Abstract
The relative contributions of net hepatic glycogenolysis (NHG) and gluconeogenesis to rates of glucose production during a physiological increment in plasma epinephrine concentrations, independent of changes in plasma insulin concentrations, were determined in seven fasting, healthy young subjects. Plasma insulin concentrations were kept constant by infusing somatostatin (0.1 μg·kg−1·min−1) and replacing basal insulin (24 pmol·m−2·min−1). Epinephrine (1.2 μg·m−2·min−1) was infused for 90 min while NHG was assessed directly by 13C magnetic resonance spectroscopy. The rate of glucose production was assessed using [6,6-2H2]glucose, and gluconeogenesis was calculated as the difference between the rate of glucose production and NHG. Plasma epinephrine concentrations increased rapidly from ∼100 to ∼2,000 pmol/l (P < 0.00001) accompanied by an increase in plasma glucose concentrations from 4.3 ± 0.2 to 13.3 ± 0.3 mmol/l at 90 min (P = 0.00001). This increase in plasma epinephrine concentration resulted in a 2.5-fold increase in glucose production (from 14.4 ± 1.0 μmol·kg−1·min−1 to 35.7 ± 2.0 μmol·kg−1·min−1, P < 0.0001), which lasted for ∼60 min (phase 1), after which glucose production decreased to 31.2 ± 1.9 μmol·kg−1·min−1 (P < 0.0001 vs. basal) during the last 30 min of the epinephrine infusion (phase 2). Hepatic glycogen concentrations decreased almost linearly during phase 1, and rates of NHG were 19.9 ± 3.0 μmol·kg−1·min−1 (P = 0.005 vs. basal), which could account for ∼60% of glucose production. During phase 2, NHG decreased to 7.3 ± 2.8 μmol·kg−1·min−1 (P = 0.02 vs. peak), accounting for only ∼20% of glucose production. In conclusion, in the presence of basal plasma insulin and glucagon concentrations, a physiological increase in plasma epinephrine concentrations stimulates glucose production with an initial, 60-min transient phase caused by stimulation of NHG and a second phase that can mostly be attributed to a twofold increase in rates of gluconeogenesis.
Keywords: net hepatic glycogenolysis, gluconeogenesis, epinephrine, humans
it is well established that hepatic glucose production increases in response to a physiological increase in plasma epinephrine concentration, but the role of epinephrine in the regulation of the relative contributions of net hepatic glycogenolysis and gluconeogenesis to glucose production in humans is unknown (14, 19). Surprisingly, only a few studies have examined the effects of epinephrine on glucose production in humans, and none of these studies have directly assessed the relative contributions of net hepatic glycogenolysis and gluconeogenesis to this process due to the difficulty of measuring these processes in vivo.
In this regard, 13C magnetic resonance spectroscopy (MRS) provides a noninvasive method to directly measure changes in liver glycogen content in humans in real time allowing for calculation of rates of net hepatic glycogenolysis (10, 15). Rates of gluconeogenesis can be calculated by subtracting rates of net hepatic glycogenolysis from rates of whole body glucose production as determined with [6,6-2H2]glucose. The purpose of the present study was to use 13C MRS in combination with [6,6-2H2]glucose to directly estimate the relative contributions of net hepatic glycogenolysis and gluconeogenesis to overall glucose production in humans during a physiological increase in plasma epinephrine concentration.
Because epinephrine not only stimulates hepatic glucose production but also directly and indirectly alters pancreatic β-cell function, we infused epinephrine in combination with somatostatin to inhibit endogenous insulin and glucagon secretion and infused insulin to maintain basal peripheral insulin concentration. Using this approach, we were able to examine the effects of epinephrine per se on the relative contributions of net hepatic glycogenolysis and gluconeogenesis to glucose production in humans.
METHODS
Subjects
We studied seven healthy, nonsmoking volunteers (5 males, 2 females, 22 ± 2 yr, body weight 68 ± 4 kg, body mass index: 21.7 ± 0.7 kg/m2). None of the subjects was taking any medication or had a family history of diabetes. Informed consent was obtained from each subject after the purpose, nature, and potential risks of the study had been explained. The protocol was reviewed and approved by the Human Investigation Committee of the Yale University School of Medicine.
