Abstract
The objectives of this study were to determine whether 1) the improvement in insulin action induced by short-term exercise training in patients with type 2 diabetes is due to an improvement in insulin sensitivity, an improvement in insulin responsiveness, or a combination of improved insulin sensitivity and responsiveness and 2) short-term exercise training results in improved suppression of hepatic glucose production by insulin. Fourteen obese patients with type 2 diabetes, age 64 ± 2 yr, underwent a two-stage hyperinsulinemic euglycemic clamp procedure, first stage 40 mU·m−2·min−1 insulin infusion, second stage 1,000 mU·m−2·min−1 insulin infusion, together with a [3-3H]glucose infusion, before and after 7 days of exercise. The training consisted of 30 min of cycling and 30 min of treadmill walking at ∼70% of maximal aerobic capacity daily for 7 days. The exercise program resulted in improvements in insulin action in the absence of weight loss. Glucose disposal rates during the euglycemic clamp were significantly increased at both hyperinsulinemic stages after training (40 mU: 1.84 ± 0.32 to 2.67 ± 0.37 mg·kg−1·min−1, P < 0.0001; 1,000 mU: 7.57 ± 0.61 to 8.84 ± 0.56 mg·kg−1·min−1, P = 0.008). Hepatic glucose production, both in the basal state (3.17 ± 0.43 vs. 2.54 ± 0.26 mg·kg−1·min−1, P = 0.05) and during the 40-mU clamp stage (1.15 ± 0.41 vs. 0.46 ± 0.20 mg·kg−1·min−1, P = 0.03), was significantly reduced after training. One week of vigorous exercise training can induce significant improvements in insulin action in type 2 diabetes. These improvements include increased peripheral insulin sensitivity and responsiveness as well as enhanced suppression of hepatic glucose production.
Keywords: hyperinsulinemic euglycemic clamp, insulin resistance, hepatic glucose production
exercise has a number of beneficial effects that can play important roles in the prevention and treatment of the insulin resistance that leads to and mediates type 2 diabetes (23). In addition to increased energy expenditure, which helps to prevent and reverse obesity, exercise has short-term effects that enhance insulin action. Exercise acutely increases muscle glucose transport, and this effect is mediated by an increase in the glucose transporter 4 (GLUT4) isoform of the glucose transporter in skeletal muscle (18). As this effect wears off, it is replaced by an increase in insulin sensitivity (19). As a result of these two adaptations, exercise results in increases in both insulin sensitivity and insulin responsiveness. Insulin sensitivity is evaluated in terms of the concentration of insulin required to induce 50% of its maximal effect on glucose transport (25). An increase in insulin sensitivity causes the insulin dose-response curve to shift to the left so that the insulin concentration required to cause 50% of its maximal effect is lower. Insulin responsiveness, in contrast, determines the magnitude of the increase in glucose transport induced by a maximally effective insulin stimulus (25). An increase in insulin responsiveness causes a larger increase in glucose transport in response to a maximal insulin stimulus and a proportional upward shift in the insulin dose-response curve.
Exercise training results in increases in insulin action in humans (9, 11, 12, 14, 20, 22). Exercise training also induces increases in muscle GLUT4 content in humans (10, 21, 22), and 7–10 days of training is sufficiently long to bring about this adaptation (15, 34). These are short-term effects of exercise that wear off in a few days after the last exercise bout (9, 27, 45). In light of this information, there has been considerable research on the effects of exercise in patients with insulin resistance and impaired or diabetic glucose tolerance. A number of studies utilized short-term exercise training, making it possible to evaluate the effects of exercise training in the absence of weight loss. In one of these studies, 7 days of exercise training for 50–60 min/day at ∼68% of maximal oxygen uptake capacity (V̇o2max), which did not result in weight loss, improved glucose tolerance from diabetic to impaired in patients with mild diabetes (39). Ten days of a similar exercise program resulted in a large improvement in insulin action, measured using a hyperglycemic clamp procedure, in obese individuals with impaired glucose tolerance (1). In two other studies on patients with type 2 diabetes, 1 wk of exercise training resulted in significant improvements in insulin-stimulated glucose disposal rates measured during euglycemic clamps (34, 51). Those authors referred to these improvements as increases in insulin sensitivity but examined only the effects of submaximally effective insulin stimuli. In the study of Winnick et al. (51), the improvement in insulin action was only on glucose disposal, with no improvement in insulin suppression of endogenous glucose production, which is predominantly of hepatic origin.
