Abstract
A key and highly regulated enzyme that is required for the biosynthesis of monounsaturated fatty acids is stearoyl-CoA desaturase (SCD), which catalyzes the D9-cis desaturation of a range of fatty acyl-CoA substrates. The preferred substrates are palmitoyl- and stearoyl-CoA, which are converted into palmitoleoyl- and oleoyl-CoA respectively. Oleate is the most abundant monounsaturated fatty acid in dietary fat and is therefore readily available. Studies of mice that have a naturally occurring mutation in the SCD-1 gene isoform as well as a mouse model with a targeted disruption of the SCD gene (SCD-1−/−) have revealed the role of de novo synthesized oleate and thus the physiological importance of SCD-1 expression. SCD-1 deficiency results in reduced body adiposity, increased insulin sensitivity, and resistance to diet-induced obesity. The expression of several genes of lipid oxidation are upregulated, whereas lipid synthesis genes are downregulated. SCD-1 was also found to be a component of the novel metabolic response to the hormone leptin. Therefore, SCD-1 appears to be an important metabolic control point, and inhibition of its expression could be of benefit for the treatment of obesity, diabetes, and other metabolic diseases. In this article, we summarize the recent and timely advances concerning the important role of SCD in the biochemistry and physiology of lipid metabolism.
stearoyl-coa desaturase (SCD) is an endoplasmic reticulum (ER) enzyme that catalyzes the biosynthesis of monounsaturated fatty acids (MUFAs) from saturated fatty acids that are either synthesized de novo or derived from the diet. SCD in conjunction with NADH, the flavoprotein cytochrome b5 reductase, and the electron acceptor cytochrome b5 as well as molecular oxygen introduces a single double bond in a spectrum of methylene-interrupted fatty acyl-CoA substrates. The preferred substrates are palmitoyl- and stearoyl-CoA, which are then converted into palmitoleoyl- and oleoyl-CoA, respectively (15, 41). These products are the most abundant MUFAs and serve as substrates for the synthesis of various kinds of lipids, including phospholipids, triglycerides (TG), cholesteryl esters, wax esters, and alkyldiacylglycerols. Apart from being the components of lipids, MUFAs have also been implicated to serve as mediators in signal transduction and cellular differentiation, including neuronal differentiation (5, 57). Recently, oleate has been shown to regulate food intake in the brain (43), and MUFAs may also influence apoptosis and mutagenesis in some tumors (19). Thus, given the multiple roles of MUFAs, variation in stearoyl-CoA desaturase activity in mammals would be expected to affect a variety of key physiological variables, including cellular differentiation, insulin sensitivity, metabolic rate, adiposity, atherosclerosis, cancer, and obesity.
Biochemistry of Stearoyl-CoA Desaturases
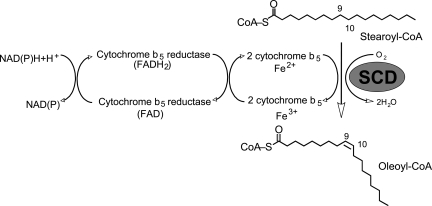
In mammals, the SCD reaction is an aerobic process requiring molecular oxygen, NAD(P)-cytochrome b5 reductase, and the electron acceptor cytochrome b5. The electrons flow from NAD(P)H via cytochrome b5 reductase, to cytochrome b5, to SCD, and finally to O2, which is reduced to H2O. The enzyme complex introduces a single double bond at the Δ9,10 postion of long-chain acyl-CoAs (Fig. 1) either from de novo synthesis or from the diet. Based on kinetic isotope data of the plant desaturase, the current hypothesis for the desaturation reaction is that the enzyme removes hydrogen atoms starting with that at the C-9 position, followed by the removal of the second hydrogen atom from the C-10 position (3, 39). This stepwise mechanism is highly specific for the position at which the double bond is introduced, implying that the C-9 and C-10 bond is accurately positioned with respect to the diiron center of the enzyme (6).
Fig. 1.
The pathway of electron transfer in the desaturation of fatty acids by stearoyl-CoA desaturase (SCD).
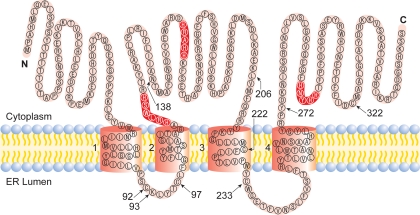
SCD-1 contains four transmembrane domains with both the NH2 and COOH termini oriented toward the cytosol. The single cytoplasmic loop and the COOH terminus contain the eight histidine (His) residues known to form the His box, which binds iron within the catalytic center of the desaturase (Fig. 2). The two ER luminal loops are relatively small compared with the cytosolic loop, which houses two of the three conserved His motifs.
Fig. 2.
Proposed model for the membrane topology of mouse SCD-1 (26). The highlighted residues represented the conserved histidine regions, which are catalytically essential. N and C represent NH2 and COOH termini, respectively. The 5 cysteines in SCD-1 are located at residues 92, 97, 222, 233, and 322. ER, endoplasmic reticulum.
Purified SCD-1 migrates as a 37-kDa band on SDS-PAGE with the His segments located at positions 119, 156, and 296. These regions are expected to be necessary to provide ligands for nonheme iron within the catalytic site of the enzyme. Unlike cytochrome b5 or cytochrome b5 reductase, SCD-1 is rapidly degraded in microsomes. The NH2-terminal 33 amino acids are sufficient to induce rapid degradation when fused to a green fluorescent protein chimera, whereas 27–358 linked to green fluorescent protein demonstrated increased stability within the ER (38). In addition to rapid protein turnover, SCD-1 gene expression is regulated by several dietary, hormonal, and environmental factors. Upon refeeding a high-carbohydrate meal, SCD-1 gene expression is rapidly induced ≤40-fold over fasting. Much of the induction is thought to be due to insulin-mediated increases in sterol regulatory element-binding protein (SREBP)-1c activation and subsequent activation of the SCD-1 gene promoter.
