Abstract
Recent evidence suggests that leptin reduces food intake via actions in the brain circuitry of food reward, such as the ventral tegmental area (VTA), as leptin receptors are present in the VTA, and leptin injection in the VTA reduces food intake. In the hypothalamus, leptin-induced anorexia requires signaling via Janus kinase-signal transducer and activator of transcription (Jak-STAT), insulin receptor substrate (IRS)-phosphatidylinositol 3-kinase (PI 3-kinase), and mammalian target of rapamycin (mTOR). In this study, we determined whether leptin activates each of these signal transduction pathways in the VTA and whether these signaling pathways are required for VTA-leptin induced anorexia. Here, we show that pSTAT3-Tyr705, a marker of leptin activation, was induced in a midbrain region containing the VTA and substantia nigra following either intracerebroventricular leptin or direct administration of leptin to the VTA, but these interventions failed to increase levels of either pAKT-Ser473 or phospho-p70S6K-Thr389, markers of IRS-PI 3-kinase and mTOR signaling, respectively. Moreover, the effect of intra-VTA leptin administration to reduce 4- and 20-h food intake and 20-h body weight was blocked by an inhibitor of Jak-2, at a dose that had no effect on food intake or body weight by itself, but not by local inhibition of either PI 3-kinase (LY-294002) or mTOR (rapamycin) in this timeframe. Taken together, these data support the hypothesis that leptin signaling in the VTA is involved in the regulation of energy balance, but, in contrast to the leptin signaling in the hypothalamus, these effects are mediated predominantly via Jak-2 signaling rather than via the IRS-PI 3-kinase or mTOR signaling pathway.
Keywords: leptin, food intake, ventral tegmental area, signal transducer and activator of transcription signaling
although energy homeostasis research has concentrated primarily on the role of the hypothalamus, an area that integrates and processes information from a variety of short- and long-term signals regarding nutritional state, another important neural network that participates in this process is the central nervous system (CNS) circuitry of reward and motivation. This circuitry processes sensory input from visual, olfactory, and other external cues and integrates it with emotional processing, decision-making, and learning (4). In addition to homeostatic mechanisms that drive the motivation for food, brain reward circuits play an important role in the decision of whether or not to eat.
One key signal that conveys information regarding peripheral energy stores to the brain is the adipocyte-derived hormone leptin (42). Leptin circulates in direct proportion to body fat stores (12), enters the CNS in proportion to its plasma level, and acts on its receptor expressed in key brain areas (2, 15) to regulate food intake and energy expenditure. Leptin is critical for the regulation of energy balance, since reduced or deficient leptin signaling causes hyperphagia and obesity (42) while, conversely, administration of leptin reduces food intake and body weight (10, 32, 40). Recent evidence suggests that, in addition to hypothalamic areas regulating energy homeostasis, leptin can also act at brain circuitry that mediates reward and motivation (19, 20, 24). Accordingly, deficient neuronal signaling by leptin may favor weight gain by affecting both homeostatic and reward circuitry governing food intake.
Like other members of the class I cytokine family, the leptin receptor has been demonstrated to activate the Janus kinase-signal transducer and activator of transcription (Jak-STAT) pathway. Leptin binding to the “long” or “signaling” isoform of the leptin receptor, leprb, activates Jak-2, which in turn phosphorylates leprb, resulting in the recruitment and tyrosine phosphorylation of STAT3 (6, 22, 39). Phospho-STAT3 dimers then translocate to the nucleus and activate the transcription of various target genes, including suppressor of cytokine signaling-3 (a signaling inhibitor that blocks leprb activation of Jak-STAT signaling) (1, 5, 39). Signaling via leprb is critical for the ability of leptin to regulate energy homeostasis, since mice that lack leprb (e.g., db/db mice) exhibit a similar phenotype to the leptin-deficient, ob/ob mouse (3). Moreover, STAT signaling has been implicated in the physiological function of leptin. A “knock-in” mouse model in which LepRbS1138 (a mutant for Tyr1138) replaces endogenous leprb to specifically disrupt leprb-STAT signaling is characterized by hyperphagia and obesity (3), whereas leptin's ability to acutely reduce food intake depends on intact STAT3 signaling (9).
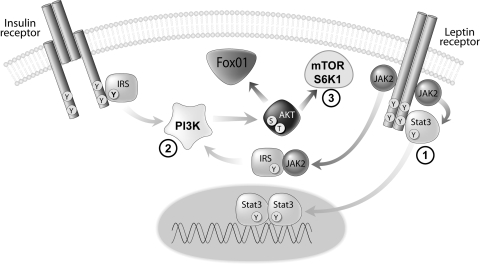
Recent data suggest that, like insulin, leptin is also capable of activating the insulin receptor substrate (IRS)-phosphatidylinositol-3-OH kinase (PI 3-kinase) pathway in the mediobasal hypothalamus (29, 36) (Fig. 1) and that this effect is also required for leptin regulation of food intake (29). In addition, leptin increases hypothalamic mammalian target of rapamycin (mTOR) activity, an enzyme that regulates growth by sensing changes in energy status, and activation of this pathway is also required for leptin-induced anorexia (13). Therefore, leptin is capable of activating each of these signal transduction pathways in the hypothalamus, and signaling via these pathways is required for the ability of leptin to reduce food intake and body weight. Beyond the hypothalamus, the long or signaling form of the leptin receptor is expressed in hippocampus, hindbrain, and in mesolimbic areas involved in reward, including the ventral tegmental area (VTA) and substantia nigra (SN) (15, 18). Because leptin administration directly in the VTA reduces food intake and body weight (24) and induces tyrosine phosphorylation of STAT-3 (20, 24), a key downstream mediator of leptin receptor signaling in this brain area, these data raise the possibility that leptin action may reduce food intake through a direct action on brain motivational/reward circuitry, in addition to leptin actions in brain areas associated with energy homeostasis.