Impaired insulin suppression of hepatic glucose production is a key defect in type 2 diabetes and a major contributor to the hyperglycemia that characterizes type 2 diabetes (2, 31, 36). Although not much is known regarding the effects of exercise on hepatic glucose production, there is considerable evidence that activation of adenosine monophosphate-activated protein kinase (AMPK) in the liver results in repression of phosphoenolpyruvate carboxykinase and glucose-6-phosphatase expression and thus of gluconeogenesis (3, 26, 32, 44). Hepatic AMPK is activated during exercise (6), which suggests the possibility that an AMPK-induced adaptive response to exercise could mediate an improvement in suppression of hepatic glucose production.
One purpose of the present study was to determine whether the improvement in peripheral insulin action induced by short-term exercise training in insulin-resistant patients with type 2 diabetes is due to enhanced insulin sensitivity, enhanced insulin responsiveness, or increases in both insulin sensitivity and responsiveness. Our second purpose was to reevaluate the effect of short-term training on the resistance of hepatic glucose production to suppression by insulin in patients with type 2 diabetes.
EXPERIMENTAL PROCEDURES
Participants.
Potential participants were screened via a medical history questionnaire, an oral glucose tolerance test, an exercise stress test, and blood chemistry profiles. Volunteers were excluded if they demonstrated any contraindications to exercise as evidenced by resting or exercise-induced ECG abnormalities, if they displayed any thyroid, cardiovascular, or hematological disease, or if they were taking any medications known to affect metabolism. In addition, only individuals with diabetic glucose tolerance as assessed by oral glucose tolerance test were accepted into the study (fasting glucose concentration >100 mg/dl and a 2-h plasma glucose >200 mg/dl). Following these screening procedures, 14 overweight/obese, diabetic volunteers (11 men, 3 women, age 64 ± 2 yr, BMI 31.9 ± 2.2 kg/m2) were enrolled in a 7-day exercise training intervention. Participants had not been previously diagnosed with diabetes and were therefore not being treated with insulin or oral hypoglycemic agents. All participants had been weight stable for the preceding 6 mo, were previously sedentary, and had not participated in any systematic exercise training in the past. In addition, all female subjects were postmenopausal. The study was approved by the Institutional Review Board at the Washington University School of Medicine and all subjects provided signed written consent in accordance with the guidelines for the protection of human subjects.
Intervention.
On 7 consecutive days, all volunteers performed 50–60 min of exercise consisting of treadmill walking and stationary cycling at 80–85% of their maximum heart rate (∼67–75% of their maximal aerobic capacity). Participants wore heart rate monitors (Polar Electro, Woodbury, NY) during each training session to monitor their individualized target heart rate. Each training session incorporated a brief standardized warmup and cooldown and included a series of stretching exercises. Every session was supervised by an exercise physiologist, and therefore, compliance with the training was carefully documented.
Dietary control.
Prior to the study, a 3-day outpatient control period was used to monitor adequate caloric intake using dietary intake questionnaires. Macronutrient composition (%calories) was ∼50% carbohydrate, 35% fat, and 15% protein. During this control period, all volunteers underwent measurements of body composition, aerobic fitness, and insulin action, as described below. These tests were repeated at the end of the study. Metabolic measurements (insulin action) were performed in the morning following an overnight fast. Poststudy metabolic measurements were performed 18–20 h after the last exercise session. During the 7-day exercise intervention, dietary intake was monitored using diet records. The records were checked on a daily basis by one of the investigators who counseled volunteers to maintain their usual dietary habits throughout the study.
Body composition.
Body mass and height were measured by standard techniques using a calibrated scale and stadiometer. Body density was determined by hydrostatic weighing, with correction for residual lung volume, and body fat mass was calculated using the equations of Brozek, as described previously (29).
Aerobic fitness.
Each participant performed an incremental treadmill exercise test to determine their V̇o2max, as described previously (46). Due to the acute effects of exercise on insulin sensitivity, the preintervention V̇o2max test was performed ≥4 days prior to other metabolic measurements. Blood pressure was monitored using a sphygmomanometer; resting and maximal heart rates were also recorded.
Insulin action.
