Abstract
Elevated plasma free fatty acids (FFA) cause insulin resistance and are thought to play a key role in mediating insulin resistance in patients with the metabolic syndrome (MTS) and type 2 diabetes mellitus (DM). Two experimental models used to study the mechanisms responsible for insulin resistance in patients are high-fat diet-fed rodents and administration of triglycerides and heparin to raise plasma FFA. As evidence that insulin resistance in high-fat diet-fed rats is due to high FFA, it has been reported that the insulin resistance is rapidly reversed by an overnight fast, a high-glucose meal, and an exercise bout. If true, these findings would invalidate the high-fat diet-fed rodent as a model for MTS or type 2 DM, because insulin resistance is not rapidly reversed by these treatments in patients. The purpose of this study was to determine whether diet-induced insulin resistance is, in fact, rapidly reversible. Incubation of muscles in vitro rapidly reversed insulin resistance induced by administration of triglycerides and heparin, but not by a high-fat diet. An overnight fast and a high-glucose meal were followed by a large increase in insulin-stimulated muscle glucose transport. However, these are adaptive responses, rather than reversals of insulin resistance, because they also occurred in muscles of insulin-sensitive, chow-fed control rats. Our results show that insulin resistance induced by high FFA, i.e., Randle glucose-fatty acid cycle, is transient. In contrast, the insulin resistance induced by a high-fat diet does not reverse rapidly.
Keywords: free fatty acids, glucose-fatty acid cycle, high-fat diet, insulin sensitivity
the most widely accepted hypothesis regarding the mechanism responsible for muscle insulin resistance in patients with the metabolic syndrome (MTS) and type 2 diabetes mellitus (DM) is that it is mediated by accumulation of fat metabolites as a result of increased uptake or decreased oxidation of fatty acids (2, 20). A number of studies have provided evidence that accumulation of fat metabolites, such as diacylglycerol, acyl-CoA, or ceramides, results in activation of serine kinases, leading to inhibition of steps in the insulin signaling pathway (9, 15). A frequently used model for studying this phenomenon is elevation of plasma free fatty acids (FFA) to very high levels by administration of triglycerides and heparin (3, 9, 17, 26, 28). As first shown by Randle and co-workers (24), a large increase in FFA results in an inhibition of insulin-stimulated glucose transport into muscle. Another commonly used experimental approach used to induce insulin resistance is feeding laboratory rodents high-fat diets (4, 11, 12, 14, 21, 23).
It now seems to be generally assumed that these two experimental models result in “fat-induced” insulin resistance by the same mechanisms, i.e., accumulation of fat metabolites as a result of high plasma FFA levels, and that both models are suitable for studying the mechanisms that mediate insulin resistance in the metabolic syndrome and type 2 DM (3, 9, 17, 26, 28). In support of the concept that high-fat diets induce insulin resistance by raising fat metabolites, it has been reported that the insulin resistance induced by a high-fat diet is rapidly reversed by interventions thought to result in lowering of muscle fatty acid metabolites: a single low-fat, high-glucose meal, an overnight fast, or a single exercise bout (1, 22).
Patients with type 2 DM have elevated plasma FFA. Administration of triglycerides and heparin has, therefore, been used as a model to study the mechanism by which elevation of FFA causes fat-induced insulin resistance. However, treatment with triglyceride and heparin results in very high plasma FFA concentrations, in the ∼1.5 to ∼3.5 mM range, which very rapidly cause inhibition of insulin-stimulated glucose uptake by the Randle effect. In contrast, patients with type 2 DM and rodents fed high-fat diets have only modestly elevated plasma FFA concentrations, in the range of ∼0.6 to ∼0.75 mM, which are too low to cause inhibition of insulin-stimulated muscle glucose uptake. This is evidenced by the finding that, in contrast to the Randle effect, which develops very rapidly, rodents fed a high-fat diet do not become insulin resistant until they have been fed the diet for 3–4 wk (19). If the insulin resistance was directly mediated by the high-fat diet-induced increase in plasma FFA, it should occur soon after the diet is started, not after 1 mo on the diet. The development of insulin resistance in response to high-fat and high-sugar diets coincides with an increase in visceral fat, and, early during the development of insulin resistance, there is a strong inverse correlation between visceral fat mass and degree of muscle insulin resistance (19).