Fig. 1.
Signal transduction mechanisms implicated in leptin action. Leptin binding activates Janus kinase (Jak-2) that leads to the binding of and tyrosine phosphorylation of signal transducer and activator of transcription (STAT3). In addition, leptin receptor signaling can activate the insulin receptor substrate (IRS)-phosphatidylinositol 3-kinase [PI 3-kinase (PI3K)] pathway and mammalian target of rapamycin (mTOR) signaling via protein kinase B (Akt).
The studies here were undertaken to examine the mechanism(s) by which leptin signaling in the VTA reduces food intake. We determined whether, similar to the hypothalamus, leptin activates the Jak-STAT, IRS-PI 3-kinase, and mTOR signal transduction pathways in the VTA/SN and whether activation of these pathways is required for VTA-leptin induced anorexia. Our data suggest that Jak-2 signaling in the VTA, a key brain area involved in food reward, is activated by leptin; that this effect is required for anorexia induced by VTA leptin action; and that leptin signaling via IRS-PI 3-kinase and mTOR do not appear to participate in VTA leptin-induced anorexia.
METHODS
Experimental Animals
Adult male Wistar rats (Harlan Laboratories Indianapolis, IN) were housed individually in a specific pathogen-free environment, maintained in a temperature-controlled room with a 12:12-h light-dark cycle, and provided with ad libitum access to water and standard laboratory chow (PMI Nutrition International), unless otherwise stated. All study protocols were approved by the Animal Care and Use Committee at the University of Washington and conducted in accordance with National Institutes of Health guidelines for the care and use of animals.
Surgery
To examine the signal transduction pathways activated by intracerebroventricular (icv) administration of leptin, animals received an indwelling stainless steel cannula to the third cerebral ventricle (3V) under isoflurane anesthesia, as previously described (34). In experiments requiring intra-VTA injections, rats were surgically implanted with bilateral 26-gauge stainless steel guide cannulas (Plastics One, Roanoke, VA) to the VTA. Stereotaxic coordinates for VTA placement based on the brain atlas of Paxinos and Watson were as follows: ±1.0 mm lateral, 5.2 mm posterior to Bregma, and 7.1 mm below the skull surface (25). The cannula was secured to the skull with stainless steel screws and dental cement and closed with an obturator. Buprenorphine hydrochloride (0.3 mg/kg; Rickett Colman Pharmaceuticals) was administered at the completion of the surgery. Animals were allowed to recover at least 7 days after surgery while daily food intake and body weight were recorded. Injections were administered using an injector (33-gauge) needle that extended 1 mm beyond the tip of the cannula over a period of 60 s in a final volume of 0.5 μl (each side). The injector was then allowed to remain for an addition 60 s before removing.
Immunohistochemical Verification of Injection Site
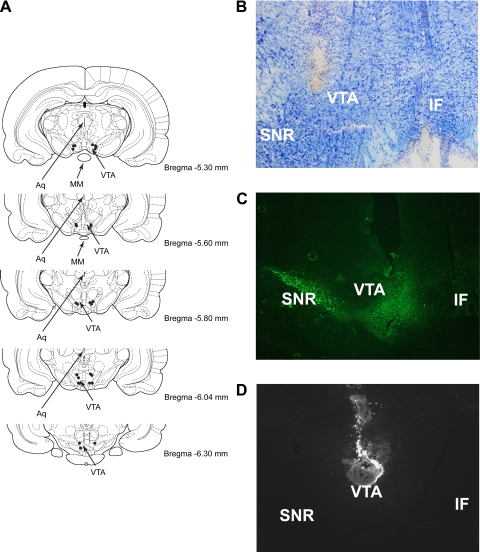
To histologically verify the injection site, coronal cryostat sections (60 μm) were taken, mounted on microscope slides, and stained with cresyl violet (a Nissl stain) as previously described (8, 37). Slides were examined under a Nikon SMZ-U stereomicroscope, and the presence of gliosis was used to determine the injection site and the placement of injector tips. The brightfield picture of cannula tract placement was imaged from the left side of the VTA using a Nikon Coolscope using a ×5 objective lens. To confirm the anatomic placement of the cannula to the VTA and to determine the extent of spread following bilateral injection, a subset of animals received a bilateral VTA injection (0.5 μl/side) of Cy3-labeled recombinant mouse leptin (Phoenix Pharmaceuticals, Belmont, CA). Later (30 min), animals were anesthetized and perfused with ice-cold PBS (Diamedix, Miami, FL) followed by 4% paraformaldehyde (PFA; Electron Microscopy Sciences, Hatfield, PA). Brains were removed, postfixed in PFA overnight, and transferred to a 25% sucrose solution in PBS for 48 h at 4°C before being frozen in isopentane cooled on dry ice. Coronal sections throughout the VTA were then slide-mounted and stored at −80°C before visualization of the Cy3-labeled leptin using fluorescence microscopy. Slides were analyzed with a Zeiss Axioplan fluorescence microscope, and all injection sites were analyzed using a ×10 objective lens. Identification of anatomic landmarks was assisted by staining cell nuclei with Hoechst 33258 (Sigma, St. Louis, MO), which was added to the mounting medium and observed with a conventional DAPI filter set and assay for tyrosine hydroxylase (TH), a marker of dopamine signaling that is expressed in the VTA. Briefly, slide-mounted sections were rinsed with 10 mM PBS at room temperature and then blocked for 60 min at room temperature with a buffer containing 5% normal goat serum in 10 mM PBS before being incubated overnight at 4°C with a mouse monoclonal anti-TH, 1:1,000 dilution (Chemicon International, Temecula, CA). Slides were rinsed with PBS and incubated for 60 min at room temperature with a secondary antibody [1:200, goat anti-mouse IgG-Cy3 (Jackson ImmunResearch, West Grove, PA)]. After being rinsed with PBS, slides were then coverslipped using an anti-fading glycerol-based mounting media (Sigma-Aldrich, St. Louis, MO). This protocol and these antibodies have been previously used for staining of TH in the brain (7, 18, 41). Slides were examined using a ×10 objective, and images were captured using a Nikon Eclipse E600 fluorescence microscope equipped with a Diagnostic Instruments Spot RT color digital camera. A summary of cannula placements (modified from Ref. 31) and representative photomicrographs of cannula placement and the injection site are shown in Fig. 2.