A hyperinsulinemic euglycemic clamp combined with a [3-3H]glucose infusion was used to measure basal and insulin-stimulated glucose metabolism, as reported previously (29). At 8:00 AM, following fasting blood sample collection, a 2-h primed (25 μCi bolus) continuous (0.25 μCi/min) infusion of [3-3H]glucose was initiated into an antecubital vein (baseline stage). At 10:00 AM, a primed continuous 40 mU·m−2·min−1 infusion of insulin began. This proceeded for 2 h (40 mU stage), at which point (12:00 PM) the infusion rate was increased to 1,000 mU·m−2·min−1 for an additional 2 h (1,000 mU stage). During the insulin infusion periods, 0.5 ml of arterialized blood was drawn from a retrograde dorsal hand line every 5 min, and plasma glucose concentrations were determined (Beckman Instruments, Fullerton, CA). A variable-rate glucose infusion (20% dextrose) was used to maintain euglycemia (90 mg/dl) according to the calculations of DeFronzo et al. (8). [3-3H]glucose was added to the cold glucose to minimize underestimating glucose rates of appearance. A continuous infusion of potassium chloride was also maintained throughout to prevent insulin-induced hypokalemia. Additional blood samples were collected at baseline and every 15 min throughout the clamp for insulin determination (33). Samples were also collected immediately before the insulin infusion and at 10-min intervals during the last 30 min of each insulinemic stage for 3-3H-specific activity determinations. Radioactivity of plasma [3-3H]glucose was measured by liquid scintillation counting after plasma deproteinization with 3 M perchloric acid, as described previously (29). Glucose rates of appearance and disappearance were calculated according to the equations of Steele (47) modified for variable-rate glucose tracer infusions (13). We refer to the rate of glucose disappearance as glucose disposal rate (GDR). Endogenous glucose production, which is predominantly hepatic glucose production (HGP), was calculated by subtracting the rate of glucose appearance from the glucose infusion rate. GDR and HGP are reported as the mean rates calculated during the final 30 min of each stage: baseline, 40 mU, and 1,000 mU. Postintervention clamps were begun within 24 h following the final exercise session.
Statistics.
All data are presented as means ± SE. Intervention effects on insulin sensitivity and substrate metabolism at the two hyperinsulinemic infusion rates were assessed using two-way (insulin step × time) repeated measures ANOVA. Bonferroni post hoc tests were applied to significant F ratios where appropriate. Body composition and aerobic fitness were analyzed using paired Student's t-tests. Due to technical problems, glucose kinetics analyses were not possible on two of the 14 volunteers. Statistical significance was accepted when P < 0.05. Analyses were carried out using StatView for Windows 5.0.1 (SAS Institute).
RESULTS
The participants exercised at 81 ± 2% of their maximum heart rate (∼68% of their V̇o2max) (48) for the 7 training days. As shown in Table 1, there were no significant changes in body weight, percent body fat, or V̇o2max in response to 1 wk of training.
Table 1.
Participant characteristics prior to and following the 7-day aerobic exercise intervention
| Prestudy | Poststudy | P Value | |
|---|---|---|---|
| Age, yr | 63±1 | ||
| Weight, kg | 90.3±5.0 | 90.3±5.1 | 0.84 |
| BMI, kg/m2 | 31.9±2.2 | 31.8±2.1 | 0.65 |
| %Body fat | 27.8±3.3 | 27.1±2.8 | 0.50 |
| V̇o2max, ml·kg−1·min−1 | 23.5±0.8 | 24.4±0.9 | 0.16 |
| FPG, mg/dl | 117±4 | 111±3 | 0.08 |
| FPI, μU/ml | 12.9±2.4 | 9.8±1.8 | 0.02 |
Data represent means ± SE; n = 14. BMI, body mass index; V̇o2max, maximal aerobic capacity; FPG, fasting plasma glucose; FPI, fasting plasma insulin.
Insulin action.
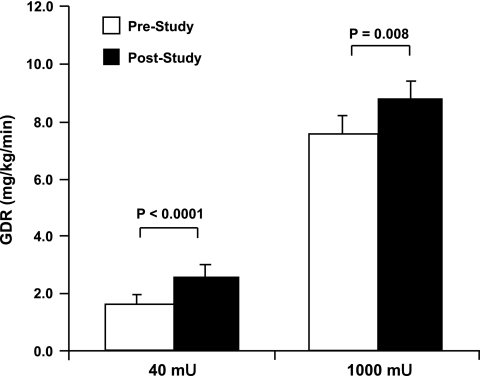
There was a decreasing trend in fasting plasma glucose concentration (P = 0.08) despite a significant decrease in fasting plasma insulin concentrations (P = 0.02) in response to the training. The steady-state plasma insulin concentration attained during the 40 mU·m−2·min−1 infusion stage of the euglycemic clamp was 70 ± 5 μU/ml before and 62 ± 5 μU/ml after the exercise program, whereas the steady-state plasma insulin levels attained during the 1,000 mU·m−2·min−1 phase of the clamp averaged 5,959 ± 493 μU/ml before and 5,617 ± 530 μU/ml after training. Plasma glucose concentration was maintained at 89 ± 1 and 90 ± 0.6 mg/dl before and at 90 ± 0.8 and 90 ± 0.5 mg/dl after training during the 40 and 1,000 mU·m−2·min−1 insulin infusion rates, respectively. In response to the 7 days of exercise, GDR increased from 1.84 ± 0.32 to 2.67 ± 0.37 mg·kg−1·min−1 during the 40-mU stage (P < 0.0001; Fig. 1) and from 7.57 ± 0.61 to 8.84 ± 0.56 mg·kg−1·min−1 during the 1,000-mU stage (P = 0.008; Fig. 1).
