Abstract
Sympathetic baroreflex sensitivity (BRS) and muscle sympathetic nerve activity (MSNA) responses during early follicular (EF) and midluteal (ML) phases of the menstrual cycle are controversial. We hypothesize an augmented sympathetic BRS and MSNA response to orthostatic stress during the ML phase of the menstrual cycle. MSNA, mean arterial pressure (MAP), and heart rate (HR) were recorded during progressive lower body negative pressure (LBNP) (−5, −10, −15, −20, −30, and −40 mmHg; 3 min/stage) in 13 healthy, eumenorrheic women (age 21 ± 1 yr). Sympathetic BRS was assessed by examining relations between spontaneous fluctuations of diastolic arterial pressure and MSNA at rest and during progressive LBNP. Plasma estradiol (42 ± 6 vs. 112 ± 12 pg/ml; P < 0.01) and progesterone (2 ± 0 vs. 10 ± 2 ng/ml; P < 0.04) were elevated during the ML phase. Resting MSNA (8 ± 1 vs. 11 ± 1 bursts/min), MAP (79 ± 2 vs. 78 ± 2 mmHg), and HR (58 ± 2 vs. 60 ± 2 beats/min) were not different during EF and ML phases. MSNA and HR increased during progressive LBNP (P < 0.001), and the increases in MSNA burst frequency (bursts/min) and HR were similar during both phases. In contrast, increases in total MSNA (arbitrary units) during progressive LBNP were augmented during the ML phase (P < 0.04), but this response does not appear to be linked to differences in sympathetic BRS. Progressive LBNP did not change MAP during either phase. Our results demonstrate an augmentation of the MSNA response to progressive LBNP during the ML phase of the menstrual cycle. These findings suggest that hormonal fluctuations of eumenorrheic women may influence sympathoexcitation during an orthostatic challenge, but not through sympathetic baroreflex-mediated pathways.
Keywords: muscle sympathetic nerve activity, arterial blood pressure, lower body negative pressure, baroreflex, estrogen
evidence suggests that orthostatic intolerance is more prevalent in women compared with men (3, 5, 7, 19, 21–23). The mechanisms responsible for this apparent sex difference are presently unclear, but altered patterns of sympathoexcitation have been suggested. Shoemaker et al. (22) reported a blunted muscle sympathetic nerve activity (MSNA) response to orthostatic stress in women compared with men, suggesting MSNA responses to an orthostatic challenge may contribute to the higher incidence of orthostatic intolerance in women. In contrast, Fu et al. (9) recently reported that sex did not affect MSNA responses to an orthostatic challenge. The influence of sex on MSNA responses to orthostatic stress remains debatable.
Shoemaker et al. (22) and Fu et al. (8) did not control for menstrual phase in their female subjects; thus, it is possible that differences between these two studies were due to hormonal fluctuations associated with the menstrual cycle. To address this concern, Fu et al. (8) recently examined MSNA responses to head up tilt (HUT) during early follicular (EF) and midluteal (ML) phases of the menstrual cycle. It was determined that MSNA responses were augmented during the ML phase, but sympathetic baroreflex sensitivity was not different between phases (8). This finding is in conflict with Minson et al. (18), who demonstrated an augmentation of the sympathetic baroreflex during the ML phase. Thus the influence of the menstrual cycle on sympathetic baroreflex sensitivity is controversial and warrants further attention. Alterations of sympathetic baroreflex sensitivity, particularly in the presence of an orthostatic stress, could help explain why women experience orthostatic intolerance more frequently than men. Additionally, the physiological significance of an augmentation of total MSNA during HUT in the ML phase is unclear.
Therefore, the purpose of this study was to examine sympathetic neural responses to progressive lower body negative pressure (LBNP) in young, healthy women during the EF and ML phases of the menstrual cycle. The reported increase of sympathetic baroreflex sensitivity during the ML phase (18), coupled with a recent report of augmented total MSNA responses to HUT during this same menstrual phase (8), led us to hypothesize an augmented sympathoexcitation to LBNP during the ML phase in young, eumenorrheic women. We hypothesized that the augmentation of MSNA during the ML phase would be governed by the sympathetic baroreflex.
METHODS
Subjects.
Thirteen healthy women (age 21 ± 1 yr, height 169 ± 3 cm, weight 70 ± 4 kg) participated in the study. All subjects were nonsmokers, nondiabetics, and were not taking oral contraceptives and/or other hormonal supplementations. All subjects were instructed to abstain from exercise and caffeine for 12 h before laboratory testing. Only subjects with consistent and regular menstrual cycles were included in the study (26–30 days in length). The Michigan Technological University Human Subjects Committee approved the experimental protocols, and all participants signed a written informed consent.