Fig. 2.
Summary of cannula placements in the VTA (A) and a representative example of cannula placement using cresyl violet staining from coronal sections at the level of the VTA using a ×5 objective lens (B). An example of cannula place analysis with anti-tyrosine hydroxylase staining to mark the VTA (C) and a fluorescent micrograph of a representative injection site of the Cy3-labeled leptin within the VTA using a ×10 objective lens (D). VTA, ventral tegmental area; SNR, substantia nigra reticularis; IF, interfascicular; Aq, aqueduct; MM, mammillary nuclei. Modified from Ref. 31.
Experimental Protocol
Effect of intra-VTA leptin on food intake and body weight.
Animals were habituated to regular handling and received a mock injection before the commencement of experiments. Before dark cycle onset (4 h), food was removed, and, 1 h later, animals received a bilateral microinjection in the VTA of either vehicle or recombinant mouse leptin obtained from Dr. A. F. Parlow (National Hormone and Peptide Program) dissolved in PBS (pH 7.9) at a dose of either 0.05, 0.25 or 0.50 μg in an injection volume of 0.5 μl for each side. Food was replaced at dark cycle onset, and intake was measured at 4 and 20 h. This paradigm was based on previously conducted experiments examining the effect of intracerebroventricular leptin to reduce food intake and body weight (29).
Determination of cell signaling pathways in VTA leptin action.
To determine the role of Jak-STAT signaling in VTA leptin action, adult male Wistar rats were surgically implanted with indwelling bilateral cannulas directed to the VTA as described above. Before dark cycle onset (4 h), food was removed. Later (2 h), animals subsequently received a bilateral VTA microinjection of either vehicle or leptin (0.50 μg), counterbalanced in combination with either a pretreatment injection of either the Jak-2 inhibitor (AG490; 1 nmol; Calbiochem, Gibbstown, NJ) or its vehicle [5% dimethyl sulfoxide (DMSO) in PBS]. This dose of inhibitor has previously been demonstrated to block the central effect of leptin to reduce food intake and body weight (28). Food was replaced at dark cycle onset and measured at 4 and 20 h later while body weight was recorded 20 h later.
To determine the role of IRS-PI 3-kinase signaling in VTA leptin action, a separate group of male Wistar rats with bilateral cannulas aimed at the VTA were injected with either vehicle or leptin (0.50 μg), counterbalanced in combination with either a pretreatment injection of either the PI 3-kinase inhibitor (LY-294002; 1 nmol; Calbiochem) or its vehicle (5% DMSO in PBS). This inhibitor has previously been demonstrated to block the ability of intracerebroventricular leptin to reduce food intake and body weight in rats at this dose (29). Injections commenced 3 h before dark cycle onset 1 h following food removal. Food was replaced at dark cycle onset and measured at 4 and 20 h later while body weight was recorded 20 h later.
To determine the role of mTOR signaling in VTA leptin action, using the same paradigm, an additional group of male Wistar rats received a bilateral injection in the VTA of either vehicle or leptin (0.50 μg), counterbalanced in combination with either a pretreatment injection of either an inhibitor of mTOR (rapamycin; 10 μg; Calbiochem) or its vehicle (100% DMSO). This dose of inhibitor was selected on the basis of previous studies demonstrating the ability of rapamycin to block leptin-induced anorexia (13, 33). Food was replaced at dark cycle onset and measured at 4 and 20 h later while body weight was recorded 20 h later.
Tissue Processing and Biochemical Analysis
Separate groups of adult male Wistar rats bearing either a single cannula to the third ventricle (mean body weight = 417 g) or a bilateral cannula directed to the VTA (mean body weight = 373 g) were fasted overnight and received an injection of either vehicle or leptin (icv: 10 μg; intra-VTA: 1 μg). At 30 and 90 min after injection, animals were killed, the brain was removed, and either a wedge of hypothalamus (defined caudally by the mamillary bodies, rostrally by the optic chiasm, laterally by the optic tract, and superiorly by the apex of the third ventricle) or tissue enriched in the VTA and SN was rapidly dissected. For VTA/SN enriched tissue, briefly, brains were removed, and coronal cuts were made at the rostral and caudal boundaries of the VTA using the mamillary bodies as a landmark. Bilateral sagittal cuts were then made using the SN as a landmark, and a horizontal cut was made ∼1 mm ventral to the aqueduct. Dissected tissues were snap-frozen and protein extracted and stored at −80°C for subsequent analysis (29). We selected these time points based on previous studies that demonstrated leptin-induced activation of the IRS-PI 3-kinase pathway at 30 min and leptin-induced activation of the mTOR pathway at 90 min (13, 29). Antibodies against phospho-Stat 3 (Tyr705), phospho (p)-protein kinase B (Akt; Ser473), and phospho-p70S6K (pS6K1), a downstream marker of mTOR activation, STAT3, Akt1, and p70S6K (S6K1) were obtained from Cell Signaling (Beverly, MA). Protein levels were quantified using the Micro BCA Protein Assay Kit (Pierce, Rockford, IL) according to the manufacturer's instruction, and equal amounts of protein were used for each condition in each assay. Western blots were performed using SDS gel electrophoresis on a 4 by 20% gradient gel and then transferred to nitrocellulose. Membranes were probed with anti-STAT3, -Akt1, -S6K1, pStat 3, pAkt, and pS6K1 antibodies (1:1,000) overnight. Quantitation of protein expression and phosphorylation was performed using Image J software.
