Abstract
The metabolic sensor AMP-activated protein kinase (AMPK) has emerged as an important link between cellular metabolic status and ion transport activity. We previously found that AMPK binds to and phosphorylates CFTR in vitro and inhibits PKA-dependent stimulation of CFTR channel gating in Calu-3 bronchial serous gland epithelial cells. To further characterize the mechanism of AMPK-dependent regulation of CFTR, whole cell patch-clamp measurements were performed with PKA activation in Calu-3 cells expressing either constitutively active or dominant-negative AMPK mutants (AMPK-CA or AMPK-DN). Baseline CFTR conductance in cells expressing AMPK-DN was substantially greater than controls, suggesting that tonic AMPK activity in these cells inhibits CFTR under basal conditions. Although baseline CFTR conductance in cells expressing AMPK-CA was comparable to that of controls, PKA stimulation of CFTR was completely blocked in AMPK-CA-expressing cells, suggesting that AMPK activation renders CFTR resistant to PKA activation in vivo. Phosphorylation studies of CFTR in human embryonic kidney-293 cells using tetracycline-inducible expression of AMPK-DN demonstrated AMPK-dependent phosphorylation of CFTR in vivo. However, AMPK activity modulation had no effect on CFTR in vivo phosphorylation in response to graded doses of PKA or PKC agonists. Thus, AMPK-dependent CFTR phosphorylation renders the channel resistant to activation by PKA and PKC without preventing phosphorylation by these kinases. We found that Ser768, a CFTR R domain residue considered to be an inhibitory PKA site, is the dominant site of AMPK phosphorylation in vitro. Ser-to-Ala mutation at this site enhanced baseline CFTR activity and rendered CFTR resistant to inhibition by AMPK, suggesting that AMPK phosphorylation at Ser768 is required for its inhibition of CFTR. In summary, our findings indicate that AMPK-dependent phosphorylation of CFTR inhibits CFTR activation by PKA, thereby tuning the PKA-responsiveness of CFTR to metabolic and other stresses in the cell.
Keywords: Cl− channels, patch-clamp, metabolism, Xenopus oocytes, cystic fibrosis, transmembrane conductance regulator
cftr, the pka-activated Cl− channel that is defective in cystic fibrosis, contains 12 predicted transmembrane helices and five cytoplasmic domains consisting of two nucleotide-binding domains (NBDs), a regulatory (R) domain containing numerous consensus PKA and PKC phosphorylation sequences, and NH2- and COOH-terminal cytoplasmic tails (25, 26). CFTR activation requires PKA-dependent phosphorylation of the R domain and ATP binding and hydrolysis at the NBDs (3, 6). In addition, protein kinase C (PKC)-dependent phosphorylation of CFTR occurs at multiple sites and is required for full PKA-dependent activation of CFTR (5, 19). It has been proposed that the requirement for ATP could allow CFTR to actively transport substrates across the plasma membrane (29), although none have been identified, and/or afford coupling of CFTR activity to cellular metabolism (24).
Studies in our laboratory first demonstrated that the metabolic-sensing kinase AMPK binds to the COOH-terminal tail of CFTR, phosphorylates CFTR in vitro, shares apical colocalization with CFTR in relevant epithelia, and inhibits CFTR Cl− channel activity in bronchial Calu-3 cells and colonic T84 cells, primarily through an inhibition of PKA-stimulated CFTR channel gating (11–13). Work in oocytes suggested that CFTR inhibition by AMPK requires the presence of both the COOH-terminal CFTR-binding sequence of AMPK-α1 and an active kinase domain (13). This direct interaction may efficiently target AMPK to CFTR and thereby enable the close coupling of CFTR to cellular metabolic status. Indeed, because AMPK activity is highly sensitive to metabolic fluctuations and other cellular stresses (15), we have proposed that regulation by AMPK provides an exquisitely sensitive and efficient mechanism to couple the activity of CFTR and other membrane transport proteins to metabolic status and potentially other cellular stresses (9, 13). Although AMPK can phosphorylate CFTR in vitro (13), the relevant site(s) and functional consequences of AMPK-dependent phosphorylation in cells have not yet been established. Another area of uncertainty is whether or how AMPK is involved in the coordinated regulation of CFTR by PKA and PKC. It is also unclear whether AMPK plays a role in the regulation of CFTR under steady-state physiological conditions in the absence of perceptible metabolic stress.
This study was undertaken to further characterize the mechanisms of AMPK-dependent regulation of CFTR, both functionally and at a molecular level. Whole cell patch-clamp studies were performed in Calu-3 cells expressing endogenous CFTR and mutant forms of AMPK to assess the effects of AMPK on both basal CFTR activity and acute PKA-dependent stimulation of the channel. Cellular 32P-orthophosphate labeling approaches were used to demonstrate AMPK-dependent phosphorylation of CFTR in vivo and assess whether AMPK may modulate PKA or PKC-dependent phosphorylation of the channel. Finally, mutation of candidate phosphorylation sites and in vitro phosphorylation assays were used to identify a dominant AMPK phosphorylation site within the R domain of CFTR that is required for AMPK-dependent CFTR inhibition.
MATERIALS AND METHODS
Reagents, chemicals, and cell culture.
Reagents and chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise noted. Bronchial serous gland epithelial (Calu-3) cells and human embryonic kidney (HEK)-293 cells were maintained in culture as described previously (1, 2, 12).
Whole cell patch-clamp studies.
