biochem Pharmacol 70: 47–58, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Duncan RK, Fuchs PA. Variation in large-conductance, calcium-activated potassium channels from hair cells along the chicken basilar papilla. J Physiol 547: 357–371, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fettiplace R, Fuchs PA. Mechanisms of hair cell tuning. Annu Rev Physiol 61: 809–834, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Fuchs PA, Evans MG. Potassium currents in hair cells isolated from the cochlea of the chick. J Physiol 429: 529–551, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giangiacomo KM, Kamassah A, Harris G, McManus OB. Mechanism of maxi-K channel activation by dehydrosoyasaponin-I. J Gen Physiol 112: 485–501, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hafidi A, Beurg M, Dulon D. Localization and developmental expression of BK channels in mammalian cochlear hair cells. Neuroscience 130: 475–484, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Hafidi A, Dulon D. Developmental expression of Ca(v)1.3 (alpha1d) calcium channels in the mouse inner ear. Brain Res Dev Brain Res 150: 167–175, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Hille B Ion Channels of Excitable Membranes. Sunderland, MA: Sinauer Associates, 1992.
- 21.Housley GD, Marcotti W, Navaratnam D, Yamoah EN. Hair cells–beyond the transducer. J Membr Biol 209: 89–118, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Hultcrantz M, Simonoska R, Stenberg AE. Estrogen and hearing: a summary of recent investigations. Acta Otolaryngol 126: 10–14, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Isaacson JS, Murphy GJ. Glutamate-mediated extrasynaptic inhibition: direct coupling of NMDA receptors to Ca(2+)-activated K+ channels. Neuron 31: 1027–1034, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Issa NP, Hudspeth AJ. Clustering of Ca2+ channels and Ca(2+)-activated K+ channels at fluorescently labeled presynaptic active zones of hair cells. Proc Natl Acad Sci USA 91: 7578–7582, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones EM, Gray-Keller M, Art JJ, Fettiplace R. The functional role of alternative splicing of Ca(2+)-activated K+ channels in auditory hair cells. Ann NY Acad Sci 868: 379–385, 1999. [DOI] [PubMed] [Google Scholar]
- 26.Jones EM, Gray-Keller M, Fettiplace R. The role of Ca2+-activated K+ channel spliced variants in the tonotopic organization of the turtle cochlea. J Physiol 518: 653–665, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kharkovets T, Hardelin JP, Safieddine S, Schweizer M, El-Amraoui A, Petit C, Jentsch TJ. KCNQ4, a K+ channel mutated in a form of dominant deafness, is expressed in the inner ear and the central auditory pathway. Proc Natl Acad Sci USA 97: 4333–4338, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim EY, Zou S, Ridgway LD, Dryer SE. Beta1-subunits increase surface expression of a large-conductance Ca2+-activated K+ channel isoform. J Neurophysiol 97: 3508–3516, 2007. [DOI] [PubMed] [Google Scholar]
- 29.King JT, Lovell PV, Rishniw M, Kotlikoff MI, Zeeman ML, McCobb DP. Beta2 and beta4 subunits of BK channels confer differential sensitivity to acute modulation by steroid hormones. J Neurophysiol 95: 2878–2888, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Knirsch M, Brandt N, Braig C, Kuhn S, Hirt B, Munkner S, Knipper M, Engel J. Persistence of Ca(v)1.3 Ca2+ channels in mature outer hair cells supports outer hair cell afferent signaling. J Neurosci 27: 6442–6451, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korovkina VP, Brainard AM, Ismail P, Schmidt TJ, England SK. Estradiol binding to maxi-K channels induces their down-regulation via proteasomal degradation. J Biol Chem 279: 1217–1223, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Langer P, Grunder S, Rusch A. Expression of Ca2+-activated BK channel mRNA and its splice variants in the rat cochlea. J Comp Neurol 455: 198–209, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Laugel GR, Dengerink HA, Wright JW. Ovarian steroid and vasoconstrictor effects on cochlear blood flow. Hear Res 31: 245–251, 1987. [DOI] [PubMed] [Google Scholar]
- 34.Layton MG, Robertson D, Everett AW, Mulders WH, Yates GK. Cellular localization of voltage-gated calcium channels and synaptic vesicle-associated proteins in the guinea pig cochlea. J Mol Neurosci 27: 225–244, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Lee JE, Kwak J, Suh CK, Shin JH. Dual effects of nitric oxide on the large conductance calcium-activated potassium channels of rat brain. J Biochem Mol Biol 39: 91–96, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Lee JH, Marcus DC. Estrogen acutely inhibits ion transport by isolated stria vascularis. Hear Res 158: 123–130, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Lee K, Rowe IC, Ashford ML. NS 1619 activates BKCa channel activity in rat cortical neurones. Eur J Pharmacol 280: 215–219, 1995. [DOI] [PubMed] [Google Scholar]
- 38.Liu YC, Lo YC, Huang CW, Wu SN. Inhibitory action of ICI-182,780, an estrogen receptor antagonist, on BK(Ca) channel activity in cultured endothelial cells of human coronary artery. Biochem Pharmacol 66: 2053–2063, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Marcotti W, Johnson SL, Kros CJ. Effects of intracellular stores and extracellular Ca(2+) on Ca(2+)-activated K(+) currents in mature mouse inner hair cells. J Physiol 557: 613–633, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McManus OB, Helms LM, Pallanck L, Ganetzky B, Swanson R, Leonard RJ. Functional role of the beta subunit of high conductance calcium-activated potassium channels. Neuron 14: 645–650, 1995. [DOI] [PubMed] [Google Scholar]