Statistical Analyses
All results are expressed as means ± SE. Comparisons between multiple groups were made using a two-way analysis of variance with a least-significant difference post hoc test for comparisons between groups. For two-group comparisons, a two-sample, unpaired Student's t-test was used. Statistical analyses were performed using Statistica (version 7.1; StatSoft). Probability values of <0.05 were considered significant.
RESULTS
Effect of Intra-VTA Pretreatment on Leptin-Induced Anorexia
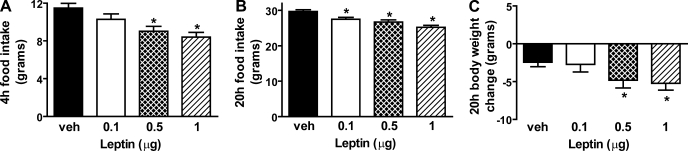
Consistent with observations of Hommel and colleagues (24), we found that bilateral intra-VTA leptin administration in rats (mean body weight = 413 g) at doses of 0.5 or 1 μg leptin significantly reduced food intake at 4 h by 17 and 25%, respectively (P < 0.05 for each) and 20 h by 8 and 14%, respectively (P < 0.05 for each). As expected, these reductions of food intake were accompanied by dose-dependent decreases of body weight in animals that received leptin relative to vehicle (−2.46 ± 0.59 g for vehicle vs. −4.83 ± 1.02 g for 0.5 μg leptin vs. −5.20 ± 0.90 g for 1 μg leptin; P < 0.05 for each) (Fig. 3).
Fig. 3.
Dose-response effect of intra-VTA leptin on food intake and body weight. A: food intake was measured 4 h after bilateral intra-VTA administration of either saline or leptin (0.1, 0.5, 1.0 μg). Food intake (B) and body weight (C) were measured in the same 4 groups of animals 20 h after treatment (n = 10–12/group). Data are means ± SE. *P < 0.05 vs. vehicle (veh).
Histological analysis using cresyl violet and TH staining (a marker of dopamine signaling expressed in the VTA) showed that the cannula tips were located in the VTA and that the injected solution diffused throughout the VTA (Fig. 2).
Role of Jak-STAT Signaling in VTA Leptin Action
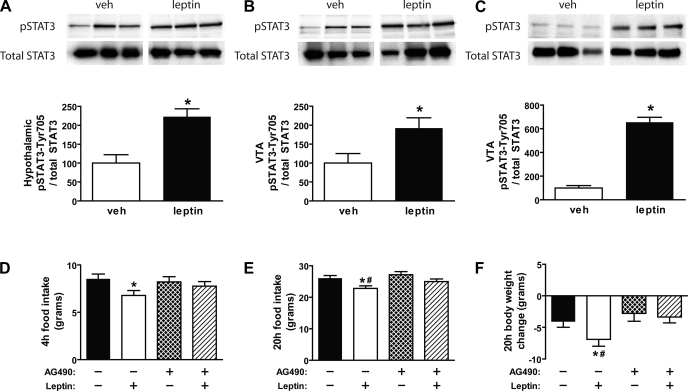
As a positive control, we first demonstrated that intracerebroventricular leptin (10 μg) induced pSTAT3 levels in the mediobasal hypothalamus relative to vehicle-treated animals (>2-fold; P < 0.05) using Western blot analysis (Fig. 4A). To determine whether leptin activates the Jak-STAT pathway in the VTA, animals received intracerebroventricular injections of leptin (10 μg), and we examined tyrosine phosphorylation of STAT3 in Western blots of VTA/SN tissue extracts. Consistent with previous findings (20, 24), we found that intracerebroventricular leptin increased pSTAT3 levels in the VTA/SN compared with vehicle-treated controls (P < 0.05) (Fig. 4B). These data suggest that central administration of leptin activates the Jak-STAT pathway in the VTA/SN but does not address whether this effect occurred directly in the VTA or via leptin action at a remote site. To determine whether leptin acts directly in the VTA to activate the Jak-STAT pathway, leptin was delivered directly in the VTA of rats via surgically implanted bilateral cannulas. As with intracerebroventricular leptin, we found that intra-VTA administration of leptin significantly induced tyrosine phosphorylation of STAT3 in the VTA/SN compared with vehicle (P < 0.05) (Fig. 4C).
Fig. 4.
Effect of icv leptin (10 μg) on induction of pSTAT3 in the hypothalamus (A) and VTA (B) using Western blot analysis (n = 5–6/group). C: Western blot analysis of pSTAT3 in the VTA of rats injected bilaterally to the VTA with either vehicle or leptin (1 μg). D: cumulative food intake measured during the first 4 h after bilateral intra-VTA pretreatment injections of either vehicle or the Jak-2 inhibitor (AG-490; 1 nmol), followed 1 h later by intra-VTA administration of either vehicle or leptin (0.5 μg) (n = 10–12/group). Food intake (E) and body weight change (F) were measured in the same groups of animals 20 h after treatment (n = 10–12/group). Data are means ± SE. P < 0.05 vs. vehicle-vehicle (*) and vs. AG-490-leptin (#).