Experimental Protocols
For 3 days before the study, subjects were given a weight-maintaining diet (30–40 kcal/kg body wt, 60% carbohydrate, 20% protein, and 20% fat) prepared by the metabolic kitchen of the Yale University-Center for Clinical Investigation Hospital Research Unit (HRU). The diet was given as breakfast, lunch, and dinner, with each meal providing one-third of the daily caloric intake. On the evening before the study, participants were admitted to the HRU, and, at 10:00 P.M., they were given an extra meal in liquid form (1,000 kcal, 60% carbohydrate with 80% as sucrose, 20% protein, and 20% fat). After this meal, the subject fasted overnight until the end of the study the following day.
At 6:00 A.M., a Teflon catheter was inserted in an antecubital vein in each arm for blood drawing and infusions, and the subjects were brought to the Magnetic Resonance Research Center. Liver volume was measured using magnetic resonance imaging with three-dimensional reconstruction performed in a 1.5-T magnet (Signa III; General Electric, Milwaukee, WI), as previously described (15). At 7:30 A.M., the subjects were placed in the supine position on the MRS bed (2.1 T, 1 m bore, Bruker I Biospec Spectrometer; Bruker Instruments, Billerica, MA), and the receiver coil was placed on the abdomen over the liver. After an initial image to confirm the position of the coil over the liver, the 2-h baseline period was begun with continuous measurements of liver glycogen concentrations (10) along with infusions of [6,6-2H2]glucose (primed: 5 mg/kg; continuous: 0.05 mg·kg−1·min−1), somatostatin (0.1 μg·kg−1·min−1, to inhibit endogenous insulin and glucagon release), and insulin (24 pmol·kg−1·min−1; to replace basal peripheral insulin concentrations). At the end of the baseline period, blood was collected for determination of plasma [6,6-2H2]glucose enrichment [atom percent enrichment (APE)] and concentrations of glucose, epinephrine, insulin, C-peptide, glucagon, and lactate.
At time 0 min, while the patients remained inside the MR Spectrometer, the epinephrine infusion was started (1.2 μg·m−2·min−1) and continued for 90 min. The 13C MRS measurements of liver glycogen concentration measurements were continued throughout the epinephrine infusion. Every 5 min, blood samples were drawn for determination of plasma glucose and lactate concentrations and every 15 min for determination of plasma [6,6-2H2]glucose enrichment, epinephrine, insulin, C-peptide, and glucagon concentrations.
Analytical Procedures
Every 5 min, plasma glucose and lactate concentrations were measured using the glucose oxidase method (YSI 2700A; Yellow Springs Instruments, Yellow Springs, CA). Plasma free fatty acid (FFA) concentrations were measured using a microfluorimetric method (8), and plasma glycerol concentrations were measured as previously described (7). Plasma immunoreactive insulin, C-peptide, and glucagon concentrations were measured using double-antibody radioimmunoassay kits (Diagnostic Systems Laboratories, Webster, TX). Plasma epinephrine concentrations were measured using a radioenzymatic assay (Amersham, Arlington Heights, IL). Plasma APE of [6,6-2H]glucose was determined every 15 min by gas chromatography-mass spectrometry of the penta-acetate derivative of plasma glucose after deproteinization and deionization as previously described (10).
13C MRS Measurements of Hepatic Glycogen Concentrations
Hepatic glycogen concentrations were monitored throughout the 120 min of the baseline period and the 90 min of the epinephrine infusion (epinephrine period) using 13C MRS, as previously described (15). Briefly, a 9-cm 13C observation coil and a 12- × 14-cm coplanar butterfly 1H decoupling coil were placed rigidly over the lateral aspect of the abdomen of the supine subject, the position of the coil over the liver was confirmed with an image, and the C1 glycogen resonance (100.6 ppm) was quantified by integration and comparison with a glycogen solution.
Calculations
During the baseline period, rates of glucose production were calculated for steady-state conditions as the ratio of the tracer-to-tracee APE multiplied by the rates of [6,6-2H2]glucose infusion, corrected for the glucose infusion rate (10). During the epinephrine period, rates of glucose production were calculated using a modification of the two-compartment model of Radziuk et al. (13) for non-steady-state conditions.