Calu-3 cell patch-clamp studies were performed essentially as described previously using an Axon 200A amplifier and PULSE+PULSEFIT software (HEKA) (12) or using a HEKA EPC-10 amplifier and PatchMaster software (HEKA). Cells were plated on glass coverslips and then transiently transfected with bi-cistronic pTracer plasmid to express green fluorescent protein (GFP) and AMPK-α1-K45R (kinase-dead, dominant-negative mutant; AMPK-DN), AMPK-γ1-R70Q (constitutively active mutant; AMPK-CA), or empty vector (12). Whole cell patch clamping was performed on GFP-positive cells 1–3 days after transfection. Standard bath and pipette solutions used were as described previously (17). The standard pipette solution also contained PKA catalytic subunit (200 U/ml; no. V5161, Promega) to activate CFTR upon break-in to whole cell mode. In additional experiments to assess basal CFTR-dependent whole cell conductance, PKA catalytic subunit was omitted from the pipette solution, and the specific CFTR blocker CFTR-Inh-172 (30 μM; Calbiochem/EMD BioSciences) was added to the bath solution following break-in to whole cell mode. CFTR-dependent whole cell conductances were measured as the reduction in whole cell conductance after CFTR-Inh-172 treatment relative to that before treatment. Whole cell conductance was calculated as the slope at −20 mV of a fifth-order polynomial fit to the current-voltage (I-V) data at each time point and corrected for whole cell capacitance to give a normalized conductance (pS/pF), as previously described (12).
In vivo phosphorylation studies.
Flp-In T-REx HEK-293 cells (Invitrogen) stably transfected to express AMPK-DN in a tetracycline (Tet)-inducible fashion were used for in vivo phosphorylation studies (1). Tet-dependent expression of the hemagglutinin (HA)-tagged AMPK-DN protein was detected by HA immunoblots. Modulation of cellular AMPK activity was quantitated using the in vitro synthetic AMPK substrate (SAMS) peptide AMPK activity assay performed on cell lysates (14). AMPK-dependent in vivo phosphorylation of CFTR was performed essentially as described previously (1). Cells were washed in phosphate-free efflux buffer and then labeled at 37°C with 0.5 mCi/ml 32P-orthophosphate during a 5-h incubation with either 2 mM metformin to acutely activate AMPK in the uninduced cells or vehicle control in cells that were Tet-induced for 3 days (AMPK inhibited). For some experiments, activators of PKA [forskolin, IBMX, and chlorophenylthio (CPT)-cAMP] or PKC (PMA) were added during the last 30 min of the labeling period. After this incubation, cells were washed twice in ice-cold phosphate-free buffer before lysis in ice-cold RIPA buffer containing phosphatase and protease inhibitors. After lysis, CFTR was immunoprecipitated from cell lysates, eluted, and subjected to SDS-PAGE followed by transfer to a nitrocellulose membrane. Membranes were immunoblotted for CFTR, and enhanced chemiluminescence was quantified by a Versadoc Imager (Bio-Rad). The same membrane was then exposed to a phospho-screen for quantitation of 32P incorporation into the CFTR band using a phospho-imager. After correction for background signal, the phosphorylation signal normalized to protein expression on the membrane was calculated and compared across conditions.
In vitro phosphorylation studies.
HEK-293 cells were transiently transfected with wild-type and various CFTR mutant constructs cloned into pcDNA3.1 (Invitrogen). After expression for 2 days, CFTR was immunoprecipitated from cell lysates for in vitro phosphorylation experiments. Purified recombinant active (α-T172D, β, γ) or inactive (α-D157A, β, γ) AMPK holoenzyme [∼2 μg; generous gift of Dr. D. Neumann (22)] was added to a reaction mix containing immunoprecipitated CFTR and 10–20 μCi [γ-32P]-ATP at room temp for 60 min, and then the CFTR was washed, eluted, and subjected to SDS-PAGE followed by transfer to a nitrocellulose membrane. The 32P incorporation into CFTR and CFTR protein expression was quantified as described in In vivo phosphorylation studies. The CFTR-10SA construct, which has 10 dominant PKA phosphorylation sites mutated to Ala (4), was subcloned from pNUT (generous gift from Dr. J. Riordan) into pcDNA3.1 for expression in HEK-293 cells. CFTR mutants were generated using the Stratagene QuikChange mutagenesis kit using either wild-type CFTR (CFTR-WT) or CFTR-10SA as a template for the reaction. All clones were confirmed by DNA sequencing.
Two-electrode voltage clamp.
Surgical removal of oocytes from Xenopus laevis was performed using protocols approved by the University of Pittsburgh Institutional Animal Use and Care Committee. One day after harvesting, oocytes were injected with either wild-type CFTR or CFTR-S768A cRNA (1 ng). Oocytes were used 2–3 days postinjection for experiments, and either potassium gluconate (KG; 32 nl of 40 mM stock) as a control or the AMP-mimetic AMPK activator potassium 5-aminoimidazole-4-carboxamide ribonucleoside monophosphate (ZMP; 32 nl of 40 mM stock) was microinjected ∼1–3 h before use as previously described (2). To stimulate CFTR current, the PKA agonists forskolin (1 μM) and IBMX (0.1 mM) were bath applied via perfusion manifold. The voltage commands used to evoke current were ramps from −20 to 60 mV as previously described (13). Data were acquired via a Warner Instruments Oocyte Clamp amplifier (OC-725C), and a PC computer running Clampex 8.1.
Statistical analysis.
Statistical analyses were performed using StatView (SAS) software. Analysis of variance (ANOVA) was used to compare data obtained from different batches of oocytes for two-electrode voltage-clamp (TEV) experiments. For other biochemical experiments, statistical analyses were performed using Student's t-tests, as described for each figure. In all cases, P < 0.05 was considered significant. Measurements are stated as means ± SE.
RESULTS
Effects of AMPK mutants on resting and PKA-stimulated CFTR conductance.
