Abstract
This study tests the hypothesis that lipids could act as an alternative fuel source in the brain during insulin-induced hypoglycemia. Male Sprague-Dawley rats were subjected to hyperinsulinemic (5 mU·kg−1·min−1) hypoglycemic (∼50 mg/dl) clamps. In protocol 1, intralipid (IL), a fat emulsion, was infused intravenously to prevent the fall in free fatty acid levels that occurs in response to hyperinsulinemic hypoglycemia. Intravenous lipid infusion did not alter the counterregulatory responses to hypoglycemia. To test whether IL could have central effects in mediating the counterregulatory response to hypoglycemia, in protocol 2 the brains of precannulated rats were intracerebroventricularly (icv) infused with IL or artificial cerebrospinal fluid (aCSF) as control. Unexpectedly, the epinephrine and glucagon response to hypoglycemia was significantly augmented with icv IL infusion. To determine whether central IL infusion could restore defective counterregulation, in protocol 3 rats were made recurrently hypoglycemic (RH) for 3 days and on the 4th day underwent hyperinsulinemic hypoglycemic clamps with icv IL or aCSF infusion. RH rats had the expected impaired epinephrine response to hypoglycemia, and icv IL infusion again significantly augmented the epinephrine response in RH rats to normal. With regard to our experimental model of hypoglycemic counterregulation, we conclude that 1) systemic lipid infusion did not alter the counterregulatory response to hypoglycemia, 2) the icv infusion of lipids markedly increased CSF FFA levels and paradoxically augmented the epinephrine and glucagon responses, and 3) the blunted sympathoadrenal response in recurrently hypoglycemic rats was completely normalized with the icv lipid infusion. It is concluded that, in the setting of insulin-induced hypoglycemia, increased brain lipids can enhance the sympathoadrenal response.
Keywords: intralipid, intracerebroventricular
hypoglycemia continues to be the rate-limiting step for achieving blood sugar control for patients with diabetes (8). Because the brain is the primary glucose sensor (2), many efforts have been made toward finding alternate fuel sources that could maintain cognitive function and/or restore the defective counterregulatory response to hypoglycemia. Although the brain is critically dependent on glucose as a substrate, many recent studies have indicated that the brain is also sensitive to fatty acids (14, 22). In particular, the intracerebroventricular (icv) infusion of fatty acids has been shown to regulate glucose homeostasis and feeding behavior (21, 23). The icv infusion of intralipid (IL; a lipid solution) was associated with a decreased sympathetic tone (6) that was shown to be mediated by the β-oxidation of the fatty acids in the brain (7). Analogous to glucose-excited and glucose-inhibited neurons in the hypothalamus (thought to be critically important in glucose sensing and the initiation of the counterregulatory response), Wang et al. (29) have identified fatty acid-excited and fatty acid-inhibited neurons in the hypothalamus.
Traditionally, the brain was thought to be exclusively dependent on glucose as a fuel source, but other nonglucose fuel sources such as lactate (3, 16, 28), ketones (1, 28), and perhaps lipids (9, 20, 24) may serve as potential alternative fuel sources, especially in the setting of hypoglycemia. In response to insulin administration, both glucose and fatty acid levels are suppressed in the peripheral circulation, and their availability for brain metabolism is reduced. Thus, in the setting of insulin-induced hypoglycemia, it may be that suppressed fatty acid levels could be sensed in the hypothalamus (29), which could influence the counterregulatory response. A more important corollary would be that perhaps the maintenance or elevation of fatty acid levels during insulin-induced hypoglycemia could, at least in part, support brain metabolism in the setting of reduced glucose availability. Consistent with this hypothesis, the intravenous infusion of IL solution during hypoglycemia demonstrated a blunting of counterregulatory response to hypoglycemia (9). It was hypothesized that this blunting was due to the extra availability of fatty acids in critical sensing areas in the brain. However, that study was not able to selectively distinguish peripheral from central nervous system actions of free fatty acid (FFA) metabolism.
In this study, the hypothesis to be tested was that a selective increase in brain lipid levels during insulin-induced hypoglycemia would provide an alternative fuel source to the brain and thereby blunt the rat's counterregulatory response to hypoglycemia. To achieve this aim, rats were infused with either IL, a lipid emulsion, or control artificial cerebrospinal fluid (aCSF) into the third cerebral ventricles of the brain during a hyperinsulinemic hypoglycemic clamp. Paradoxically, the results demonstrate that IL acts in the brain to augment, and not suppress, the counterregulatory response. Furthermore, the impaired sympathoadrenal response in recurrently hypoglycemic rats was fully normalized with icv IL infusion. Since hypoglycemia is rate limiting for insulin therapy in people with diabetes, and people with diabetes have an impaired counterregulatory response to hypoglycemia, these findings reveal a potential role for lipids to act in the brain to augment/restore the counterregulatory response.