Experimental design.
Subjects were tested once during the EF phase (3 ± 0 days after start of menstruation) and again during the ML phase (23 ± 0 days after start of menstruation). The initial testing phase of the menstrual cycle was randomized. On the days of testing, the subjects reported for a blood draw to document levels of estradiol and progesterone. All subjects were tested during the same time of day for both trials. The experiment was conducted with subjects lying comfortably in the supine position. Baseline values were recorded for 5 min. Following baseline, 3-min stages of progressive LBNP were applied at −5, −10, −15, −20, −30, and −40 mmHg. MSNA, heart rate (HR), and beat-to-beat arterial blood pressure were acquired throughout the experiment.
LBNP.
All subjects wore a neoprene skirt designed to form an airtight seal between the subject and the LBNP chamber. The application of LBNP (below the iliac crest) resulted in a redistribution of blood away from the upper body to the abdomen and lower extremities. Progressive LBNP was applied at −5, −10, −15, −20, −30, and −40 mmHg in 3-min stages after a 5-min baseline. These levels of LBNP do not typically induce presyncope, but subjects were monitored closely, and the experiment would have been stopped if any presyncopal signs were observed [i.e., symptoms of lightheadedness, nausea, diaphoresis, or a decrease of systolic arterial pressure (SAP) to <80 mmHg]. No subjects experienced presyncope during the present study.
Measurements.
Multifiber recordings of MSNA were made by inserting a tungsten microelectrode in the peroneal nerve of a resting leg. A reference electrode was inserted subcutaneously 2–3 cm from the recording electrode. Both electrodes were connected to a differential preamplifier, and then to an amplifier (total gain of 80,000) where the nerve signal was band-pass filtered (700–2,000 Hz), and integrated (time constant, 0.1) to obtain a mean voltage display of the nerve activity. Satisfactory recordings of MSNA were defined by spontaneous, pulse synchronous bursts that increased during end-expiratory apnea, and did not change during auditory stimulation.
Arterial blood pressure was measured using two techniques. Resting arterial blood pressure was measured three consecutive times (separated by ∼1-min intervals) using an automated sphygmomanometer and reported as a mean value. Beat-to-beat arterial blood pressure was recorded continuously via a Finometer (Finapres Medical Systems, Amsterdam, The Netherlands). The Finometer allowed us to determine precise changes in blood pressure that occur during LBNP, whereas the sphygmomanometer allows us to compare baseline arterial pressure during the EF and ML phases. Arterial blood pressures are expressed as systolic (SAP), diastolic (DAP), and mean (MAP) arterial pressures. HR was recorded using a three-lead electrocardiogram. A total of 10 women successfully completed all LBNP levels during both the EF and ML phases of the menstrual cycle (3 subjects were unable to revisit the laboratory on the corresponding phase of testing). Quality tracings of MSNA were successfully recorded during both the EF and ML phase, and throughout the entire progressive LBNP protocol, in a total of seven subjects.
Data analysis.
Data were imported and analyzed in a software program (WinCPRS; Absolute Aliens, Turku, Finland) on a Windows-formatted computer. R-waves were detected and marked in the time series. Muscle sympathetic bursts were automatically detected on the basis of amplitude using a signal-to-noise ratio of 3:1, within a 0.5-s search window centered on the 1.3-s expected burst peak latency from the previous R-wave. Potential bursts were displayed and edited by one trained investigator. The integral of all sympathetic bursts occurring during the initial 3-min baseline period was calculated and divided by the number of bursts to derive an average control burst integrated area that could be compared across time. Subsequent bursts that were equal to average bursts occurring during baseline were assigned a value of 100. MSNA was expressed as bursts per minute and total burst activity (total activity = total number of bursts multiplied by the averaged normalized burst area). HR, blood pressure, and MSNA are reported as the average for the corresponding stage (i.e., 5 min for baseline and 3 min for each LBNP stage).
Sympathetic baroreflex sensitivity was determined by examining the relations between spontaneous fluctuations in DAP and MSNA (10–13, 20). This analysis is described in detail by Keller et al. (12). Briefly, diastolic blood pressures for each cardiac cycle were grouped into 3-mmHg intervals (bins). Burst incidence, total MSNA, and burst strength for each DAP bin were calculated and plotted against the corresponding DAP. The slopes of these relationships were determined using linear regression analysis. All linear regression analyses were weighted for the number of cardiac cycles within each DAP bin, and a minimum r-value of 0.5 was used as the criteria for accepting slopes. For the burst incidence analysis, burst frequency was expressed as bursts per 100 cardiac cycles. Comparing burst incidence for the various DAP bins allowed us to determine the number of bursts generated at a particular DAP level. The burst incidence analysis only examined burst frequency (expressed as bursts/100 cardiac cycles) and did not take into account the area of the burst. In contrast, the total MSNA and burst strength analyses allow for an assessment of burst area. For the total MSNA analysis, the total burst area for all cardiac cycles within a given DAP bin was calculated and divided by the number of cardiac cycles that occurred within that bin. If a burst did not occur, the burst area was assigned a value of zero for that particular cardiac cycle. For the burst strength analysis, the slope between MSNA area and DAP was examined only when a burst occurred. Therefore, cardiac cycles with a burst area of zero (i.e., cardiac cycles without a burst) were not included for this analysis.