SCD Isoforms
The genes for SCD have been cloned from different species, including yeast, Drosophila, C. elegans, sheep, hamster, rat, mice, and human (22, 28, 40, 53). In the mouse, four isoforms (SCD-1, SCD-2, SCD-3, and SCD-4) have been identified, whereas in the rat, two isoforms have been characterized. In many different mouse strains, all of the SCD genes are localized in close proximity on chromosome 19 and code for a transcript of ∼4.9 kb. Humans express two δ-9 desaturase genes designated SCD-1 and SCD-5, with the former exhibiting nearly ubiquitous expression and the latter being restricted primarily to the brain and pancreas. The SCD-1 gene is located on chromosome 10 and spans 24 kb with six exons. Chromosome 17 contains a transcriptionally inactive SCD-1 psuedogene. SCD-5 is located at 4q21.1 and spans 169 kb with five exons and was initially identified during a genetic screen for familial cleft lip. Although the mouse isoforms share 85–88% identity at their amino acid sequence, their 5′-flanking regions differ somewhat, resulting in divergent tissue-specific gene expression (41). Under normal dietary conditions, SCD-1 mRNA is highly expressed in white adipose tissue, brown adipose tissue, meibomium gland, Harderian gland, and prepeutial gland, and SCD-1 expression is dramatically induced in liver tissue and heart in response to high-carbohydrate diet (33, 35, 37). SCD-2 is expressed predominantly in brain and is developmentally induced during the neonatal myelinating period (30). Similarly to SCD-1, SCD-2 mRNA is expressed to a lesser extent in kidney, spleen, heart, and lung, where it is induced in response to a high-carbohydrate diet. In addition, SCD-2 expression has been reported to be required for 3T3-L1 preadipocyte differentiation (10). In some tissues, such as the adipose and eyelid, both SCD-1 and SCD-2 genes are expressed, whereas in the skin, Harderian and preputial glands SCD-1, SCD-2, and SCD-3 gene isoforms are expressed. SCD-4 is expressed mainly in the heart, where it is induced by high-carbohydrate feeding and liver X receptor (LXR)α agonists but not repressed by polyunsaturated fatty acids (34). In skin, SCD-1 expression is restricted to the undifferentiated sebocytes, whereas SCD-3 is expressed mainly in the differentiated sebocytes and SCD-2 is expressed in hair follicles. Expression of mouse SCD-4 in the heart and the human SCD-1 gene both give rise to two mRNA transcripts of 3.9 and 5.2 and of 3.4 and 2.8 kb, respectively, which arise as a consequence of the two polyadenlyation signals, indicating that the two differently expressed transcripts encode the same SCD polypeptide (34). The function of the polyadenylation is not known but could be, in addition to the transcriptional control, a means by which the two transcripts differ in stability or translatability, thus allowing for rapid and efficient changes in cellular environment. The reason for having two or more SCD isoforms in the same tissue is not known but seems to be related to the substrate specificity of the isomers and their regulation by hormonal and dietary factors through tissue-specific expression.
SCD Gene Expression
The promoter of SCD-1 contains several putative transcription factor binding sites that either positively or negatively regulate SCD-1 gene transcription. Although SCD-1 expression is high in white and brown adipose tissue on a chow diet in mice, its level in liver, heart, and skeletal muscle increases dramatically with a high-carbohydrate diet. Additionally, high-saturated as well as monounsaturated-fat diets can increase SCD-1 expression, although not to the extent of the lipogenic effect of a high-carbohydrate diet (58).
SREBP-1c.
SCD-1 expression is induced by nuclear SREBP-1 via the SRE element in its promoter. There are two isoforms of SREBP-1 (SREBP-1a and -1c); SREBP-1a and -1c arise through alternative splicing of a single gene, and both are involved in the regulation of genes of fatty acid biosynthesis, whereas SREBP-2 seems to be involved more in cholesterol homeostasis. SREBPs are synthesized as immature 125-kDa ER membrane-associated proteins with two membrane-spanning domains. The COOH terminus binds to SREBP cleavage-activating protein (SCAP), and in sterol-depleted cells the SREBP-SCAP complex is then transported to the golgi membrane (3a). Once inside the golgi, site 1 and site 2 proteases sequentially cleave SREBP at two sites, thereby releasing and activating the 65-kDa mature basic helix-loop-helix transcription factor. Mature SREBP then translocates to the nucleus, where it activates transcription of target genes including fatty acid synthase and SCD-1 as well as several others, including SREBP-1 itself. However, in mice with a targeted disruption of SCD-1, there is a failure to process SREBP-1c. In fact, these mice fail to induce lipogenesis when on a high-fructose diet, and although oleate supplementation can partially restore SREBP-1c activation, lipogenesis remains suppressed relative to wild-type mice (31).
LXRs.
LXRs (LXRα and LXRβ) belong to the nuclear hormone receptor superfamily and form heterodimers with selected ligands, such as oxysterols. Upon binding with ligand, the complex activates transcription of target genes involved in reverse cholesterol transport. One such target of LXR activation is SCD-1, and treatment with the synthetic LXR ligand T0901317 has been shown to increase SCD-1 gene expression in mouse liver and human aortic endothelial cells by >10- and 3.5-fold, respectively (11, 48). The induction of SCD-1 by T0901317 increased TG secretion in WT 129S6/SvEv mice but failed to induce an increase in plasma TG in SCD-1−/− mice despite the fact that both hepatic TG content and plasma cholesterol were elevated (24). In aortic endothelial cells, LXRβ activation increased SCD-1 mRNA and protein expression, which served to protect the cells from saturated fatty acid-induced lipotoxicity. Thus, alone (11) or in conjunction with SREBP-1c, LXR activation promotes the activation of SCD-1 expression among a wide range of tissues.
Peroxisome proliferator-activated receptors.
Peroxisome proliferator-activated receptors (PPARs) are another class of nuclear receptor transcription factors that interact with LXR and retinoid X receptors. There are three isoforms, PPARα expressed in liver, kidney, heart, muscle, and adipose tissue, where it is responsible for inducing lipid catabolic genes. The normal refeeding response after fasting induces SCD-1 gene expression and activity within 12–24 h. However, in mice with a targeted deletion of PPARα, the ability to increase SCD-1 expression was completely attenuated, with only a modest increase in activity at 12 h. This effect was not due to changes in the intrinsic LXR activity but was likely attributable to a loss of LXR/PPARα dimerization (20), which has been demonstrated previously (58). Next, PPARβ/δ is ubiquitously expressed and regulates the expression of genes involved in β-oxidation. The synthetic PPARβ/δ agonist GW0742 significantly blunted the expression of SCD-1 and other lipogenic target genes in vitro (49). Finally, PPARγ is involved in adipocyte differentiation and regulates genes involved in lipid storage. Genetic deletion of both PPARγ alleles causes embryonic lethality; fortunately, the haploinsufficiency associated with heterozygous deletion increases insulin sensitivity and reduces high-fat diet-induced obesity (24, 29). The class of antidiabetic drugs known as thiazolidinediones (TZDs) acts as a synthetic ligand for PPARγ. Three major forms of TZDs are troglitazone, rosiglitazone, and pioglitazone, and all three activate PPARγ-mediated gene expression. SCD-1 is a target of PPARγ; however, unlike rosiglitazone and pioglitazone, troglitazone actually represses both SCD-1 gene and protein expression (23). It is unclear how the three TZDs are able to exert their differential effects on SCD-1 expression; however, it may be due to differences in tissue isoform expression (i.e., PPARγ1 vs. PPARγ2 vs. PPARγ3), the potency of the ligand, or differences in conformational arrangement of the different TZD ligands and PPAR isoforms.
Estrogen receptor.
The estrogen receptor is another class of nuclear hormone receptors that exists in two forms, estrogen receptor-α and estrogen receptor-β. When bound by ligand, they form either αα or ββ homodimers or αβ heterodimers to regulate transcription of a wide array of genes, including those involved in lipid metabolism. In ovariectomized rats, in which the ligand 17β-estradiol is absent, or estrogen receptor knockout mice, there is a profound increase in lipogenic gene expression in liver and adipose tissue. Under these conditions, SCD-1 expression has been shown to increase more than fivefold, with concomitant increases in adiposity and insulin resistance (7, 46). Until recently, the underlying mechanism by which estrogen reduced lipogenesis was unknown; however, Bryzgalova et al. (8) were able to demonstrate direct repression of SCD-1 promoter activity in the presence of estrogen. In addition, they were also able to demonstrate the ability of estrogen therapy in high-fat-fed mice to repress SCD-1 expression by ∼80%. However, it remains unresolved whether this is a direct effect of estrogen receptor-α binding to SCD-1 promoter or whether an indirect partner is involved.