MATERIALS AND METHODS
Rats and housing.
Sprague-Dawley rats were obtained from Charles River Laboratories (Wilmington, MA) at 9 wk of age, weighing between 250 and 350 g. Rats were housed in the Washington University Animal Care Facility and maintained on a 12:12-h light-dark cycle. The animals were fed standard rat chow. All experimental protocols were approved by the Animal Studies Committee at the Washington University School of Medicine.
icv Surgery.
Approximately 2 wk before the experiments, rats destined for central lipid infusion underwent surgery for implantation of an icv catheter. The animals were anesthetized through continuous administration of 1–5% isoflurane through an Isotec vaporizer. After visualization of bregma, stereotaxic equipment (Benchmark Digital, St. Louis, MO) was used to implant and secure a stainless steel guide cannula (PlasticsOne, Roanoke, VA) to the third cerebral ventricle (−2.8 mm anterior/posterior, 0.0 mm mediolateral, and −9.5 mm dorsoventral). After the surgeries, rats were given a subcutaneous bolus (0.05 mg/kg) of buprenorphine (Hospira, Lake Forest, IL) for postoperative pain control and were returned to cages for recovery.
Intravascular cannulation.
Anesthesia was induced with an intraperitoneal injection of 87 mg/kg ketamine and 2.6 mg/kg xylazine. A Micro-Renathane (Braintree Scientific, Boston, MA) catheter was inserted into the left common carotid artery, and two catheters were implanted into the right jugular vein. To prevent clotting, catheters were filled with 40% polyvinylpyrrolidone (Sigma-Aldrich, St. Louis, MO) in heparin (1,000 U/ml; Baxter Healthcare, Deerfield, IL) solution. Following surgery and a buprenorphine injection (0.05 mg/kg), rats were allowed to recover for 1 wk prior to the hyperinsulinemic hypoglycemic clamp.
Experimental setup.
On the evening before the clamp, food was removed for a 15-h fast before the beginning of the experiment. Rats were allowed water ad libitum. On the morning of the experiment the vascular catheters were externalized and flushed, and then a continuous slow infusion (0.1 μl/min) of heparinized saline was started, using a Model 101 infusion pump (KD Scientific), to keep the catheters patent.
Protocol 1: systemic IL infusion.
To assess the effects of systemic fat emulsion infusion on the counterregulatory response to hypoglycemia, IL (20%; Sigma-Aldrich) or saline (control) was infused intravenously at 4 μl/min using a Model 101 infusion pump. After a 90-min rest period, a basal blood sample was taken at time zero. Intravenous insulin (Humulin R; Eli Lilly, Indianapolis, IN) was then administered (50 mU/kg bolus + 5 mU·kg−1·min−1) with glucose (50% dextrose; Hospira). For this systemic IL protocol, the intravenous delivery of IL (iv + IL) was increased threefold (to 12 μl/min) at time zero to prevent FFA levels from decreasing in response to insulin infusion. Intravenous saline (iv + SAL) was increased to 12 μl/min in control experiments. During the first half-hour, blood glucose (BG) samples were taken every 10 min, and glucose infusion rates were varied throughout the clamp to achieve a target BG of 50 mg/dl. Blood samples were taken for metabolite and hormonal analyses at minutes 30, 60, 90, 120, and 180.
Protocol 2: icv IL infusion.
For experiments in which IL was infused centrally, on the morning of the clamp, an internal cannula (PlasticsOne) was inserted into the icv guide cannula, extending 1 mm beyond the guide cannula. An icv infusion of either IL fat emulsion (20%; Sigma-Aldrich) or an aCSF as control was begun at 0.1 μl/min using a Model 101 infusion pump. After the icv infusion was begun, the animal was left undisturbed for 90 min. This period allowed the animal to rest from the stress of being handled and allowed the fatty acids to reach desirable levels in the brain. The two groups of icv-treated animals (icv + aCSF and icv + IL) underwent a hyperinsulinemic hypoglycemic clamp, as described above. At the end of the experiments, animals were briefly anesthetized with isofluorane to obtain samples of cerebrospinal fluid (CSF) by cervical spine needle insertion. Correct placement of the icv cannula was confirmed with Evans blue dye administration.
Protocol 3: recurrent hypoglycemia.