The high-fat diet-fed rat develops the rodent equivalent of the metabolic syndrome that can progress to type 2 DM (10) and is used to study development of these pathologies on the basis of the assumption that they are similar in rodents and humans. Unfortunately, neither the metabolic syndrome nor type 2 DM is reversed by a single low-fat high-glucose meal, an overnight fast, or a single bout of exercise, as has been reported to occur in rats fed a high-fat diet (1, 22). Instead, prolonged calorie restriction and/or exercise training is needed to bring about an improvement in insulin action. Therefore, if insulin resistance is, in fact, rapidly reversed in rats fed a high-fat diet, this is actually not a good experimental model for studying the mechanisms involved in development of the metabolic syndrome.
The purpose of the present study was to test the hypothesis that, in contrast to the Randle effect, the muscle insulin resistance induced by a high-fat diet is not rapidly reversed. We repeated the experiments of Oakes et al. (22) and also determined the effect of in vitro muscle incubation on reversal of the insulin resistance.
METHODS
Materials.
2-Deoxy-[1,2-3H]glucose (2-DG) was purchased from American Radiolabeled Chemicals (St. Louis, MO). [14C]mannitol was obtained from ICN Radiochemicals (Irvine, CA). All other chemicals were obtained from Sigma Aldrich (St. Louis, MO).
Animal care and treatments.
This research was approved by the Animal Studies Committee of Washington University School of Medicine. Male Wistar rats (∼60 g body wt; Charles River, Wilmington, MA) were housed two per cage with a 12:12-h light-dark cycle; food and water were provided ad libitum. Some rats were fed a high-fat diet [23% of calories from protein (294 g/kg casein), 50% of calories from fat (180 g/kg lard and 100 g/kg corn oil), and 27% of calories from carbohydrates (346.7 g/kg sucrose), 22 g/kg of vitamin mix (Teklad Premier no. 40077), 51 g/kg of mineral mix (Teklad Premier no. 170915), 5 g of methionine (Teklad Premier no. 10850), and 1.3 g/kg of choline chloride] for 5 wk. Other animals were fed a Purina rodent laboratory chow (28.7% of calories from protein, 12.4% of calories from fat, and 58.9% of calories from carbohydrate). The high-fat diet contained ∼5.1 kcal/g, and the chow diet contained ∼3.3 kcal/g.
Experiments designed to determine whether high-fat diet-induced insulin resistance is rapidly reversible in vivo.
The high-fat diet-fed rats and chow-fed controls were assigned to four subgroups. An untreated control group was fed their usual diet. A second group was fed a high-glucose meal (10% of calories from corn oil, 69% of calories from glucose, and 21% of calories from protein) at 5 PM. A third group received no meal. The fourth group of high-fat diet-fed rats were used in an experiment in which we incubated muscles in vitro (see below) to determine whether the high-fat diet-induced muscle insulin resistance could be reversed.
The rats in the three treatment groups described above were used for measurement of insulin-stimulated 2-DG transport between 12 and 3 PM on the next day. The treatments of the rats are the same as those described by Oakes et al. (22), except we included chow-fed controls. The animals were anesthetized with pentobarbital sodium (5 mg/100 g body wt ip), and the epitrochlearis and soleus muscles were removed (16). The animals were euthanized by exsanguination. The soleus muscles were split longitudinally into strips before incubation and measurement of glucose transport activity.
Experiment designed to determine whether insulin resistance induced by a high-fat diet or an acute increase in plasma FFA is rapidly reversible in vitro.
Chow-fed rats were given 1 ml of corn oil per 100 g body wt by stomach tube in the morning after an overnight fast. After 3 h, they were injected with heparin (100 U/100 g body wt sc). A second heparin injection was given 2 h later. At 1 h after the second heparin injection, the animals were anesthetized with pentobarbital sodium (5 mg/100 g body wt ip), and the epitrochlearis and soleus muscles were removed. The soleus muscles were split longitudinally into strips before incubation.
Muscle insulin resistance “washout” incubations.
Muscles of rats fed the high-fat diet or rats given oil and heparin were incubated in flasks containing 2 ml of oxygenated Krebs-Henseleit buffer (KHB) supplemented with 8 mM glucose, 32 mM mannitol, and 0.1% bovine serum albumin in a gas phase of 95% O2-5% CO2. The flasks were placed in a shaking incubator maintained at 35°C for 20 min, 2.0 h, or 5 h. During the last 20 min of incubation, some of the muscles were treated with insulin (2 mU/ml).
Measurement of 2-DG transport.