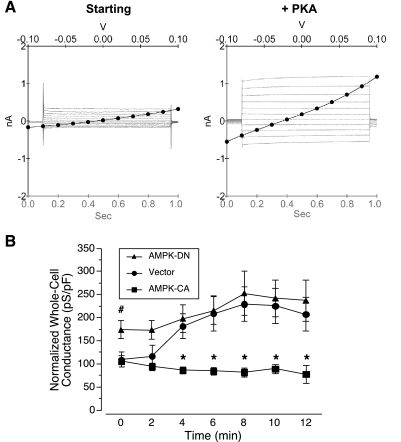
To determine the effects of AMPK activity on baseline CFTR conductance and the acute stimulation of CFTR by PKA, whole cell patch-clamp measurements of CFTR conductance were performed on Calu-3 cells transiently transfected with constitutively active (γ1-R70Q; AMPK-CA) or dominant-negative (α1-K45R; AMPK-DN) AMPK mutants, or with vector alone (vector) (Fig. 1). The pipette solution contained PKA catalytic subunit, which, after break-in to whole cell mode at time 0, could then diffuse into the cells and activate CFTR conductance. Representative I-V and current-time sweeps from before and after PKA stimulation in vector-transfected cells are shown (Fig. 1A). Vector-transfected cells displayed an increase in whole cell conductance of >100% within 8–10 min after break-in to whole cell mode (Fig. 1B). In cells expressing AMPK-DN, the baseline conductance after breaking in was significantly greater than in control, suggesting that AMPK has a tonic level of activity under control conditions that inhibits CFTR. Indeed, the elevated basal whole cell conductance in AMPK-DN-expressing cells is largely attributable to CFTR, because additional experiments demonstrated greater reduction in basal whole cell conductance following treatment with the CFTR channel blocker CFTR-Inh-172 (30 μM) in AMPK-DN-expressing cells as compared with vector-transfected cells (reduction of 2.75 ± 0.74 nS vs. 0.87 ± 0.44 nS, respectively; or % inhibition of the basal conductance by 76.4 ± 12.2 vs. 41.4 ± 8.1, respectively; P < 0.05, unpaired t-test, n = 5–6 experiments for each condition). In the AMPK-DN-expressing cells, there was only slight further activation of CFTR with PKA stimulation to a level that was not significantly different from that measured in control cells. In cells expressing the AMPK-CA mutant, the ability of PKA to stimulate whole cell CFTR conductance was completely blocked, suggesting that chronic AMPK activation may render CFTR in a state where it can no longer become effectively activated by PKA acutely. This result is consistent with our earlier finding that chronic AMPK activation inhibited CFTR gating by stabilizing a channel-closed state of CFTR in these cells (12). Alternatively, it is conceivable that AMPK activation could inhibit PKA-mediated phosphorylation of CFTR in vivo, thereby accounting for the inhibition (see below).
Fig. 1.
Effects of modulating AMPK activity on whole cell conductance responses of Calu-3 cells to PKA stimulation. A: representative current-voltage (I-V) plots with superimposed current-time sweeps from vector-transfected cells are shown just after break-in to the whole cell mode at time 0 (left) and after 10 min of PKA catalytic subunit diffusion from the pipette solution into the cell (right). B: time course of mean (± SE) whole cell conductances normalized to whole cell capacitance measured at the start of each experiment in Calu-3 cells transfected with either dominant-negative AMPK mutant (AMPK-DN; α1-K45R), constitutively active AMPK mutant (AMPK-CA; γ1-R70Q), or vector (pTracer). AMPK-CA inhibited PKA-dependent activation of CFTR (*P < 0.05 vs. vector control at same time points, unpaired t-test). Conductance in cells expressing AMPK-DN was greater than vector control at time 0 (#P < 0.05, unpaired t-test; n = 5–7 experiments for each condition).
AMPK-dependent phosphorylation of CFTR in vivo.
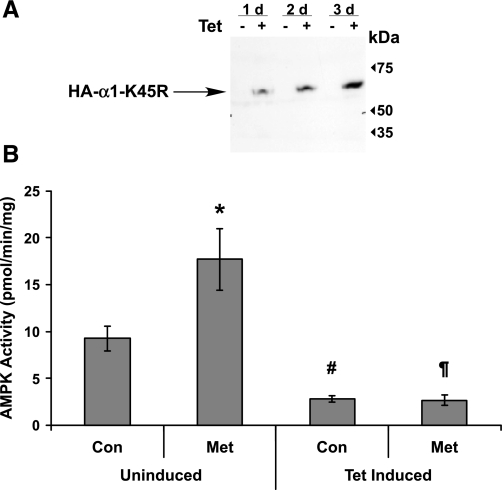
Poor transfection/transduction efficiency makes it difficult to specifically modulate AMPK activity in a population of Calu-3 cells. Therefore, to test whether AMPK-dependent phosphorylation of CFTR occurs in cells, we developed HEK-293 cells that are stably transfected with AMPK-DN (α1-K45R) under a Tet-inducible promoter, and we transiently transfected CFTR into these cells 2 days before in vivo phosphorylation experiments. Control experiments demonstrated that uninduced cells do not express detectable AMPK-DN protein and that three days of Tet induction is optimal for protein expression (Fig. 2A). As measured by an in vitro SAMS peptide kinase assay performed on cell lysates (11), AMPK was acutely activated in uninduced cells by a 5-h treatment with the AMPK activator metformin (2 mM), an effect that was blocked in Tet-induced cells overexpressing AMPK-DN (Fig. 2B). Moreover, AMPK activity was significantly inhibited in the Tet-induced cells relative to that of uninduced controls (Fig. 2B). We used these conditions to produce a wide range of AMPK activities for in vivo phosphorylation studies by comparing in vivo 32P incorporation into CFTR in cells that were Tet-induced for 3 days with that in metformin-treated uninduced cells. With cellular AMPK activation, CFTR phosphate labeling increased modestly, but reproducibly, by ∼40% relative to baseline levels in AMPK-inhibited cells (Fig. 3B), indicating that significant AMPK-dependent phosphorylation of CFTR occurs constitutively in vivo. The significant residual phosphorylation signal in the absence of AMPK activity likely represents CFTR phosphorylation by other kinases (e.g., PKA and PKC).
Fig. 2.