A third group of rats underwent recurrent hypoglycemia (RH) experiments. One week after systemic catheter implantation, RH was induced with 3 consecutive days of subcutaneous injections of regular human insulin (1st day 6 U/kg, 2nd day 5 U/kg, 3rd day 4 U/kg; Eli Lilly). Food was withheld, and tail vein glucose levels were measured hourly. This protocol consistently maintained blood glucose levels in the 40–50 mg/dl range for 3 h/day. Hypoglycemia was terminated by a subcutaneous injection (100 μl) of 50% dextrose (Hospira), and animals were allowed free access to food. On the 4th day, RH rats were subjected to the hyperinsulinemic hypoglycemic clamp with icv infusions of either aCSF (RH + icv + aCSF) or IL solutions (RH + icv + IL), as described above.
Analytical methods.
BG was measured throughout the experiment (BD Logic, Franklin Lakes, NJ). Catecholamine analysis was performed by a single isotope-derived (radioenzymatic) method (26). Radioimmunoassays were performed for glucagon (Linco Research, St. Charles, MO) and corticosterone (MP Biomedicals, Orangeburg, NY). Insulin levels were measured through an insulin ELISA kit (Crystal Chem, Downers Grove, IL). The concentration of FFAs was assayed with a NEFA C Kit (Wako Chemicals, Richmond, VA).
Data analysis.
Data are presented as means ± SE. Statistical analysis was by one- or two-way ANOVA using SigmaStat 3.1 (Systat Software, San Jose, CA). P < 0.05 was set as the criterion for statistical significance.
RESULTS
Protocol 1: systemic IL infusion.
Six rats received intravenous IL (iv + IL), and six control rats received intravenous saline (iv + SAL). Body weights of both iv + SAL (308 ± 13) and iv + IL (316 ± 13) were not different. In response to insulin infusion, BG levels dropped to ∼55 mg/dl in both groups [P = not significant (NS)] within 30 min (Fig. 1A). Consistent with the known effects of IL infusion to induce systemic insulin resistance, the glucose infusion rates were significantly lower (P < 0.01) in the iv + IL-treated rats (Fig. 1B). Following insulin infusion, insulin levels rose ∼10-fold above basal but equally in both groups (Fig. 1C). In response to insulin infusion, FFA levels were suppressed in iv + SAL by 61% during the last half of the clamp (Fig. 1D). By experimental design, systemic IL treatment (iv + IL) prevented the fall in plasma FFA. Indeed, with iv + IL, FFA levels increased significantly above basal, by 40% (Fig. 1D). During the hypoglycemic clamp, epinephrine, norepinephrine, glucagon, and corticosterone levels increased to similar levels with both treatment groups (P = NS; Fig. 2, A–H).
Fig. 1.
Plasma glucose levels (A), glucose infusion rates (B), insulin levels (C), and free fatty acid (FFA) levels (D) in the basal period and during a hyperinsulinemic (5 mU·kg−1·min−1) hypoglycemic (∼50 mg/dl) clamp in rats treated with intravenous saline (iv + SAL; ▴) or intravenous intralipid (iv + IL; ▵).
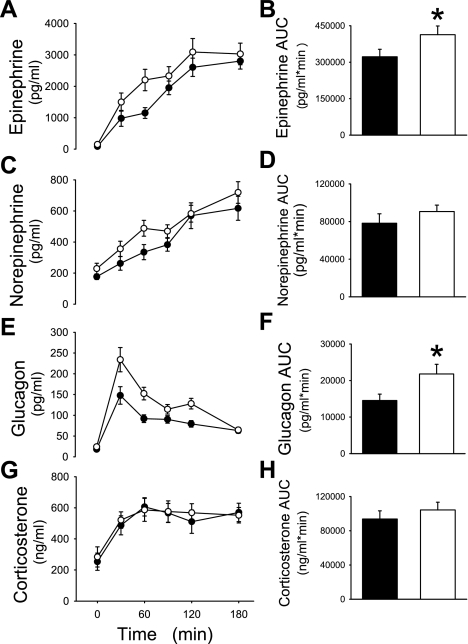
Fig. 2.
Plasma epinephrine (A), norepinephrine (C), glucagon (E), and corticosterone levels (G) and their respective areas under curve (AUC; B, D, F, and H) are shown during a hyperinsulinemic (5 mU·kg−1·min−1) hypoglycemic (∼50 mg/dl) clamp in rats treated with iv + SAL (▴ and filled bars) or iv + IL (▵ and open bars).
Protocol 2: icv IL infusion.