Fig. 1.
Effects of the 7-day exercise training program on insulin sensitivity and responsiveness. Glucose disposal rates (GDR) during the 40 (P < 0.0001) and 1,000 mU·m−2·min−1 (P = 0.008) hyperinsulinemic clamp stages were significantly greater following the 7-day program. P values indicate pre- vs. poststudy comparisons. White bars represent prestudy GDR; black bars represent poststudy GDR. Data are means ± SE; n = 14.
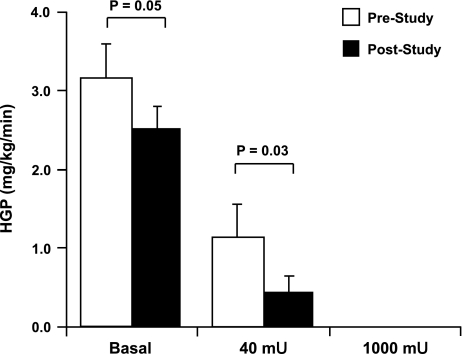
Basal HGP was lower following 7 days of exercise: 3.17 ± 0.43 vs. 2.54 ± 0.26 mg·kg−1·min−1 (P = 0.05; Fig. 2). HGP during the 40-mU insulin stage was also decreased following exercise: 1.15 ± 0.41 vs. 0.46 ± 0.20 mg·kg−1·min−1 (P = 0.03; Fig. 2). Compared with basal, the exercise program resulted in greater suppression of HGP during the 40-mU stage (−69.1 ± 9.1 vs. −84.5 ± 4.3%; P = 0.05). HGP was completely suppressed during the 1,000-mU stage both before and after the exercise program (Fig. 2). These findings provide evidence for an increased insulin suppression of HGP after the 7 days of exercise training.
Fig. 2.
Effects of the 7-day exercise training program on endogenous glucose production. [3-3H]glucose was used to assess glucose kinetics at baseline and during each stage of hyperinsulinemia. Exercise training led to a decrease in basal endogenous hepatic glucose production (HGP) (P = 0.05) plus a reduction in insulin-stimulated HGP during the 40 mU·m−2·min−1 hyperinsulinemic stage of the clamp (P = 0.03). HGP was completely suppressed during the 1,000 mU·m−2·min−1 stage prior to and after training. P values indicate pre- vs. poststudy comparisons. White bars represent prestudy HGP; black bars represent poststudy HGP. Data are means ± SE; n = 12.
DISCUSSION
The results of this study show that 7 days of vigorous exercise training that did not result in weight loss induced increases in both insulin sensitivity and responsiveness of glucose disposal. Previous studies on healthy young humans and on laboratory rodents have shown that exercise can result in increases in insulin sensitivity and responsiveness (9, 18). Numerous studies have also shown that exercise training can improve insulin action on peripheral glucose disposal in patients with type 2 diabetes and obese insulin-resistant individuals with impaired glucose tolerance (4, 11, 12, 34, 46, 51). However, in these studies, this improvement was referred to as an increase in insulin sensitivity, and insulin responsiveness was not evaluated. Our results show that an increase in insulin responsiveness is also a major contributor to the improvement in insulin action induced by short-term exercise training in patients with type 2 diabetes.
Most of the glucose removed from blood in response to insulin is taken up by skeletal muscle, so it seems reasonable to assume that the improvements in insulin sensitivity and responsiveness that occurred in this study were due to exercise-induced adaptations in muscle. It is well documented that exercise training induces increased expression of the GLUT4 isoform of the glucose transporter in muscle of humans and laboratory rodents (10, 15, 19, 21, 22, 34). Studies on rodent skeletal muscle have shown that this adaptive increase in GLUT4 results in a proportional increase in the number of GLUT4 glucose transporters that are translocated to the cell surface in response to a given insulin stimulus (38). This appears to be the mechanism by which exercise results in an increase in insulin responsiveness. Exercise also induces an increase in muscle insulin sensitivity (19). It has been shown that the same submaximal insulin stimulus results in translocation of more GLUT4 to the cell surface in muscle following exercise than in the absence of exercise (16). The mechanisms underlying the increase in muscle insulin sensitivity have not been established, but some evidence suggests that it may be mediated by enhanced insulin signaling (14, 20, 28).