We next sought to determine whether signaling via the Jak-STAT pathway is required for VTA-leptin induced anorexia. To test this hypothesis, animals (mean body weight = 392 g) received a bilateral VTA pretreatment injection of the Jak-2 inhibitor, AG-490, before intra-VTA administration of leptin. Consistent with our initial observation, leptin administration directly in the VTA reduced food intake at both 4 and 20 h (Fig. 4, D and E), and this was accompanied by decreased body weight at 20 h relative to vehicle-vehicle treated animals (P < 0.05 for each) (Fig. 4F). However, intra-VTA pretreatment of the Jak-2 inhibitor, AG-490, at a dose (1 nmol) that had no effect on food intake or body weight when given by itself, blocked the ability of leptin in the VTA to reduce 4- and 20-h food intake and to decrease body weight.
Finally, we determined whether the Jak-2 inhibitor, AG-490, blocked leptin-induced activation of pSTAT3 in the VTA. To test this, we used the same experimental paradigm as in the feeding studies where nonfasted animals received a bilateral VTA pretreatment injection of the Jak-2 inhibitor, AG-490, before intra-VTA administration of leptin. As expected, tyrosine phosphorylation of STAT3 normalized to total STAT3 was induced in the VTA in vehicle-leptin compared with vehicle-vehicle controls (198.3 ± 9.8 vs. 100.0 ± 26.9 units; P < 0.05), and this effect was blocked by an intra-VTA pretreatment of the Jak-2 inhibitor (129.9 ± 22.0 for AG-490-leptin vs. 198.3 ± 9.8 units for vehicle-leptin; P < 0.05), at a dose of AG-490 that had no effect on pSTAT3 levels by itself compared with vehicle-vehicle treated controls [P = not significant (NS)].
Role of IRS-PI 3-Kinase Signaling in VTA Leptin Action
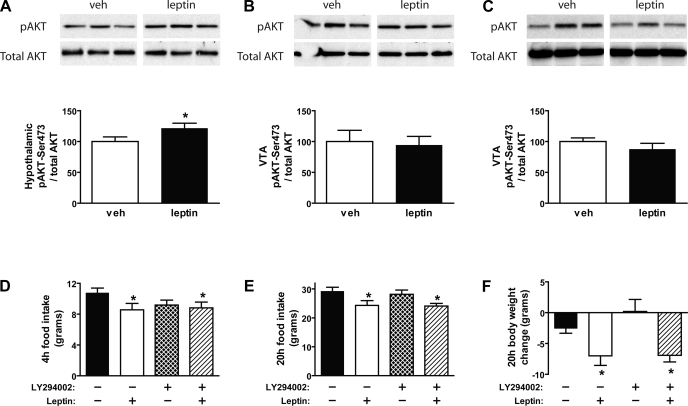
Consistent with our previously published data, we demonstrated that, in addition to activation of pSTAT3 (Fig. 4A), intracerebroventricular administration of leptin induced serine phosphorylation of Akt relative to vehicle-treated animals in the mediobasal hypothalamus (P < 0.05) (Fig. 5A). To determine whether leptin in the VTA signals via the IRS-PI 3-kinase pathway, we examined in a separate group of animals: 1) whether either intracerebroventricular leptin or direct administration of leptin in the VTA increases pAkt (a downstream marker of PI 3-kinase activation) in the VTA/SN and 2) whether intra-VTA pretreatment with the PI 3-kinase inhibitor, LY-294002, blocks VTA leptin-induced anorexia. Although central administration of leptin resulted in activation of pSTAT3 in the VTA (Fig. 4B), it failed to induce pAkt in the VTA at 30 min (Fig. 5B). As expected, leptin failed to activate pAkt at the 90-min time point (data not shown) (29). Similarly, while direct administration of leptin to the VTA activated the Jak-STAT pathway (Fig. 4C), it again failed to activate pAkt in this brain area at 30 min after injection (Fig. 5C).
Fig. 5.
Effect of icv leptin (10 μg) on induction of phospho-Akt (pAkt) in the hypothalamus (A) and VTA (B) using Western blot analysis (n = 5–6/group). C: Western blot analysis of pAkt in the VTA of rats injected bilaterally to the VTA with either vehicle or leptin (1 μg). D: cumulative food intake measured during the first 4 h after bilateral intra-VTA pretreatment injections of either vehicle or the PI 3-kinase inhibitor (LY-294002; 1 nmol), followed 1 h later by intra-VTA administration of either vehicle or leptin (0.5 μg) (n = 10–12/group). Food intake (E) and body weight change (F) were measured in the same groups of animals 20 h after treatment (n = 10–12/group). Data are means ± SE. *P < 0.05 vs. vehicle-vehicle.
Consistent with this outcome, the ability of intra-VTA administration of leptin (mean body weight = 409 g) to reduce 4-h and 20-h food intake and decrease body weight relative to vehicle-vehicle treated animals (P < 0.05 for each) (Fig. 5, D–F) was not attenuated by intra-VTA pretreatment with the PI 3-kinase inhibitor, LY-294002 (P = NS), at a dose (1 nmol) previously shown to block leptin-induced anorexia when given intracerebroventricularly (29) while having no independent effect on food intake or body weight (Fig. 5, D–F).