Muscles were rinsed for 10 min at 29°C in 2 ml of oxygenated KHB containing 40 mM mannitol and insulin, if it was present in the previous incubation. After the rinse step to remove glucose, muscles were incubated for 20 min at 29°C in flasks containing 2 ml of oxygenated KHB with 4 mM 2-DG (1.5 μCi/ml) and 36 mM [14C]mannitol (0.2 μCi/ml) and insulin, if it was present in the previous incubation, in a shaking incubator (13). The muscles were blotted and clamp frozen and then processed for determination of extracellular space and intracellular 2-DG accumulation, as described previously (27).
In the rats that were fasted or fed a high-glucose meal, glucose transport activity was measured as described above, except insulin concentration during a 1-h preincubation and during the measurement of 2-DG transport was 60 μU/ml.
Plasma FFA.
Plasma FFA concentrations were measured using a Wako NEFA-HR(2) Microtiter Procedure kit (Wako Diagnostics, Richmond, VA).
Statistics.
Values are means ± SE. The significance of the differences between two groups was assessed using Student's unpaired t-test. For multiple comparisons, significance was determined by one-way analysis of variance followed by post hoc comparison using Fisher's least significant difference method.
RESULTS AND DISCUSSION
Reversal of muscle insulin resistance in vitro.
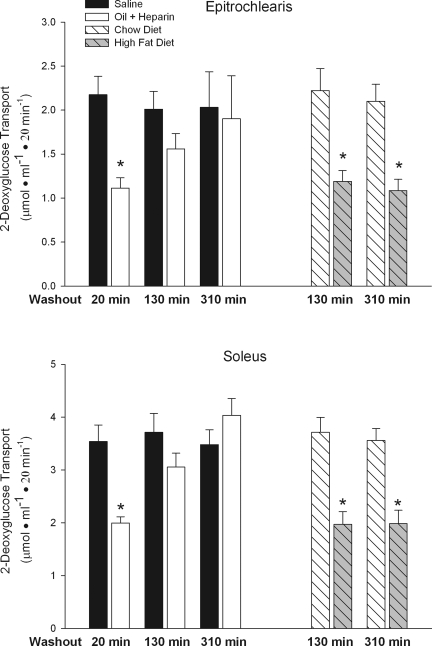
Muscle of rats treated with triglycerides and heparin to raise plasma FFA and rats fed a high-fat diet were incubated in the absence of FFA to evaluate the reversibility of fat-induced insulin resistance. As shown in Fig. 1, both experimental interventions resulted in severe insulin resistance of the glucose transport process in the epitrochlearis, a fast-twitch white muscle, and the soleus, a slow-twitch red muscle. In the muscles of the rats given triglycerides and heparin, most of the insulin resistance was washed out in 130 min, and the insulin resistance was completely washed out within 310 min. In contrast, the 310-min washout had no effect on the insulin resistance of the muscles of the rats fed a high-fat diet for 5 wk. This finding shows that the insulin resistance caused by a high-fat diet is not due to the Randle cycle effect, which is mediated acutely by exposure of muscles to a very high concentration of fatty acids and is rapidly reversed.
Fig. 1.
A 310-min in vitro washout of epitrochlearis and soleus muscles reverses insulin resistance induced by administration of triglycerides and heparin to rats, but not insulin resistance induced by feeding rats a high-fat diet. Values are means ± SE for 10–20 muscles per group. *P < 0.01 vs. control; chow diet is control for high-fat diet experiment, and saline is control for oil + heparin experiment.
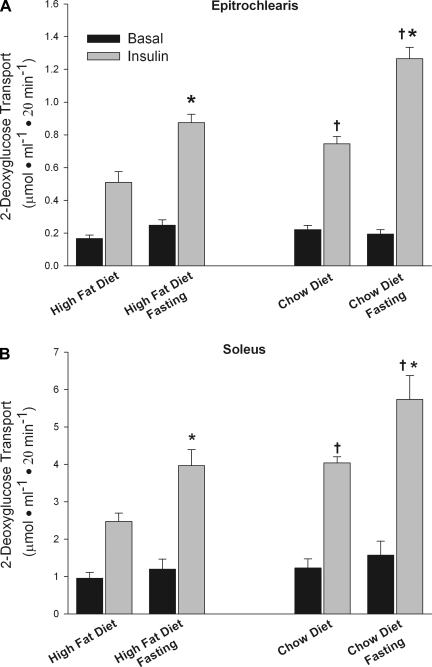
Effect of an overnight fast.