Rates of net hepatic glycogenolysis were calculated from the slope of the best linear fit of glycogen concentrations over time. Rates of net hepatic glycogenolysis (mmol·kg−1·min−1) were calculated by multiplication with the liver volume and division by the body weight. Rates of gluconeogenesis were calculated as the difference between rates of whole body glucose production and rates of net hepatic glycogenolysis (10, 15).
Statistical Tests
Data are given as means ± SE. Statistical comparisons within and between groups were performed by the use of the paired Student's test.
RESULTS
Plasma Metabolite Concentrations
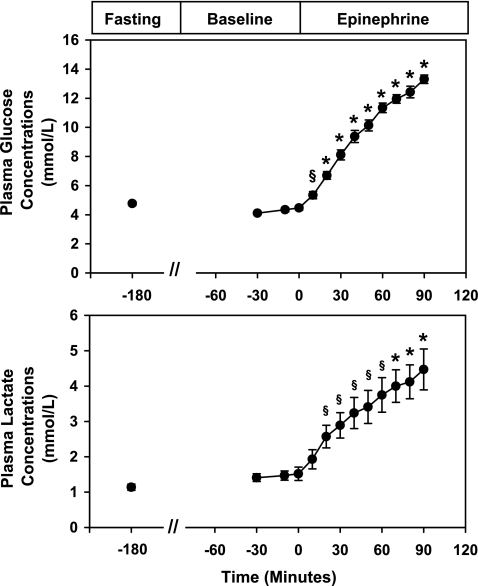
The time courses of plasma concentrations of glucose and lactate are shown in Fig. 1. During the 2-h baseline period after start of the somatostatin infusion, plasma glucose concentrations remained constant at 4.3 ± 0.2 mmol/l and lactate at 1.5 ± 0.2 mmol/l. At time 0 min when the epinephrine infusion was begun, plasma glucose concentrations immediately increased and reached a peak of 13.3 ± 0.3 mmol/l by the end of the epinephrine infusion (P = 0.00001 vs. baseline) (Fig. 1). After 20 min of epinephrine infusion, plasma lactate concentrations began to increase and by the end of the epinephrine infusion reached a peak of 4.6 ± 0.7 mmol/l (P < 0.00001 vs. baseline). During the epinephrine infusion, plasma concentrations of FFA increased approximately fivefold from 265 ± 41 to 1,093 ± 136 μmol/l (P < 0.0002), and plasma glycerol concentrations increased approximately fourfold (basal period: 42 ± 5 μmol/l vs. epinephrine period: 135 ± 18 μmol/l; P = 0.02).
Fig. 1.
Effect of epinephrine on plasma glucose and lactate concentrations. Values are expressed as means ± SE. §P < 0.01 and *P < 0.001.
Hormone Concentrations
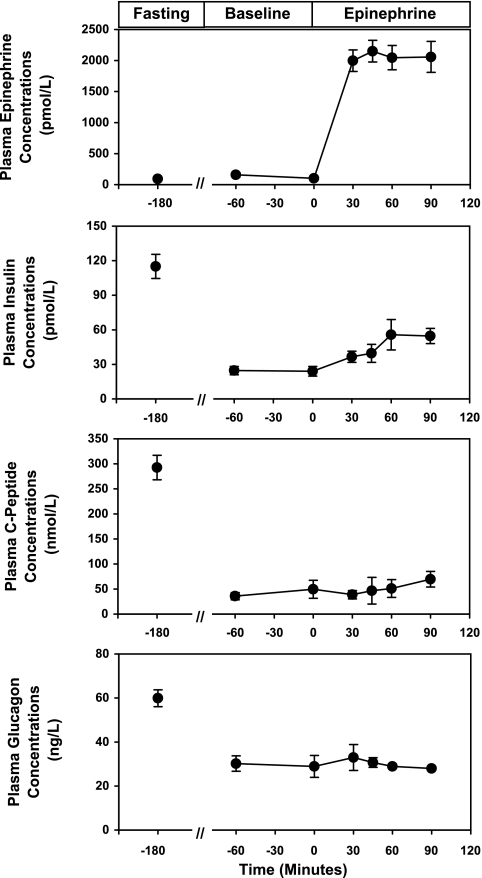
The plasma concentrations of epinephrine, insulin, C-peptide, and glucagon are shown in Fig. 2. There were no changes in plasma epinephrine concentrations from fasting to the baseline period. In contrast, once the somatostatin infusion was started (time = −120 min), peripheral plasma concentrations of insulin decreased from 115 ± 11 to 24 ± 3 pmol/l (P < 0.0001), C-peptide concentrations decreased from 293 ± 24 to 42 ± 8 pmol/l (P < 0.0001), and plasma glucagon concentrations decreased from 60 ± 3 to 29 ± 3 ng/l (P < 0.0001). At time 0 min, when the epinephrine infusion was begun, plasma epinephrine concentrations immediately increased from 125 ± 130 to 2,058 ± 250 pmol/l (P < 0.0001), whereas peripheral plasma insulin, C-peptide, and glucagon concentrations remained suppressed, despite the epinephrine-induced hyperglycemia. The average heart rate increased from 64 ± 2 beats/min during the basal period to 82 ± 3 beats/min during the epinephrine infusion (P < 0.0001).