Role of mTOR Signaling in VTA Leptin Action
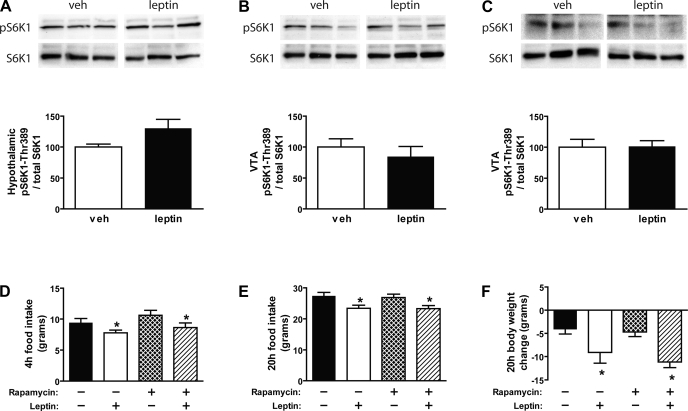
Although intracerebroventricular administration of leptin induced both pSTAT3 (Fig. 4A) and pAkt (Fig. 5A) in the mediobasal hypothalamus, leptin tended to increase pS6K1, a downstream marker of mTOR activation in this same brain area relative to vehicle-treated controls (P = 0.069) (Fig. 6A). To investigate whether the mTOR pathway mediates leptin action in the VTA, in an additional group of animals, we determined whether administration of leptin activates pS6K1 in the VTA/SN and whether pretreatment with the mTOR inhibitor, rapamycin, blocks VTA leptin-induced anorexia. Neither intracerebroventricular (Fig. 6B) nor direct intra-VTA (Fig. 6C) administration of leptin induced pS6K1 in the VTA/SN at 90 min, suggesting that leptin does not activate the mTOR signaling pathway in the VTA.
Fig. 6.
Effect of icv leptin (10 μg) on induction of phospho-p70S6K (pS6K1) in the hypothalamus (A) and VTA (B) using Western blot analysis (n = 5–6/group). C: Western blot analysis of pS6K1 in the VTA of rats injected bilaterally to the VTA with either vehicle or leptin (1 μg). D: cumulative food intake measured during the first 4 h after bilateral intra-VTA pretreatment injections of either vehicle or the mTOR inhibitor (rapamycin; 10 μg), followed 1 h later by intra-VTA administration of either vehicle or leptin (0.5 μg) (n = 10–12/group). Food intake (E) and body weight change (F) were measured in the same groups of animals 20 h after treatment (n = 10–12/group). Data are means ± SE. *P < 0.05 vs. vehicle-vehicle.
As expected, leptin administration in the VTA (mean body weight = 431 g) significantly reduced 20-h food intake and body weight relative to vehicle-vehicle treated animals (P < 0.05 for each). However, intra-VTA pretreatment with the mTOR inhibitor, rapamycin, at a dose (10 μg) that did not affect food intake or body weight on its own, failed to block the anorexic effect of leptin on either food intake (Fig. 6, D and E) or body weight (P = NS) (Fig. 6F).
DISCUSSION
Previous observations support the hypothesis that leptin regulates feeding not only in brain areas involved with energy homeostasis (hypothalamus) but in circuitry that mediates reward and motivation (VTA) (19, 20, 24). In this study, we demonstrate that 1) Jak-STAT signaling in the VTA/SN, a key brain area involved in reward, is activated by leptin; 2) this effect involves a local action of leptin in the VTA; 3) this effect is required for the ability of leptin in the VTA to reduce food intake and body weight; and 4) leptin signaling via IRS-PI 3-kinase and mTOR does not appear to participate in VTA leptin-induced anorexia. These findings extend earlier work and highlight differences in signal transduction mechanisms employed by leptin in distinct brain areas controlling feeding behavior.
Although the hypothalamus clearly plays a key role to mediate leptin effects on food intake, growing evidence suggests that extrahypothalamic sites are also important in this process. Leptin receptors are expressed not only in the hypothalamus but the hindbrain and VTA (15, 18), and intracerebroventricular administration of leptin induces pSTAT3, a downstream marker of leptin activation, in each of these brain areas (20, 23, 24, 39). Although these effects could possibly be explained by an indirect effect of leptin, our data support work by Hommel and colleagues (24) and demonstrate that leptin can act directly on leptin receptors in the VTA, since delivery of leptin directly in this brain area potently increases its pSTAT3 content. The effect of centrally administered leptin to reduce food intake and body weight may therefore involve activation of leptin receptor signaling in extrahypothalamic sites, such as the VTA.
The effect of leptin in the VTA to reduce food intake and body weight was blocked by local pretreatment using the Jak-2 inhibitor, AG-490. Because leptin-induced activation of pSTAT3 in the VTA was also blocked by this inhibitor, taken together, these findings implicate Jak-2 signaling in the feeding effects of leptin in the VTA and diminish the likelihood that this action of leptin involves a nonspecific mechanism. However, although we and others have shown that the Jak-2 inhibitor AG-490 blocks leptin-induced activation of pSTAT3 (14), we cannot rule out the possibility that the effects are independent of Jak-STAT signaling. Moreover, although subpopulations of mesolimbic dopaminergic neurons are involved in controlling locomotor activity and exploratory behavior (27), the changes in feeding and body weight by leptin do not seem to involve changes in ambulatory activity (24). Taken together, these studies suggest that leptin directly activates the Jak-STAT pathway in the VTA, a key brain area involved in reward, and signaling via Jak-2 is required for the VTA leptin-induced anorexia.