The average rate of glucose disposal during the 40 mU·m−2·min−1 insulin infusion was 1.84 mg·kg−1·min−1 before training, which is 24% of that attained during the maximal insulin stimulus, which averaged 7.57 ± 0.61 mg·kg−1·min−1. After training, the rate of glucose disposal during the 40 mU·m−2·min−1 insulin infusion rate averaged 2.67 mg·kg−1·min−1, which is 30% of that attained during the maximal insulin stimulus, which averaged 8.84 ± 0.56 mg·kg−1·min−1. Thus, whereas the actual rate of glucose disposal during the 40 mU·m−2·min−1 glucose disposal rate was 45% higher after training, the rate of glucose disposal expressed relative to the maximal rate was 25% higher. Evaluated another way, a 17% increase in insulin responsiveness would result in a 17% upward shift of the insulin-glucose dose-response curve. So, the increase in insulin responsiveness of 17% should, by itself, have resulted in an increase in GDR from 1.84 to 2.15 mg·kg−1·min−1 during the 40 mU·m−2·min−1 insulin infusion. The actual GDR after training was 2.67 mg·kg−1·min−1, i.e., 24% higher. This finding provides evidence for an increase in insulin sensitivity, whereas the 17% increase in the GDR at the maximally effective insulin concentration provides evidence for an increase in insulin responsiveness as a result of the 7-day exercise program.
An important point regarding the improvements in insulin sensitivity and responsiveness induced by exercise is that they are short-term effects that wear off in 3–4 days (9, 27, 45). That these short-lived effects might also be related to transient exercise-induced changes in skeletal muscle mitochondrial function has been suggested by Kacerovsky-Bielesz et al. (24). Therefore, frequent exercise is needed to maintain the beneficial effects of exercise. Furthermore, even rather vigorous training that does not result in negative energy balance and fat loss improves but does not normalize insulin action or glucose tolerance (4, 7, 22, 35). The obesity/insulin resistance-mediated form of type 2 diabetes is a completely reversible disease in its early stage, before there is severe pancreatic β-cell damage that causes insulin deficiency. The best evidence for this statement is the rapid reversal of insulin resistance and diabetes in patients who have undergone gastric bypass surgery (5, 43). Therefore, it would be interesting in future studies to determine whether long-term exercise training that induces a significant negative energy balance can reverse type 2 diabetes. This would require a study design in which calorie intake is not increased to compensate for the increase in energy expenditure. To our knowledge, there have been no such studies on patients with type 2 diabetes. However, a number of studies on nondiabetic middle-aged people have shown that this approach is feasible and, in addition to improving insulin action, can bring about large decreases in visceral and total abdominal fat (35, 37, 40, 41, 50).
Hepatic insulin resistance resulting in increased glucose production is a key defect in type 2 diabetes and is an important factor contributing to development and maintenance of hyperglycemia (2, 31, 36). AMPK inhibits gluconeogenesis by suppressing expression of the key gluconeogenic enzymes phosphoenolpyruvate carboxykinase and glucose-6-phosphatase (3, 26, 44). Exercise results in activation of AMPK in the liver (6) and should, therefore, induce an adaptive response that results in decreased HGP. Therefore, we reevaluated the effect of short-term exercise training on insulin suppression of HGP. We found that HGP in the basal state and at a plasma insulin concentration of ∼60–70 μU/ml was significantly reduced after 7 days of exercise. Thus, it appears that a reduction in HGP may contribute to the beneficial effect of exercise training on glucose homeostasis in type 2 diabetes. Although the precise mechanism explaining exercise-induced changes in hepatic insulin sensitivity is largely unknown, recent work suggests that liver lipid partitioning may play a role. Hepatic lipid content and insulin suppression of HGP are related (42). Furthermore, exercise and diet-induced weight loss interventions reduce hepatic steatosis (17, 30, 49), yet reductions in total body weight and adiposity confound the direct hepatic effects of exercise in these studies. Preliminary data collected in our laboratory indicates that a 7-day exercise protocol identical to that employed in this study does not alter hepatic steatosis in overweight individuals with fatty liver (unpublished observations).
In conclusion, the results of this study show that vigorous exercise training for only 7 days results in significant improvements in insulin action in insulin-resistant patients with type 2 diabetes. These improvements in insulin action involve enhanced sensitivity and responsiveness of peripheral glucose uptake, presumably by muscle, to insulin as well as an increased inhibition of HGP by insulin.