Statistical analysis.
All data were analyzed statistically using commercial software (SPSS 15.0; SPSS, Chicago, IL). A two-way repeated-measures ANOVA was used to determine if changes in MSNA (bursts/min and total activity), MAP, HR, and DAP-MSNA slopes occurred during each intervention (baseline, −5, −10, −15, −20, −30, and −40 mmHg) and across trials (EF and ML phases). Resting variables were compared using paired t-tests. Means were considered significantly different when P < 0.05, and results are expressed as means ± SE.
RESULTS
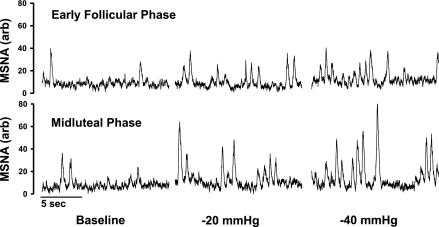
Resting baseline values during the EF and ML phases are presented in Table 1. Plasma estradiol and progesterone were significantly increased during the ML phase (P < 0.05). Resting MSNA, MAP, and HR were not different between the EF and ML phases. Representative neurograms of MSNA during the EF and ML phases are illustrated in Fig. 1, which demonstrates a more dramatic increase of burst area when LBNP was performed in the ML phase.
Table 1.
Baseline values for the early follicular and midluteal phases of the menstrual cycle
| Variable | EF | ML | P Value |
|---|---|---|---|
| Estrogen, pg/ml | 42±5 | 109±13* | 0.003 |
| Progesterone, ng/ml | 2±0 | 9±3* | 0.038 |
| MSNA, bursts/min | 8±1 | 11±1 | 0.254 |
| SAP, mmHg | 105±3 | 104±3 | 0.569 |
| DAP, mmHg | 66±1 | 65±2 | 0.549 |
| MAP, mmHg | 79±2 | 78±2 | 0.403 |
| HR, beats/min | 58±2 | 60±2 | 0.128 |
Values are means ± SE; n = 10 subjects unless noted. MSNA, muscle sympathetic nerve activity (n = 7); SAP, systolic arterial pressure; DAP, diastolic arterial pressure; MAP, mean arterial blood pressure; HR, heart rate; EF, early follicular; ML, midluteal.
P < 0.05 vs. corresponding EF value.
Fig. 1.
Representative neurograms obtained from one subject during baseline, −20 mmHg lower body negative pressure (LBNP), and −40 mmHg LBNP of the early follicular and midluteal phases of the menstrual cycle. Although the menstrual cycle did not alter burst frequency between phases, the total activity based on muscle sympathetic nerve activity (MSNA) area increased during the midluteal phase.
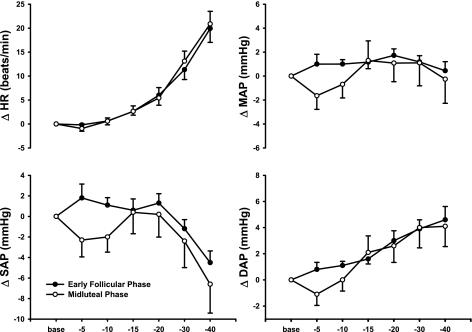
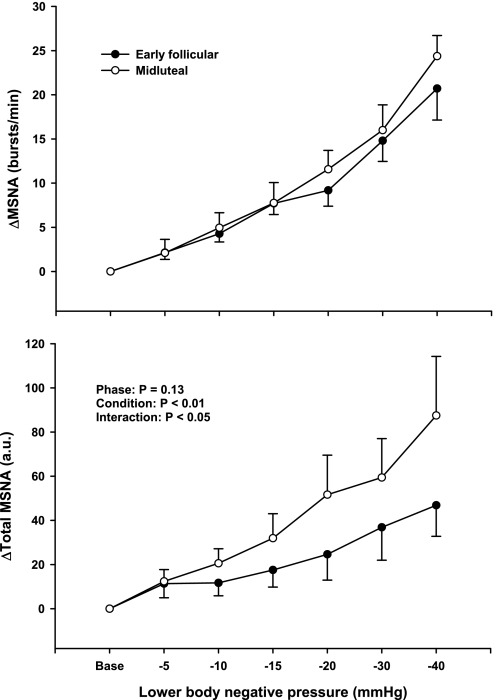
Figure 2 demonstrates that hemodynamic responses to progressive LBNP were not different between the EF and ML phases of the menstrual cycle. Progressive LBNP increased HR (P < 0.001) but did not change SAP, DAP, or MAP during the EF and ML phases of the menstrual cycle. SAP tended to decrease and DAP tended to increase during higher levels of LBNP, but these responses did not reach statistical significance. Figure 3 demonstrates that progressive LBNP increased MSNA burst frequency (bursts/min; P < 0.001) and total MSNA (arbitrary units; P < 0.001). Increases in MSNA during progressive LBNP were augmented during the ML phase when expressed as total MSNA (interaction, P < 0.04), but were not different between phases when expressed as burst frequency (Fig. 3).