Rats were treated with icv infusions of aCSF (icv + aCSF; n = 11) or with IL (icv + IL; n = 11). Rats had similar weights (icv + aCSF 319 ± 12 g, icv + IL 327 ± 10 g, P = NS). In the basal period, there were no significant differences between levels of glucose, insulin, FFA, epinephrine, norepinephrine, glucagon, or corticosterone.
In response to insulin infusion, BG levels dropped to ∼50 mg/dl in both groups (P = NS) within 30 min (Fig. 3A). BG concentrations were carefully maintained at matched levels of hypoglycemia with the simultaneous intravenous glucose infusion (Fig. 3B). Following insulin infusion, insulin levels rose ∼10-fold in both groups (Fig. 3C). Consistent with previous results (above), insulin suppressed plasma FFA levels by ∼60% by the end of the clamp (P = NS between groups; Fig. 3D). icv + IL treatment significantly increased the net epinephrine response [area under the curve (AUC)] compared with control icv + aCSF-treated rats (P < 0.01; Fig. 4, A and B). The glucagon response was also significantly higher with IL treatment compared with aCSF controls (P < 0.05; Fig. 4, E and F). Norepinephrine and corticosterone levels rose significantly in response to hypoglycemia but similarly in both treatment groups (Fig. 4, C, D, G, and H).
Fig. 3.
Plasma glucose levels (A), glucose infusion rates (B), insulin levels (C), and FFA levels (D) in the basal period and during a hyperinsulinemic (5 mU·kg−1·min−1) hypoglycemic (∼50 mg/dl) clamp in rats treated with intracerebroventricular artificial cerebrospinal fluid (icv + aCSF; •) or intracerebroventricular intralipid (icv + IL; ○).
Fig. 4.
Plasma epinephrine (A), norepinephrine (C), glucagon (E), and corticosterone levels (G) and their respective AUC (B, D, F, and H) are shown during a hyperinsulinemic (5 mU·kg−1·min−1) hypoglycemic (∼50 mg/dl) clamp in rats treated with icv + aCSF (• and filled bars) or icv + IL (○ and open bars). *P < 0.05
As expected with icv lipid infusion, FFA levels in the CSF of the icv IL-infused rats were 13-fold higher than aCSF-treated rats (icv + IL 0.58 ± 0.11 mmol/l, icv + aCSF 0.04 ± 0.03 mmol/l, P < 0.001). However, this difference in brain CSF FFA levels was not observed in the peripheral plasma because there was no significant difference in systemic FFA levels between the groups (Fig. 3D).
Protocol 3: icv + IL infusion in recurrently hypoglycemic rats.
To determine whether icv + IL would also enhance the sympathoadrenal response to hypoglycemia in a model of defective counterregulation, a third group of rats was treated with 3 days of RH prior to hypoglycemic clamps on day 4. Recurrently hypoglycemic rats were treated with icv infusions of aCSF (RH + icv + aCSF; n = 10) or with IL (RH + icv + IL; n = 12). Rats had similar weights (RH + icv + aCSF 332 ± 11 g, RH + icv + IL 324 ± 9 g, P = NS). In the basal period, there were no significant differences between levels of glucose, insulin, FFA, epinephrine, norepinephrine, glucagon, or corticosterone.
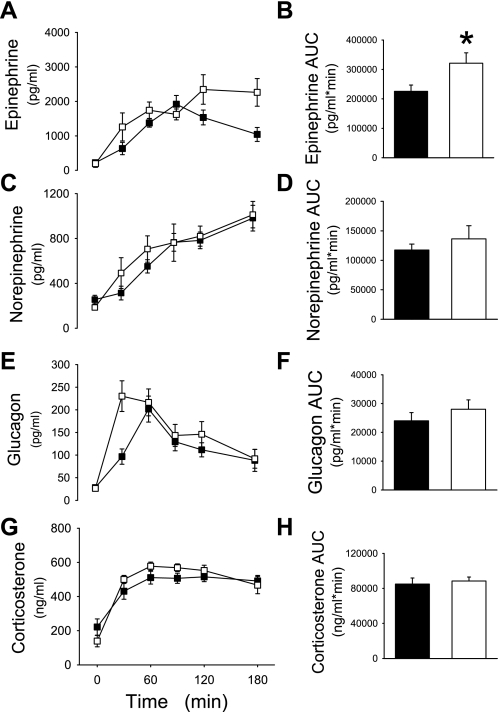
In response to insulin infusion, BG levels dropped to ∼50 mg/dl in both groups (P = NS; Fig. 5A), and both groups had similar rates of glucose infusion (Fig. 5B). Following insulin infusion, insulin levels rose ∼10-fold above basal but equally in all groups (Fig. 5C). Consistent with previous results (above), insulin suppressed plasma FFA levels by 60% by the end of the clamp (P = NS between groups; Fig. 5D). Validating the model of RH as a model of impaired counterregulation, the net epinephrine response was 28% lower in RH + icv + aCSF rats (2.3 ± 0.2 × 105 pg·ml−1·min−1) compared with control icv + aCSF rats (3.2 ± 0.3 × 105 pg·ml−1·min−1, P < 0.01) (Figs. 4B and 6B). In the RH rats, icv + IL treatment significantly increased the epinephrine response (AUC) above control aCSF-treated rats (RH + icv + IL 3.2 ± 0.4 × 105 pg·ml−1·min−1 vs. RH + icv + aCSF 2.3 ± 0.2 × 105 pg·ml−1·min−1; Fig. 6B). Indeed, IL infusion completely normalized the epinephrine response in RH rats (RH + icv + IL 3.2 ± 0.4 × 105 pg·ml−1·min−1 vs. control icv + aCSF 3.2 ± 0.3 × 105 pg·ml−1·min−1, P = NS). The glucagon, norepinephrine, and corticosterone levels rose significantly in response to hypoglycemia but similarly in both treatment groups (Fig. 6, C–H).