Human embryonic kidney (HEK)-293 Flp-In T-REx cells express hemagglutinin (HA)-tagged, AMPK-DN (α1-K45R) in a tetracycline (Tet)-inducible fashion. A: immunoblot demonstrating time course of expression after Tet-induction. d, Day. B: AMPK activity in uninduced vs. 3-day-induced cells ± 2 mM metformin (Met), an AMPK activator [*P < 0.05 and #P < 0.001 compared with uninduced control (Con); ¶P < 0.001 compared with uninduced Met; unpaired t-tests, n = 4–17].
Fig. 3.
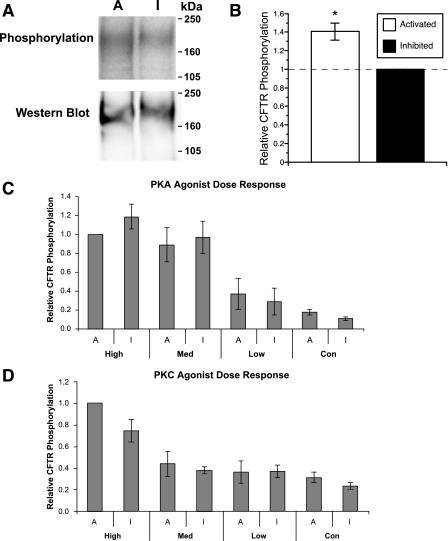
In vivo phosphorylation of CFTR expressed in HEK-293 Flp-In T-REx AMPK-DN cells with AMPK either activated (A = uninduced + metformin) or inhibited (I = Tet-induced). A: representative phospho-screen image (top) and immunoblot (bottom) of CFTR on the same membrane. B: summary of mean (± SE) relative CFTR phosphorylation with AMPK activated versus inhibited (*P = 0.005; paired t-test, n = 9). C: relative phosphorylation with graded cellular PKA stimulation (high, 10 μM forskolin + 1 mM IBMX + 250 μM chlorophenylthio-cAMP; Med, 1 μM forskolin + 0.1 mM IBMX; low, 0.1 μM forskolin; n = 4 replicate experiments). D: relative phosphorylation with graded cellular PKC stimulation (high, 100 nM PMA; Med, 10 nM PMA; low, 1 nM PMA; n = 5 replicate experiments). None of the paired samples at any given level of agonist stimulation were significantly different from each other except for the pooled unstimulated controls, as shown in B.
CFTR likely exists within a complex of closely associated proteins, including those involved in its PKA- and PKC-mediated phosphorylation (17, 21). PKA-mediated phosphorylation activates CFTR gating by destabilizing a closed channel state(s) (30), whereas AMPK appears to inhibit CFTR gating by stabilizing a closed channel state(s), the opposite effect (12). Also, constitutive PKC phosphorylation of CFTR is necessary for robust PKA-dependent CFTR activation (5, 19). Thus, we considered the possibility that AMPK inhibits CFTR channel gating by either inhibiting the activity of these kinases or by specifically interfering with their phosphorylation of CFTR in vivo. To test whether modulation of endogenous AMPK activity significantly affects PKA- or PKC-dependent CFTR phosphorylation in vivo, we examined 32P incorporation into CFTR after graded stimulation with low-, medium-, or high-dose agonists of PKA (forskolin, IBMX, and CPT-cAMP; Fig. 3C) or PKC (PMA; Fig. 3D), with AMPK either activated or inhibited. To enable results to be compared across experiments, CFTR phosphorylation levels were normalized to those in the presence of high-dose PKA or PKC agonist treatment with AMPK activated. CFTR phosphorylation was enhanced in a dose-dependent fashion with increasing concentrations of PKA agonists (Fig. 3C) or of PMA to increase PKC stimulation (Fig. 3D) Notably, AMPK activity modulation had no significant effect on the degree of CFTR phosphorylation at any given stimulation level.
AMPK phosphorylation of CFTR in vitro.
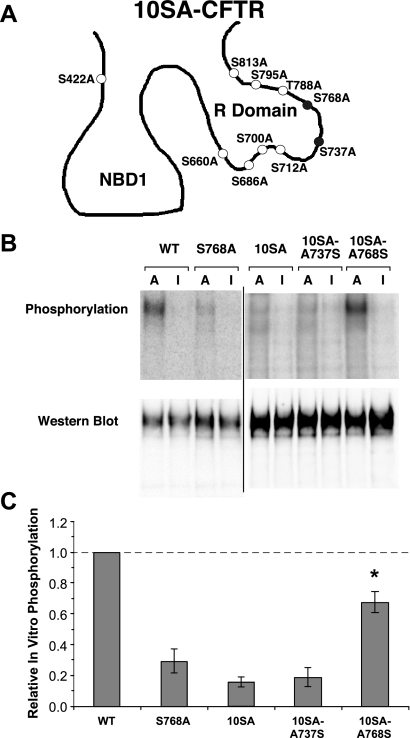
To examine direct, AMPK-mediated phosphorylation of CFTR in vitro, highly purified, recombinant active (α1-T172D, β1, γ1) and kinase-dead (α1-D157A, β1, γ1) mutants of human AMPK holoenzyme were used in vitro to phosphorylate either WT or mutant (10SA) CFTR in the presence of [γ-32P]-ATP (Fig. 4B). The ratio of the phosphorylation signal to the immunoblot signal in each lane was compared across conditions to derive relative phosphorylation levels for each condition (Fig. 4C). Virtually no background phosphorylation occurred in the presence of inactive AMPK (“I”), suggesting that the 32P labeling was specific for AMPK in this assay. Robust AMPK-dependent phosphorylation of CFTR-WT was observed (Fig. 4B, left). To determine whether AMPK phosphorylation site(s) in CFTR overlap with known phosphorylation sites of other kinases (e.g., PKA), we compared CFTR-WT phosphorylation with that of a CFTR mutant harboring Ala mutations at 10 important PKA phosphorylation sites, CFTR-10SA (4). A substantial ∼80% reduction in AMPK-dependent in vitro phosphorylation was observed in the CFTR-10SA mutant, suggesting that AMPK-dependent phosphorylation site(s) in CFTR overlap with those of PKA. Because the effect of AMPK is to inhibit CFTR, this result led us to speculate that AMPK phosphorylation might occur at one or both of two reported inhibitory PKA sites on CFTR, Ser737 or Ser768 (8, 27, 28).