Fig. 2.
Changes in heart rate (HR) and systolic (SAP), diastolic (DAP), and mean (MAP) arterial pressures during progressive LBNP. Subjects were examined during the early follicular (EF) and midluteal (ML) phases of the menstrual cycle. Progressive LBNP elicited increases in HR, but did not change SAP, DAP, or MAP. Hemodynamic responses were similar between menstrual phases (n = 10 subjects).
Fig. 3.
Changes in MSNA during progressive LBNP. Subjects were examined during the EF and ML phases of the menstrual cycle. Progressive LBNP elicited similar increases of MSNA burst frequency during the EF and ML phases. In contrast, progressive LBNP elicited a greater increase in total MSNA activity [arbitrary units (AU)] during the ML phases of the menstrual cycle. Reported as means ± SE (n = 7).
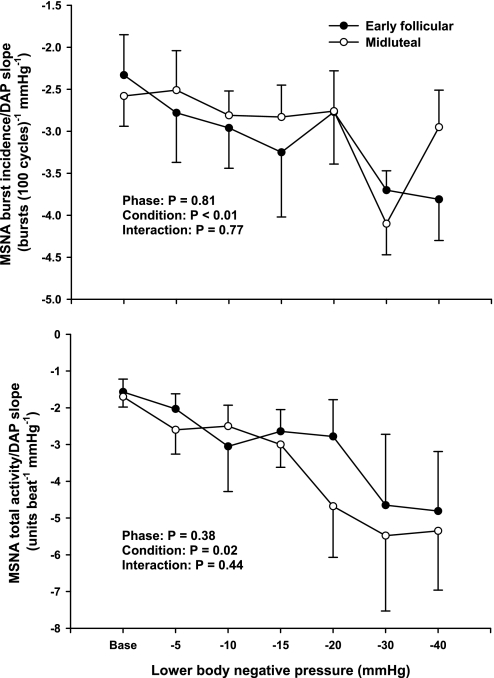
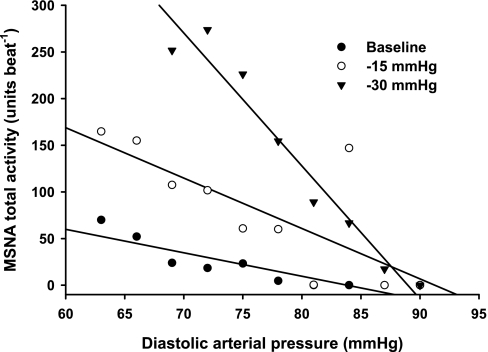
Sympathetic baroreflex sensitivities during EF and ML phases of the menstrual cycle are presented in Table 2. Progressive LBNP significantly increased sympathetic baroreflex sensitivity during both EF and ML phases (burst incidence condition, P < 0.05; total MSNA condition, P < 0.01). Increases of sympathetic baroreflex sensitivity during LBNP were not different between phases, whether assessed as burst incidence or total MSNA (Fig. 4). Linear regression slopes for “burst strength” did not yield correlation coefficients >0.5; thus, they are not reported. Representative data from one subject are presented in Fig. 5, illustrating that sympathetic baroreflex sensitivities increase (as a function of the MSNA-DAP slope) during progressive LBNP.
Table 2.