Fig. 5.
Rats underwent 3 days of recurrent hypoglycemia (RH) with insulin injections (∼5 U/kg) to reduce blood glucose to ∼50 mg/dl. On the 4th day, a hyperinsulinemic (5 mU·kg−1·min−1) hypoglycemic (∼50 mg/dl) clamp was performed in rats treated with icv aCSF (RH + icv + aCSF; ▪) or icv IL (RH + icv + IL; □). Plasma glucose levels (A), glucose infusion rates (B), insulin levels (C), and FFA levels (D) are shown.
Fig. 6.
Rats underwent 3 days of RH (as outlined in Fig. 5). Plasma epinephrine (A), norepinephrine (C), glucagon (E), and corticosterone levels (G) and their respective AUC are shown during a hyperinsulinemic (5 mU·kg−1·min−1) hypoglycemic (∼50 mg/dl) clamp in rats treated with RH + icv + aCSF (▪ and filled bars) or RH + icv + IL (□ and open bars). *P < 0.05.
DISCUSSION
Although the brain is critically dependent on glucose as a nutrient, recent data has suggested that the brain may also sense and respond to fatty acids (6, 7, 14, 21–24, 29). The ability of the brain to utilize nonglucose substrates may be particularly important when circulating glucose fuel sources are low, as they are during insulin-induced hypoglycemia. Our data indicate that the fall in systemic fatty acids does not contribute significantly to the counterregulatory response to hypoglycemia because the maintenance of elevated FFA levels with systemic lipid infusion did not alter the counterregulatory response. Paradoxically, the icv infusion of lipids during hypoglycemia augmented the sympathoadrenal response. Furthermore, in the recurrent hypoglycemia model of impaired sympathoadrenal response to hypoglycemia, icv lipids fully normalized the defective sympathoadrenal response to hypoglycemia.
The results demonstrating that systemic lipid infusion did not alter the counterregulatory response to hypoglycemia are consistent with previous studies showing that raising systemic lipid levels had either no effect (11, 24) or a slight inhibitory effect (9) on the counterregulatory response to hypoglycemia. However, the icv lipid infusion protocol allowed for selective increase of FFAs in the brain, isolating the central nervous effects. Surprisingly, icv lipid infusion increased the counterregulatory hormone responses to hypoglycemia. These responses were unexpected because we initially suspected that, by providing an alternative fuel source, icv lipid infusion would suppress the counterregulatory response to hypoglycemia. Interestingly, although the differential effect attributed to icv lipid infusion produced relatively small incremental responses, the responses (particularly the epinephrine response) were statistically significant and consistent in both icv lipid-infused groups. That is, icv lipid infusion increased the epinephrine AUC response by 28% (P < 0.05) in rats, and icv lipid infusion increased the epinephrine AUC response by 42% (P < 0.05) in recurrently hypoglycemic rats.
In the absence of lipid infusion, FFA levels in the CSF were about 6% of the levels in the plasma, consistent with reports in the literature (12). It may be that failure for systemic lipid infusion to augment the counterregulatory response is related to the failure of systemic lipid infusion (in protocol 1) to increase brain FFA levels as markedly as those achieved with icv lipid infusion (as in protocol 2). Alternatively, the proximity of icv lipid infusion to lipid-sensing neurons in the ventromedial hypothalamus (29) may account for the augmentation of the counterregulatory response not observed with intravenous lipid infusion.