Fig. 4.
In vitro phosphorylation of CFTR-wild type (WT) and various mutants by purified active (A) vs. inactive (I) AMPK holoenzyme. A: schematic representation of the CFTR-10SA construct with the two inhibitory residues shaded black. NBD1, nucleotide-binding domain 1; R domain, regulatory domain. B: representative phospho-screen images and corresponding Western blots of the various CFTR mutants. The WT and S768A images shown are from a different membrane than the rest of the mutants shown. C: in vitro phosphorylation, corrected for CFTR protein expression and normalized to that of CFTR-WT (*P < 0.01, unpaired t-tests compared with the S768A, 10SA, and 10SA-A737S mutants; n = 4–6 separate experiments). There were no significant differences in phosphorylation intensity among the S768A, 10SA, and 10SA-A737S mutants (P > 0.10, unpaired t-tests). However, the phosphorylation intensities of all three of these mutants were dramatically reduced when compared with either WT or the 10SA-A768S mutant (P < 0.01 for all comparisons, unpaired t-tests). Thus, the Ser768 site appears to be the only relevant one among the 10 sites for AMPK phosphorylation in vitro.
Ser768 is a major site of AMPK-dependent phosphorylation of CFTR in vitro.
Further experiments were undertaken to evaluate the specific sites of AMPK-dependent phosphorylation of CFTR. Because the functional effect of AMPK is to inhibit CFTR, and Ser737 and Ser768 have been described as “inhibitory” PKA phosphorylation sites (8, 27, 28), we initially generated mutant full-length CFTR constructs with corresponding point mutations in a wild-type CFTR background, as well as revertants in the CFTR-10SA background, and cloned these into mammalian and oocyte expression vectors. These constructs were used to test whether AMPK phosphorylates CFTR in vitro at either of these two inhibitory PKA phosphorylation sites in the R domain (Fig. 4A). AMPK-dependent phosphorylation of CFTR-S768A was substantially reduced (by ∼70%) compared with that of wild-type CFTR, to levels similar to that of the CFTR-10SA mutant (Fig. 4, B and C). Furthermore, when Ala at position 768 was mutated back to Ser in the 10SA background (CFTR-10SA-A768S), AMPK-dependent phosphorylation of CFTR was largely restored. In contrast, AMPK-mediated phosphorylation was not restored in the CFTR-10SA-A737S mutant. These results strongly implicate that Ser768 is a major site of AMPK-dependent phosphorylation of CFTR in vitro. It was reported previously that CFTR-S768A exhibits a very high open probability, whereas the phospho-mimic Ser-to-Asp S768D CFTR mutant has a very low open probability relative to wild-type CFTR following PKA stimulation (8, 27, 28). These findings are consistent with the idea that AMPK phosphorylation at this residue may cause inhibition of PKA-stimulated CFTR gating.
Mutation of Ser768 in CFTR prevents AMPK-dependent inhibition of CFTR following PKA stimulation.
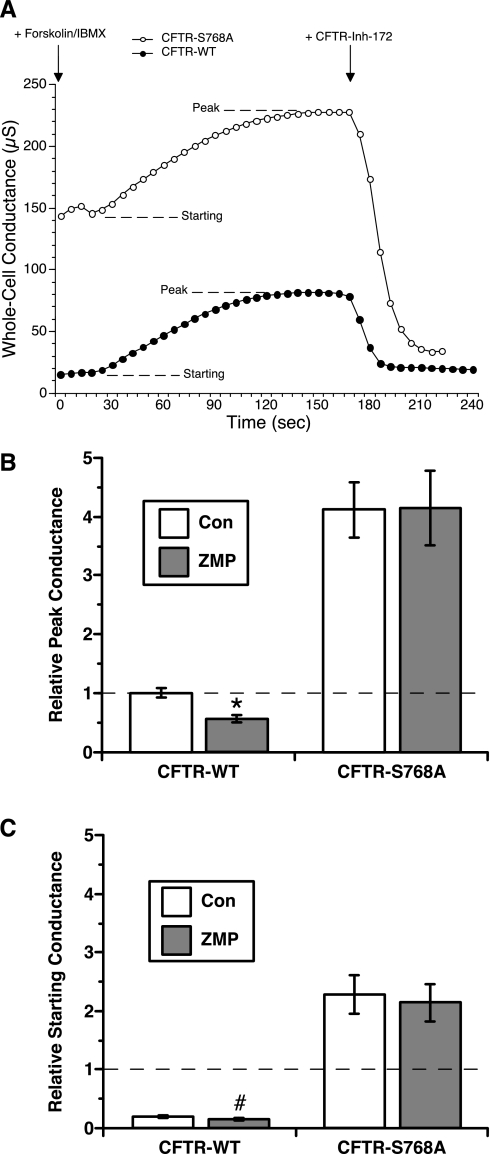
The relevance of Ser768 for AMPK modulation of CFTR activity was further investigated in TEV studies of Xenopus oocytes expressing either wild-type CFTR or CFTR-S768A. Whole cell conductance was measured before and after stimulation with 1 μM forskolin + 100 μM IBMX in oocytes that had been injected 1–3 h before TEV measurements with either an AMPK activator (ZMP) or a control solution (KG) (Table 1 and Fig. 5A). Previous work indicates that AMPK is endogenously expressed in Xenopus oocytes, because overexpression of the dominant-negative AMPK-α1-K45R mutant in oocytes blocked the ZMP-mediated inhibition of epithelial Na+ channel current (2). A substantial ∼50% decrease in peak CFTR-WT conductance was observed in ZMP-injected oocytes compared with KG-injected controls (P < 0.01; Fig. 5B). Peak currents following stimulation with PKA agonists were significantly greater in oocytes expressing CFTR-S768A than in those expressing CFTR-WT, consistent with previous results (8). Of note, ZMP was without effect on the peak conductance of oocytes expressing CFTR-S768A, suggesting that this mutant is resistant to AMPK-dependent gating inhibition. In addition, baseline, unstimulated CFTR conductances were enhanced by approximately fivefold in oocytes expressing CFTR-S768A (Fig. 5C), suggesting that CFTR-WT conductance is tonically inhibited in oocytes by Ser768 phosphorylation. Taken together, these results suggest that AMPK-dependent phosphorylation at Ser768 is necessary for its regulation of CFTR activity.