Sympathetic baroreflex sensitivity during the EF and ML phases of the menstrual cycle
| Menstrual Phase | Baseline | −5 mmHg | −10 mmHg | −15 mmHg | −20 mmHg | −30 mmHg | −40 mmHg | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Burst incidence slope, bursts·100 heart beat−1·mmHg−1 | |||||||||||||
| EF | −2.3±0.5 | −2.8±0.6 | −3.0±0.5 | −3.2±0.8 | −2.8±0.6 | −3.7±0.2 | −3.8±0.5 | ||||||
| (0.70±0.07) | (0.74±0.03) | (0.70±0.05) | (0.67±0.07) | (0.63±0.08) | (0.69±0.09) | (0.66±0.09) | |||||||
| ML | −2.6±0.4 | −2.5±0.5 | −2.8±0.3 | −2.8±0.4 | −2.8±0.5 | −4.1±0.4 | −2.9±0.4 | ||||||
| (0.69±0.05) | (0.67±0.10) | (0.62±0.09) | (0.61±0.11) | (0.48±0.11) | (0.62±0.13) | (0.50±0.14) | |||||||
| Total MSNA slope, units·beat−1·mmHg−1 | |||||||||||||
| EF | −1.6±0.4 | −2.0±0.4 | −3.1±1.2 | −2.6±0.6 | −2.8±1.0 | −4.6±1.9 | −4.8±1.6 | ||||||
| (0.75±0.06) | (0.74±0.05) | (0.78±0.07) | (0.78±0.04) | (0.75±0.08) | (0.87±0.03) | (0.90±0.03) | |||||||
| ML | −1.7±0.3 | −2.6±0.7 | −2.5±0.6 | −3.0±0.6 | −4.7±1.4 | −5.5±2.1 | −5.3±1.6 | ||||||
| (0.73±0.04) | (0.75±0.05) | (0.63±0.08) | (0.73±0.06) | (0.81±0.08) | (0.79±0.08) | (0.79±0.03) | |||||||
Values are means ± SE; n = 7 subjects. Correlation coefficients for linear regression slopes are provided in parentheses. Progressive lower body negative pressure increased sympathetic baroreflex sensitivity during both the EF and ML phases of the menstrual cycle (burst incidence condition, P < 0.05; total MSNA condition, P < 0.01), but responses were not different between phases.
Fig. 4.
Linear relations between MSNA and DAP during EF and ML phases of the menstrual cycle. Progressive LBNP elicited significant reductions in the MSNA-DAP slopes during both the EF and ML phases of the menstrual cycle, indicating increased sympathetic baroreflex sensitivities during orthostatic stress. The increases in sympathetic baroreflex sensitivity were not different between the EF and ML phases. Reported as means ± SE (n = 7).
Fig. 5.
Representative linear regression slopes from one subject during baseline, −15 mmHg, and −30 mmHg LBNP. Slopes became more negative with increasing levels of LBNP, indicating sympathetic baroreflex sensitivity increased with increasing levels of orthostatic stress. Data presented are from the EF phase, since results were similar between the EF and ML phases.
DISCUSSION
This study investigated sympathetic neural and cardiovascular responses to orthostatic stress during the EF and ML phases of the menstrual cycle. Based on previous data indicating increased sympathetic baroreflex sensitivity during the ML phase of the menstrual cycle (18), we hypothesized an augmented sympathoexcitation to LBNP during this phase in young, eumenorrheic women. Our findings support this hypothesis by demonstrating an augmentation of MSNA (when expressed as total activity) during the ML phase of the menstrual cycle. However, this augmented sympathoexcitation during the ML phase does not appear to be due to increased sympathetic baroreflex sensitivity as originally hypothesized. Increases of sympathetic baroreflex sensitivity during orthostatic stress were similar between the EF and ML phases. Our findings, taken in conjunction with a recent report by Fu et al. (8), provide strong evidence that orthostatic stress elicits altered patterns of sympathoexcitation during the menstrual cycle, but these altered patterns are not modulated by sympathetic baroreflex sensitivity. The augmentation of total MSNA during the ML phase occurred despite no change in MSNA burst frequency, suggesting that the fluctuations of reproductive hormones during the menstrual cycle preferentially modulate the neural recruitment patterns as opposed to simply the number of bursts produced during an orthostatic challenge. Furthermore, increased sympathetic baroreflex sensitivity during LBNP demonstrated in the present study challenges a recent report indicating no change in sympathetic baroreflex sensitivity during progressive LBNP (10).