Role of SCD-1 in Regulation of Lipid Biosynthesis
Endogenous expression of SCD-1 and its subsequent synthesis of oleate is now widely believed to be required for normal physiological function despite the fact that mammalian diets supply abundant levels of oleate. Using the asebia mouse strains (abj and ab2j) that have a naturally occurring mutation in SCD-1 (54) as well as a mouse model with a targeted disruption of SCD-1, our laboratory showed that SCD-1−/− mice are deficient in TG, cholesterol esters, wax esters, and alkyldiacylglycerols (37, 42). The levels of palmitoleate (16:1) and oleate (18:1) are reduced in the plasma and tissue lipid fractions of SCD-1−/− mice, whereas palmitate and stearate are increased. These changes are correlated with a decrease in desaturation index (18:1/18:0 or 16:1/16:0 ratio) in liver tissue and plasma.
Normally, a high-carbohydrate diet fed to mice or rats induces the expression of the hepatic SCD-1 gene and other lipogenic genes through the insulin-mediated SREBP-1c-dependent mechanism. Activation of SREBP-1c results in an increased synthesis of MUFAs and hepatic TG. One of our recent observations is that SCD-1−/− mice on a very low-fat high-carbohydrate diet (VLF) fail to process SREBP1c precursor into the mature, nuclear form. As a result, these mice fail to increase de novo lipogenesis yet are able to accumulate hepatic TG and cholesterol esters (16). Despite their improvement in plasma lipid levels, apoB clearance is diminished in SCD-1−/− mice on a VLF diet. The resultant increase in LDL retention and subsequent hypercholesterolemia lead to the appearance of lipoprotein X and cholestasis (16). However, supplementation of the VLF diet with canola oil was sufficient to restore normal lipoprotein profile. Thus, the reversal of the cholesterol phenotype after MUFA supplementation indicates the necessity of SCD-1 activity in regulating sufficient clearance of circulating lipoproteins. Finally, supplementation of the VLF diet with high levels of triolein or tripalmitolein can normalize cholesterol ester levels, but the triglyceride levels cannot be returned to the levels found in the wild-type mouse.
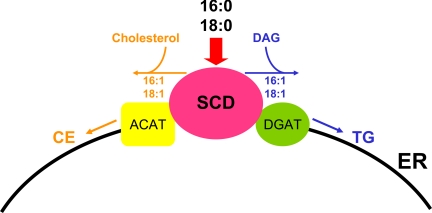
In addition, SCD-1−/− mice have very low levels of triglycerides in the very low-density lipoprotein (VLDL) and LDL fractions compared with their wild-type counterparts. Furthermore, the rate of VLDL-TG secretion, as measured by inhibition of VLDL clearance using Triton, was dramatically reduced in SCD-1−/− mice (11); however, transient transfections of an SCD-1 expression vector into Chinese hamster ovary cells result in increased SCD-1 activity and esterification of cholesterol to cholesterol esters (36). These observations reveal that endogenously synthesized MUFAs by SCD most likely serve as the main substrates for the synthesis of hepatic TG and cholesterol esters. The enzymes involved in the de novo synthesis of TG and cholesterol ester, including SCD, acyl-CoA:cholesterol acyltransferase (ACAT), diacylglycerol acyltransferase (DGAT), and microsomal glycerol phosphate acyltransferase (GPAT), are located in the ER membrane. A possible physiological explanation for the requirement of SCD expression in the synthesis of the TG and cholesterol esters is the production of more easily accessible MUFAs within the vicinity of ACAT, DGAT, and microsomal GPAT. In fact, SCD-1 was shown to colocalize with DGAT2 in the ER (25). These enzymes probably exist in a complex promoting substrate channeling and fuel partitioning into various metabolic pathways (Fig. 3). Another possibility is that the MUFAs are incorporated into the TG, but they are immediately hydrolyzed and the fatty acids oxidized.
Fig. 3.
Proposed model of channeling of endogenously synthesized monounsaturated fatty acids from SCD-1 to diacylglycerol acyltransferase (DGAT) in the synthesis of triglycerides (TG) or acyl-CoA:cholesterol acyltransferase (ACAT) in the synthesis of cholesterol esters (CE). Palmitate (16:0) and stearate (18:0) from the diet or de novo synthesis of fatty acids are desaturated by SCD-1 and channeled to DGAT or ACAT in TG or cholesterol ester synthesis in the ER, respectively.
The human SCD-1 gene structure and tissue-specific regulation is very similar to that of mouse SCD-1. Overexpression of SCD-1 in humans may be involved in the etiology of hypertriglyceridemia (HTG), atherosclerosis, and diabetes. Although there is strong evidence that many of these syndromes are heritable, the genetics of these disorders are not well understood. Recently, however, SCD-1 activity (e.g., desaturation index) was associated with familial combined hyperlipidemia. There was such a strong correlation among pedigrees that heritability was estimated at ∼48% (27). However, it is likely that HTG is a complex trait and that multiple genes influence the expression of the phenotype. In addition, the penetrance of HTG is affected by diet, insulin sensitivity, and obesity, which may mask heritability estimates.
The SCD-1−/− mouse as well as the asebia mutant mice show cutaneous abnormalities with atrophic sebaceous glands and narrow eye fissure with atrophic meibomian glands, suggesting an important role of MUFAs in skin homeostasis (37). Sebaceous glands are embedded in the skin over most of the body and are more concentrated in the scalp, face, forehead, and eyes. In the absence of sebaceous glands, mice have patchy, abnormal skin and abnormal corneas. It is known that the major function of sebaceous gland and meibomian gland is to secrete lipid complex lubricants, termed sebum and mebum, respectively. They contain, respectively, wax esters, TG, and cholesterol esters. These fluids prevent the evaporation of moisture from the skin and the eyeball. The skin and the eyelid of SCD-1−/− mice are deficient in TG, cholesterol esters, and wax esters. However, the level of cholesterol is increased (37). Thus, under the conditions of high cellular cholesterol, SCD-1 gene expression would indirectly protect the cell from the harmful effects of free cholesterol by providing MUFAs for the conversion into cholesterol ester by ACAT for storage. In addition, the presence of normal levels of MUFAs would maintain a more appropriate ratio of cholesterol to other lipids and maintain cell membrane integrity (59). Because excess free cholesterol has been known to lead to cell death, it is tempting to speculate that atrophy of the sebaceous and meibomian glands observed in the SCD-1−/− mouse may be due to an increase in the amount of cellular free cholesterol in these glands rather than the reduced levels of sebum and meibum. However, it should be noted that glycerol, but not TG, was effective in restoring stratum corneum function in asebia mice (17).