In hypothalamus, leptin activates the Jak-STAT pathway (39), and signaling via this pathway is critical for leptin to regulate energy homeostasis (3). However, in addition to this pathway, leptin has been demonstrated to activate both the IRS-PI 3-kinase (29, 36) and mTOR (13) signal transduction pathways, and signaling via these pathways is required for leptin-induced anorexia. Consistent with this, we demonstrate that intracerebroventricular administration of leptin significantly induced activation of pSTAT3 and pAkt in the mediobasal hypothalamus while tending to increase pS6K1, a marker of mTOR activation. Here, we report that both central and direct injection of leptin in the VTA potently activate the Jak-STAT pathway in the area of the VTA, although we cannot rule out the possibility that leptin may also act in the adjacent SN. Indeed, previous studies have demonstrated the expression of leptin receptors and TH in both the substantia nigra pars compacta and the substantia nigra reticularis (15, 18), although their role in feeding is unknown. Nevertheless, we observed no detectable effect of leptin on either the IRS-PI 3-kinase or mTOR signal transduction pathways in this brain area, and neither of these pathways appears to play a role in leptin's anorexic effects in the VTA. Previous studies have demonstrated that mTOR is ubiquitously expressed throughout the CNS and is activated in the VTA by metabotropic glutamate receptors (26). The failure of leptin to activate pAkt, a downstream target of PI 3-kinase signaling in the VTA, is at odds with previous evidence that leptin activates PI 3-kinase signaling in the hypothalamus and that intra-VTA administration increases the local content of phosphatidylinositol 3,4,5-triphosphate, the phospholipid product of PI 3-kinase activation (19). In those previous studies, PI 3-kinase activation was assessed very rapidly after administration of leptin. Differences in leptin dose, and histochemical vs. biochemical assessment of enzyme activity, may also explain divergent outcomes of these studies. Here, the absence of any measurable effect of a PI 3-kinase inhibitor to reduce the feeding effects of leptin in the VTA suggests that the IRS-PI 3-kinase pathway in the VTA is not required for leptin-induced anorexia over a longer time course.
Several lines of research suggest that leptin modulation of brain reward circuitry alters performance for rewarding stimuli. Central administration of leptin reduces the rewarding properties of food and other stimuli, measured using behavioral paradigms, including place preference conditioning (the seeking of a place associated with a reward) (16), the motivation to work for a food reward (17), and the rewarding effect of electrical brain stimulation (21). Interestingly, these effects are seen at doses that are subthreshold for decreasing 24-h food intake. Furthermore, conditions associated with reduced leptin signaling, such as fasting, enhance the rewarding properties of both food and certain drugs (11, 21, 35, 38). The VTA has been postulated as an extrahypothalamic site that mediates these effects, since the ability of intra-VTA administration of the mu-opioid agonist to stimulate feeding is blocked by direct acute injection of leptin in the VTA (19). Consistent with the hypothesis that leptin signaling in the VTA modulates feeding and reward, conditional knockdown of Lepr signaling in the VTA of rats using and RNAi strategy increases sensitivity to sucrose and a high-fat diet (24). In our study, we examined the ability of leptin to reduce intake of standard laboratory chow. Whether direct administration of leptin in the VTA reduces aspects of food reward, and whether this effect requires signaling via the Jak-STAT, IRS-PI 3-kinase, or mTOR signaling pathway remains to be elucidated.
One potential mechanism by which leptin signaling in the VTA reduces food intake is thought to involve dopaminergic neurons. Fluorescent immunohistochemical and double-label in situ hybridization studies demonstrate that leptin receptor protein (18) and mRNA (24) are expressed in VTA dopaminergic neurons and that peripheral administration of leptin activates pSTAT3 in dopaminergic neurons (20). Furthermore, electrophysiological studies show that leptin inhibits dopaminergic neuronal activation in normal animals (24). However, leptin increases TH mRNA in the VTA of ob/ob mice (20). Therefore, the effects of leptin on dopamine function in the regulation of food intake remain to be completely elucidated (30).
Perspectives and Significance
Our data suggest that Jak-2 signaling in the VTA is important for VTA leptin-induced anorexia. However, compared with the hypothalamus, leptin action in the VTA reduces food intake and body weight via mechanisms that are likely independent of the IRS-PI 3-kinase and mTOR signaling pathways. In keeping with a “distributed” model of neural networks that process and integrate adiposity signals, our data therefore extend previous evidence that the ability of leptin to regulate feeding involves extrahypothalamic sites, including the VTA, and that heterogeneity exists in signal transduction mechanisms downstream of the leptin receptor that mediate the feeding effects of leptin in different brain areas.
GRANTS
This work was supported by Department of Veterans Affairs and National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-40963 (D. P. Figlewicz) and the Royalty Research Fund and the Exploratory Center for Obesity Research at the University of Washington (G. J. Morton). G. J. Morton is supported by a Naomi Berrie Investigator in Diabetes Research Award from Columbia University.
Supplementary Material
Acknowledgments
We thank Hong Nguyen, Alex Cubelo, and Ezekial Maloney for technical assistance and Dr. Michael W. Schwartz for support and valuable input.