Fig. 2.
Effect of epinephrine on plasma epinephrine, insulin, C-peptide, and glucagon concentrations. Values are expressed as means ± SE.
Rates of Glucose Production, Net Hepatic Glycogenolysis, and Gluconeogenesis
Baseline period.
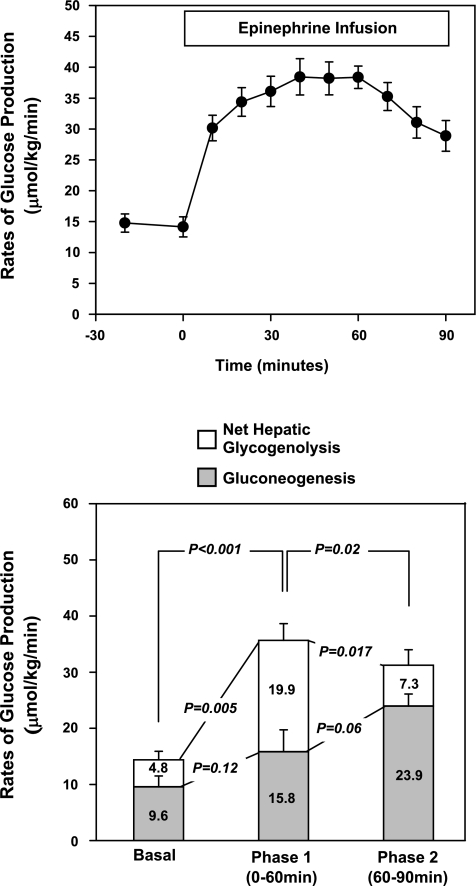
During the baseline period, whole body glucose production was 14.4 ± 1.0 μmol·kg−1·min−1 with net hepatic glycogenolysis accounting for 36 ± 11% (4.8 ± 1.5 μmol·kg−1·min−1) and gluconeogenesis for 64 ± 11% (9.6 ± 2.0 μmol·kg−1·min−1) (Fig. 3).
Fig. 3.
Top: time course for the change in glucose production under basal conditions and during an epinephrine infusion. Bottom: effect of epinephrine on rates of glucose production, net hepatic glycogenolysis, and gluconeogenesis. Values are expressed as means ± SE. Significance is indicated by paired Student's t-test result.
Epinephrine period.
Upon starting the epinephrine infusion, whole body glucose production immediately increased and reached a maximum of 35.7 ± 2.0 μmol·kg−1·min−1 (P = 0.0002 vs. basal) at 60 min. During phase 1 of the epinephrine infusion, plasma glucose concentrations increased to a peak of 11.6 ± 0.3 mmol/l (P < 0.001 vs. basal) (Fig. 1). During phase 2 of the epinephrine infusion (60–90 min), glucose production slowly decreased but remained twofold higher than during the baseline period (31.2 ± 1.9 μmol·kg−1·min−1; P = 0.02 vs. peak and P < 0.0001 vs. basal) (Fig. 3, top).
