Abstract
To determine how ascorbic acid moves from the bloodstream into tissues, we assessed transfer of the vitamin across the barrier generated by EA.hy926 endothelial cells when these were cultured on semipermeable filter supports. Ascorbate transfer from the luminal to the abluminal compartment was time dependent, inhibited by anion channel blockers and by activation of protein kinase A, but was increased by thrombin. Ascorbate transfer occurred by a paracellular route, since it did not correlate with intracellular ascorbate contents and was not rectified or saturable. Nonetheless, intracellular ascorbate inhibited the transfer of both ascorbate and radiolabeled inulin across the endothelial barrier. The increase in barrier function due to ascorbate was dependent on its intracellular concentration, significant by 15 min of incubation, prevented by the cytoskeletal inhibitor colchicine, associated with F-actin stress fiber formation, and not due to collagen deposition. These results show that ascorbate traverses the endothelial barrier by a paracellular route that is regulated by cell metabolism, ion channels, and ascorbate itself. Since the latter effect occurred over the physiological range of ascorbate plasma concentrations, it could reflect a role for the vitamin in control of endothelial barrier function in vivo.
Keywords: paracellular transport, ascorbate transport, anion channels, endothelial permeability
vitamin c, or ascorbic acid, enters the bloodstream in mammals from the liver, either having been absorbed in the intestine (all mammals) or synthesized in the liver (excepting humans, higher primates, and a few other species). From the blood, ascorbate must traverse the endothelial barrier to reach organs that require it, such as brain, muscle, skin, and hematopoietic tissues. How the vitamin does this, especially in organs with tight endothelial barriers, is unknown. It is known that ascorbate enters all cells, including the endothelium, by one of two mechanisms. The first involves uptake by a specific sodium- and energy-dependent transport protein, termed the sodium-dependent vitamin C transporter (SVCT), of which there are two known isoforms. The SVCT1, which is the product of the SLC23A1 gene, is present primarily in epithelial brush borders and mediates intestinal absorption and renal reabsorption of the vitamin (34). The SVCT2 is the product of the SLC23A2 gene and is found in most other cell types. Both transporters generate a steep gradient of ascorbate across the plasma membrane from plasma or interstitium into cells (34). Although intracellular ascorbate concentrations in endothelial cells of large or small vessels have not been measured directly, both primary (6, 12) and immortalized (22) endothelial cells in culture show sodium- and energy-dependent ascorbate uptake. This transport is likely on the SVCT2 (31) and sustains low millimolar intracellular ascorbate levels in cells cultured with physiological ascorbate concentrations (12, 22). The second mechanism by which ascorbate can enter cells is via uptake of dehydroascorbic acid (DHA) on the ubiquitously expressed GLUT-type glucose transporters (37). DHA is the two-electron-oxidized form of ascorbate that, once inside cells, is rapidly reduced to ascorbate (23). Since ascorbate itself is not transported on glucose transporters (38) and since it is an anion at physiological pH, it tends to be trapped within the cell and can accumulate at least transiently to low millimolar concentrations, even in cells such as erythrocytes that do not express the SVCT proteins (24, 27). If, as seems likely, these two routes of ascorbate uptake do generate relatively high intracellular ascorbate concentrations in endothelial cells, it is plausible that this ascorbate is transported across the abluminal or basolateral cell membrane into the underlying interstitium. If so, then one would predict a significant efflux of ascorbate from endothelial cells.
Ascorbate efflux has been described in endothelial cells (9, 35), as well as in hepatocytes (35) and brain cells under glutamate-induced excitotoxic stress (28). We (25) recently showed that cultured EA.hy926 endothelial cells have substantial rates of ascorbate efflux that are countered by reuptake on the SVCT transporter. If this efflux relates to vectorial transport of ascorbate, then it should be evident as transendothelial ascorbate movement when the cells are cultured on porous filter supports. To test this hypothesis and to determine whether such efflux might be regulated, we used EA.hy926 endothelial cells. These cells are a hybridoma line derived from human umbilical vein endothelial cells that retain endothelial cell features and orientation in that they exhibit a cobblestone appearance with formation of capillary-like tubes in culture (3), express factor VIII antigen (11), oxidatively modify human low-density lipoprotein (30), and have calcium-dependent endothelial nitric oxide synthase activity (17, 30). We found that these cells in culture on porous filters impeded the movement of ascorbate across the filter in a manner sensitive to agents known to affect endothelial barrier function. However, contrary to our hypothesis, transendothelial ascorbate movement was not due to efflux from the cells, but rather to paracellular movement of ascorbate between the cells. Furthermore, culture with ascorbate tightened the endothelial barrier to transfilter movement of ascorbate. These results were confirmed in primary cultures of endothelial cells derived from human dermal capillaries. The findings suggest a role for ascorbate in maintaining the integrity of the endothelial barrier.
MATERIALS AND METHODS
Materials.
Sigma-Aldrich Chemical (St. Louis, MO) supplied the reagent chemicals, including ascorbate, catalase, cAMP, DHA, HEPES, thrombin, 5-nitro-2-(3-phenylpropylamino)benzoic acid (NPPB), niflumic acid, probenecid, bumetanide, 3-isobutyl-1-methylxanthine (IBMX), forskolin, superoxide dismutase, and sulfinpyrazone. Agents listed after thrombin were initially dissolved in a small amount of dimethyl sulfoxide and then diluted with culture medium such that the final dimethyl sulfoxide concentration was 0.8%. Perkin-Elmer Life and Analytical Sciences (Boston, MA) supplied the d-[1-14C]mannitol (50 mCi/mmol) and [carboxyl-14C]inulin (molecular weight range 5,000–5,500, 2 mCi/g).
Cell culture.
EA.hy926 cells were a gift from Dr. Cora Edgell (University of North Carolina, Chapel Hill, NC). The cells were cultured in Dulbecco's minimal essential medium and 10% (vol/vol) heat-inactivated fetal bovine serum, which contained 20 mM d-glucose and HAT media supplement (Sigma-Aldrich Chemical). Primary cultures of human dermal microvascular endothelial cells were obtained from ScienCell Research Laboratories (Carlsbad, CA; catalog no. 2000) and were cultured in endothelial cell medium (catalog no. 1001). Cells were cultured to confluence at 37°C in humidified air containing 5% CO2.
Assay of transfilter ascorbate transport.
Endothelial cells were seeded on BD Falcon polyethylene terephthalate cell culture inserts (4.2 cm2; BD Biosciences, Franklin Lakes, NJ) in six-well plates that contained 0.4-μm pores at a density of 2 ± 0.2 × 106 pores/cm2. After culture at moderate seeding density for 4 days with 1.7 ml of medium in the upper well and 2.8 ml of medium in the lower well, the cells were confluent under phase-contrast microscopy. The cells were used as noted at 4–7 days in culture for assay of ascorbate uptake and transmembrane passage. Ascorbate, d-[1-14C]mannitol, or [carboxyl-14C]inulin and other agents were added above the cells/filter except as otherwise noted, and incubation was carried out at 37°C, typically for 1 h. Aliquots of the medium above and below the cells/filter were taken for assay of ascorbate or for liquid scintillation counting of radiolabeled mannitol or inulin. When intracellular ascorbate was to be measured, the cells on the filter were gently rinsed three times with Krebs-Ringer HEPES buffer (KRH) that consisted of 20 mM HEPES, 128 mM NaCl, 5.2 mM KCl, 1 mM NaH2PO4, 1.4 mM MgSO4, and 1.4 mM CaCl2, pH 7.4. After removal of the last rinse, the cell monolayer was treated with 0.1 ml of 25% (wt/vol) metaphosphoric acid, detached from the filter with a rubber spatula, and then treated with 0.35 ml of 0.1 M Na2HPO4 and 0.05 mM EDTA, pH 8.0. The lysate was removed and centrifuged at 3°C for 1 min at 13,000 g, and the supernatant was taken for assay of ascorbate as described below.