Table 1.
Whole cell CFTR conductance before and after addition of PKA agonists with or without AMPK activation in Xenopus oocytes
| Starting Conductance, μS | Peak Conductance, μS | |
|---|---|---|
| CFTR-WT + KG | 12.4±1.4 | 67.0±9.3 |
| CFTR-WT + ZMP | 8.5±0.9 | 30.5±3.8 |
| CFTR-S768A + KG | 146.9±13.0 | 256.6±15.6 |
| CFTR-S768A + ZMP | 128.5±9.0 | 238.7±13.7 |
Values are means ± SE of whole cell two-electrode voltage clamp conductances measured before and at the peak after infusion of 1 μM forskolin and 100 μM IBMX. Data are derived from measurements on 21–23 individual oocytes (from 5 separate batches) for each condition performed 2–3 days following cRNA injection and 1–3 h after injection of potassium gluconate (KG) or 5-aminoimidazole-4-carboxamide ribonucleoside monophosphate (ZMP). WT, wild type.
Fig. 5.
Two-electrode voltage-clamp (TEV) measurements of whole cell conductances during PKA agonist stimulation in WT- versus mutant CFTR-expressing Xenopus oocytes with or without AMPK activation. A: representative whole cell conductance recordings from control [potassium gluconate (KG)]-injected oocytes expressing either CFTR-WT or CFTR-S768A. Traces demonstrate the absolute whole cell conductance values measured, the time courses of activation after treatment with 1 μM forskolin + 100 μM IBMX (with starting and peak levels indicated), and the sensitivity to inhibition by treatment at the indicated time with the specific CFTR inhibitor CFTR-Inh-172 (30 μM). B: mean (± SE) peak conductances normalized to the mean control (KG)-injected, CFTR-WT peak conductance for that experimental day and batch in CFTR-WT versus CFTR-S768A-expressing oocytes injected with either control (KG) or AMPK activator [5-aminoimidazole-4-carboxamide ribonucleoside monophosphate (ZMP)]. A significant decrease in relative peak conductance was observed for ZMP-injected, CFTR-WT-expressing oocytes (*P < 0.01) but not for ZMP-injected CFTR-S768A-expressing oocytes. C: mean (± SE) starting conductances for the four experimental conditions relative to the mean KG-injected, CFTR-WT peak conductance for that experimental day and batch. CFTR-S768A-expressing oocytes had a dramatically elevated prestimulation starting conductance relative to that of CFTR-WT-expressing oocytes. There was also a ∼40–50% decrease in starting conductance of CFTR-WT-expressing oocytes injected with ZMP versus KG (#P < 0.05), whereas there was no difference in starting conductance between the two conditions for CFTR-S768A-expressing oocytes.
DISCUSSION
In this study we focused on defining the mechanisms underlying CFTR regulation by the metabolic-sensing kinase AMPK. We found that bronchial Calu-3 cells expressing dominant-negative AMPK have substantially elevated baseline CFTR-dependent whole cell conductance relative to controls before any exogenous PKA stimulation of the channel (Fig. 1). This intriguing result suggests that there is tonic inhibition of CFTR by AMPK in the absence of any perceptible metabolic stress in these cells. Conversely, PKA stimulation of CFTR conductance was fully blocked in Calu-3 cells expressing a constitutively active form of AMPK (Fig. 1). We previously showed that CFTR is a target for AMPK-mediated phosphorylation in vitro (13). Here, using inducible expression of mutant AMPK to modulate AMPK activity in HEK-293 cells, we have demonstrated AMPK-dependent phosphorylation of CFTR in vivo (Figs. 2 and 3). Importantly, in vivo CFTR phosphorylation by graded doses of either PKA or PKC agonists was not affected by AMPK activity modulation, indicating that AMPK modulation of CFTR channel activity is not mediated by alterations of the ability of either of these two kinases to phosphorylate CFTR (Fig. 3). Taken together, these results suggest that cellular AMPK activity and AMPK-dependent phosphorylation of CFTR renders the channel in a state or conformation that is resistant to gating activation by PKA phosphorylation.
Our in vitro phosphorylation assays using wild-type and various mutants of CFTR demonstrated that Ser768 is a major AMPK phosphorylation site in CFTR (Fig. 4). Of note, this is a previously described “inhibitory” PKA phosphorylation site in the R domain of CFTR (8, 27, 28). The amino acid sequence in the vicinity of this site conforms reasonably well to the loose substrate recognition motif reported for AMPK (ISVISTGPTLQARRRQSVLNL; where S is the phosphorylated residue, basic residues are present at the P-3 and P-4 positions, and hydrophobic residues are present at the P+4, P-7, P-13, and P-16 positions) (16). Consistent with previous observations (8), we found that mutation of this site to Ala (S768A) greatly enhanced baseline, unstimulated conductance of CFTR expressed in Xenopus oocytes, and it reduced the relative activation of CFTR by PKA agonists (Fig. 5). The apparent constitutive activation of CFTR-S768A is reminiscent of that observed for CFTR-WT in cells with AMPK activity inhibited by overexpression of AMPK-DN (Fig. 1). These observations suggest that AMPK tonically inhibits CFTR in the absence of PKA stimulation via AMPK-dependent phosphorylation of CFTR at Ser768. The importance of AMPK phosphorylation at Ser768 is further demonstrated by the observation that whereas PKA stimulation of CFTR-WT conductance was inhibited by the AMPK activator ZMP, PKA stimulation of CFTR-S768A was insensitive to ZMP. Thus, AMPK-dependent phosphorylation at Ser768 appears to be necessary for AMPK regulation of CFTR activity.