Rates of net hepatic glycogenolysis are shown in Fig. 3, bottom. During phase 1 of the epinephrine infusion, rates of net hepatic glycogenolysis increased from 4.8 ± 1.5 μmol·kg−1·min−1 to a peak at 19.9 ± 3.0 μmol·kg−1·min−1 (P = 0.005 vs. basal), which could account for 58 ± 10% of rates of glucose production.
During phase 2 of the epinephrine infusion, rates of net hepatic glycogenolysis decreased to 7.3 ± 2.8 μmol·kg−1·min−1 (P = 0.017 vs. peak, P = not significant vs. basal) to account for 21 ± 9% of glucose production.
During phase 1 of the epinephrine infusion, rates of gluconeogenesis remained relatively constant (P = 0.12 vs. basal) and could account for 42 ± 10% of rates of glucose production. During phase 2 of the epinephrine infusion (60–90 min), rates of gluconeogenesis increased dramatically and peaked at 23.9 ± 2.2 μmol·kg−1·min−1 (P = 0.02 vs. baseline) to account for 79 ± 9% of glucose production.
DISCUSSION
The effects of a physiological increase in plasma epinephrine concentration on rates of whole body glucose production were examined in combination with 13C MRS measurements of rates of net hepatic glycogenolysis to assess the relative contributions of net hepatic glycogenolysis and gluconeogenesis to glucose production. Infusing epinephrine, at a rate of 1.2 μg·m−2·min−1, increased plasma epinephrine concentrations to ∼2,000 pmol/l, which is equivalent to the levels observed during exercise at 60–80% of V̇o2max (11), and increased the average heart rate ∼30% from resting. This increment in plasma epinephrine concentration increased glucose production in a biphasic manner with an initial ∼2.5-fold increase during the first 60 min of the infusion (phase 1), which could be accounted for by a fourfold increase in rates of net hepatic glycogenolysis and no appreciable changes in rates of gluconeogenesis.
This was followed by a second phase of 30 min (60–90 min), during which rates of glucose production decreased by ∼15% compared with the peak, but still were slightly more than twofold higher than basal rates. During this second phase, net hepatic glycogenolysis decreased to ∼20% of glucose production and gluconeogenesis increased ∼2.5-fold from basal to account for ∼80% of whole body glucose production.
Previous studies in humans have examined the effects of epinephrine on glucose production, but no study has yet quantified the individual contributions of net hepatic glycogenolysis and gluconeogenesis to glucose production. Rizza et al. (4), using somatostatin to prevent increases in plasma insulin and glucagon levels during the epinephrine administration, found a transient but sustained increase in glucose production, similar to our results, but did not estimate the relative contribution of hepatic glycogenolysis and gluconeogenesis to glucose production. Using arterial-hepatic venous difference, Sacca et al. (16) estimated net splanchnic glucose output during an epinephrine infusion. Plasma epinephrine levels were not measured in that study, and the authors did not control the endogenous production of insulin and glucagon with somatostatin. In response to this epinephrine infusion, plasma glucose concentration rose slowly to a peak of ∼6.7 mmol/l after 60 min and then remained stable for the last 30 min. At the same time, net splanchnic glucose output increased rapidly during the first 30 min to a peak of ∼22 μmol·kg−1·min−1, and then decreased gradually to basal rates during the last 60 min of the epinephrine infusion. This early decrease in glucose production contrasts with the sustained increase in the rate of glucose production observed in our study, which peaks at ∼36 μmol·kg−1·min−1, and is most likely due to the significant compensatory rise in plasma insulin concentrations in response to the hyperglycemia. These authors also examined the incorporation of [U-14C]alanine into [14C]glucose during these studies and found that it was delayed, leading them to conclude that the initial increase in glucose production by epinephrine was due to glycogenolysis and that gluconeogenesis was activated at a later time. However, they were not able to quantify the relative contributions of these processes to hepatic glucose production using this approach (16).