The studies of SCD gene expression in the mouse Harderian gland revealed the role of SCD in the biosynthesis of another class of lipids, the alkyl-2,3-diacylglycerol (35). The Harderian gland that was first described by Johann J. Harder toward the end of 17th century is located in the orbit of the eye (47). The major products from the gland vary between the different species of mammals. In rodents, the gland synthesizes lipids, indoles, and porphyrins, which are secreted by an exocytotic mechanism. The major lipid synthesized by the mouse Harderian gland is 1-alkyl-2,3-diacylglycerol (ADG). ADG is a lubricant of the eyeball and is crucial in facilitating the movement of the eyelid along with mebum from the meibomian gland. In our recent study, SCD-1−/− mice exhibited a deficiency in ADG and n-9 eicosenate (20:1n-9), which is the main MUFA of ADG. It was found that 20:1n-9 is an elongation product of 18:1n-9. The feeding of diets with high levels of oleate or eicosenate did not result in an increase of 20:1n-9 and failed to restore the deficiency in ADG. Therefore, endogenously synthesized oleate by SCD-1 as was observed for synthesis of TG in liver is essential for the biosynthesis of ADG and eicosenate in the mouse Harderian gland. The reasons for incorporating specific very long-chain MUFAs in the ADG of the mouse Harderian gland are not clear, but it is possible that they are required to maintain the correct physical properties of the fluids for normal eye function. There were very low levels of n-3 and n-6 polyunsaturated fatty acids in the Harderian gland, indicating that the lipids of this gland are composed mainly of saturated fatty acids and MUFAs of the n-9 series. C18:1 is a major component of the Harderian gland membranes, and a decrease in the level of C18:1 would reduce membrane fluidity of the gland. Consistent with this observation, the Harderian gland isolated from SCD-1−/− mice was very rigid, which may be due to decreased membrane fluidity. Therefore, the Harderian gland could be a useful model in studying the metabolism of MUFAs of the n-9 series and the roles they play in physiological processes. Humans do not have the Harderian gland, but because this structure is part of the retinal axis in mice, it is likely that this tissue has similar functions in the human retina. Consistent with this stipulation, high SCD expression has been reported in human retinal pigment epithelial cells (52), and its expression may play an important role in the pathophysiology of these cells.
Role of SCD-1 in Regulation of Fatty Acid Oxidation
Due to the impairment in acyl-CoA desaturation in SCD-1−/− mice, investigators sought to determine whether whole animal energy homeostasis was altered and whether dietary MUFAs would ameliorate the deficiency in desaturation. Although the growth curves of male SCD-1−/− mice were similar to that of the wild-type sibs on chow diet, a high-fat diet revealed large differences in weight gain in both males and females. On average, SCD-1−/− mice consumed 10–15% more food than wild-type mice, although they were leaner and accumulated less fat in their adipose tissue. The epididymal fat pad mass was markedly reduced in male SCD-1−/− vs. wild-type mice on a chow diet and a high-fat diet. Decreases in fat pads weights were observed in subcutaneous, mesenteric, and retroperitoneal on both chow and high-fat diets. The livers of the wild-type and SCD-1−/− mice were grossly normal and of similar mass on a chow diet. However, on a high-fat diet the livers of the wild-type were lighter in color than knockout mice, suggesting steatosis. Masses of white adipose depots in SCD-1−/− mice were universally decreased compared with wild type, regardless of diet. Thus, SCD-1−/− mice were resistant to diet-induced weight gain and fat accumulation despite increased food intake (42). Plasma leptin was measured to determine whether changes in levels of plasma leptin could account for the protection from weight gain, increased energy expenditure, and insulin sensitivity in SCD-1−/− mice. Plasma leptin was significantly reduced in SCD-1−/− mice relative to the wild-type controls. Thus, the protection from adiposity is present despite lower leptin levels in SCD-1−/− mice.
The reduction in plasma lipids among SCD-1-deficient mice is mediated in large part through increased β-oxidation. Carnitine palmitoyl-transferase I (CPT I) is the enzyme responsible for transporting activated fatty acids (fatty acyl-CoA) from the cytosol into the mitochondria, and it is a major site of regulation in the β-oxidation pathway (21). SCD-1 −/− mice have increased gene and protein expression of CPT I in skeletal muscle and liver as well as increased CPT I activity (13, 14). CPT I catalyzes the transfer of the thiol ester (CO-S) bond of the acyl-coenzyme-A (CoA-SH) to carnitine, which allows the fatty acyl-carnitine to cross the innermitochondrial membrane. Once inside the mitochondria, CPT II catalyzes the transfer of the thiol ester bond of the fatty acyl-carnitine to CoA-SH, thereby reforming the activated acyl-CoA inside the mitochondrial matrix (21). Because this process increases the flux of fatty acids into the β-oxidation pathway, it is a significant contributor to the clearance of plasma lipids.
Indirect calorimetry was used to investigate whether the resistance to weight gain was due to increased energy expenditure. SCD-1−/− mice exhibited consistently higher rates of oxygen consumption (higher metabolic rates) than their wild-type littermates throughout the light and dark cycles. It was hypothesized that the increased energy expenditure in SCD-1−/− mice was due to increased lipid catabolism. Although ketone bodies were undetectable in plasma from either strain during postprandial conditions, β-hydroxybutyrate levels were consistently much higher in knockout mice following a 4-h fast, indicating a higher rate of β-oxidation in SCD-1−/− mice. DNA microarrays were employed to identify genes whose expression was altered in the liver of SCD-1−/− mice. Two hundred mRNAs that were significantly different between the livers of SCD-1−/− and wild-type mice were identified.
Lipid oxidation genes such as acyl-CoA oxidase (ACO), very long-chain acyl-CoA dehydrogenase (VLCAD), CPT I, and fasting-induced adipocyte factor (FIAF) were upregulated, whereas lipid synthesis genes such as SREBP-1, FAS, and mitochondrial GPAT were downregulated, in SCD-1−/− mice (42). SREBP-1c is the main SREBP-1 isoform expressed in liver and regulates the expression of lipogenic genes. Insulin levels, dietary carbohydrate, fatty acids, and cholesterol regulate the SREBP-1 gene expression and protein maturation. Thus, the downregulation of SREBP-1 gene expression in SCD-1−/− mice could have numerous effects on various metabolic pathways regulated by SREBP-1. For instance, the induction of SREBP-1 by insulin greatly enhances the synthesis and secretion of TG by the liver. However, in the SCD-1−/− mouse, carbohydrate feeding fails to induce SREBP-1 and lipogenic gene expression to the same level found in wild-type mice (4). The signals generated in SCD-1−/− mice that lead to reduced lipogenesis are currently being studied.
CPT, ACO, VLCAD, and FIAF are known targets of PPARα (47) and contain PPARα response regions in their promoters (47). Since PPARα mRNA level is unchanged, the upregulation of enzymes of fatty acid β-oxidation in the SCD-1−/− mice must be downstream of PPARα transcription. Thus, the characteristics exhibited by the SCD-1−/− mice are consistent with presence of a PPARα activator with reduced activity in wild-type mice. The SCD-1−/− mice exhibit an increase in the contents of saturated fatty acids (C16:0 and C18:0; Fig. 4), whereas the contents of the polyunsaturated fatty acids of the n-6 and n-3 are not changed. Changes in the levels of saturated fats may indirectly stimulate the burning of fat by mitochondria. The other possible mechanism is that SCD-1 deficiency leads to an alteration in the levels of lipid metabolites that activate the PPARα. However, when the SCD-1−/− mice were crossed with the PPARα-null mice to generate a double-knockout mouse (SCD-1−/−/PPARα−/−), the fatty acid β-oxidation genes remained elaveted, suggesting that the activation of these genes in the SCD-1−/− mice is independent of PPARα (47). Although increased fat oxidation in the SCD-1−/− could have an advantage in promoting decrease in body weight, the deficiency could also increase free radical species, a potential sequelae of increased oxidative metabolism. The generation of free radicals in these mice is currently being studied.