Although AMPK phosphorylation at Ser768 appears to be necessary, it is unlikely to be sufficient by itself to account for AMPK-dependent inhibition of CFTR. Of note, PKA can and likely does phosphorylate CFTR at Ser768 (8), yet the overall effect of PKA by itself is to activate CFTR gating. Therefore, there may be additional factors involved in the AMPK-CFTR regulatory interaction [e.g., accessory proteins and/or AMPK-dependent phosphorylation of other site(s) on CFTR or other proteins]. Indeed, a residual AMPK-mediated phosphorylation of 25–30% was observed in CFTR-S768A (Fig. 4), suggesting that other AMPK phosphorylation sites in CFTR may exist. An additional accessory or regulatory protein that might help mediate the AMPK-dependent inhibition of CFTR is the nucleoside diphosphate kinase A, which has been reported to associate with AMPK and CFTR (26a).
As this manuscript was in the final stages of preparation, another study reached similar conclusions regarding CFTR regulation by AMPK and the importance of AMPK-dependent phosphorylation of Ser768 in this mechanism (20). Our results extend the findings of this recent study, which used pharmacological approaches to modulate AMPK activity in Xenopus oocytes. Here we have demonstrated, through expression of dominant-negative or activating AMPK mutants, that AMPK exerts an important inhibitory role on CFTR activity. This AMPK-dependent inhibition of CFTR can occur both tonically and during acute stimulation by PKA agonists in an endogenous CFTR-expressing epithelial cell line (Fig. 1). In contrast to the findings of Kongsuphol et al. (20), however, our results do not implicate AMPK-dependent phosphorylation of the other R domain PKA “inhibitory” site, Ser737. This difference may result from the fact that we have examined AMPK-dependent phosphorylation in the context of full-length CFTR, rather than with an isolated R domain construct, as in Ref. 20. Moreover, our results indicate for the first time that AMPK-dependent in vivo phosphorylation of CFTR occurs in intact cells, and furthermore that AMPK activity modulation does not affect the ability of either PKA or PKC to phosphorylate CFTR. Rather, AMPK appears to inhibit the ability of PKA phosphorylation to have its normal functional effects on channel gating. These findings suggest an antagonistic relationship between AMPK and PKA with respect to CFTR function. Of note, additional regulatory relationships between PKA and AMPK function have been reported. For example, cAMP-dependent signaling pathways can regulate AMPK activity via phosphorylation of the catalytic α-subunit (18). In addition, we have recently found that AMPK activation inhibits the cAMP/PKA-dependent accumulation of the vacuolar H+-ATPase at the apical membrane of epididymal clear cells (10, 23).
AMPK may be important in regulating CFTR not only under conditions of cellular metabolic stress, but also during normal physiological situations. Specifically, our observation that AMPK exerts effects that prevent tonic CFTR activation under normal conditions (Fig. 1) suggests that AMPK may play an important role in limiting CFTR activity in the absence of PKA and other agonist stimulation. Indeed, these data suggest that AMPK, rather than PKA, may be the physiologically relevant kinase that phosphorylates Ser768 in CFTR in vivo under resting conditions. A better understanding of the roles of AMPK under physiological versus pathological conditions in regulating CFTR function is an important goal for further study. As previously suggested (8), inhibitory phosphorylation at Ser768 may ensure activation of CFTR by strong signals while dampening its response to weak signals, thereby improving the signal-to-noise ratio in the PKA stimulation pathway.
GRANTS
This work was supported by the Cell Physiology Core of the National Institutes of Health Grant P30-DK-079307 “Pittsburgh Kidney Research Center.” It was also supported by grants from the National Institutes of Health (T32-HL-007563 to J. D. King, R01-GM-074999 to D. D. Mak, and K08-DK-059477 and R01-DK-075048 to K. R. Hallows) and from the Cystic Fibrosis Foundation (FOSKETT04G0 to J. K. Foskett and HALLOW06P0 to K. R. Hallows).
Acknowledgments
We thank Dietbert Neumann for the generous gift of purified active and inactive recombinant AMPK holoenzyme and John Riordan for the gift of the pNUT-CFTR-10SA plasmid.