In the present study, the effects of epinephrine to stimulate rates of net hepatic glycogenolysis were transient but could not be accounted for by depletion of hepatic glycogen stores. After 60 min of epinephrine infusion (phase 2), rates of net hepatic glycogenolysis declined despite liver glycogen concentrations of 248 ± 17 mmol/l, which are similar to the levels of hepatic glycogen after an overnight fast but approximately sixfold higher than after a prolonged fast of 64 h (42 ± 9 mmol/l) (15). This observation of an evanescent effect of a continuous hormone stimulation on rates of net hepatic glycogenolysis is similar to what has previously been observed for glucagon (6). This reduction in hepatic glucose production is likely due to the ability of hyperglycemia per se to inhibit hepatic glucose production (18) through inhibition of glycogen phosphorylase activity (9, 17, 19), as well as the intrinsic post-G-coupled receptor downregulation of signaling (4, 5). Epinephrine, at this physiological concentration (∼2,000 pmol/l), appears to be as potent as glucagon (at physiological levels of ∼300 ng/l) to stimulate net hepatic glycogenolysis in humans, as reflected by similar maximal rates of net hepatic glycogenolysis in response to epinephrine and to glucagon [19.9 ± 3.0 and 21.7 μmol·kg−1·min−1, respectively (6)]. It is possible that the contribution of epinephrine on net hepatic glycogenolysis may represent an underestimate of the effects of epinephrine on net hepatic glycogenolysis due to portal vein hypoglucagonemia during the epinephrine infusion.
In the second phase of epinephrine infusion (60–90 min), when net hepatic glycogenolysis had waned, rates of gluconeogenesis increased by 150% and accounted for most of the increased glucose production (∼80%). This likely reflects epinephrine's ability to stimulate gluconeogenesis through upregulation of transcription factors and cofactors involved in the regulation of hepatic gluconeogenesis (e.g., cAMP response element-binding protein, peroxisome proliferator-activated receptor-γ coactivator-1α) (12), resulting in an increased expression of key gluconeogenic enzymes (phosphoenolpyruvate carboxykinase, glucose-6-phosphatase) (1). In addition, the increase in plasma lactate concentrations, due to epinephrine-induced muscle glycogenolysis (3), and the increase in plasma glycerol concentrations were more pronounced during the second phase of epinephrine administration and provided increased gluconeogenic substrate supply to the liver during this time period. The significant increase in plasma FFA concentrations during the epinephrine infusion is consistent with epinephrine-induced lipolysis, and, as previously shown in humans, this acute increase in plasma FFA may also have contributed to a direct stimulation of gluconeogenesis (2). Thus the increase in gluconeogenic substrates, combined with increased gluconeogenic enzyme expression/activation, might explain the later increased rate of gluconeogenesis observed in this study.
In summary, our studies show that, under conditions of basal peripheral insulin and glucagon concentrations, a physiological increase in epinephrine concentration in humans induces a sustained and biphasic increase in glucose production. Using natural abundance 13C MRS, we demonstrate that, during the first 60 min of epinephrine infusion, activation of hepatic glycogenolysis is responsible for the increase in glucose production. We also demonstrate that the activation of net hepatic glycogenolysis is only transient and that, during the later phase of epinephrine infusion (60–90 min), rates of net hepatic glycogenolysis return to basal. As that point, increased rates of glucose production are sustained due to a 2.5-fold increase in gluconeogenesis.
GRANTS
These studies were supported by National Institutes of Health Grants R01 DK-49230, P01 DK-68229, R01 AG-23286, and M01 RR-00125. G. I. Shulman is an investigator at the Howard Hughes Medical Institute.
Acknowledgments
We thank Joan Boyer, Veronica Walton, Donna Caseria, Yanna Kosover, and the staff of the YCCI for expert technical assistance with these studies.
Present address for V. Lebon: CEA-MIRCen, 92265 Fontenay-au-Roses Cedex, France.