Transfer of an agent across the filter plus cell matrix deposited during 4 or more days in culture (no cells present) was measured as described elsewhere (36). Briefly, after the initial transfer experiment was completed, the medium above and below the cells was removed and the cell layer was rinsed once in 2 ml of KRH. This was followed by treatment of the cell layer with 2 ml of 20 mM ammonium hydroxide. After 10 min of incubation with gentle swirling at 37°C, the cells lifted off the membrane and were removed by three rinses in 2 ml of KRH. Transfer of the same concentration of ascorbate or radiolabeled agent from above the filter to below was measured again for 1 h at 37°C in the absence of cells.
Calculation of permeability coefficients.
The permeability coefficients for ascorbate, d-[1-14C]mannitol, and [carboxyl-14C]inulin were calculated as previously described (32). This involved first determining the volume (V) or space occupied by the agent that was cleared from the luminal to the abluminal side of the cells/filter as
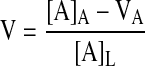 |
(1) |
where [A]A is the abluminal molecule concentration, VA is the volume of the abluminal chamber, and [A]L is the final luminal concentration of the molecule. The final concentrations of the agents above and below the filter were used in the calculation, since these were conveniently obtained at the end of the transfer, and since there was a <20% decrease from initial concentrations during the course of the transfer experiment (results not shown). Clearance of all three agents was linear over the 1-h duration of the transfer experiment (see e.g., Fig. 1B), so the rate of transfer (dV/dt) was calculated based on the 1-h time point. The permeability (P) of each molecule across the cells and filter was calculated as
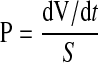 |
(2) |
where S is the surface area of the filter on which the cells were cultured. Equation 2 was used to calculate the measured permeability of the cells and filter (PF+EC) as well as that of the filter plus any matrix laid down by the cells (PF). The permeability of the endothelial cell layer alone (PEC) was then calculated from
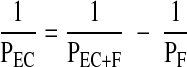 |
(3) |
Fig. 1.
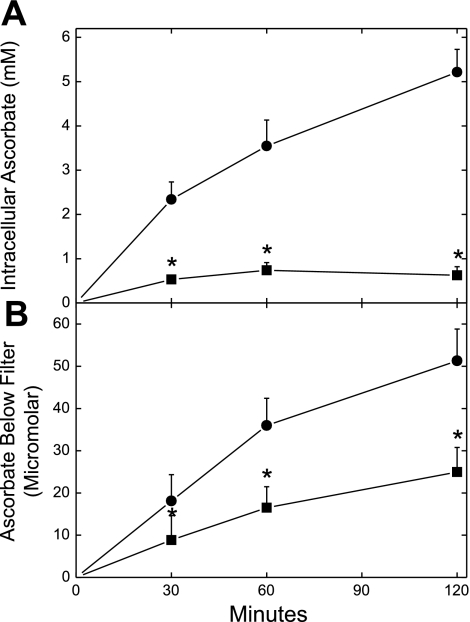
Ascorbate loading and transendothelial movement in EA.hy926 cells. Cells in culture on porous membrane filters for 4 days were incubated at 37°C for the times indicated with 300 μM ascorbate in the absence (•) or presence (▪) of 2 mM sulfinpyrazone, both added above the cells at the same time. Aliquots of the medium below the cells and filter were removed for assay of ascorbate (B), and the cells were rinsed 3 times in Krebs-Ringer HEPES buffer (KRH) before extraction for assay of intracellular ascorbate as described in materials and methods (A). Results are shown from 5 experiments. *P < 0.05 compared with the sample not treated with sulfinpyrazone.
Assay of ascorbate.
Cell samples for assay of ascorbate were prepared as described above. Ascorbate was also measured in culture medium by adding 0.1 ml of medium to 0.1 ml of 25% metaphosphoric acid (wt/vol), mixing, neutralizing with 0.35 ml of the above-described phosphate-EDTA buffer, and centrifuging to remove any precipitated solids before assay of ascorbate. Assay of ascorbic acid was performed in duplicate using high-performance liquid chromatography as previously described (26). Intracellular concentrations of ascorbate were calculated based on the intracellular distribution space of 3-O-methylglucose in EA.hy926 cells. This was previously determined in EA.hy926 cells to be 3.6 ± 1.2 μl/mg protein (17) and was considered to be similar in dermal capillary endothelial cells.
F-actin staining.
After treatments as noted, EA.hy926 cells were rinsed three times with phosphate-buffered saline (PBS; 140 mM NaCl and 12.5 mM sodium phosphate, pH 7.4). The cells were then fixed for 20 min at 37°C in PBS that contained 4% paraformaldehyde, rinsed in PBS, and permeabilized for 10 min at 23°C with 1% Triton X-100 in PBS. The cells were stained with rhodamine-phalloidin (R415; Molecular Probes, Eugene, OR) at a concentration of 2 U/ml for 10 min at 23°C in PBS that contained 1% (wt/vol) bovine serum albumin.
Data analysis.
Results are means + SE. Statistical comparisons were made using SigmaStat 2.0 software (Jandel Scientific, San Rafael, CA). Differences between treatments were assessed using two-way analysis of variance with post hoc testing using Dunnett's test.
RESULTS
Although EA.hy926 endothelial cells contained little or no detectible ascorbate in culture (results not shown), they progressively took up the vitamin when cultured on filters for 2 h at 37°C to concentrations as high as 5 mM (Fig. 1A, circles). This uptake was inhibited ∼80% by 2 mM sulfinpyrazone (Fig. 1A, squares). Ascorbate also accumulated in the well below the filter in a time-dependent manner that was inhibited ∼50% by sulfinpyrazone (Fig. 1B).