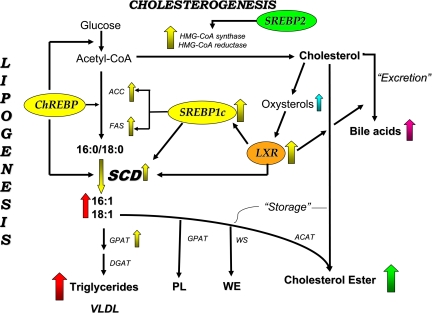
Fig. 4.
Regulation of SCD-1 expression during de novo lipogenesis. Excess glucose converted to acetyl-CoA is channeled into de novo lipid synthesis through the products of sterol regulatory element-binding protein (SREBP)-1c and liver X receptor (LXR) activity. Increased SREBP-1c and LXR activity upregulate SCD-1 gene expression, whereby the conversion of 16:0 and 18:0 to 16:1 and 18:1 is increased. The products of SCD-1 activity are incorporated into phospholipids (PL), wax esters (WE), and CE. HMG-CoA, 3-hydroxy-3-methylglutaryl coenzyme A; ACC, acetyl-CoA carboxylase; FAS, fatty acid synthase; GPAT, glycerophosphate acyltransferase; WS, wax ester synthase.
If SCD-1 is blocked, cells may no longer generate normal levels of monounsaturated fats, which are required for normal synthesis of VLDL and TG (Fig. 5). If that happens, saturated fatty acids might build up. Scientists reported in the 1970s that fatty acids inhibit acetyl-CoA carboxylase-1 and -2 (ACC1 and ACC2, respectively), which produce malonyl-CoA. Such a buildup of saturated fatty acids might act to decrease malonyl-CoA levels and stimulate fatty acid import into mitochondria and in turn the burning of fat. Malonyl-CoA is known to inhibit the catabolism of fats, and one possibility is that somehow SCD-1 controls malonyl-CoA levels. In this mechanism the saturated fatty acyl-CoAs would allosterically inhibit ACC, thus reducing cellular levels of malonyl-CoA (52, 53). Malonyl-CoA is required for fatty acid biosynthesis and also inhibits the mitochondrial CPT shuttle system, the rate-limiting step in the import and oxidation of fatty acids in mitochondria (54, 55). Thus, reduced levels of SCD-1 would lead to a decrease in the cellular levels of malonyl-CoA and derepress fatty acid oxidation. The findings in the SCD-1−/− mice are therefore similar to those observed in mice lacking ACC2, which also have increased fatty acid oxidation in skeletal muscle and possess a lean phenotype (56).
Fig. 5.
The effect of loss of SCD on lipid metabolism and fuel storage. In the presence of SCD-1, hepatic saturated fatty acids (SFA) are converted into monounsaturated fatty acids, which increase SREBP-1c maturation, lipogenesis, and TG/CE synthesis. In the absence of SCD-1 (SCD-1−/−), the inability to desaturate hepatic SFA leads to an inability to upregulate de novo lipogenesis via SREBP-1c and storage as TG. Therefore, β-oxidation and uncoupling (not shown) increase to decrease TG and VLDL.
Other mechanisms that could account for the increased energy expenditure in the SCD-1-deficient mice have been reported. For instance, SCD-1 deficiency has been shown to be associated with increased activity of AMP-activated protein kinase, an enzyme that stimulates fatty acid oxidation following leptin administration. (57). Another possibility is that a deficiency in SCD-1 would alter phospholipid composition, thereby impacting on membrane properties or signal transduction. SCD-1 deficiency could also be associated with direct or indirect effects on uncoupling proteins that are involved in thermogenesis. Finally, the fur and lipid abnormalities associated with defects in SCD-1-deficient mice could be associated with increased energy dissipation.
Role of SCD-1 in Regulation of Carbohydrate Metabolism
Reduced adipose tissue mass could elicit either insulin resistance or insulin sensitivity, as demonstrated in several animal models. Fasting insulin levels are lower in the male SCD-1−/− mice on chow diet compared with the wild-type mice. On a high-fat diet, insulin levels are similar between the two groups. Fasting glucose levels were similar between the SCD-1−/− and wild-type mice. However, after a 30-min glucose load, both male and female SCD-1−/− mice tend to have lower fasting plasma glucose levels and show improved glucose tolerance compared with wild-type mice. In addition, the glucose-lowering effect of insulin is greater in the SCD-1−/− mice than in wild-type mice. Recently, it was demonstrated that loss of SCD-1 function in mice leads to increased basal state tyrosine phosphorylation of insulin receptor (IR), insulin receptor substrate (IRS)-1, and IRS-2 in muscle (50). There are several mechanisms by which SCD-1 deficiency could lead to increased basal tyrosine autophosphorylation of the IR despite lower levels of plasma insulin in the SCD-1−/− mice. The mechanism that is consistent with the current results is that loss of SCD-1 function resulted in the downregulation of the expression of the protein-tyrosine phosphatase (PTP), an enzyme that catalyzes the rapid dephosphorylation of the IR and IRS-1 (50). The downregulation of the PTP-1B expression and activity is responsible for the sustained IR autophosphorylation despite reduced levels of plasma insulin in the SCD-1−/− mice. Insulin-mediated glucose uptake was also higher in the soleus muscle from SCD-1−/−, suggesting that the insulin receptor is more responsive to insulin in the SCD-1−/− than in SCD-1+/+ mice. Consistent with these observations, PTP-1B knockout mice exhibit increased tyrosine phosphorylation of the IR and IRS-1 in muscle (50). The PTP-1B−/− mice also show increased insulin sensitivity and are also resistant to diet-induced obesity. Thus, the phenotypes exhibited by the PTP-1B−/− mice in many ways are similar to those of the SCD-1−/− mice (50). It is not known at present whether PTP-1B is a downstream target of SCD-1 expression or whether the decrease observed in expression is a secondary consequence of altered lipid homeostasis (e.g., due to changes in intracellular lipid levels) as a result of SCD-1 deficiency. Thus, SCD-1−/− mice have increased insulin sensitivity despite their apparent lipodystrophy. The other possible mechanism that could lead to increased insulin signaling is that an alteration in the properties of the cell membrane, which is composed largely of lipid, activates the IR. Oleate is the major MUFA found in membrane phospholipids, and the ratio of saturated to monounsaturated fatty acids has been implicated in alteration of membrane fluidity (45). It is proposed that the decrease in the MUFA content of the membrane phospholipids in the SCD-1−/− mice is compensated by polyunsaturated fatty acids, causing a greater increase in membrane fluidity due to the presence of more double bonds in the fatty acyl chain. Recent data show that the degree of insulin resistance in rodents and humans is inversely correlated to the amount of polyunsaturated fatty acids within skeletal muscle phospholipid (45). The increased membrane fluidity would enhance insulin receptor aggregation, thus increasing its phosphorylation upon insulin binding. However, more studies will be required to demonstrate a direct correlation between insulin sensitivity and membrane fluidity.