REFERENCES
- 1.Barthel A, Schmoll D. Novel concepts in insulin regulation of hepatic gluconeogenesis. Am J Physiol Endocrinol Metab 285: E685–E692, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Chen X, Iqbal N, Boden G. The effects of free fatty acids on gluconeogenesis and glycogenolysis in normal subjects. J Clin Invest 103: 365–372, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cori CF, Schmidt G, Cori GT. The synthesis of a polysaccharide from glucose-1-phosphate in muscle extract. Science 89: 464–465, 1939. [DOI] [PubMed] [Google Scholar]
- 4.Hutson NJ, Brumley FT, Assimacopoulos FD, Harper SC, Exton JH. Studies on the alpha-adrenergic activation of hepatic glucose output. I. Studies on the alpha-adrenergic activation of phosphorylase and gluconeogenesis and inactivation of glycogen synthase in isolated rat liver parenchymal cells. J Biol Chem 251: 5200–5208, 1976. [PubMed] [Google Scholar]
- 5.Liljenquist JE, Bomboy JD, Lewis SB, Sinclair-Smith BC, Felts PW, Lacy WW, Crofford OB, Liddle GW. Effect of glucagon on net splanchnic cyclic AMP production in normal and diabetic men. J Clin Invest 53: 198–204, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magnusson I, Rothman DL, Gerard DP, Katz LD, Shulman GI. Contribution of hepatic glycogenolysis to glucose production in humans in response to a physiological increase in plasma glucagon concentration. Diabetes 44: 185–189, 1995. [DOI] [PubMed] [Google Scholar]
- 7.Mayerson AB, Hundal RS, Dufour S, Lebon V, Befroy D, Cline GW, Enocksson S, Inzucchi SE, Shulman GI, Petersen KF. The effects of rosiglitazone on insulin sensitivity, lipolysis, and hepatic and skeletal muscle triglyceride content in patients with type 2 diabetes. Diabetes 51: 797–802, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miles J, Glasscock R, Aikens J, Gerich J, Haymond M. A microfluorometric method for the determination of free fatty acids in plasma. J Lipid Res 24: 96–99, 1983. [PubMed] [Google Scholar]
- 9.Petersen KF, Laurent D, Rothman DL, Cline GW, Shulman GI. Mechanism by which glucose and insulin inhibit net hepatic glycogenolysis in humans. J Clin Invest 101: 1203–1209, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petersen KF, Price T, Cline GW, Rothman DL, Shulman GI. Contribution of net hepatic glycogenolysis to glucose production during the early postprandial period. Am J Physiol Endocrinol Metab 270: E186–E191, 1996. [DOI] [PubMed] [Google Scholar]
- 11.Petersen KF, Price TB, Bergeron R. Regulation of net hepatic glycogenolysis and gluconeogenesis during exercise: impact of type 1 diabetes. J Clin Endocrinol Metab 89: 4656–4664, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Postic C, Dentin R, Girard J. Role of the liver in the control of carbohydrate and lipid homeostasis. Diabetes Metab 30: 398–408, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Radziuk J, Norwich KH, Vranic M. Experimental validation of measurements of glucose turnover in nonsteady state. Am J Physiol Endocrinol Metab Gastrointest Liver Physiol 234: E84–E93, 1978. [DOI] [PubMed] [Google Scholar]
- 14.Rizza R, Haymond M, Cryer P, Gerich J. Differential effects of epinephrine on glucose production and disposal in man. Am J Physiol Endocrinol Metab 237: E356–E362, 1979. [DOI] [PubMed] [Google Scholar]
- 15.Rothman DL, Magnusson I, Katz LD, Shulman RG, Shulman GI. Quantitation of hepatic glycogenolysis and gluconeogenesis in fasting humans with 13C NMR. Science 254: 573–576, 1991. [DOI] [PubMed] [Google Scholar]
- 16.Sacca L, Vigorito C, Cicala M, Corso G, Sherwin RS. Role of gluconeogenesis in epinephrine-stimulated hepatic glucose production in humans. Am J Physiol Endocrinol Metab 245: E294–E302, 1983. [DOI] [PubMed] [Google Scholar]
- 17.Shiota M, Moore MC, Galassetti P, Monohan M, Neal DW, Shulman GI, Cherrington AD. Inclusion of low amounts of fructose with an intraduodenal glucose load markedly reduces postprandial hyperglycemia and hyperinsulinemia in the conscious dog. Diabetes 51: 469–478, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Shulman GI, Liljenquist JE, Williams PE, Lacy WW. Glucose disposal during insulinopenia in somatostatin-treated dogs. The roles of glucose and glucagon. J Clin Invest 62: 487–491, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevenson RW, Steiner KE, Connolly CC, Fuchs H, Alberti KG, Williams PE, Cherrington AD. Dose-related effects of epinephrine on glucose production in conscious dogs. Am J Physiol Endocrinol Metab 260: E363–E370, 1991. [DOI] [PubMed] [Google Scholar]