As shown in Fig. 2, the insulin-induced increase in glucose transport activity was ∼75% greater after an overnight fast than in the fed state. However, this increase in glucose uptake is not due to a reversal of high-fat diet-induced insulin resistance but, rather, is mediated by an effect of fasting on insulin action. This is evidenced by the finding that the increase in insulin action induced by fasting was similar in relative magnitude in muscles from the rats fed the high-fat and the rats fed chow. As a result, the muscles of the high-fat diet-fed rats were just as insulin resistant vs. those of the chow-fed rats in the fasted as in the fed state. This finding did not come as a surprise to us, because we routinely measure glucose transport activity in muscles of rats after an overnight fast and have, nevertheless, been able to detect large decreases in insulin-stimulated muscle glucose transport in the rats fed the high-fat diet vs. the rats fed chow (12, 19). Also, glucose tolerance tests, euglycemic clamps, and other measures of insulin resistance used in patients are routinely performed after an overnight fast. It is of interest that the fasting resulted in an increase in insulin-stimulated glucose transport, despite a fasting-induced increase in plasma FFA to 0.83 ± 0.06 mM. In 1979, Goodman and Ruderman (8) reported that fasting/short-term starvation results in increased insulin action in muscle.
Fig. 2.
An overnight fast increases insulin-stimulated glucose transport in epitrochlearis and soleus muscles of rats fed a high-fat or a chow diet. Values are means ± SE for 5–7 muscles per group. *P < 0.01, fasting vs. fed. †P < 0.01, chow vs. high-fat diet.
Oakes et al. (22) also found that insulin-stimulated muscle glucose transport was markedly improved in insulin-resistant rats fed a high-fat diet on the day following a bout of exercise. They attributed this improvement to a rapid reversal of fat-induced insulin resistance. However, as with fasting, this improvement is an adaptive response, not a reversal of insulin resistance. We did not think that repeating this study was necessary or justified, because we showed previously that exercise is followed on the next day by a 60–100% increase in insulin-stimulated glucose transport in rats that had been eating a chow diet (6, 25). Similarly, Gao et al. (5) and Kern et al. (18) found that exercise improved insulin action in obese rats but did not normalize it compared with controls. They found that insulin action was just as severely impaired in exercised obese rats vs. exercised lean rats as in sedentary obese vs. lean sedentary rats.
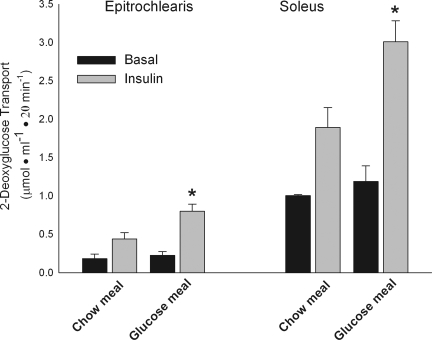
Bell et al. (1) and Oakes et al. (22) also showed that feeding insulin-resistant, high-fat diet-fed rats a high-glucose meal is followed by a large increase in insulin-stimulated muscle glucose uptake on the next afternoon. They attributed this remarkable effect to a reversal of high-fat diet-induced insulin resistance as a result of decreased muscle lipid availability, evidenced by a 22% decrease in muscle triglyceride concentration. However, because they did not study a comparably treated control group, it seemed possible that, as with fasting and exercise, the improvement in insulin action could have been mediated by an adaptive response, rather than by a reversal of insulin resistance. We, therefore, evaluated the effect of a high-glucose meal in rats that had been maintained on a chow control diet. As shown in Fig. 3, insulin-stimulated muscle glucose uptake was markedly increased in muscles of control rats fed the glucose meal on the previous evening.
Fig. 3.
Feeding rats a high-glucose meal in the evening is followed by an increase in insulin-stimulated glucose transport activity, measured on the next afternoon, in epitrochlearis and soleus muscles. Values are means ± SE for 5–7 muscles per group. *P < 0.02 vs. chow meal.
This finding shows that, as with fasting and exercise, the improvement in insulin action following a high-glucose meal is an adaptive response, not a reversal of fat-induced insulin resistance. That a high-glucose meal is followed by a large increase in insulin action on muscle glucose transport seems surprising, particularly in light of the fact that feeding rodents high-sugar/high-caloric-density diets over the long term causes insulin resistance. Our current working hypothesis is that this acute phenomenon is mediated by a large spike in insulin secretion induced by the glucose. It is generally thought that the well-documented increase in muscle insulin sensitivity that follows a bout of exercise is mediated by a specific effect of exercise. However, we have obtained evidence that any stimulus that increases glucose transport in muscle by translocation of glucose transporters (GLUT4) to the cell surface is followed by an increase in insulin sensitivity, and, as with exercise, treatment of muscles with insulin is followed by a large increase in insulin sensitivity (7). In this context, it seems possible that the increase in insulin sensitivity that follows a high-glucose meal is due to an insulin-induced increase in insulin sensitivity.