Fu et al. (8) recently examined MSNA responses to HUT during the EF and ML phases, and reported that the sympathetic baroreflex sensitivities were not different between menstrual phases during resting conditions, 30° HUT, or 60° HUT. This finding is in conflict with Minson et al. (18), who demonstrated a significant increase of sympathetic baroreflex sensitivity during the ML phase. Our results support the findings of Fu et al. (8), since we report that progressive LBNP elicits similar increases in sympathetic baroreflex sensitivity during EF and ML phases of the menstrual cycle. Thus evidence is accumulating to suggest that menstrual phase does not influence sympathetic baroreflex sensitivity in young, healthy eumenorrheic women. Differences between Minson et al. (18) and the present study/Fu et al. (8) are likely due to the technique used to assess sympathetic baroreflex sensitivity. Minson et al. (18) assessed sympathetic baroreflex sensitivity by measuring changes in arterial blood pressure and MSNA during intravenous bolus injections of sodium nitroprusside and phenylephrine (i.e., modified Oxford technique). With the use of this technique, sympathetic baroreflex sensitivity was determined by comparing MSNA and DAP slopes. In contrast, the present study and Fu et al. (8) assessed sympathetic baroreflex sensitivity by examining the linear regression slopes between spontaneous fluctuations of MSNA and DAP. This method does not induce as dramatic increases and decreases in DAP as the modified Oxford technique, yet it typically examines arterial blood pressure fluctuations within a range of 20 mmHg, which appears to be a good reflection of arterial baroreflex control of MSNA under actual physiological conditions.
It is important to note that both the present study and the recent study by Fu et al. (8) demonstrate that orthostatic stress induces an augmentation of total MSNA during the ML phase of the menstrual cycle. Interestingly, both studies also report no change in burst frequency between phases during orthostatic stress. Thus, despite two different approaches (i.e., LBNP vs. HUT), it appears that the menstrual cycle alters sympathetic neural responses to orthostatic stress when the area of a burst is considered. The physiological significance of this alteration of neural recruitment during an orthostatic challenge in women during high and low hormonal phases remains unclear. Fu et al. (8) suggested differences in norepinephrine spillover during EF and ML phases may contribute to differences in total MSNA and burst frequency responses, but norepinephrine spillover was not measured. We agree, and our data allow us to expand upon this concept further. Fu et al. (8) examined the linear regression slope between total MSNA and DAP, but did not report burst incidence and burst strength slopes. In contrast, the present study analyzed total MSNA, burst incidence, and burst strength slopes. Figure 4 demonstrates that, when examined as burst incidence, changes in the linear regression slopes during the ML phase were generally more positive compared with the EF phase. In contrast, changes in the linear regression slopes during the ML phase were generally more negative compared with the EF phase. Burst strength slopes could not be reported because the correlation coefficients were well below our threshold of r = 0.5, but the directional shift of the total MSNA-DAP slope data with respect to the burst incidence-DAP slope data clearly indicates the average area of a burst (i.e., burst strength) was greater during the ML phase. Thus our data suggest that the amount of norepinephrine release per efferent sympathetic burst, not burst frequency, is altered by the menstrual cycle during an orthostatic challenge. Examinations of norepinephrine spillover during orthostatic stress in the high and low hormonal phases of the menstrual cycle are warranted. Additionally, measurements of single nerve fiber activity could provide valuable insight (16).
The present study contributes further by providing data during low and high levels of baroreceptor unloading. Specifically, the lower levels of the progressive LBNP protocol (i.e., −5 and −10 mmHg) are generally believed to primarily engage the cardiopulmonary baroreceptors, while the higher levels of LBNP (−15, −20, −30, and −40 mmHg) are generally recognized as levels that engage both the cardiopulmonary and arterial baroreceptors (24). Interestingly, our data reveal that MSNA responses to LBNP begin to differ between the EF and ML phases at the higher levels of baroreceptor unloading. The failure to observe phase differences in MSNA during −5 and −10 mmHg suggests that arterial baroreceptors may play a greater role than the cardiopulmonary baroreceptors in the augmentation of orthostatically mediated MSNA during the ML phase of the menstrual cycle.
Our progressive LBNP protocol increased sympathetic baroreflex sensitivities during both the EF and ML phases, and these increases were not different across phases. Ichinose et al. (10) recently reported that progressive LBNP did not increase sympathetic baroreflex sensitivity. Differences between our results and the findings of Ichinose et al. (10) are not immediately apparent, but may be due to the blood pressure binning techniques used. Ichinose et al. (10) utilized 1-mmHg DAP bins, and the present study utilized 3-mmHg bins, as outlined by Keller et al. (12). However, in addition to our reported 3-mmHg bin data, we also examined our data using 1-mmHg and 2-mmHg bins (data not included) to allow for more direct comparison with Ichinose et al. (10). Correlation coefficients for the 2-mmHg bin did not differ from the 3-mmHg bin, but correlations were generally a bit lower (but still above the threshold of r = 0.5). Slopes produced using 2-mmHg bins vs. 3-mmHg bins did not differ; thus, we chose to present our 3-mmHg data. In contrast, the 1-mmHg binning produced extremely poor correlation coefficients for our data, preventing us from utilizing data from the 1-mmHg bin as an index of sympathetic baroreflex sensitivity. We are uncertain why our correlation coefficients differed so much from Ichinose et al. (10), who demonstrated robust correlations with the 1-mmHg binning technique. Nevertheless, our data indicate an increase of sympathetic baroreflex sensitivity during progressive LBNP that was not observed by Ichinose et al. (10). Furthermore, previous studies have demonstrated an increase of sympathetic baroreflex sensitivity during mild levels of LBNP (11, 15). The increased sympathetic baroreflex sensitivities observed during LBNP in the present study and others (11, 15) are suggestive that alterations of sympathetic baroreflex sensitivity during orthostatic stress are important in the maintenance of orthostatic tolerance. Our results indicate that such adjustments are not different between the EF and ML phases of the menstrual cycle.