GRANTS
This research was supported by National Institute on Aging Program Project Grant AG-05562, Clinical Research Center Grant M01-RR-00036, and Diabetes Research and Training Center Grant AM-20579. J. P. Kirwan and D. M. Wojta were supported by Institutional National Research Service Award AG-00078. Additional support was provided by National Institute on Aging Grant AG-12834.
Acknowledgments
Present address of M. A. Staten: Div. of Diabetes, Endocrinology, & Metabolic Diseases, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, 6707 Democracy Blvd., Bethesda, MD 20817 (e-mail: statenm@mail.nih.gov). Present address of D. M. Wojta: The Ohio State University, Mansfield Campus, 1760 University Dr., Mansfield, OH 44906 (e-mail: wojta.1@osu.edu).
REFERENCES
- 1.Arciero PJ, Vukovich MD, Holloszy JO, Racette SB, Kohrt WM. Comparison of short-term diet and exercise on insulin action in individuals with abnormal glucose tolerance. J Appl Physiol 86: 1930–1935, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Basu R, Chandramouli V, Dicke B, Landau B, Rizza R. Obesity and type 2 diabetes impair insulin-induced suppression of glycogenolysis as well as gluconeogenesis. Diabetes 54: 1942–1948, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Berasi SP, Huard C, Li D, Shih HH, Sun Y, Zhong W, Paulsen JE, Brown EL, Gimeno RE, Martinez RV. Inhibition of gluconeogenesis through transcriptional activation of EGR1 and DUSP4 by AMP-activated kinase. J Biol Chem 281: 27167–27177, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Bruce CR, Kriketos AD, Cooney GJ, Hawley JA. Disassociation of muscle triglyceride content and insulin sensitivity after exercise training in patients with Type 2 diabetes. Diabetologia 47: 23–30, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA 292: 1724–1737, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Carlson CL, Winder WW. Liver AMP-activated protein kinase and acetyl-CoA carboxylase during and after exercise. J Appl Physiol 86: 669–674, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Cox KL, Burke V, Morton AR, Beilin LJ, Puddey IB. Independent and additive effects of energy restriction and exercise on glucose and insulin concentrations in sedentary overweight men. Am J Clin Nutr 80: 308–316, 2004. [DOI] [PubMed] [Google Scholar]
- 8.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol Endocrinol Metab Gastrointest Physiol 237: E214–E223, 1979. [DOI] [PubMed] [Google Scholar]
- 9.Dela F, Mikines KJ, Von Linstow M, Secher NH, Galbo H. Effect of training on insulin-mediated glucose uptake in human muscle. Am J Physiol Endocrinol Metab 263: E1134–E1143, 1992. [DOI] [PubMed] [Google Scholar]
- 10.Dela F, Ploug T, Handberg A, Petersen LN, Larsen JJ, Mikines KJ, Galbo H. Physical training increases muscle GLUT4 protein and mRNA in patients with NIDDM. Diabetes 43: 862–865, 1994. [DOI] [PubMed] [Google Scholar]
- 11.Dengel DR, Pratley RE, Hagberg JM, Rogus EM, Goldberg AP. Distinct effects of aerobic exercise training and weight loss on glucose homeostasis in obese sedentary men. J Appl Physiol 81: 318–325, 1996. [DOI] [PubMed] [Google Scholar]
- 12.Ferrara CM, Goldberg AP, Ortmeyer HK, Ryan AS. Effects of aerobic and resistive exercise training on glucose disposal and skeletal muscle metabolism in older men. J Gerontol A Biol Sci Med Sci 61: 480–487, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Finegood DT, Bergman RN, Vranic M. Estimation of endogenous glucose production during hyperinsulinemic-euglycemic glucose clamps. Comparison of unlabeled and labeled exogenous glucose infusates. Diabetes 36: 914–924, 1987. [DOI] [PubMed] [Google Scholar]
- 14.Frösig C, Rose AJ, Treebak JT, Kiens B, Richter EA, Wojtaszewski JF. Effects of endurance exercise training on insulin signaling in human skeletal muscle: interactions at the level of phosphatidylinositol 3-kinase, Aft, and AS160. Diabetes 56: 2093–2102, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Gulve EA, Spina RJ. Effect of 7–10 days of cycle ergometer exercise on skeletal muscle GLUT-4 protein content. J Appl Physiol 79: 1562–1566, 1995. [DOI] [PubMed] [Google Scholar]