The series of protein phosphorylations on the signaling molecules downstream of the IR culminates in the uptake of glucose into cells by the glucose transporter GLUT4. The mechanism by which GLUT4-containing vesicles become activated and dock at the plasma membrane remains a controversial issue. However, Akt/PKB activated by phosphatidylinositol (PI) 3-kinase phosohorylation has been implicated in the process. Akt also appears to participate in the insulin-signaling pathway by phosphorylating glycogen synthase kinase-3 to promote glycogen synthesis via glycogen synthase. Increased association of PI 3-kinase with IRS-1 and IRS-2 and increased serine and threonine phosphorylation of total Akt, as well as a significant increase in both total and active forms of glycogen synthase, were found in the muscle of SCD-1−/− mice. The glycogen content was higher in the muscle of SCD-1−/− mice, and histological analysis of muscle tissue revealed the presence of more glycogen granules in SCD-1−/− mice than in wild type. These results indicate that increased insulin signaling followed by increased glucose transport and increased glycogen synthesis ultimately leads to increased glycogen accumulation in the muscle of SCD-1−/− mice. Interestingly, it was also reported that muscle glycogen phosphorylase activity was also higher in the SCD-1−/− mice (50). Increased glycogen phosphorylase activity would have been expected to promote glycogenolysis. However, overexpression of glycogen phosphorylase in cultured human skeletal muscle cells increased the level of glycogen synthase (2), which suggests that the amount of glycogen synthase and phosphorylase is controlled in a reciprocal manner by glycogen levels. Thus, the increase in both glycogen synthase and phosphorylase activities would also imply that there is increased glycogen metabolism in the SCD-1−/− mice. This observation together with our previous results showing that SCD-1−/− mice have a higher metabolic rate and oxygen consumption in both the dark and light cycles (42) provide strong evidence that SCD plays a major role in lipid and carbohydrate metabolism.
Finally, with the use of tissue-specific deletion in mice, the contribution of hepatic SCD-1 has been assessed (32). In this study, the physiological role of SCD-1 in liver by creating liver-specific Scd1-knockout (LKO) mice was investigated. It was found that LKO mice were protected from high-carbohydrate, but not high-fat, diet-induced adiposity and liver steatosis. Consistent with the prevention of carbohydrate-induced adiposity, LKO mice displayed a marked decrease in the rate of lipogenesis with decreased expression of SREBP-1c and carbohydrate response element-binding protein and its target genes. Liver-specific SCD-1 deficiency also caused a severe impairment of gluconeogenesis, resulting in hypoglycemia and depletion of lipogenic carbohydrate metabolites such as G6P and X5P. These results demonstrate a vital role of hepatic SCD-1 in carbohydrate-induced adiposity and lipogenesis. The studies also exemplify how SCD-1 and oleate levels may communicate with glucose to regulate hepatic gluconeoegenesis and energy homeostasis.
Conclusion
The vast majority of our understanding of SCD is derived from rodent models; however, several recent investigations have attempted to utilize the plasma desaturation index (MUFA/saturated fatty acid ratio) in humans. A recent review summarized the role of SCD-1 in human metabolic disease (51), where there appears to be an inverse relationship between 18:1/18:0 or 16:1/16:0 and insulin sensitivity (1, 12, 44, 55, 56). Humans express two isoforms of SCD (SCD-1 and SCD-5), yet only SCD-1 is thought to contribute to desaturation in lipogenic tissues. Finally, little is known with respect to the substrate specificity (i.e., 18:0 vs. 16:0) within various tissues, and this is of direct importance because recent investigations have established a role for adipose-derived palmitoleic acid (16:1n7) as a lipokine that regulates whole body lipid metabolism (9).
The recent studies using the knockout mouse models have revealed the phenotypes generated as a result of SCD-1 gene deficiency. It is reported here and through recent reviews on SCD that this enzyme is critical in the biosynthesis of TG, cholesterol esters, wax esters, and ADG. It is also suggested that SCD-1 deficiency either directly or indirectly induces a signal that partitions fatty acids toward oxidation rather than synthesis. SCD-1 deficiency leads to leanness and increased metabolic rate and insulin sensitivity. SCD-1 is a component of the novel metabolic response of leptin signaling, upregulates insulin-signaling components, and affects glycogen metabolism. Therefore, SCD-1 appears to be an important metabolic control point and is emerging as a promising therapeutic target that could be used in the treatment of obesity, diabetes, and other metabolic diseases.
GRANTS
This work was supported by Grants DK-62388 (to J. M. Ntambi) and DK-007665 (to C. M. Paton) from the National Institute of Diabetes and Digestive and Kidney Diseases.