In addition to the counterregulatory effects of lipid action in the brain, the cognitive effects of lipids in the brain have also been investigated and again have yielded divergent results. Intravenous intralipid infusion, which suppressed the counterregulatory response, did not improve cognitive performance in nondiabetic subjects (9). Conversely, oral consumption of a medium-chain triglyceride solution did not alter the counterregulatory response but (again paradoxically) did improve memory performance in hypoglycemic diabetic subjects (24). Although the differing cognitive tests, lipid treatment regimens, routes of administration, and patient populations may account for these divergent results, these studies suggest regional differences between glucose-sensing regions and the cognitive regions of the brain. Thus, in the setting of hypoglycemia, brain fatty acid metabolism may play a unique role in independently regulating the counterregulatory response, cognitive performance, and the extent of hypoglycemia unawareness.
The initial hypothesis was that central intralipid infusion would decrease the counterregulatory response to hypoglycemia, consistent with clinical studies (9). Paradoxically, there was an increase in the counterregulatory response to hypoglycemia. The mechanism for this paradoxical reaction to intralipid infusion remains unknown. One possible mechanism is based on Randle's glucose/fatty acid cycle whereby increased FFA supply limits glucose uptake/oxidation in muscle, liver, and pancreatic β-cells (25). If a similar negative correlation exists in the brain, the relative abundance of FFAs produced by icv infusion of intralipid would result in limited glucose uptake/oxidation, leading to augmented counterregulatory response. Interestingly, the activity of fatty acid-sensing neurons in the hypothalamus was influenced by ambient glucose concentrations, consistent with the supposition regarding an interaction between glucose and fatty acid-sensing neurons within the hypothalamus (29). Second, the increased availability of brain fatty acids for metabolism may have activated hypothalamic AMP kinase activity (15, 31), which is also important in generating the counterregulatory response to hypoglycemia (18, 19). Third, increased fatty acid may have activated GABA receptors (30) also known to mediate the counterregulatory response (4, 5). Fourth, the increased brain fatty acids may have activated (opened) hypothalamic ATP-sensitive K+ channels (14, 23), which play a critical role in central nervous system (CNS) glucose sensing and the triggering of the counterregulatory response to hypoglycemia (10, 17). Also, CNS fatty acids may have augmented the counterregulatory response by direct actions on other ion channels, such as potassium channels (32), chloride channels (27), and calcium channels (13). Analogous to specialized glucose-excited and glucose-inhibited neurons in the hypothalamus, fatty acid-excited neurons appear to be chloride ion channel dependent, whereas fatty acid-inhibited neurons appear to be mediated by ATP-sensitive K+ channels (20).
Although the use of intralipid to increase FFA concentrations has also been validated in previous studies (6, 7, 9), a few caveats about the data and their interpretation should be discussed. First, the interpretations above assume that the intralipid effects are due solely to increases to the brain FFA concentration. The 20% intralipid emulsion used in these experiments is an oil-in-water emulsion containing soybean oil, egg phospholipids, and glycerol. These experiments were not designed to test whether the different components of intralipid could have had a role in altering the CNS glucose sensing/counterregulation. Second, although all groups were matched for equivalent volumes of icv infusion, there exists the possibility that the augmented counterregulatory response associated with icv intralipid was due to a nonspecific central stressor effect of the lipid infusion. Arguing against a nonspecific effect of icv intralipid infusion, it should be noted that, following 90 min of infusion, basal stress hormone levels were not elevated with icv intralipid infusion compared with icv aCSF infusion. Basal stress hormone levels (e.g., epinephrine, norepinephrine, and corticosterone) were very low and consistent with other studies in basal, nonstressed rats (17). Although we cannot rule out the possibility of a nonspecific effect of icv lipid infusion, this effect would likely have been very small relative to the 25-fold increase in epinephrine levels induced by hypoglycemia. Thus, as opposed to a major nonspecific CNS effect, the results indicate that, in the setting of insulin-induced hypoglycemia, increased lipids act specifically in the brain, perhaps by altering glucose sensing and/or neuronal activity, leading to an enhanced sympathoadrenal response to hypoglycemia.
In summary, these results demonstrate that central but not systemic lipid infusion can act in the brain to augment the counterregulatory response. Since hypoglycemia is rate limiting for insulin therapy in people with diabetes, and people with diabetes have an impaired counterregulatory response to hypoglycemia, these findings identify a potential role for lipids to act in the brain to augment/restore the counterregulatory response. Novel approaches to increase lipid substrates to the brain could be investigated as potential therapies to augment/restore the counterregulatory response to hypoglycemia in at-risk patients.