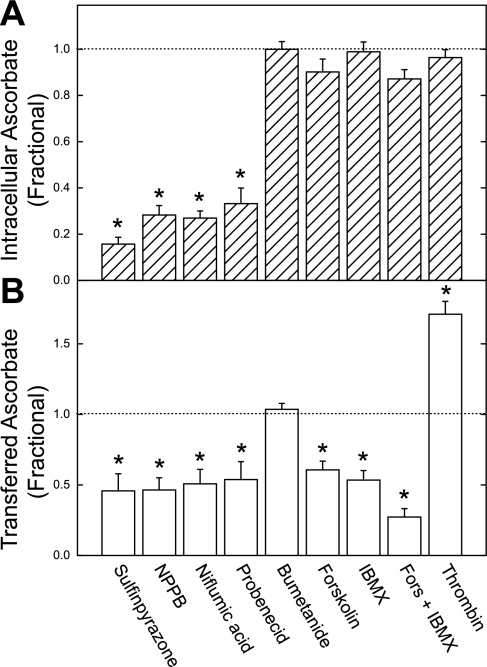
Inhibition of ascorbate movement across the endothelial cell barrier by sulfinpyrazone prompted testing of other agents at concentrations known to affect anion channels, anion transporters, and related functions in endothelial cells (8, 29). As shown in Fig. 2, the anion channel inhibitors sulfinpyrazone, NPPB, niflumic acid, and probenecid decreased both entry of ascorbate into the cells and the transfer of ascorbate across the endothelial cell barrier. The anion transport inhibitor bumetanide had no affect on either intracellular ascorbate or ascorbate transfer. Agents known to increase intracellular cAMP (IBMX) or activate protein kinase A directly (forskolin) had no effect on intracellular ascorbate but decreased ascorbate transfer with a partially additive effect. Thrombin, which increases permeability of endothelial layers (8), also had no effect on intracellular ascorbate but markedly increased the transfer of ascorbate across the endothelial cells. No effect on ascorbate transfer over 1 h was observed when catalase (5,900 U/ml) or superoxide dismutase (2,900 U/ml) were added to the upper chamber, making it very unlikely that the transfer was due to radical species generated by redox cycling of ascorbate with iron in the culture medium (results not shown). Together, these results show that ascorbate transfer across the endothelial cell layer does not always follow its accumulation of ascorbate within cells. The discrepancies between ascorbate content and transendothelial transfer raise the possibility that intracellular ascorbate is not the source of transferred ascorbate.
Fig. 2.
Agent effects on intracellular and transferred ascorbate. EA.hy926 cells cultured on filters for 4 days were treated in culture medium above the filters with 300 μM ascorbate and the agents shown at the following concentrations: sulfinpyrazone, 2 mM; 5-nitro-2-(3-phenylpropylamino)benzoic acid (NPPB), 0.3 mM; niflumic acid, 0.3 mM; probenecid, 2 mM; bumetanide, 0.3 mM; forskolin (Fors), 0.1 mM; IBMX, 0.1 mM; and thrombin, 0.15 IU/ml. After 1 h, medium below the cells was sampled for assay of ascorbate (B), and the cells were rinsed 3 times in KRH and taken for assay of intracellular ascorbate (A). Results are shown from 4–5 experiments, expressed as a fraction of the ascorbate concentration found in cells treated only with ascorbate. *P < 0.05 compared with control (indicated by the horizontal dashed line). Absolute values for control samples treated only with ascorbate were as follows: intracellular ascorbate, 3.8 ± 0.2 mM; transferred ascorbate, 34 ± 2.4 μM in the bottom well.
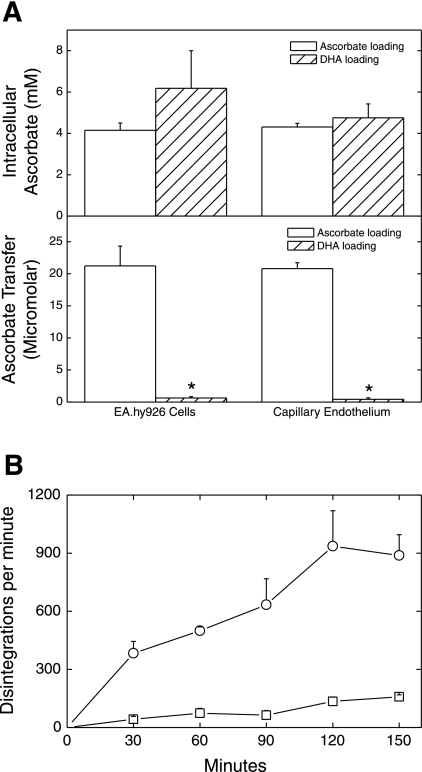
To directly assess whether ascorbate crosses the endothelial cell layer and filter through or around the cells, transfer of extracellular ascorbate from above to below the cells and filter was compared with transfer of intracellular ascorbate that was derived from DHA loading of the cells. In this experiment, both EA.hy926 cells and human dermal capillary endothelial cells were treated with 300 μM ascorbate or DHA, followed by measurement of ascorbate in the cells as well as below the cells after 1 h. Ascorbate in both cell types increased to similar low millimolar concentrations with both ascorbate and DHA loading (Fig. 3A, top). Whereas incubation of the cells with ascorbate above the filter for 1 h resulted in an ascorbate concentration below the filter similar to that observed at 1 h in Fig. 1, very little ascorbate appeared below the filter in DHA-loaded cells of either type (Fig. 3A, bottom). Although endothelial cells did not release intracellular ascorbate across the filters, EA.hy926 cells that had been loaded with radioactive ascorbate did release it into the medium above the filter. This is shown in Fig. 3B as a comparison of rates of radiolabel appearance above and below the filter. Ascorbate efflux into the compartment above the cells and filter was much greater than efflux into the compartment below the cells. Together, these results indicate that efflux of intracellular ascorbate contributes little to its transendothelial movement. Thus ascorbate must traverse the endothelial cell barrier either through a novel transcellular pathway or around the cells via a paracellular route.
Fig. 3.
Intracellular ascorbate does not contribute to its transendothelial movement. A: EA.hy926 and dermal capillary endothelial cells cultured for 4 days on membrane filters were gently rinsed 3 times with KRH, both above and below the filter. They were then incubated in KRH containing 5 mM d-glucose with 300 μM initial concentrations of either ascorbate or dehydroascorbic acid (DHA), added above the cells. After 1 h, ascorbate concentrations were measured in the cells (top) or in the medium below the cells and filter (bottom). Results are shown from 5 experiments. *P < 0.001 compared with ascorbate treatment. B: KRH-rinsed cells were incubated with 3 μM radiolabeled ascorbate (0.02 μCi) for 1 h and then rinsed above and below 3 times in KRH. Fresh KRH was added back to the same levels, and efflux of ascorbate was followed at the indicated times by sampling medium above and below the cells and filter for radioactivity. The 2 curves were significantly different for the 4 experiments shown.