REFERENCES
- 1.Attie AD, Krauss RM, Gray-Keller MP, Brownlie A, Miyazaki M, Kastelein JJ, Lusis AJ, Stalenhoef AF, Stoehr JP, Hayden MR, Ntambi JM. Relationship between stearoyl-CoA desaturase activity and plasma triglycerides in human and mouse hypertriglyceridemia. J Lipid Res 43: 1899–1907, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Baque S, Guinovart JJ, Gomez-Foix AM. Overexpression of muscle glycogen phosphorylase in cultured human muscle fibers causes increased glucose consumption and nonoxidative disposal. J Biol Chem 271: 2594–2598, 1996. [DOI] [PubMed] [Google Scholar]
- 3.Behrouzian B, Savile CK, Dawson B, Buist PH, Shanklin J. Exploring the hydroxylation-dehydrogenation connection: novel catalytic activity of castor stearoyl-ACP delta-9 desaturase. J Am Chem Soc 124: 3277–3283, 2002. [DOI] [PubMed] [Google Scholar]
- 3a.Bengoechea-Alonso MT, Ericsson J. SREBP in signal transduction: cholesterol metabolism and beyond. Curr Opin Cell Biol 19: 215–222, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Biddinger SB, Almind K, Miyazaki M, Kokkotou E, Ntambi JM, Kahn CR. Effects of diet and genetic background on sterol regulatory element-binding protein-1c, stearoyl-CoA desaturase 1, and the development of the metabolic syndrome. Diabetes 54: 1314–1323, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Bradley RL, Fisher FM, Maratos-Flier E. Dietary fatty acids differentially regulate production of TNF-alpha and IL-10 by murine 3T3-L1 adipocytes. Obesity (Silver Spring) 16: 938–944, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broadwater JA, Laundre BJ, Fox BG. Desaturation of trans-octadecenoyl-acyl carrier protein by stearoyl-acyl carrier protein delta9 desaturase. J Inorg Biochem 78: 7–14, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Bryzgalova G, Gao H, Ahren B, Zierath JR, Galuska D, Steiler TL, Dahlman-Wright K, Nilsson S, Gustafsson JA, Efendic S, Khan A. Evidence that oestrogen receptor-alpha plays an important role in the regulation of glucose homeostasis in mice: insulin sensitivity in the liver. Diabetologia 49: 588–597, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Bryzgalova G, Lundholm L, Portwood N, Gustafsson JA, Khan A, Efendic S, Dahlman-Wright K. Mechanisms of antidiabetogenic and body weight-lowering effects of estrogen in high-fat diet-fed mice. Am J Physiol Endocrinol Metab 295: E904–E912, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 134: 933–944, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christianson JL, Nicoloro S, Straubhaar J, Czech MP. Stearoyl-CoA desaturase 2 is required for peroxisome proliferator-activated receptor gamma expression and adipogenesis in cultured 3T3-L1 cells. J Biol Chem 283: 2906–2916, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Chu K, Miyazaki M, Man WC, Ntambi JM. Stearoyl-coenzyme A desaturase 1 deficiency protects against hypertriglyceridemia and increases plasma high-density lipoprotein cholesterol induced by liver X receptor activation. Mol Cell Biol 26: 6786–6798, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corpeleijn E, Feskens E, Jansen E, Mensink M, Saris W, de Bruin T, Blaak E. Improvements in glucose tolerance and insulin sensitivity after lifestyle intervention are related to changes in serum fatty acid profile and desaturase activities: the SLIM study. Diabetologia 49: 2392–2401, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Dobrzyn P, Dobrzyn A, Miyazaki M, Cohen P, Asilmaz E, Hardie DG, Friedman JM, Ntambi JM. Stearoyl-CoA desaturase 1 deficiency increases fatty acid oxidation by activating AMP-activated protein kinase in liver. Proc Natl Acad Sci USA 101: 6409–6414, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dobrzyn P, Sampath H, Dobrzyn A, Miyazaki M, Ntambi JM. Loss of stearoyl-CoA desaturase 1 inhibits fatty acid oxidation and increases glucose utilization in the heart. Am J Physiol Endocrinol Metab 294: E357–E364, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Enoch HG, Catala A, Strittmatter P. Mechanism of rat liver microsomal stearyl-CoA desaturase. Studies of the substrate specificity, enzyme-substrate interactions, and the function of lipid. J Biol Chem 251: 5095–5103, 1976. [PubMed] [Google Scholar]
- 16.Flowers MT, Groen AK, Oler AT, Keller MP, Choi Y, Schueler KL, Richards OC, Lan H, Miyazaki M, Kuipers F, Kendziorski CM, Ntambi JM, Attie AD. Cholestasis and hypercholesterolemia in SCD1-deficient mice fed a low-fat, high-carbohydrate diet. J Lipid Res 47: 2668–2680, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Fluhr JW, Mao-Qiang M, Brown BE, Wertz PW, Crumrine D, Sundberg JP, Feingold KR, Elias PM. Glycerol regulates stratum corneum hydration in sebaceous gland deficient (asebia) mice. J Invest Dermatol 120: 728–737, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Hardy S, Langelier Y, Prentki M. Oleate activates phosphatidylinositol 3-kinase and promotes proliferation and reduces apoptosis of MDA-MB-231 breast cancer cells, whereas palmitate has opposite effects. Cancer Res 60: 6353–6358, 2000. [PubMed] [Google Scholar]
- 20.Hebbachi AM, Knight BL, Wiggins D, Patel DD, Gibbons GF. Peroxisome proliferator-activated receptor alpha deficiency abolishes the response of lipogenic gene expression to re-feeding: restoration of the normal response by activation of liver X receptor alpha. J Biol Chem 283: 4866–4876, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Jogl G, Hsiao Y, Tong L. Structure and function of carnitine acyltransferases. Ann NY Acad Sci 1033: 17–29, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Kaestner KH, Ntambi JM, Kelly TJ Jr, Lane MD. Differentiation-induced gene expression in 3T3-L1 preadipocytes. A second differentially expressed gene encoding stearoyl-CoA desaturase. J Biol Chem 264: 14755–14761, 1989. [PubMed] [Google Scholar]
- 23.Kim YC, Gomez FE, Fox BG, Ntambi JM. Differential regulation of the stearoyl-CoA desaturase genes by thiazolidinediones in 3T3-L1 adipocytes. J Lipid Res 41: 1310–1316, 2000. [PubMed] [Google Scholar]
- 24.Kubota N, Terauchi Y, Miki H, Tamemoto H, Yamauchi T, Komeda K, Satoh S, Nakano R, Ishii C, Sugiyama T, Eto K, Tsubamoto Y, Okuno A, Murakami K, Sekihara H, Hasegawa G, Naito M, Toyoshima Y, Tanaka S, Shiota K, Kitamura T, Fujita T, Ezaki O, Aizawa S, Nagai R, Tobe K, Kimura S, Kadowaki T. PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol Cell 4: 597–609, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Man WC, Miyazaki M, Chu K, Ntambi J. Colocalization of SCD1 and DGAT2: implying preference for endogenous monounsaturated fatty acids in triglyceride synthesis. J Lipid Res 47: 1928–1939, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Man WC, Miyazaki M, Chu K, Ntambi JM. Membrane topology of mouse stearoyl-CoA desaturase 1. J Biol Chem 281: 1251–1260, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Mar-Heyming R, Miyazaki M, Weissglas-Volkov D, Kolaitis NA, Sadaat N, Plaisier C, Pajukanta P, Cantor RM, de Bruin TW, Ntambi JM, Lusis AJ. Association of stearoyl-CoA desaturase 1 activity with familial combined hyperlipidemia. Arterioscler Thromb Vasc Biol 28: 1193–1199, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mihara K Structure and regulation of rat liver microsomal stearoyl-CoA desaturase gene. J Biochem 108: 1022–1029, 1990. [DOI] [PubMed] [Google Scholar]