- 16.Hansen PA, Nolte LA, Chen MM, Holloszy JO. Increased GLUT-4 translocation mediates enhanced insulin sensitivity of muscle glucose transport after exercise. J Appl Physiol 85: 1218–1222, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Hickman IJ, Jonsson JR, Prins JB, Ash S, Purdie DM, Clouston AD, Powell EE. Modest weight loss and physical activity in overweight patients with chronic liver disease results in sustained improvements in alanine aminotransferase, fasting insulin, and quality of life. Gut 53: 413–419, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holloszy JO A forty-year memoir of research on the regulation of glucose transport into muscle. Am J Physiol Endocrinol Metab 284: E453–E467, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Holloszy JO Exercise-induced increase in muscle insulin sensitivity. J Appl Physiol 99: 338–343, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Houmard JA, Shaw CD, Hickey MS, Tanner CJ. Effect of short-term exercise training on insulin-stimulated PI 3-kinase activity in human skeletal muscle. Am J Physiol Endocrinol Metab 277: E1055–E1060, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Houmard JA, Shinebarger MH, Dolan PL, Leggett-Frazier N, Bruner RK, McCammon MR, Israel RG, Dohm GL. Exercise training increases GLUT-4 protein concentration in previously sedentary middle-aged men. Am J Physiol Endocrinol Metab 264: E896–E901, 1993. [DOI] [PubMed] [Google Scholar]
- 22.Hughes VA, Fiatarone MA, Fielding RA, Kahn BB, Ferrara CM, Shepherd P, Fisher EC, Wolfe RR, Elahi D, Evans WJ. Exercise increases muscle GLUT-4 levels and insulin action in subjects with impaired glucose tolerance. Am J Physiol Endocrinol Metab 264: E855–E862, 1993. [DOI] [PubMed] [Google Scholar]
- 23.Ivy JL, Zderic TW, Fogt DL. Prevention and treatment of non-insulin-dependent diabetes mellitus. Exerc Sport Sci Rev 27: 1–35, 1999. [PubMed] [Google Scholar]
- 24.Kacerovsky-Bielesz G, Chmelik M, Ling C, Pokan R, Szendroedi J, Farukuoye M, Kacerovsky M, Schmid AI, Gruber S, Wolzt M, Moser E, Pacini G, Smekal G, Groop L, Roden M. Short-term exercise training does not stimulate skeletal muscle ATP synthesis in relatives of humans with type 2 diabetes. Diabetes. In Press. [DOI] [PMC free article] [PubMed]
- 25.Kahn CR Insulin resistance, insulin insensitivity, and insulin unresponsiveness: a necessary distinction. Metabolism 27, Suppl 2: 1893–1902, 1978. [DOI] [PubMed] [Google Scholar]
- 26.Kim YD, Park KG, Lee YS, Park YY, Kim DK, Nedumaran B, Jang WG, Cho WJ, Ha J, Lee IK, Lee CH, Choi HS. Metformin inhibits hepatic gluconeogenesis through AMP-activated protein kinase-dependent regulation of the orphan nuclear receptor SHP. Diabetes 57: 306–314, 2008. [DOI] [PubMed] [Google Scholar]
- 27.King DS, Baldus PJ, Sharp RL, Kesl LD, Feltmeyer TL, Riddle MS. Time course for exercise-induced alterations in insulin action and glucose tolerance in middle-aged people. J Appl Physiol 78: 17–22, 1995. [DOI] [PubMed] [Google Scholar]
- 28.Kirwan JP, Del Aguila LF, Hernandez JM, Williamson DL, O'Gorman DJ, Lewis R, Krishnan RK. Regular exercise enhances insulin activation of IRS-1-associated PI3-kinase in human skeletal muscle. J Appl Physiol 88: 797–803, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Kohrt WM, Kirwan JP, Staten MA, Bourey RE, King DS, Holloszy JO. Insulin resistance in aging is related to abdominal obesity. Diabetes 42: 273–281, 1993. [PubMed] [Google Scholar]
- 30.Larson-Meyer DE, Heilbronn LK, Redman LM, Newcomer BR, Frisard MI, Anton S, Smith SR, Alfonso A, Ravussin E. Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care 29: 1337–1344, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Magnusson I, Rothman DL, Katz LD, Shulman RG, Shulman GI. Increased rate of gluconeogenesis in type II diabetes mellitus. A 13C nuclear magnetic resonance study. J Clin Invest 90: 1323–1327, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsumoto M, Accili D. The tangled path to glucose production. Nat Med 12: 33–34; discussion 34, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Morgan CR, Lazarow A. Immunoassay of insulin: two antibody system. Diabetes 12: 115–126, 1963. [Google Scholar]
- 34.O'Gorman DJ, Karlsson HK, McQuaid S, Yousif O, Rahman Y, Gasparro D, Glund S, Chibalin AV, Zierath JR, Nolan JJ. Exercise training increases insulin-stimulated glucose disposal and GLUT4 (SLC2A4) protein content in patients with type 2 diabetes. Diabetologia 49: 2983–2992, 2006. [DOI] [PubMed] [Google Scholar]