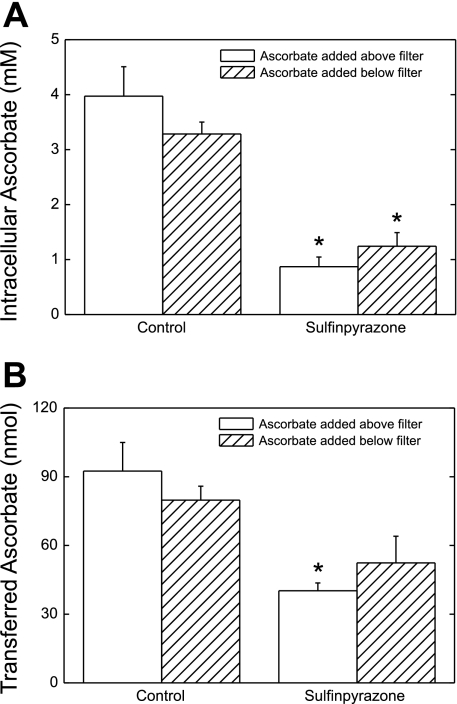
If ascorbate moves across the cell layer via a transcellular channel or pore, across tight junctions, or even in openings between the visually confluent cells, then its movement should not be rectified or saturable. The former was tested in two types of experiment. First, cells were incubated in culture medium with the same concentration of ascorbate applied either above or below the cells for 1 h, and the appearance of ascorbate in the cells and on the opposite side of the cells and filter was measured. As shown in Fig. 4A, bars at left, intracellular ascorbate concentrations were similar whether cells were incubated with ascorbate added above or below the cells/filter. Sulfinpyrazone (always added above the cells) decreased intracellular ascorbate to the same extent, regardless of the side of ascorbate addition (Fig. 4A, bars at right). When the total amount of ascorbate transferred across the cells/filter was measured, transfer in either direction was similar (Fig. 4B, bars at left), although the inhibition by sulfinpyrazone of ascorbate transfer from below to above the cells/filter did not reach statistical significance (Fig. 4B, bars at right). In the second type of experiment to test bidirectional transfer asymmetry, cells cultured on filter supports were incubated in culture medium for 2 h with equal 300 μM ascorbate concentrations added to both sides of the filter, rather than on just one side as in the experiments shown in Fig. 4. There was no differential accumulation of ascorbate below or above the filter, although the ascorbate concentrations in the cells increased to 3.5 mM (results not shown). These results suggest that ascorbate transfer across the cells/filter is driven by the ascorbate concentration gradient, regardless of its direction.
Fig. 4.
Bidirectional transfer of ascorbate. Cells cultured on filters for 4 days were incubated for 1 h with ascorbate (∼330 μM) added to culture medium either above or below the cells and filter, as noted. Sulfinpyrazone (2 mM) was added as indicated above the cells at the beginning of the incubation. After the incubation, medium below the cells was sampled for assay of ascorbate that had crossed the membrane (B). Cells were rinsed 3 times in KRH and taken for assay of ascorbate (A). Concentrations of ascorbate above and below the cells measured at the end of these experiments were 0.35 ± 0.02 and 0.32 ± 0.02 mM, respectively. Results are shown from 6 experiments. *P < 0.05 compared with the sample not treated with sulfinpyrazone.
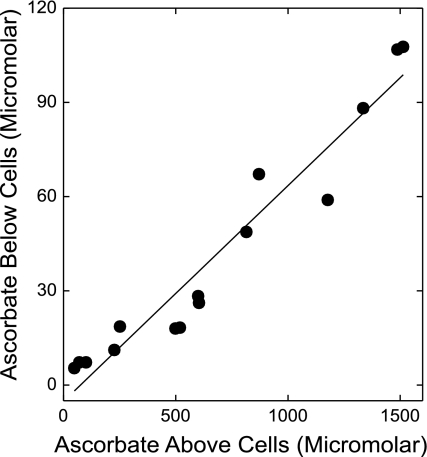
Ascorbate transfer from above to below the cells/filter was not saturable with increasing ascorbate concentrations added above the cells. This is shown in Fig. 5 as a plot of the sulfinpyrazone-inhibited component of ascorbate transferred across the cells/filter against the measured final ascorbate concentration above the cells. Linear regression of these data yielded a correlation coefficient of 0.97 (P < 0.001). Similar results were obtained when transfer of ascorbate was not corrected for the inhibition by sulfinpyrazone (r = 0.96, P < 0.001).
Fig. 5.
Failure of ascorbate transfer across cells and filter to saturate. Cells cultured on filters for 4 days were incubated at 37°C in culture medium that contained increasing ascorbate concentrations, in either the absence or presence of 2 mM sulfinpyrazone. After 1 h, ascorbate was measured above and below the cells/filter. Shown are the ascorbate concentrations in the well below the cells minus the corresponding concentrations generated in the presence of sulfinpyrazone, plotted as a function of the final ascorbate concentrations in the wells above the cells. Results are from 5 experiments, with the solid line showing the best fit by linear regression (r = 0.97, P < 0.001).
To compare ascorbate transfer with that of other molecules known to cross the endothelial barrier by a paracellular route, transfer of radiolabeled d-[1-14C]mannitol and [carboxyl-14C]inulin was measured. The former was chosen because, although uncharged, it has a molecular weight similar to that of ascorbate and is not appreciably taken up by cells. On the other hand, the cell-impermeant [carboxyl-14C]inulin carries a negative charge but is a much larger linear molecule (molecular weight average 5,000–5,500). To allow direct comparison of transfer rates of the three molecules, we calculated permeability coefficients as described in materials and methods and shown in Fig. 6 for cells plus filter (A) and cells alone (B). Transfer of all the agents was substantially retarded by the cells, since the ratios of the permeabilities of the filters after cell removal to that of the filters plus cells were as follows: ascorbate, 3.0 ± 0.3 (n = 8 determinations); mannitol, 2.2 ± 0.2 (n = 14 determinations); and inulin, 2.0 ± 0.2 (n = 7 determinations). Comparisons of basal transfer rates of the three agents showed that rates of ascorbate and mannitol transfer across both cells plus filter and across cells alone were similar, whereas transfer of inulin was slower than that of both the smaller molecules across cells plus filters and across cells alone (P < 0.05 for both comparisons). These results fit with expectations based on the different molecular weights of the molecules.
Fig. 6.
Comparison of the permeabilities of ascorbate, mannitol, and inulin. Cells that had been in culture on filters for 4 days were incubated for 1 h in the absence (control) or presence of 300 μM ascorbate as well as [carboxyl-14C]inulin (14C-inulin; 10 μM) or d-[1-14C]mannitol (14C-mannitol; 300 μM), as noted. Where indicated, 0.15 IU/ml thrombin or 2 mM sulfinpyrazone were also added at the beginning of the incubation. After 1 h, medium was sampled for assay of the respective agent above and below the cells and filter. The cells were removed as described in materials and methods, and the transfer assay was repeated. Permeabilities of each agent were calculated for the cells and filter (A) and for the cells alone (B). Asc, ascorbate; SPZ, sulfinpyrazone. Results are shown from at least 7 experiments with each agent. *P < 0.05 compared with treatment with ascorbate alone in each respective group.
As expected from results shown earlier, ascorbate permeability was increased by thrombin and decreased by sulfinpyrazone, both across cells plus filter (Fig. 6A) and across the cells alone (Fig. 6B). The permeability coefficient for mannitol transfer was unaffected by a 1-h preincubation with 300 μM ascorbate for transfer. Although thrombin modestly but significantly increased mannitol transfer in cells also treated with ascorbate, there was no effect of sulfinpyrazone. In contrast to mannitol, when cells were preincubated for 1 h with ascorbate, the permeability coefficients for inulin both across cells and filter and across cells alone were significantly decreased. Compared with ascorbate treatment alone, thrombin enhanced and sulfinpyrazone diminished both permeability coefficients. These results show that after 4 days in culture, a 1-h treatment with ascorbate modestly tightened the cell-derived endothelial barrier to large molecules such as inulin, whereas ascorbate transfer was much more sensitive to modulation by thrombin and sulfinpyrazone than transfer of either inulin or mannitol.