The graded LBNP protocol used in the present study did not elicit presyncopal symptoms in any subjects. This is not surprising considering subjects were exposed to a maximum of −40 mmHg and the relative short duration of the protocol. Thus it is unclear if sympathetic baroreflex sensitivities are similar between menstrual phases as subjects approach presyncope. Fu et al. (8) reported that 5 of 11 women experienced presyncope during the EF phase, whereas only 3 experienced presyncope during the ML phase. Three recent studies indicate no differences in orthostatic tolerance during high and low hormone phases of the menstrual cycle, but these studies did not assess sympathetic baroreflex sensitivity (2, 4, 17). Instead, cardiovagal baroreflex sensitivity was assessed and determined to be similar during the various phases of the menstrual cycle (2, 4, 17). The influence of menstrual phase on sympathetic baroreflex sensitivities during a maximal orthostatic stress test remains unknown.
Finally, the influence of the menstrual cycle on resting MSNA has been controversial. Ettinger et al. (6) was the first to report MSNA during altered phases of the menstrual cycle and reported no differences between the EF and follicular phases of the menstrual cycle. In contrast, Minson et al. (18) reported increased levels of resting MSNA during the ML phase compared with the EF phase. More recently, several studies have reported no changes in resting MSNA during the EF and ML phases (1, 8, 14). The present study once again reports no change in resting MSNA; thus, evidence is accumulating to suggest that hormonal fluctuations during the menstrual cycle do not modify MSNA at rest.
There are three limitations worth discussing. First, our MSNA sample size is modest (n = 7). Nevertheless, this number allowed us to detect significant differences in total MSNA responses to LBNP during the two menstrual phases. Additional MSNA signals would have been unlikely to change MSNA burst frequency responses, which were remarkably similar during EF and ML phases. Second, our resting MSNA levels are low (i.e., 8–10 bursts/min), but this is not uncommon with young, eumenorrheic women. In fact, the resting MSNA levels reported in the present study are similar to previous studies examining resting MSNA in young, eumenorrheic women (1, 14) . Third, the interpretation of the MSNA-DAP slope analysis is limited to the spontaneous fluctuations of blood pressure (i.e., ∼20-mmHg range). Pharmacological manipulations of blood pressure, or the neck-chamber technique, would produce a wider range of blood pressure changes, but this would be outside the normal physiological range. Furthermore, examination of spontaneous fluctuations allows for analysis of burst incidence slopes. Thus there are advantages and limitations to using the spontaneous MSNA-DAP slope method. We limit our interpretations to sympathetic baroreflex sensitivity at or around the normal operating point of the baroreflex function curve.
In conclusion, our results demonstrate an augmentation of MSNA responses to LBNP during the ML phase of the menstrual cycle. This augmentation of MSNA during the ML phase is not linked to increases of sympathetic baroreflex sensitivity. The present study, coupled with the findings of Fu et al. (8), suggest that the increased incidence of orthostatic stress in women does not appear to be due to fluctuations of sympathetic baroreflex sensitivity during the menstrual cycle. Furthermore, the augmented total MSNA response to orthostatic stress during the ML phase of the menstrual cycle is in agreement with Fu et al. (8), but the physiological significance of this response remains unclear.
GRANTS
This project was supported by National Heart, Lung, and Blood Institute Grant HL-088689.
Acknowledgments
We thank Jessica Cross and Christopher Schwartz for technical assistance.