Plasma FFA concentrations.
The oil meal followed by heparin injection increased plasma FFA concentrations to 2.46 ± 0.52 mM (mean ± SE for 6 rats). Plasma FFA concentration in chow-fed rats averaged 0.25 ± 0.02 mM compared with 0.65 ± 0.08 mM in rats fed the high-fat diet for 2 days and 0.63 ± 0.08 mM in rats fed the high-fat diet for 5 wk (means ± SE for 6 rats per group). We have found that the development of muscle insulin resistance in rats fed a high-fat diet coincides with a significant increase in the weight of intra-abdominal fat depots (19), which occurs between 3 and 4 wk, depending on diet composition/palatability, which determines rate of weight gain. Thus the high-fat diet-induced increase in plasma FFA is present for ≥2 wk without causing insulin resistance (19). This finding, together with the results of the in vitro insulin resistance washout study, demonstrates that high-fat diet-induced insulin resistance is not mediated by the Randle glucose-fatty acid cycle effect, which is transient and requires a much higher FFA concentration. We think that it is highly probable that the insulin resistance in patients with type 2 DM is also not mediated by the Randle cycle effect, inasmuch as their plasma FFA levels are in the same range as those of rats fed a high-fat diet.
The present results do not argue for or against the concept that the metabolic syndrome and type 2 DM are mediated by fat-induced insulin resistance. However, they show that there are two different types of fat-induced insulin resistance and that raising plasma FFA to extremely high levels by administration of triglycerides and heparin is not an appropriate model for studying the mechanisms responsible for insulin resistance in patients with the metabolic syndrome and/or type 2 DM.
GRANTS
This research was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-18986. C. Hancock was supported by an American Diabetes Association Mentor-Based Postdoctoral Fellowship.
Acknowledgments
We are grateful to Victoria Reckamp for expert assistance with preparation of the manuscript.
REFERENCES
- 1.Bell KS, Schmitz-Peiffer C, Lim-Fraser M, Biden TJ, Cooney GJ, Kraegen EW. Acute reversal of lipid-induced muscle insulin resistance is associated with rapid alteration in PKC-φ localization. Am J Physiol Endocrinol Metab 279: E1196–E1201, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Boden G Obesity and free fatty acids. Endocrinol Metab Clin North Am 37: 635–646, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boden G, Lebed B, Schatz M, Homko C, Lemieux S. Effects of acute changes of plasma free fatty acids on intramyocellular fat content and insulin resistance in healthy subjects. Diabetes 50: 1612–1617, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Chalkley SM, Hettiarachchi M, Chisholm DJ, Kraegen EW. Long-term high-fat feeding leads to severe insulin resistance but not diabetes in Wistar rats. Am J Physiol Endocrinol Metab 282: E1231–E1238, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Gao J, Sherman WM, McCune SA, Osei K. Effects of acute running exercise on whole body insulin action in obese male SHHF/Mcc-facp rats. J Appl Physiol 77: 534–541, 1994. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Roves PM, Han DH, Song Z, Jones TE, Hucker KA, Holloszy JO. Prevention of glycogen supercompensation prolongs the increase in muscle GLUT4 after exercise. Am J Physiol Endocrinol Metab 285: E729–E736, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Geiger PC, Han DH, Wright DC, Holloszy JO. How muscle insulin sensitivity is regulated: testing of a hypothesis. Am J Physiol Endocrinol Metab 291: E1258–E1263, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Goodman MN, Ruderman NB. Insulin sensitivity of rat skeletal muscle: effects of starvation and aging. Am J Physiol Endocrinol Metab Gastrointest Physiol 236: E519–E523, 1979. [DOI] [PubMed] [Google Scholar]
- 9.Griffin ME, Marcucci MJ, Cline WJ, Bell K, Barucci N, Lee D, Goodyear LJ, Kraegen EW, White MF, Shulman GI. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C-θ and alterations in the insulin signaling cascade. Diabetes 48: 1270–1274, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Han DH, Hansen PA, Host HH, Holloszy JO. Insulin resistance of muscle glucose transport in rats fed a high-fat diet: a reevaluation. Diabetes 46: 1761–1767, 1997. [DOI] [PubMed] [Google Scholar]