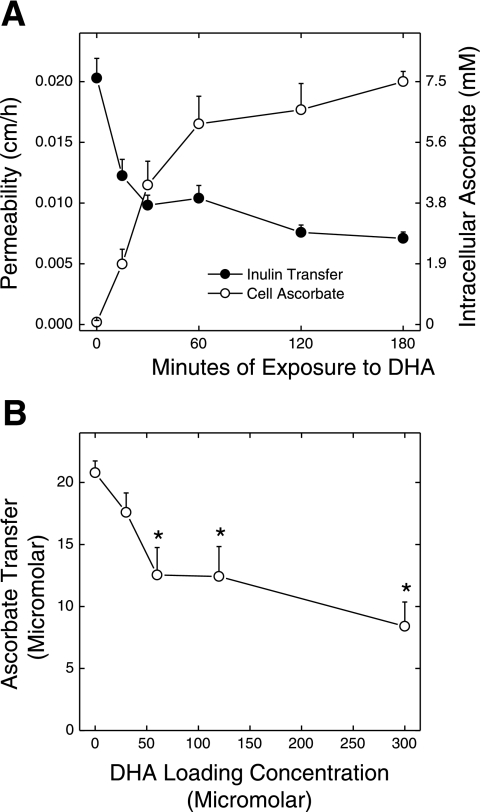
Culture of cells for 6–7 days before the transfer experiment resulted in tighter endothelial cell junctions, since the permeability of the cells to inulin decreased from 0.044 ± 0.005 cm/h (Fig. 6B) to 0.020 ± 0.002 cm/h (Fig. 7A, filled circles). When such cells were rapidly loaded with ascorbate as DHA before and during the 1-h inulin transfer assay, inulin transfer was decreased in a time-dependent manner (Fig. 7A, filled circles). This decrease was significant by 15 min and by 30 min fell to <50% of the transfer rate seen in the cells that did not contain ascorbate. The decrease in endothelial cell layer permeability to inulin changed inversely with the increase in ascorbate content of the cells over the time course studied (Fig. 7A, open circles). Thus culture of cells for longer than 4 days tightened the endothelial barrier to inulin and resulted in a greater relative effect of ascorbate loading to further increase the barrier function. Furthermore, the inhibition of paracellular transfer of inulin closely followed the intracellular ascorbate content of the cells.
Fig. 7.
Time course of and concentration dependence of inhibition of inulin transfer by ascorbate. A: EA.hy926 cells that had been plated on filters for 6 days in culture were rinsed in KRH and incubated at 37°C in KRH containing 5 mM d-glucose and 300 μM DHA for the times indicated. At 120 min of incubation, [carboxyl-14C]inulin (10 μM) was added. After an additional 60 min, medium above and below the filter was sampled for radioactive counting. The cells were removed as described in materials and methods, and the transfer assay was repeated with calculation of inulin permeability across the cells (•). In cells treated exactly the same except that radiolabeled inulin was not added, intracellular ascorbate was measured (○). Results are shown from 6 experiments of inulin transfer and 3 experiments in which ascorbate uptake was measured. All values were significantly different from the zero time control for both assays at P < 0.05. B: dermal capillary fibroblasts that had been cultured 6 days to confluence on membrane filters were treated with the indicated concentration of DHA for 30 min. Ascorbate (300 μM) was added to the upper chamber for 1 h, followed by measurement of the appearance of ascorbate in the lower chamber. Results are shown from 6 experiments. *P < 0.05 compared with cells not treated with DHA.
To determine whether intracellular ascorbate tightens the endothelial barrier in primary cultures of capillary endothelium, we loaded dermal capillary endothelial cells with ascorbate by incubation for 30 min with increasing concentrations of DHA and then treated cells for 1 h with 300 μM ascorbate added to the upper chamber. As shown in Fig. 7B, appearance of ascorbate in the lower chamber was progressively decreased by increasing concentrations of added DHA.
The results of Fig. 7A show that the effect of ascorbate to tighten the endothelial barrier to inulin transfer persisted at least 3 h. To determine whether the barrier-tightening effect of ascorbate persists longer and whether persistence of the ascorbate effect might be related to ascorbate-induced collagen deposition in the membrane pores, we treated confluent cells after 4 days of culture for an additional 18 h with increasing concentrations of ascorbate. Assay of ascorbate permeability was then measured 1 h after a single addition of 300 μM ascorbate above the cells. It is important to note that after such overnight culture following a single addition of ascorbate, very little ascorbate (<2 μM) could be detected either above or below the filter, although ascorbate was retained by the cells (results not shown). Thus there was little or no extracellular ascorbate present to interfere with the 1-h transfer assay. This allowed direct addition of 300 μM fresh ascorbate for the transfer assay to all the wells without having to disturb the cells by rinses to remove residual ascorbate. Overnight culture with increasing concentrations of ascorbate from 30 to 300 μM progressively decreased the permeability of ascorbate across cells and filter (Fig. 8A) and across the cells alone (Fig. 8B) in the transfer assay. A significant decrease was observed at an initial loading concentration of 30 μM for both cells and filter and cells alone, with a maximal decrease of 70% for cells alone at 300 μM ascorbate. The permeability across the filter and matrix laid down by the cells was not affected by ascorbate loading (Fig. 8C), showing that the changes observed were due to the cells and not to increased collagen deposition in response to ascorbate.
Fig. 8.
Inhibition of cell permeability to ascorbate by overnight culture of EA.hy926 cells with ascorbate. Cells in culture on filters for 4 days were treated with a single addition of ascorbate at the indicated concentration, followed by further culture for 18 h before a second addition of ascorbate to 300 μM above the cells and assay of ascorbate transfer in the presence and absence of cells for 1 h at 37°C as described in materials and methods. Results are shown from 7 experiments as permeabilities of the cells plus filter (A), of the cells alone (B), and the filter plus remaining matrix (C). *P < 0.05 compared with cells not treated overnight with ascorbate.
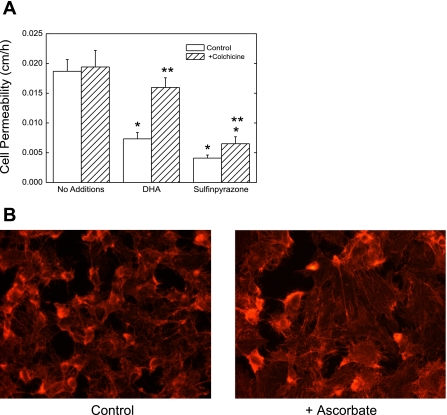
If ascorbate increases endothelial resistance to its own passage (or to that of inulin) by a paracellular route, then it might do so by closing spaces between the cells. This would require changes in cell shape and should be inhibited by impairing cytoskeletal function. This was tested with the use of the microtubule inhibitor colchicine. Colchicine did not significantly affect the uptake of 300 μM ascorbate over 1 h of incubation, in that intracellular ascorbate concentrations were 3.9 ± 0.3 mM in the absence of colchicine and 3.1 ± 0.5 mM in the presence of 10 μM colchicine. However, whereas 10 μM colchicine had no effect on the transfer of radiolabeled inulin in the absence of ascorbate, it completely prevented the ability of intracellular ascorbate to decrease the transfer rate of inulin in cells that had been cultured for 6 days (Fig. 9A). A similar preventive effect of colchicine was also observed for transfer of ascorbate after 4 days of culture (results not shown). Inhibition of inulin transfer by sulfinpyrazone was also partially prevented by colchicine (Fig. 9A). Further evidence that intracellular ascorbate affects the cytoskeleton in endothelial cells is shown in Fig. 9B, in which cells cultured for 1 h with ascorbate and stained for F-actin with rhodamine-phalloidin showed appearance of stress fibers and increased flattening compared with cells not treated with ascorbate.