REFERENCES
- 1.Banks AS, Davis SM, Bates SH, Myers MG Jr. Activation of downstream signals by the long form of the leptin receptor. J Biol Chem 275: 14563–14572, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Baskin DG, Breininger JF, Schwartz MW. Leptin receptor mRNA identifies a subpopulation of neuropeptide Y neurons activated by fasting in rat hypothalamus. Diabetes 48: 828–833, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, Neel BG, Schwartz MW, Myers MG Jr. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature 421: 856–859, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Berthoud HR, Morrison C. The brain, appetite, obesity. Annu Rev Psychol 59: 55–92, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Bjorbaek C, Lavery HJ, Bates SH, Olson RK, Davis SM, Flier JS, Myers MG Jr. SOCS3 mediates feedback inhibition of the leptin receptor via Tyr985. J Biol Chem 275: 40649–40657, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Bjorbaek C, Uotani S, da Silva B, Flier JS. Divergent signaling capacities of the long and short isoforms of the leptin receptor. J Biol Chem 272: 32686–32695, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Blevins JE, Chelikani PK, Haver AC, Reidelberger RD. PYY(3-36) induces Fos in the arcuate nucleus and in both catecholaminergic and non-catecholaminergic neurons in the nucleus tractus solitarius of rats. Peptides 29: 112–119, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blevins JE, Stanley BG, Reidelberger RD. Brain regions where cholecystokinin suppresses feeding in rats. Brain Res 860: 1–10, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Buettner C, Pocai A, Muse ED, Etgen AM, Myers MG Jr, Rossetti L. Critical role of STAT3 in leptin's metabolic actions. Cell Metab 4: 49–60, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science 269: 546–549, 1995. [DOI] [PubMed] [Google Scholar]
- 11.Carroll ME, France CP, Meisch RA. Food deprivation increases oral and intravenous drug intake in rats. Science 205: 319–321, 1979. [DOI] [PubMed] [Google Scholar]
- 12.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, Caro JF. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 334: 292–295, 1996. [DOI] [PubMed] [Google Scholar]
- 13.Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science 312: 927–930, 2006. [DOI] [PubMed] [Google Scholar]
- 14.De Minicis S, Seki E, Oesterreicher C, Schnabl B, Schwabe RF, Brenner DA. Reduced nicotinamide adenine dinucleotide phosphate oxidase mediates fibrotic and inflammatory effects of leptin on hepatic stellate cells. Hepatology 48: 2016–2026, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol 395: 535–547, 1998. [PubMed] [Google Scholar]
- 16.Figlewicz DP, Bennett J, Evans SB, Kaiyala K, Sipols AJ, Benoit SC. Intraventricular insulin and leptin reverse place preference conditioned with high-fat diet in rats. Behav Neurosci 118: 479–487, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Figlewicz DP, Bennett JL, Naleid AM, Davis C, Grimm JW. Intraventricular insulin and leptin decrease sucrose self-administration in rats. Physiol Behav 89: 611–616, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Figlewicz DP, Evans SB, Murphy J, Hoen M, Baskin DG. Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain Res 964: 107–115, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Figlewicz DP, MacDonald Naleid A, Sipols AJ. Modulation of food reward by adiposity signals. Physiol Behav 91: 473–478, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron 51: 811–822, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Fulton S, Woodside B, Shizgal P. Modulation of brain reward circuitry by leptin. Science 287: 125–128, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Ghilardi N, Skoda RC. The leptin receptor activates janus kinase 2 and signals for proliferation in a factor-dependent cell line. Mol Endocrinol 11: 393–399, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Grill HJ, Schwartz MW, Kaplan JM, Foxhall JS, Breininger J, Baskin DG. Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology 143: 239–246, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 51: 801–810, 2006. [DOI] [PubMed] [Google Scholar]
- 25.MacDonald AF, Billington CJ, Levine AS. Alterations in food intake by opioid and dopamine signaling pathways between the ventral tegmental area and the shell of the nucleus accumbens. Brain Res 1018: 78–85, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Mameli M, Balland B, Lujan R, Luscher C. Rapid synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area. Science 317: 530–533, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Mogenson GJ, Yang CR. The contribution of basal forebrain to limbic-motor integration and the mediation of motivation to action. Adv Exp Med Biol 295: 267–290, 1991. [DOI] [PubMed] [Google Scholar]
- 28.Morrison CD, White CL, Wang Z, Lee SY, Lawrence DS, Cefalu WT, Zhang ZY, Gettys TW. Increased hypothalamic protein tyrosine phosphatase 1B contributes to leptin resistance with age. Endocrinology 148: 433–440, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG Jr, Schwartz MW. Intracellular signalling Key enzyme in leptin-induced anorexia. Nature 413: 794–795, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Palmiter RD Is dopamine a physiologically relevant mediator of feeding behavior? Trends Neurosci 30: 375–381, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic, 1998. [DOI] [PubMed]
- 32.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269: 540–543, 1995. [DOI] [PubMed] [Google Scholar]
- 33.Ropelle ER, Pauli JR, Fernandes MF, Rocco SA, Marin RM, Morari J, Souza KK, Dias MM, Gomes-Marcondes MC, Gontijo JA, Franchini KG, Velloso LA, Saad MJ, Carvalheira JB. A central role for neuronal AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) in high-protein diet-induced weight loss. Diabetes 57: 594–605, 2008. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz MW, Sipols AJ, Marks JL, Sanacora G, White JD, Scheurink A, Kahn SE, Baskin DG, Woods SC, Figlewicz DP, Porte D Jr. Inhibition of hypothalamic neuropeptide Y gene expression by insulin. Endocrinology 130: 3608–3616, 1992. [DOI] [PubMed] [Google Scholar]
- 35.Shizgal P, Fulton S, Woodside B. Brain reward circuitry and the regulation of energy balance. Int J Obes Relat Metab Disord 25, Suppl 5: S17–S21, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Spanswick D, Smith MA, Groppi VE, Logan SD, Ashford ML. Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature 390: 521–525, 1997. [DOI] [PubMed] [Google Scholar]
- 37.Stanley BG, Lanthier D, Leibowitz SF. Multiple brain sites sensitive to feeding stimulation by opioid agonists: a cannula-mapping study. Pharmacol Biochem Behav 31: 825–832, 1988. [DOI] [PubMed] [Google Scholar]
- 38.Stuber GD, Evans SB, Higgins MS, Pu Y, Figlewicz DP. Food restriction modulates amphetamine-conditioned place preference and nucleus accumbens dopamine release in the rat. Synapse 46: 83–90, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Vaisse C, Halaas JL, Horvath CM, Darnell JE Jr, Stoffel M, Friedman JM. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet 14: 95–97, 1996. [DOI] [PubMed] [Google Scholar]
- 40.Weigle DS, Bukowski TR, Foster DC, Holderman S, Kramer JM, Lasser G, Lofton-Day CE, Prunkard DE, Raymond C, Kuijper JL. Recombinant ob protein reduces feeding and body weight in the ob/ob mouse. J Clin Invest 96: 2065–2070, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams DL, Schwartz MW, Bastian LS, Blevins JE, Baskin DG. Immunocytochemistry and laser capture microdissection for real-time quantitative PCR identify hindbrain neurons activated by interaction between leptin and cholecystokinin. J Histochem Cytochem 56: 285–293, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 372: 425–432, 1994. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.