GRANTS
Research support from the Juvenile Diabetes Research Foundation and the National Institute of Diabetes and Digestive and Kidney Diseases (DK-073683) is acknowledged. S. C. Haywood and A. J. Bree were supported by the Howard Hughes Medical Institute as part of a Washington University Summer Undergraduate Research Fellowship.
Acknowledgments
We thank Ronaldo Perez for expert technical support and Dr. Philip Cryer and Dr. Cryer's laboratory for performing the catecholamine determinations.
REFERENCES
- 1.Amiel SA, Archibald HR, Chusney G, Williams AJ, Gale EA. Ketone infusion lowers hormonal responses to hypoglycaemia: evidence for acute cerebral utilization of a non-glucose fuel. Clin Sci (Lond) 81: 189–194, 1991. [DOI] [PubMed] [Google Scholar]
- 2.Biggers DW, Myers SR, Neal D, Stinson R, Cooper NB, Jaspan JB, Williams PE, Cherrington AD, Frizzell RT. Role of brain in counterregulation of insulin-induced hypoglycemia in dogs. Diabetes 38: 7–16, 1989. [DOI] [PubMed] [Google Scholar]
- 3.Borg MA, Tamborlane WV, Shulman GI, Sherwin RS. Local lactate perfusion of the ventromedial hypothalamus suppresses hypoglycemic counterregulation. Diabetes 52: 663–666, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Chan O, Cheng H, Herzog R, Czyzyk D, Zhu W, Wang A, McCrimmon RJ, Seashore MR, Sherwin RS. Increased GABAergic tone in the ventromedial hypothalamus contributes to suppression of counterregulatory responses after antecedent hypoglycemia. Diabetes 57: 1363–1370, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan O, Zhu W, Ding Y, McCrimmon RJ, Sherwin RS. Blockade of GABA(A) receptors in the ventromedial hypothalamus further stimulates glucagon and sympathoadrenal but not the hypothalamo-pituitary-adrenal response to hypoglycemia. Diabetes 55: 1080–1087, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Clément L, Cruciani-Guglielmacci C, Magnan C, Vincent M, Douared L, Orosco M, Assimacopoulos-Jeannet F, Pénicaud L, Ktorza A. Intracerebroventricular infusion of a triglyceride emulsion leads to both altered insulin secretion and hepatic glucose production in rats. Pflugers Arch 445: 375–380, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Cruciani-Guglielmacci C, Hervalet A, Douared L, Sanders NM, Levin BE, Ktorza A, Magnan C. Beta oxidation in the brain is required for the effects of non-esterified fatty acids on glucose-induced insulin secretion in rats. Diabetologia 47: 2032–2038, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Cryer PE Diverse causes of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med 350: 2272–2279, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Evans ML, Matyka K, Lomas J, Pernet A, Cranston IC, Macdonald I, Amiel SA. Reduced counterregulation during hypoglycemia with raised circulating nonglucose lipid substrates: evidence for regional differences in metabolic capacity in the human brain? J Clin Endocrinol Metab 83: 2952–2959, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Evans ML, McCrimmon RJ, Flanagan DE, Keshavarz T, Fan X, McNay EC, Jacob RJ, Sherwin RS. Hypothalamic ATP-sensitive K + channels play a key role in sensing hypoglycemia and triggering counterregulatory epinephrine and glucagon responses. Diabetes 53: 2542–2551, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Fanelli C, Calderone S, Epifano L, De Vincenzo A, Modarelli F, Pampanelli S, Perriello G, De Feo P, Brunetti P, Gerich JE. Demonstration of a critical role for free fatty acids in mediating counterregulatory stimulation of gluconeogenesis and suppression of glucose utilization in humans. J Clin Invest 92: 1617–1622, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goto M, Spitzer JJ. Fatty acid profiles of various lipids in the cerebrospinal fluid. Proc Soc Exp Biol Med 136: 1294–1296, 1971. [DOI] [PubMed] [Google Scholar]
- 13.Honen BN, Saint DA, Laver DR. Suppression of calcium sparks in rat ventricular myocytes and direct inhibition of sheep cardiac RyR channels by EPA, DHA and oleic acid. J Membr Biol 196: 95–103, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Lam TK, Pocai A, Gutierrez-Juarez R, Obici S, Bryan J, Aguilar-Bryan L, Schwartz GJ, Rossetti L. Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat Med 11: 320–327, 2005. [DOI] [PubMed] [Google Scholar]