Fig. 9.
Cytoskeletal effects of ascorbate on inulin transfer and cellular actin. A: EA.hy926 cells in culture for 6 days were rinsed 3 times in KRH and incubated 37°C with KRH containing 5 mM d-glucose, 0.3 mM DHA, or 2 mM sulfinpyrazone (the latter 2 in the upper well above the filter). After 1 h of incubation, [carboxyl-14C]inulin (10 μM) was added. After an additional 60 min, the transfer assay was carried out with calculation of inulin permeability across the cells. Results are shown from 5 experiments. *P < 0.05 compared with control. **P < 0.05 compared with treatment without colchicine. B: EA.hy926 cells were cultured on glass slides to near confluence for 4 days and treated for 1 h with or without 300 μM ascorbate as indicated, followed by staining of F-actin with rhodamine-phalloidin as described in materials and methods. Results are shown from 1 experiment typical of 2 such performed.
DISCUSSION
Despite the fact that ascorbate accumulates to low millimolar concentrations in cultured endothelial cells, this intracellular ascorbate does not appreciably move across the endothelial barrier. Rather, ascorbate transits the barrier by a paracellular route that is governed by the tightness and possibly the function of cell-to-cell contacts. Moreover, intracellular ascorbate increases this barrier function in a manner that is independent of ascorbate effects on collagen deposition but dependent on changes in the cell cytoskeleton.
Ascorbate is known to efflux from cultured endothelial cells (35), especially in response to increases in intracellular calcium (9). It follows that this efflux could reflect vectorial ascorbate transport from the abluminal or basolateral side of the cells (25). Indeed, when cultured on permeable supports, both EA.hy926 cells and human dermal capillary endothelial cells took up ascorbate from the medium above the cells and allowed a small amount of ascorbate to pass to the abluminal compartment below the cells. However, numerous features of this passage indicated that intracellular ascorbate was not the source of ascorbate appearing in the abluminal compartment: 1) intracellular ascorbate concentrations did not correlate with the amount of ascorbate transferred, 2) ascorbate transit across cells and filter required a concentration gradient between the two compartments, 3) transit occurred to a similar extent in both directions, 4) transit was not saturable with increasing ascorbate concentrations, 5) radiolabeled intracellular ascorbate effluxed to the luminal, not the abluminal compartment, and 6) ascorbate transit did not occur when intracellular ascorbate was selectively increased by loading with DHA. These results are compatible with ascorbate movement by diffusion across a gap between cells or through tight junctions or other connections between the cells.
Failure to find that ascorbate leaves the abluminal side of the cells was surprising, given the steep concentration gradient generated by function of the SVCT2 to bring ascorbate into the cells. These findings may have relevance for epithelial cells, such as those that govern ascorbate uptake in the intestine and its reuptake in the kidney. For example, Caco-2 cells, which are a colon carcinoma cell line that is frequently used as a model of the intestinal epithelial barrier, express the SVCT1 in the apical cell membrane (7, 21). When the SVCT2 is present, it is expressed in the basolateral membrane (7). Since both transporters are thought to transport ascorbate in one direction into cells (34), this orientation for both transporters would only bring ascorbate into the cells. Whether and how intracellular ascorbate exits across the basolateral membrane of epithelial cells is unknown, but presumably this would involve efflux on a yet to be described transporter or channel that is lacking in vascular endothelial cells.
Ascorbate could move around endothelial cells by one of two routes. First, it could diffuse across gaps between the cells. It is known that there are small 0.5- to 2-μm gaps between 5–10% of apparently confluent endothelial cells in culture and that the longer the cells are grown in culture, the tighter the junctions become (1). We observed this phenomenon with regard to inulin transfer across the endothelial cell barrier. Ascorbate and larger molecules could thus move freely across any gaps between the cells with little sieving effect. Their movement would be governed by the size of such gaps, by permeability of the filter membrane and any cell-deposited matrix, by intrinsic diffusion rates of the molecules, and by effects of unstirred layers (32).
The second route for paracellular ascorbate movement is diffusion across cellular connections that form a ring of attachment of endothelial cells with their neighbors (2). Endothelial cell-to-cell junctions, although not as tight as those found in epithelial cells (1), are nonetheless complex and made up of tight junctions, gap junctions, adherence junctions, and syndesmos (4, 10). The molecular architecture of these cell connections is complex, with multiple proteins interacting between adjacent cells, which results in restriction of transendothelial diffusion of solutes, thus creating a barrier function (4). In vivo, such intercellular connections vary in complexity and permeability along the vascular tree, from well-organized and numerous in endothelial cells of large arteries and veins, where there is strict control of permeability, to near absence in postcapillary venules, where exchange of plasma constituents is efficient (10). As reviewed by Bazzoni (4), it has been hypothesized that intercellular connections and tight junctional strands in particular represent an impermeable structure that nonetheless contains pores or channels that may be regulated. Opening and closing of these pores in response to intracellular events such as changes ion conductance, cAMP concentrations, or cell swelling might regulate the passage of small ions (33). In addition, such pores also may have charge selectivity that could govern transfer of an anion such as ascorbate.
Paracellular movement of large molecules such as albumin or high-molecular-weight dextrans likely occurs through gaps or fenestrations between endothelial cells. It has been well documented that these gaps (and thus flux across them) are increased by anion channel blockers (16), decreased by elevated intracellular cAMP (8, 16, 19), and increased by vasoactive agents such as thrombin (8). Our results regarding ascorbate transfer across endothelial cells are in partial agreement with earlier studies using larger molecules. In contrast to the results of Griffin (16) in pulmonary artery endothelial cells, where radiolabeled albumin permeability was increased by anion channel blockers, in the present work several anion channel inhibitors (sulfinpyrazone, NPPB, niflumic acid, and probenecid) decreased ascorbate transfer across the EA.hy926 cell barrier. These inhibitors also decreased ascorbate uptake by inhibiting the SVCT2, but as noted above, intracellular ascorbate was not moved across the endothelium. The anion transporter inhibitor bumetanide had no effect in the previous (16) or in the present study. The opposite effects of anion channel blockade in the two studies may relate to differences in blocker specificity, cell types, and culture conditions, but both are likely due to channel-induced changes in cell volume and adherence as observed for cAMP, as discussed next.
cAMP-induced decreases in endothelial cell layer permeability to large molecules are thought to be due to changes in cell shape and volume that result in gap closure between endothelial cells (2), resembling those seen in endothelial cells in vivo (5). These changes are accompanied by rearrangements in F-actin and myosin stress fibers in the cells (19) and are completely reversed by treatment with the microtubule inhibitor colchicine (2). Our results for ascorbate transfer agree with earlier studies in that ascorbate transfer was decreased by IBMX, which inhibits cAMP breakdown, as well as by forskolin, which directly activates the cAMP-dependent protein kinase A.