- 35.O'Leary VB, Marchetti CM, Krishman RK, Stetzer BP, Gonzalez F, Kirwan JP. Exercise-induced reversal of insulin resistance in obese elderly is associated with reduced visceral fat. J Appl Physiol 100: 1584–1589, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paquot N, Scheen AJ, Dirlewanger M, Lefèbvre PJ, Tappy L. Hepatic insulin resistance in obese non-diabetic subjects and in type 2 diabetic patients. Obes Res 10: 129–134, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Racette SB, Weiss EP, Villareal DT, Arif H, Steger-May K, Schechtman KB, Fontana L, Klein S, Holloszy JO; Washington University School of Medicine CALERIE Group. One year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissue. J Gerontol A Biol Sci Med Sci 61: 943–950, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ren JM, Semenkovich CF, Gulve EA, Gao J, Holloszy JO. Exercise induces rapid increases in GLUT4 expression, glucose transport capacity, and insulin-stimulated glycogen storage in muscle. J Biol Chem 269: 14396–14401, 1994. [PubMed] [Google Scholar]
- 39.Rogers MA, Yamamoto C, King DS, Hagberg JM, Ehsani AA, Holloszy JO. Improvement in glucose tolerance after 1 wk of exercise in patients with mild NIDDM. Diabetes Care 11: 613–618, 1988. [DOI] [PubMed] [Google Scholar]
- 40.Ross R, Dagnone D, Jones PJ, Smith H, Paddags A, Hudson R, Janssen I. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann Intern Med 133: 92–103, 2000. [DOI] [PubMed] [Google Scholar]
- 41.Ross R, Janssen I, Dawson J, Kungl AM, Kuk JL, Wong SL, Nguyen-Duy TB, Lee S, Kilpatrick K, Hudson R. Exercise-induced reduction in obesity and insulin resistance in women: a randomized controlled trial. Obes Res 12: 789–798, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Ryysy L, Häkkinen AM, Goto T, Vehkavaara S, Westerbacka J, Halavaara J, Yki-Jarvinen H. Hepatic fat content and insulin action on free fatty acids and glucose metabolism rather than insulin absorption are associated with insulin requirements during insulin therapy in type 2 diabetic patients. Diabetes 49: 749–758, 2000. [DOI] [PubMed] [Google Scholar]
- 43.Schauer PR, Ikramuddin S, Gourash W, Ramanathan R, Luketich J. Outcomes after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Ann Surg 232: 515–529, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, DePinho RA, Montminy M, Cantley LC. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 310: 1642–1646, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Short KR, Vittone JL, Bigelow ML, Proctor DN, Rizza RA, Coenen-Schimke JM, Nair KS. Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes 52: 1888–1896, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Solomon TP, Sistrun SN, Krishnan RK, Del Aguila LF, Marchetti CM, O'Carroll SM, O'Leary VB, Kirwan JP. Exercise and diet enhance fat oxidation and reduce insulin resistance in older obese adults. J Appl Physiol 104: 1313–1319, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steele RC Influence of glucose loading and of injected insulin on hepatic glucose output. Ann NY Acad Sci 82: 420–430, 1959. [DOI] [PubMed] [Google Scholar]
- 48.Swain DP, Abernathy KS, Smith CS, Lee SJ, Bunn SA. Target heart rates for the development of cardiorespiratory fitness. Med Sci Sports Exerc 26: 112–116, 1994. [PubMed] [Google Scholar]
- 49.Ueno T, Sugawara H, Sujaku K, Hashimoto O, Tsuji R, Tamaki S, Torimura T, Inuzuka S, Sata M, Tanikawa K. Therapeutic effects of restricted diet and exercise in obese patients with fatty liver. J Hepatol 27: 103–107, 1997. [DOI] [PubMed] [Google Scholar]
- 50.Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, Klein S, Holloszy JO. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am J Clin Nutr 84: 1033–1042, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Winnick JJ, Sherman WM, Habash DL, Stout MB, Failla ML, Belury MA, Schuster DP. Short-term aerobic exercise training in obese humans with type 2 diabetes mellitus improves whole-body insulin sensitivity through gains in peripheral, not hepatic insulin sensitivity. J Clin Endocrinol Metab 93: 771–778, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]