- 11.Han X, Ploug T, Galbo H. Effect of diet on insulin- and contraction-mediated glucose transport and uptake in rat muscle. Am J Physiol Regul Integr Comp Physiol 269: R544–R551, 1995. [DOI] [PubMed] [Google Scholar]
- 12.Hancock CR, Han DH, Chen M, Terada S, Yasuda T, Wright DC, Holloszy JO. High fat diets cause insulin resistance despite an increase in muscle mitochondria. Proc Natl Acad Sci USA 105: 7815–7820, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen PA, Gulve EA, Holloszy JO. Suitability of 2-deoxyglucose for in vitro measurement of glucose transport activity in skeletal muscle. J Appl Physiol 76: 979–985, 1994. [DOI] [PubMed] [Google Scholar]
- 14.Hansen PA, Han DH, Marshall BA, Nolte LA, Chen MM, Mueckler M, Holloszy JO. A high fat diet impairs stimulation of glucose transport in muscle. Functional evaluation of potential mechanisms. J Biol Chem 273: 26157–26163, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Hegarty BD, Furler SM, Ye J, Cooney GJ, Kraegen EW. The role of intramuscular lipid in insulin resistance. Acta Physiol Scand 178: 373–383, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Henriksen EJ, Bourey RE, Rodnick KJ, Koranyi L, Permutt MA, Holloszy JO. Glucose transporter protein content and glucose transport capacity in rat skeletal muscles. Am J Physiol Endocrinol Metab 259: E593–E598, 1990. [DOI] [PubMed] [Google Scholar]
- 17.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C and IκB-α. Diabetes 51: 2005–2011, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Kern M, Tapscott EB, Downes DL, Frisell WR, Dohm GL. Insulin resistance induced by high-fat feeding is only partially reversed by exercise training. Pflügers Arch 417: 79–83, 1990. [DOI] [PubMed] [Google Scholar]
- 19.Kim JY, Nolte LA, Hansen PA, Han DH, Ferguson K, Thompson PA, Holloszy JO. High-fat diet-induced muscle insulin resistance: relationship to visceral fat mass. Am J Physiol Regul Integr Comp Physiol 279: R2057–R2065, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Kraegen EW, Cooney GJ, Ye J, Thompson AL. Triglycerides, fatty acids and insulin resistance–hyperinsulinemia. Exp Clin Endocrinol Diabetes 109: S516–S526, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Kraegen EW, James DE, Storlien LH, Burleigh KM, Chisholm DJ. In vivo insulin resistance in individual peripheral tissues of the high fat fed rat: assessment of euglycaemic clamp plus deoxyglucose administration. Diabetologia 29: 192–198, 1986. [DOI] [PubMed] [Google Scholar]
- 22.Oakes ND, Bell KS, Furler SM, Camilleri S, Saha AK, Ruderman NB, Chisholm DJ, Kraegen EW. Diet-induced muscle insulin resistance in rats is ameliorated by acute dietary lipid withdrawal or a single bout of exercise: parallel relationship between insulin stimulation of glucose uptake and suppression of long-chain fatty acyl-CoA. Diabetes 46: 2022–2028, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Oakes ND, Cooney GJ, Camilleri S, Chisholm DJ, Kraegen EW. Mechanisms of liver and muscle insulin resistance induced by chronic high-fat feeding. Diabetes 46: 1768–1774, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose-fatty acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1: 785–789, 1963. [DOI] [PubMed] [Google Scholar]
- 25.Ren JM, Semenkovich CF, Gulve EA, Gao J, Holloszy JO. Exercise induces rapid increases in GLUT4 expression, glucose transport capacity, and insulin-stimulated glycogen storage in muscle. J Biol Chem 269: 14396–14401, 1994. [PubMed] [Google Scholar]
- 26.Ye JM, Dzamko N, Cleasby ME, Hegarty BD, Furler SM, Cooney GJ, Kraegen EW. Direct demonstration of lipid sequestration as a mechanism by which rosiglitazone prevents fatty-acid-induced insulin resistance in the rat: comparison with metformin. Diabetologia 47: 1306–1313, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Young DA, Uhl JJ, Cartee GD, Holloszy JO. Activation of glucose transport in muscle by prolonged exposure to insulin: effects of glucose and insulin concentration. J Biol Chem 261: 16049–16053, 1986. [PubMed] [Google Scholar]
- 28.Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, Atcheson B, White MF, Kraegen EW, Shulman GI. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate 1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem 277: 50230–50236, 2002. [DOI] [PubMed] [Google Scholar]