Vasoactive agents such as thrombin increase endothelial barrier permeability to large molecules (8, 14), as was observed in the current study for ascorbate transfer. The effect of thrombin requires its enzymatic activity (8) and is explained by its ability to increase gaps between endothelial cells (13, 14), probably as a result of changes in actin stress fibers in the cells (14). Whereas these findings fit with the notion that ascorbate transfer is due to modification of cell adherence and formation or loss of gaps between the cells, the present results suggest that ascorbate transfer may also occur through apposed junctions between the cells.
Comparison of monolayer permeabilities between ascorbate, mannitol, and inulin fit the pattern expected on the basis of their molecular size. The permeability of inulin across both cells and filter was lower than observed for mannitol and ascorbate after 4 days in culture. However, at this time in culture, only ascorbate transfer was very sensitive to stimulation by thrombin and inhibition by sulfinpyrazone. As discussed below, we found that ascorbate itself decreased its own transfer across the endothelial cell barrier. Including ascorbate during the transfer assay did result in modest decreases in inulin transfer after 4 days of culture, which were significantly greater after 6 days in culture. Indeed, by 6 days in culture, inulin permeability had decreased to rates similar to those measured for albumin in other types of endothelial cells [0.02–022 cm/h (1, 32)]. Nonetheless, the differential sensitivity of ascorbate transfer compared with that of inulin at 4 days does suggest that ascorbate may transit the endothelial layer, at least in part, via a route different from that of mannitol or inulin. This effect may relate to charge and size selectivity of cellular junctions. Although polydisperse carboxydextran and dextran showed similar rates of movement across cultured fetal bovine aortic endothelial cells (1), implying lack of charge selectivity, this may not apply to a smaller anion such as ascorbate, which could enter channels, such as through tight junctions, not accessible to larger molecules. Tight junctions in epithelial cells have indeed been shown to have ion size and charge selectivity ions (33). Whether a similar selectivity applies to ascorbate transfer in endothelial cells is of course speculative, but this could explain the current results and provide a unique and regulated pathway for ascorbate transfer across the endothelium.
Utoguchi et al. (36) showed that ascorbate can enhance endothelial barrier function during prolonged culture. In their experiments, bovine artery endothelial cells were cultured for 5 days on porous membrane filters (0.45-μm pore size) with daily addition of ascorbate. Movement of both molecular weight 4,000 and 70,000 fluorescein dextran across the cells and filter was impeded by loading ascorbate concentrations over the physiological plasma concentration range (10–100 μM). This ascorbate-induced increase in barrier function was completely reversed in the presence of inhibitors of collagen synthesis, leading to the conclusion that collagen deposition was required for the increase in endothelial barrier function. However, the ascorbate-induced decrease in permeability of the cells alone was relatively greater than that of the filter and matrix, indicating that there might be an effect on the cells beyond that due to collagen deposition. Our studies differ from those of Utoguchi et al. (36) in that changes in endothelial barrier were measured after a single addition of ascorbate and occurred over minutes, not days. Significant ascorbate-dependent decreases in endothelial cell permeability were evident by 15 min, and even after 18 h ascorbate did not affect permeability of the filters plus matrix, ruling out collagen deposition as a cause. Although collagen deposition can affect endothelial barrier function, the present results define a novel role for ascorbate to acutely tighten the endothelial cell barrier. Since intracellular ascorbate also decreased its own transfer in primary cultures of endothelial cells derived from human dermal capillaries, and since ascorbate transfer across the endothelium probably occurs in capillary beds, the observed effect may be of physiological relevance.
The mechanism by which intracellular ascorbate acutely enhances endothelial barrier function remains to be determined. The results of earlier studies by Utoguchi et al. (36) showed that dithiothreitol did not mimic the effect of ascorbate, suggesting that an antioxidant effect is not involved. Nonetheless, it has been shown that oxidant stress due to hydrogen peroxide (20) treatment or to redox cycling of menadione (18) in endothelial cell cultures increases transendothelial permeability. In addition to a an antioxidant action, ascorbate also serves as a cofactor for cellular dioxygenase enzymes involved in hydroxylation of proline and other residues, such as on collagen or hypoxia-inducible factor-1α (HIF-1α) (15). Whatever the mechanism of the ascorbate effect, it is associated with changes in the cytoskeleton, as reflected by its inhibition by colchicine and by an increase in F-actin stress fibers. Although changes in stress fiber structure are well-established indicators of cytoskeletal effects, their formation or dissipation cannot be used to predict changes in endothelial barrier function. For example, both increased cAMP (19) and thrombin (14) decrease stress fiber formation, yet they have opposite effects on endothelial barrier function. How ascorbate might acutely affect the cytoskeleton remains to be determined.
In conclusion, this work provides evidence that ascorbate leaves the blood to enter tissues by a paracellular route between vascular endothelial cells and that this movement is regulated by brief treatment with ascorbate concentrations over the physiological plasma range.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-050435 and by Cell Culture Core of the Vanderbilt Diabetes Research and Training Center Grant DK-020593.