- 15.López M, Lage R, Saha AK, Pérez-Tilve D, Vázquez MJ, Varela L, Sangiao-Alvarellos S, Tovar S, Raghay K, Rodríguez-Cuenca S, Deoliveira RM, Castañeda T, Datta R, Dong JZ, Culler M, Sleeman MW, Alvarez CV, Gallego R, Lelliott CJ, Carling D, Tschöp MH, Diéguez C, Vidal-Puig A. Hypothalamic fatty acid metabolism mediates the orexigenic action of ghrelin. Cell Metab 7: 389–399, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Maran A, Cranston I, Lomas J, Macdonald I, Amiel SA. Protection by lactate of cerebral function during hypoglycaemia. Lancet 343: 16–20, 1994. [DOI] [PubMed] [Google Scholar]
- 17.McCrimmon RJ, Evans ML, Fan X, McNay EC, Chan O, Ding Y, Zhu W, Gram DX, Sherwin RS. Activation of ATP-sensitive K+ channels in the ventromedial hypothalamus amplifies counterregulatory hormone responses to hypoglycemia in normal and recurrently hypoglycemic rats. Diabetes 54: 3169–3174, 2005. [DOI] [PubMed] [Google Scholar]
- 18.McCrimmon RJ, Fan X, Cheng H, McNay E, Chan O, Shaw M, Ding Y, Zhu W, Sherwin RS. Activation of AMP-activated protein kinase within the ventromedial hypothalamus amplifies counterregulatory hormone responses in rats with defective counterregulation. Diabetes 55: 1755–1760, 2006. [DOI] [PubMed] [Google Scholar]
- 19.McCrimmon RJ, Fan X, Ding Y, Zhu W, Jacob RJ, Sherwin RS. Potential role for AMP-activated protein kinase in hypoglycemia sensing in the ventromedial hypothalamus. Diabetes 53: 1953–1958, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Migrenne S, Magnan C, Cruciani-Guglielmacci C. Fatty acid sensing and nervous control of energy homeostasis. Diabetes Metab 33: 177–182, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Morgan K, Obici S, Rossetti L. Hypothalamic responses to long-chain fatty acids are nutritionally regulated. J Biol Chem 279: 31139–31148, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Obici S, Feng Z, Arduini A, Conti R, Rossetti L. Inhibition of hypothalamic carnitine palmitoyltransferase-1 decreases food intake and glucose production. Nat Med 9: 756–761, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L. Central administration of oleic acid inhibits glucose production and food intake. Diabetes 51: 271–275, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Page KA, Williamson A, Yu N, McNay EC, Dzuira J, McCrimmon RJ, Sherwin RS. Medium-chain fatty acids improve cognitive function in intensively treated type 1 diabetic patients and support in vitro synaptic transmission during acute hypoglycemia. Diabetes 58: 1237–1244, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Randle PJ Regulatory interactions between lipids and carbohydrates: the glucose fatty acid cycle after 35 years. Diabetes Metab Rev 14: 263–283, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Shah SD, Clutter WE, Cryer PE. External and internal standards in the single-isotope derivative (radioenzymatic) measurement of plasma norepinephrine and epinephrine. J Lab Clin Med 106: 624–629, 1985. [PubMed] [Google Scholar]
- 27.Tewari KP, Malinowska DH, Sherry AM, Cuppoletti J. PKA and arachidonic acid activation of human recombinant ClC-2 chloride channels. Am J Physiol Cell Physiol 279: C40–C50, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Veneman T, Mitrakou A, Mokan M, Cryer P, Gerich J. Effect of hyperketonemia and hyperlacticacidemia on symptoms, cognitive dysfunction, and counterregulatory hormone responses during hypoglycemia in normal humans. Diabetes 43: 1311–1317, 1994. [DOI] [PubMed] [Google Scholar]
- 29.Wang R, Cruciani-Guglielmacci C, Migrenne S, Magnan C, Cotero VE, Routh VH. Effects of oleic acid on distinct populations of neurons in the hypothalamic arcuate nucleus are dependent on extracellular glucose levels. J Neurophysiol 95: 1491–1498, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Witt MR, Nielsen M. Characterization of the influence of unsaturated free fatty acids on brain GABA/benzodiazepine receptor binding in vitro. J Neurochem 62: 1432–1439, 1994. [DOI] [PubMed] [Google Scholar]
- 31.Xue B, Kahn BB. AMPK integrates nutrient and hormonal signals to regulate food intake and energy balance through effects in the hypothalamus and peripheral tissues. J Physiol 574: 73–83, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng HF, Li XL, Jin ZY, Sun JB, Li ZL, Xu WX. Effects of unsaturated fatty acids on calcium-activated potassium current in gastric myocytes of guinea pigs. World J Gastroenterol 11: 672–675, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]