- 29.Miles PD, Barak Y, He W, Evans RM, Olefsky JM. Improved insulin-sensitivity in mice heterozygous for PPAR-gamma deficiency. J Clin Invest 105: 287–292, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyazaki M, Dobrzyn A, Elias PM, Ntambi JM. Stearoyl-CoA desaturase-2 gene expression is required for lipid synthesis during early skin and liver development. Proc Natl Acad Sci USA 102: 12501–12506, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyazaki M, Dobrzyn A, Man WC, Chu K, Sampath H, Kim HJ, Ntambi JM. Stearoyl-CoA desaturase 1 gene expression is necessary for fructose-mediated induction of lipogenic gene expression by sterol regulatory element-binding protein-1c-dependent and -independent mechanisms. J Biol Chem 279: 25164–25171, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Miyazaki M, Flowers MT, Sampath H, Chu K, Otzelberger C, Liu X, Ntambi JM. Hepatic stearoyl-CoA desaturase-1 deficiency protects mice from carbohydrate-induced adiposity and hepatic steatosis. Cell Metab 6: 484–496, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Miyazaki M, Gomez FE, Ntambi JM. Lack of stearoyl-CoA desaturase-1 function induces a palmitoyl-CoA Delta6 desaturase and represses the stearoyl-CoA desaturase-3 gene in the preputial glands of the mouse. J Lipid Res 43: 2146–2154, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Miyazaki M, Jacobson MJ, Man WC, Cohen P, Asilmaz E, Friedman JM, Ntambi JM. Identification and characterization of murine SCD4, a novel heart-specific stearoyl-CoA desaturase isoform regulated by leptin and dietary factors. J Biol Chem 278: 33904–33911, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Miyazaki M, Kim HJ, Man WC, Ntambi JM. Oleoyl-CoA is the major de novo product of stearoyl-CoA desaturase 1 gene isoform and substrate for the biosynthesis of the Harderian gland 1-alkyl-2,3-diacylglycerol. J Biol Chem 276: 39455–39461, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Miyazaki M, Kim YC, Gray-Keller MP, Attie AD, Ntambi JM. The biosynthesis of hepatic cholesterol esters and triglycerides is impaired in mice with a disruption of the gene for stearoyl-CoA desaturase 1. J Biol Chem 275: 30132–30138, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Miyazaki M, Man WC, Ntambi JM. Targeted disruption of stearoyl-CoA desaturase1 gene in mice causes atrophy of sebaceous and meibomian glands and depletion of wax esters in the eyelid. J Nutr 131: 2260–2268, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Mziaut H, Korza G, Ozols J. The N terminus of microsomal delta-9 stearoyl-CoA desaturase contains the sequence determinant for its rapid degradation. Proc Natl Acad Sci USA 97: 8883–8888, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagai J, Bloch K. Synthesis of oleic acid by Euglena gracilis. J Biol Chem 240: C3702–C3703, 1965. [PubMed] [Google Scholar]
- 40.Ntambi JM, Buhrow SA, Kaestner KH, Christy RJ, Sibley E, Kelly TJ Jr, Lane MD. Differentiation-induced gene expression in 3T3-L1 preadipocytes. Characterization of a differentially expressed gene encoding stearoyl-CoA desaturase. J Biol Chem 263: 17291–17300, 1988. [PubMed] [Google Scholar]
- 41.Ntambi JM Regulation of stearoyl-CoA desaturase by polyunsaturated fatty acids and cholesterol. J Lipid Res 40: 1549–1558, 1999. [PubMed] [Google Scholar]
- 42.Ntambi JM, Miyazaki M, Stoehr JP, Lan H, Kendziorski CM, Yandell BS, Song Y, Cohen P, Friedman JM, Attie AD. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc Natl Acad Sci USA 99: 11482–11486, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L. Central administration of oleic acid inhibits glucose production and food intake. Diabetes 51: 271–275, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Paillard F, Catheline D, Duff FL, Bouriel M, Deugnier Y, Pouchard M, Daubert JC, Legrand P. Plasma palmitoleic acid, a product of stearoyl-coA desaturase activity, is an independent marker of triglyceridemia and abdominal adiposity. Nutr Metab Cardiovasc Dis 18: 436–440, 2008. [DOI] [PubMed] [Google Scholar]
- 45.Pan DA, Hulbert AJ, Storlien LH. Dietary fats, membrane phospholipids and obesity. J Nutr 124: 1555–1565, 1994. [DOI] [PubMed] [Google Scholar]
- 46.Paquette A, Wang D, Jankowski M, Gutkowska J, Lavoie JM. Effects of ovariectomy on PPAR alpha, SREBP-1c, and SCD-1 gene expression in the rat liver. Menopause 15: 1169–1175, 2008. [DOI] [PubMed] [Google Scholar]
- 47.Payne AP The harderian gland: a tercentennial review. J Anat 185: 1–49, 1994. [PMC free article] [PubMed] [Google Scholar]
- 48.Peter A, Weigert C, Staiger H, Rittig K, Cegan A, Lutz P, Machicao F, Haring HU, Schleicher E. Induction of stearoyl-CoA desaturase protects human arterial endothelial cells against lipotoxicity. Am J Physiol Endocrinol Metab 295: E339–E349, 2008. [DOI] [PubMed] [Google Scholar]
- 49.Qin X, Xie X, Fan Y, Tian J, Guan Y, Wang X, Zhu Y, Wang N. Peroxisome proliferator-activated receptor-delta induces insulin-induced gene-1 and suppresses hepatic lipogenesis in obese diabetic mice. Hepatology 48: 432–441, 2008. [DOI] [PubMed] [Google Scholar]
- 50.Rahman SM, Dobrzyn A, Dobrzyn P, Lee SH, Miyazaki M, Ntambi JM. Stearoyl-CoA desaturase 1 deficiency elevates insulin-signaling components and down-regulates protein-tyrosine phosphatase 1B in muscle. Proc Natl Acad Sci USA 100: 11110–11115, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sampath H, Ntambi JM. Role of stearoyl-CoA desaturase in human metabolic disease. Future Lipidology 3: 163–173, 2008. [Google Scholar]
- 52.Samuel W, Kutty RK, Nagineni S, Gordon JS, Prouty SM, Chandraratna RA, Wiggert B. Regulation of stearoyl coenzyme A desaturase expression in human retinal pigment epithelial cells by retinoic acid. J Biol Chem 276: 28744–28750, 2001. [DOI] [PubMed] [Google Scholar]
- 53.Stukey JE, McDonough VM, Martin CE. The OLE1 gene of Saccharomyces cerevisiae encodes the delta 9 fatty acid desaturase and can be functionally replaced by the rat stearoyl-CoA desaturase gene. J Biol Chem 265: 20144–20149, 1990. [PubMed] [Google Scholar]
- 54.Sundberg JP, Boggess D, Sundberg BA, Eilertsen K, Parimoo S, Filippi M, Stenn K. Asebia-2J [Scd1(ab2J)]: a new allele and a model for scarring alopecia. Am J Pathol 156: 2067–2075, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Warensjö E, Risérus U, Vessby B. Fatty acid composition of serum lipids predicts the development of the metabolic syndrome in men. Diabetologia 48: 1999–2005, 2005. [DOI] [PubMed] [Google Scholar]
- 56.Warensjö E, Ingelsson E, Lundmark P, Lannfelt L, Syvänen AC, Vessby B, Risérus U. Polymorphisms in the SCD1 gene: associations with body fat distribution and insulin sensitivity. Obesity (Silver Spring) 15: 1732–1740, 2007. [DOI] [PubMed] [Google Scholar]
- 57.Yonezawa T, Haga S, Kobayashi Y, Katoh K, Obara Y. Unsaturated fatty acids promote proliferation via ERK1/2 and Akt pathway in bovine mammary epithelial cells. Biochem Biophys Res Commun 367: 729–735, 2008. [DOI] [PubMed] [Google Scholar]
- 58.Yue L, Ye F, Gui C, Luo H, Cai J, Shen J, Chen K, Shen X, Jiang H. Ligand-binding regulation of LXR/RXR and LXR/PPAR heterodimerizations: SPR technology-based kinetic analysis correlated with molecular dynamics simulation. Protein Sci 14: 812–822, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang S, Skalsky Y, Garfinkel DJ. MGA2 or SPT23 Is required for transcription of the delta9 fatty acid desaturase gene, OLE1, and nuclear membrane integrity in Saccharomyces cerevisiae. Genetics 151: 473–483, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]