REFERENCES
- 1.Albelda SM, Sampson PM, Haselton FR, McNiff JM, Mueller SN, Williams SK, Fishman AP, Levine EM. Permeability characteristics of cultured endothelial cell monolayers. J Appl Physiol 64: 308–322, 1988. [DOI] [PubMed] [Google Scholar]
- 2.Antonov AS, Lukashev ME, Romanov YA, Tkachuk VA, Repin VS, Smirnov VN. Morphological alterations in endothelial cells from human aorta and umbilical vein induced by forskolin and phorbol 12-myristate 13-acetate: a synergistic action of adenylate cyclase and protein kinase C activators. Proc Natl Acad Sci USA 83: 9704–9708, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauer J, Margolis M, Schreiner C, Edgell CJ, Azizkhan J, Lazarowski E, Juliano RL. In vitro model of angiogenesis using a human endothelium-derived permanent cell line: contributions of induced gene expression, G-proteins, and integrins. J Cell Physiol 153: 437–449, 1992. [DOI] [PubMed] [Google Scholar]
- 4.Bazzoni G Endothelial tight junctions: permeable barriers of the vessel wall. Thromb Haemost 95: 36–42, 2006. [PubMed] [Google Scholar]
- 5.Bensch KG, Davison PM, Karasek MA. Factors controlling the in vitro growth pattern of human microvascular endothelial cells. J Ultrastruct Res 82: 76–89, 1983. [DOI] [PubMed] [Google Scholar]
- 6.Best KA, Holmes ME, Samson SE, Mwanjewe J, Wilson JX, Dixon SJ, Grover AK. Ascorbate uptake in pig coronary artery endothelial cells. Mol Cell Biochem 271: 43–49, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Boyer JC, Campbell CE, Sigurdson WJ, Kuo SM. Polarized localization of vitamin C transporters, SVCT1 and SVCT2, in epithelial cells. Biochem Biophys Res Commun 334: 150–156, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Casnocha SA, Eskin SG, Hall ER, McIntire LV. Permeability of human endothelial monolayers: effect of vasoactive agonists and cAMP. J Appl Physiol 67: 1997–2005, 1989. [DOI] [PubMed] [Google Scholar]
- 9.Davis KA, Samson SE, Best K, Mallhi KK, Szewczyk M, Wilson JX, Kwan CY, Grover AK. Ca2+-mediated ascorbate release from coronary artery endothelial cells. Br J Pharmacol 147: 131–139, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dejana E, Corada M, Lampugnani MG. Endothelial cell-to-cell junctions. FASEB J 9: 910–918, 1995. [PubMed] [Google Scholar]
- 11.Edgell CJ, McDonald CC, Graham JB. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc Natl Acad Sci USA 80: 3734–3737, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ek A, Ström K, Cotgreave IA. The uptake of ascorbic acid into human umbilical vein endothelial cells and its effect on oxidant insult. Biochem Pharmacol 50: 1339–1346, 1995. [DOI] [PubMed] [Google Scholar]
- 13.Galdal KS, Evensen SA, Brosstad F. Effects of thrombin on the integrity of monolayers of cultured human endothelial cells. Thromb Res 27: 575–584, 1982. [DOI] [PubMed] [Google Scholar]
- 14.Garcia JG, Siflinger-Birnboim A, Bizios R, del Vecchio PJ, Fenton JW, Malik AB. Thrombin-induced increase in albumin permeability across the endothelium. J Cell Physiol 128: 96–104, 1986. [DOI] [PubMed] [Google Scholar]
- 15.Grano A, De Tullio MC. Ascorbic acid as a sensor of oxidative stress and a regulator of gene expression: the Yin and Yang of vitamin C. Med Hypotheses 69: 953–954, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Griffin MP Role for anions in pulmonary endothelial permeability. J Appl Physiol 83: 615–622, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Jones W, Li X, Perriott LM, Whitesell RR, May JM. Uptake, recycling, and antioxidant functions of α-lipoic acid in endothelial cells. Free Radic Biol Med 33: 83–93, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Lagrange P, Romero IA, Minn A, Revest PA. Transendothelial permeability changes induced by free radicals in an in vitro model of the blood-brain barrier. Free Radic Biol Med 27: 667–672, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Langeler EG, van Hinsbergh VW. Norepinephrine and iloprost improve barrier function of human endothelial cell monolayers: role of cAMP. Am J Physiol Cell Physiol 260: C1052–C1059, 1991. [DOI] [PubMed] [Google Scholar]
- 20.Lee HS, Namkoong K, Kim DH, Kim KJ, Cheong YH, Kim SS, Lee WB, Kim KY. Hydrogen peroxide-induced alterations of tight junction proteins in bovine brain microvascular endothelial cells. Microvasc Res 68: 231–238, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Maulen NP, Henriquez EA, Kempe S, Carcamo JG, Smid-Kotsas A, Bachem M, Grunen A, Bustamante ME, Nualart F, Vera JC. Upregulation and polarized expression of the sodium-ascorbic acid transporter SVCT1 in post-confluent differentiated CaCo-2 cells. J Biol Chem 278: 9035–9041, 2003. [DOI] [PubMed] [Google Scholar]
- 22.May JM, Qu ZC. Transport and intracellular accumulation of vitamin C in endothelial cells: relevance to collagen synthesis. Arch Biochem Biophys 434: 178–186, 2005. [DOI] [PubMed] [Google Scholar]
- 23.May JM, Qu ZC, Neel DR, Li X. Recycling of vitamin C from its oxidized forms by human endothelial cells. Biochim Biophys Acta 1640: 153–161, 2003. [DOI] [PubMed] [Google Scholar]
- 24.May JM, Qu ZC, Qiao H, Koury MJ. Maturational loss of the vitamin C transporter in erythrocytes. Biochem Biophys Res Commun 360: 295–298, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.May JM, Qu ZC. Ascorbic acid efflux and re-uptake in endothelial cells: maintenance of intracellular ascorbate. Mol Cell Biochem 325: 79–88, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.May JM, Qu ZC, Mendiratta S. Protection and recycling of α-tocopherol in human erythrocytes by intracellular ascorbic acid. Arch Biochem Biophys 349: 281–289, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Mendiratta S, Qu ZC, May JM. Erythrocyte ascorbate recycling: antioxidant effects in blood. Free Radic Biol Med 24: 789–797, 1998. [DOI] [PubMed] [Google Scholar]
- 28.Miele M, Boutelle MG, Fillenz M. The physiologically induced release of ascorbate in rat brain is dependent on impulse traffic, calcium influx and glutamate uptake. Neuroscience 62: 87–91, 1994. [DOI] [PubMed] [Google Scholar]
- 29.Nilius B, Droogmans G. Amazing chloride channels: an overview. Acta Physiol Scand 177: 119–147, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Pech-Amsellem MA, Myara I, Pico I, Maziere C, Maziere JC, Moatti N. Oxidative modifications of low-density lipoproteins (LDL) by the human endothelial cell line EA.hy926. Experientia 52: 234–238, 1996. [DOI] [PubMed] [Google Scholar]
- 31.Qiao H, May JM. Development of ascorbate transport in brain capillary endothelial cells in culture. Brain Res 1208: 79–86, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siflinger-Birnboim A, del Vecchio PJ, Cooper JA, Blumenstock FA, Shepard JM, Malik AB. Molecular sieving characteristics of the cultured endothelial monolayer. J Cell Physiol 132: 111–117, 1987. [DOI] [PubMed] [Google Scholar]
- 33.Tang VW, Goodenough DA. Paracellular ion channel at the tight junction. Biophys J 84: 1660–1673, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsukaguchi H, Tokui T, Mackenzie B, Berger UV, Chen XZ, Wang YX, Brubaker RF, Hediger MA. A family of mammalian Na+-dependent l-ascorbic acid transporters. Nature 399: 70–75, 1999. [DOI] [PubMed] [Google Scholar]
- 35.Upston JM, Karjalainen A, Bygrave FL, Stocker R. Efflux of hepatic ascorbate: a potential contributor to the maintenance of plasma vitamin C. Biochem J 342: 49–56, 1999. [PMC free article] [PubMed] [Google Scholar]
- 36.Utoguchi N, Ikeda K, Saeki K, Oka N, Mizuguchi H, Kubo K, Nakagawa S, Mayumi T. Ascorbic acid stimulates barrier function of cultured endothelial cell monolayer. J Cell Physiol 163: 393–399, 1995. [DOI] [PubMed] [Google Scholar]
- 37.Vera JC, Rivas CI, Fischbarg J, Golde DW. Mammalian facilitative hexose transporters mediate the transport of dehydroascorbic acid. Nature 364: 79–82, 1993. [DOI] [PubMed] [Google Scholar]
- 38.Wilson JX Regulation of vitamin C transport. Annu Rev Nutr 25: 105–125, 2005. [DOI] [PubMed] [Google Scholar]