- 41.Meltser I, Tahera Y, Simpson E, Hultcrantz M, Charitidi K, Gustafsson JA, Canlon B. Estrogen receptor beta protects against acoustic trauma in mice. J Clin Invest 118: 1563–1570, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Navarro-Antolin J, Levitsky KL, Calderon E, Ordonez A, Lopez-Barneo J. Decreased expression of maxi-K+ channel beta1-subunit and altered vasoregulation in hypoxia. Circulation 112: 1309–1315, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Nicolas MT, Barhanin J, Reyes R, Dememes D. Cellular localization of TWIK-1, a two-pore-domain potassium channel in the rodent inner ear. Hear Res 181: 20–26, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Nishimaru K, Eghbali M, Lu R, Marijic J, Stefani E, Toro L. Functional and molecular evidence of MaxiK channel beta1 subunit decrease with coronary artery ageing in the rat. J Physiol 559: 849–862, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oliver D, Taberner AM, Thurm H, Sausbier M, Arntz C, Ruth P, Fakler B, Liberman MC. The role of BKCa channels in electrical signal encoding in the mammalian auditory periphery. J Neurosci 26: 6181–6189, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Orio P, Rojas P, Ferreira G, Latorre R. New disguises for an old channel: MaxiK channel beta-subunits. News Physiol Sci 17: 156–161, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Pantelias AA, Monsivais P, Rubel EW. Tonotopic map of potassium currents in chick auditory hair cells using an intact basilar papilla. Hear Res 156: 81–94, 2001. [DOI] [PubMed] [Google Scholar]
- 48.Perez GJ Dual effect of tamoxifen on arterial KCa channels does not depend on the presence of the beta1 subunit. J Biol Chem 280: 21739–21747, 2005. [DOI] [PubMed] [Google Scholar]
- 49.Pyott SJ, Meredith AL, Fodor AA, Vazquez AE, Yamoah EN, Aldrich RW. Cochlear function in mice lacking the BK channel alpha, beta1, or beta4 subunits. J Biol Chem 282: 3312–3324, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Ramanathan K, Fuchs PA. Modeling hair cell tuning by expression gradients of potassium channel beta subunits. Biophys J 82: 64–75, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramanathan K, Michael TH, Fuchs PA. Beta subunits modulate alternatively spliced, large conductance, calcium-activated potassium channels of avian hair cells. J Neurosci 20: 1675–1684, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramanathan K, Michael TH, Jiang GJ, Hiel H, Fuchs PA. A molecular mechanism for electrical tuning of cochlear hair cells. Science 283: 215–217, 1999. [DOI] [PubMed] [Google Scholar]
- 53.Roberts WM, Jacobs RA, Hudspeth AJ. Colocalization of ion channels involved in frequency selectivity and synaptic transmission at presynaptic active zones of hair cells. J Neurosci 10: 3664–3684, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ruttiger L, Sausbier M, Zimmermann U, Winter H, Braig C, Engel J, Knirsch M, Arntz C, Langer P, Hirt B, Muller M, Kopschall I, Pfister M, Munkner S, Rohbock K, Pfaff I, Rusch A, Ruth P, Knipper M. Deletion of the Ca2+-activated potassium (BK) alpha-subunit but not the BKbeta1-subunit leads to progressive hearing loss. Proc Natl Acad Sci USA 101: 12922–12927, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Samaranayake H, Saunders JC, Greene MI, Navaratnam DS. Ca(2+) and K(+) (BK) channels in chick hair cells are clustered and colocalized with apical-basal and tonotopic gradients. J Physiol 560: 13–20, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shmygol A, Noble K, Wray S. Depletion of membrane cholesterol eliminates the Ca2+-activated component of outward potassium current and decreases membrane capacitance in rat uterine myocytes. J Physiol 581: 445–456, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Skinner LJ, Enee V, Beurg M, Jung HH, Ryan AF, Hafidi A, Aran JM, Dulon D. Contribution of BK Ca2+-activated K+ channels to auditory neurotransmission in the guinea pig cochlea. J Neurophysiol 90: 320–332, 2003. [DOI] [PubMed] [Google Scholar]
- 58.Thurm H, Fakler B, Oliver D. Ca2+-independent activation of BKCa channels at negative potentials in mammalian inner hair cells. J Physiol 569: 137–151, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Turi A, Kiss AL, Mullner N. Estrogen downregulates the number of caveolae and the level of caveolin in uterine smooth muscle. Cell Biol Int 25: 785–794, 2001. [DOI] [PubMed] [Google Scholar]
- 60.Valverde MA, Rojas P, Amigo J, Cosmelli D, Orio P, Bahamonde MI, Mann GE, Vergara C, Latorre R. Acute activation of Maxi-K channels (hSlo) by estradiol binding to the beta subunit. Science 285: 1929–1931, 1999. [DOI] [PubMed] [Google Scholar]
- 61.Van Hauwe P, Coucke PJ, Ensink RJ, Huygen P, Cremers CW, Van Camp G. Mutations in the KCNQ4 K+ channel gene, responsible for autosomal dominant hearing loss, cluster in the channel pore region. Am J Med Genet 93: 184–187, 2000. [DOI] [PubMed] [Google Scholar]
- 62.Waka N, Knipper M, Engel J. Localization of the calcium channel subunits Cav1.2 (alpha1C) and Cav2.3 (alpha1E) in the mouse organ of Corti. Histol Histopathol 18: 1115–1123, 2003. [DOI] [PubMed] [Google Scholar]
- 63.Zhang H, Schools GP, Lei T, Wang W, Kimelberg HK, Zhou M. Resveratrol attenuates early pyramidal neuron excitability impairment and death in acute rat hippocampal slices caused by oxygen-glucose deprivation. Exp Neurol 212: 44–52, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]