REFERENCES
- 1.Bhalla V, Oyster NM, Fitch AC, Wijngaarden MA, Neumann D, Schlattner U, Pearce D, Hallows KR. AMP-activated kinase inhibits the epithelial Na+ channel through functional regulation of the ubiquitin ligase Nedd4–2. J Biol Chem 281: 26159–26169, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Carattino MD, Edinger RS, Grieser HJ, Wise R, Neumann D, Schlattner U, Johnson JP, Kleyman TR, Hallows KR. Epithelial sodium channel inhibition by AMP-activated protein kinase in oocytes and polarized renal epithelial cells. J Biol Chem 280: 17608–17616, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Carson MR, Travis SM, Welsh MJ. The two nucleotide-binding domains of cystic fibrosis transmembrane conductance regulator (CFTR) have distinct functions in controlling channel activity. J Biol Chem 270: 1711–1717, 1995. [DOI] [PubMed] [Google Scholar]
- 4.Chang XB, Tabcharani JA, Hou YX, Jensen TJ, Kartner N, Alon N, Hanrahan JW, Riordan JR. Protein kinase A (PKA) still activates CFTR chloride channel after mutagenesis of all 10 PKA consensus phosphorylation sites. J Biol Chem 268: 11304–11311, 1993. [PubMed] [Google Scholar]
- 5.Chappe V, Hinkson DA, Zhu T, Chang XB, Riordan JR, Hanrahan JW. Phosphorylation of protein kinase C sites in NBD1 and the R domain control CFTR channel activation by PKA. J Physiol 548: 39–52, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng SH, Rich DP, Marshall J, Gregory RJ, Welsh MJ, Smith AE. Phosphorylation of the R domain by cAMP-dependent protein kinase regulates the CFTR chloride channel. Cell 66: 1027–1036, 1991. [DOI] [PubMed] [Google Scholar]
- 8.Csanady L, Seto-Young D, Chan KW, Cenciarelli C, Angel BB, Qin J, McLachlin DT, Krutchinsky AN, Chait BT, Nairn AC, Gadsby DC. Preferential phosphorylation of R-domain serine 768 dampens activation of CFTR channels by PKA. J Gen Physiol 125: 171–186, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hallows KR Emerging role of AMP-activated protein kinase in coupling membrane transport to cellular metabolism. Curr Opin Nephrol Hypertens 14: 464–471, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Hallows KR, Alzamora R, Li H, Gong F, Smolak C, Neumann D, Pastor-Soler NM. AMP-activated protein kinase inhibits alkaline pH- and PKA-induced apical vacuolar H+-ATPase accumulation in epididymal clear cells. Am J Physiol Cell Physiol 296: C672–C681, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hallows KR, Kobinger GP, Wilson JM, Witters LA, Foskett JK. Physiological modulation of CFTR activity by AMP-activated protein kinase in polarized T84 cells. Am J Physiol Cell Physiol 284: C1297–C1308, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Hallows KR, McCane JE, Kemp BE, Witters LA, Foskett JK. Regulation of channel gating by AMP-activated protein kinase modulates cystic fibrosis transmembrane conductance regulator activity in lung submucosal cells. J Biol Chem 278: 998–1004, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Hallows KR, Raghuram V, Kemp BE, Witters LA, Foskett JK. Inhibition of cystic fibrosis transmembrane conductance regulator by novel interaction with the metabolic sensor AMP-activated protein kinase. J Clin Invest 105: 1711–1721, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamilton SR, Stapleton D, O'Donnell JB Jr, Kung JT, Dalal SR, Kemp BE, Witters LA. An activating mutation in the gamma1 subunit of the AMP-activated protein kinase. FEBS Lett 500: 163–168, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Hardie DG, Hawley SA. AMP-activated protein kinase: the energy charge hypothesis revisited. Bioessays 23: 1112–1119, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Hardie DG, Scott JW, Pan DA, Hudson ER. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett 546: 113–120, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Huang P, Trotter K, Boucher RC, Milgram SL, Stutts MJ. PKA holoenzyme is functionally coupled to CFTR by AKAPs. Am J Physiol Cell Physiol 278: C417–C422, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Hurley RL, Barre LK, Wood SD, Anderson KA, Kemp BE, Means AR, Witters LA. Regulation of AMP-activated protein kinase by multisite phosphorylation in response to agents that elevate cellular cAMP. J Biol Chem 281: 36662–36672, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Jia Y, Mathews CJ, Hanrahan JW. Phosphorylation by protein kinase C is required for acute activation of cystic fibrosis transmembrane conductance regulator by protein kinase A. J Biol Chem 272: 4978–4984, 1997. [DOI] [PubMed] [Google Scholar]
- 20.Kongsuphol P, Cassidy D, Hieke B, Treharne KJ, Schreiber R, Mehta A, Kunzelmann K. Mechanistic insight into control of CFTR by AMPK. J Biol Chem 284: 5645–5653, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liedtke CM, Yun CH, Kyle N, Wang D. Protein kinase C epsilon-dependent regulation of cystic fibrosis transmembrane regulator involves binding to a receptor for activated C kinase (RACK1) and RACK1 binding to Na+/H+ exchange regulatory factor. J Biol Chem 277: 22925–22933, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Neumann D, Woods A, Carling D, Wallimann T, Schlattner U. Mammalian AMP-activated protein kinase: functional, heterotrimeric complexes by co-expression of subunits in Escherichia coli. Protein Expr Purif 30: 230–237, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Pastor-Soler NM, Hallows KR, Smolak C, Gong F, Brown D, Breton S. Alkaline pH- and cAMP-induced V-ATPase membrane accumulation is mediated by protein kinase A in epididymal clear cells. Am J Physiol Cell Physiol 294: C488–C494, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quinton PM, Reddy MM. Control of CFTR chloride conductance by ATP levels through non-hydrolytic binding. Nature 360: 79–81, 1992. [DOI] [PubMed] [Google Scholar]
- 25.Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, Drumm ML, Iannuzzi MC, Collins FS, Tsui LC. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245: 1066–1073, 1989. [DOI] [PubMed] [Google Scholar]
- 26.Rommens JM, Iannuzzi MC, Kerem B, Drumm ML, Melmer G, Dean M, Rozmahel R, Cole JL, Kennedy D, Hidaka N, Zsiga M, Buchwald M, Riordan JR, Tsui LC, Collins FS. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science 245: 1059–1065, 1989. [DOI] [PubMed] [Google Scholar]
- 26a.Treharne KJ, Best OG, Mehta A. The phosphorylation status of membrane-bound nucleoside diphosphate kinase in epithelia and the role of AMP. Mol Cell Biochem. In press. [DOI] [PMC free article] [PubMed]
- 27.Vais H, Zhang R, Reenstra WW. Dibasic phosphorylation sites in the R domain of CFTR have stimulatory and inhibitory effects on channel activation. Am J Physiol Cell Physiol 287: C737–C745, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Wilkinson DJ, Strong TV, Mansoura MK, Wood DL, Smith SS, Collins FS, Dawson DC. CFTR activation: additive effects of stimulatory and inhibitory phosphorylation sites in the R domain. Am J Physiol Lung Cell Mol Physiol 273: L127–L133, 1997. [DOI] [PubMed] [Google Scholar]
- 29.Wine JJ Cystic fibrosis: how do CFTR mutations cause cystic fibrosis? Curr Biol 5: 1357–1359, 1995. [DOI] [PubMed] [Google Scholar]
- 30.Winter MC, Welsh MJ. Stimulation of CFTR activity by its phosphorylated R domain. Nature 389: 294–296, 1997. [DOI] [PubMed] [Google Scholar]