REFERENCES
- 1.Carter JR, Lawrence JE. Effects of the menstrual cycle on sympathetic neural responses to mental stress in humans. J Physiol 585: 635–641, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Claydon VE, Younis NR, Hainsworth R. Phase of the menstrual cycle does not affect orthostatic tolerance in healthy women. Clin Auton Res 16: 98–104, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Convertino VA Gender differences in autonomic functions associated with blood pressure regulation. Am J Physiol Regul Integr Comp Physiol 275: R1909–R1920, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Courtar DA, Spaanderman ME, Janssen BJ, Peeters LL. Orthostatic stress response during the menstrual cycle is unaltered in formerly preeclamptic women with low plasma volume. Reprod Sci 14: 66–72, 2007. [DOI] [PubMed] [Google Scholar]
- 5.el-Bedawi KM, Hainsworth R. Combined head-up tilt and lower body suction: a test of orthostatic tolerance. Clin Auton Res 4: 41–47, 1994. [DOI] [PubMed] [Google Scholar]
- 6.Ettinger SM, Silber DH, Gray KS, Smith MB, Yang QX, Kunselman AR, Sinoway LI. Effects of the ovarian cycle on sympathetic neural outflow during static exercise. J Appl Physiol 85: 2075–2081, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Fritsch-Yelle JM, Charles JB, Jones MM, Beightol LA, Eckberg DL. Spaceflight alters autonomic regulation of arterial pressure in humans. J Appl Physiol 77: 1776–1783, 1994. [DOI] [PubMed] [Google Scholar]
- 8.Fu Q, Okazaki K, Shibata S, Shook RP, Vangunday TB, Galbreath MM, Reelick MF, Levine BD. Menstrual cycle effects on sympathetic neural responses to upright tilt. J Physiol 587: 2019–2031, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu Q, Witkowski S, Okazaki K, Levine BD. Effects of gender and hypovolemia on sympathetic neural responses to orthostatic stress. Am J Physiol Regul Integr Comp Physiol 289: R109–R116, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Ichinose M, Saito M, Fujii N, Kondo N, Nishiyasu T. Modulation of the control of muscle sympathetic nerve activity during severe orthostatic stress. J Physiol 576: 947–958, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ichinose M, Saito M, Kitano A, Hayashi K, Kondo N, Nishiyasu T. Modulation of arterial baroreflex dynamic response during mild orthostatic stress in humans. J Physiol 557: 321–330, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keller DM, Cui J, Davis SL, Low DA, Crandall CG. Heat stress enhances arterial baroreflex control of muscle sympathetic nerve activity via increased sensitivity of burst gating, not burst area, in humans. J Physiol 573: 445–451, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kienbaum P, Karlssonn T, Sverrisdottir YB, Elam M, Wallin BG. Two sites for modulation of human sympathetic activity by arterial baroreceptors? J Physiol 531: 861–869, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawrence JE, Ray CA, Carter JR. Vestibulosympathetic reflex during the early follicular and midluteal phases of the menstrual cycle. Am J Physiol Endocrinol Metab 294: E1046–E1050, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lucini D, Furlan R, Villa P, Mosqueda-Garcia R, Diedrich A, Robertson D, Malliani A, Porta A, Pagani M. Altered profile of baroreflex and autonomic responses to lower body negative pressure in chronic orthostatic intolerance. J Hypertens 22: 1535–1542, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Macefield VG, Wallin BG, Vallbo AB. The discharge behaviour of single vasoconstrictor motoneurones in human muscle nerves. J Physiol 481: 799–809, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meendering JR, Torgrimson BN, Houghton BL, Halliwill JR, Minson CT. Menstrual cycle and sex affect hemodynamic responses to combined orthostatic and heat stress. Am J Physiol Heart Circ Physiol 289: H631–H642, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation 101: 862–868, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Montgomery LD, Kirk PJ, Payne PA, Gerber RL, Newton SD, Williams BA. Cardiovascular responses of men and women to lower body negative pressure. Aviat Space Environ Med 48: 138–145, 1977. [PubMed] [Google Scholar]
- 20.Ogoh S, Fisher JP, Raven PB, Fadel PJ. Arterial baroreflex control of muscle sympathetic nerve activity in the transition from rest to steady-state dynamic exercise in humans. Am J Physiol Heart Circ Physiol 293: H2202–H2209, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Robertson D The epidemic of orthostatic tachycardia and orthostatic intolerance. Am J Med Sci 317: 75–77, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Shoemaker JK, Hogeman CS, Khan M, Kimmerly DS, Sinoway LI. Gender affects sympathetic and hemodynamic response to postural stress. Am J Physiol Heart Circ Physiol 281: H2028–H2035, 2001. [DOI] [PubMed] [Google Scholar]
- 23.White DD, Gotshall RW, Tucker A. Women have lower tolerance to lower body negative pressure than men. J Appl Physiol 80: 1138–1143, 1996. [DOI] [PubMed] [Google Scholar]
- 24.Zoller RP, Mark AL, Abboud FM, Schmid PG, Heistad DD. The role of low pressure baroreceptors in reflex vasoconstrictor responses in man. J Clin Invest 51: 2967–2972, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]