Abstract
The enzymes acyl-coenzyme A (CoA):cholesterol acyltransferases (ACATs) are membrane-bound proteins that utilize long-chain fatty acyl-CoA and cholesterol as substrates to form cholesteryl esters. In mammals, two isoenzymes, ACAT1 and ACAT2, encoded by two different genes, exist. ACATs play important roles in cellular cholesterol homeostasis in various tissues. This chapter summarizes the current knowledge on ACAT-related research in two areas: 1) ACAT genes and proteins and 2) ACAT enzymes as drug targets for atherosclerosis and for Alzheimer's disease.
Keywords: acyl-coenzyme A:cholesterol acyltransferase inhibitors, atherosclerosis, Alzheimer's disease
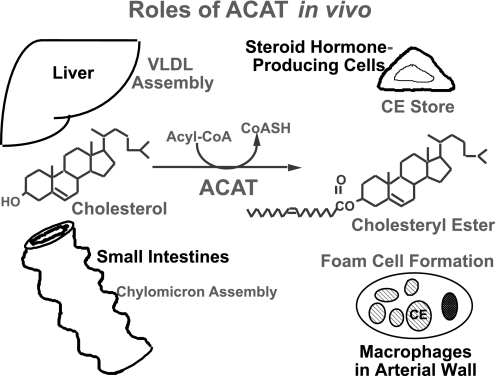
cholesterol is a lipid molecule present in the membranes of all mammalian cells and is needed for their growth and viability. Excess cellular cholesterol is stored as cholesteryl esters. The conversion of cholesterol to cholesteryl esters (CE) is catalyzed by the enzyme acyl-coenzyme A (CoA):cholesterol acyltransferase (ACAT) (Fig. 1). In most cell types, CE are present only in low levels, mainly as cytoplasmic lipid droplets. In plasma, CE are part of the neutral lipid cargo present in the intestinal chylomicrons and in the hepatic very low-density lipoproteins (VLDL). In steroidogenic tissues such as adrenals, CE serve as the cholesterol reservoir for producing steroid hormones. In the disease atherosclerosis, chronic accumulation of CE in macrophages causes these cells to appear foamy and is a hallmark of early stages in atherosclerosis. For reasons described above, ACAT has been considered as a drug target for therapeutic intervention against atherosclerosis and other human diseases (Fig. 1).
Fig. 1.
Roles of acyl-CoA:cholesterol acyltransferase (ACAT) in vivo (courtesy of Prof. Akira Miyazaki, Showa University). CE, cholesteryl ester; CoASH, free reduced CoA.
ACAT Genes and Proteins
Gene ACAT1 and its mRNAs.
The first ACAT gene was identified in 1993 (16). The ACAT family consists of ACAT1, ACAT2 (3, 10, 67, 93, 100), and acyl-CoA:diacylglycerol acyltransferase 1 (DGAT1) (11). These enzymes are founding members of the membrane-bound O-acyltransferase (MBOAT) enzyme family. MBOATs are multispan membrane enzymes that utilize long-chain or medium-chain fatty acyl-CoA and a hydrophobic substance as their substrates (39). These enzymes contain an invariant histidine (H) within a long stretch of hydrophobic residues and a near-invariant asparagine (N) within a stretch of modestly hydrophilic residues (39, 94). In addition to ACAT and DGAT1, the additional MBOATs include grelin octanoyl-CoA acyltransferase (38, 94) and lysophospholipid acyltransferases (reviewed in Ref. 81). Human Acat1 (hAcat1) is located on two different chromosomes, chromosomes 1 and 7, with each chromosome containing a distinct promoter: P1, contiguous with exons 1–16, is located in chromosome 1; and P7, contiguous with the optional long exon Xa, is located in chromosome 7 (55). This novel arrangement is almost unprecedented in the mammalian genome and is species specific. In mice, although the Acat1 gene is also located in chromosome 1, it does not contain the optional exon Xa present in hAcat1. The gene Acat1 is not under the control of the transcription factors sterol regulatory element-binding proteins, which control the expression of many genes involved in cellular lipid metabolism (33). In response to cellular cholesterol depletion/repletion, the alteration of ACAT1 activity occurs within 1–2 h (15), i.e., at a time frame much faster than what is needed for transcriptional control mechanism(s) to be in full operation.
There are at least three human Acat1 mRNAs. The 2.8- and 3.6-knt Acat1 mRNAs contain a short 5′-untranslated region (UTR) and comprise most (70–80%) of the total Acat1 mRNAs that are produced from the hAcat1 P1 promoter. These mRNAs are translated as a single 50-kDa protein band in SDS-PAGE, and the resultant protein is the major ACAT1 isoenzyme observed in various human cells and tissues (14). The 4.3-knt Acat1 mRNA instead contains the alternate long 5′-UTR. This mRNA is produced from two different chromosomes by a novel RNA recombination event. The 5′-UTR contains an alternative initiation codon and an extended in-frame sequence, enabling the chimeric mRNA to produce a larger ACAT1 isoform. In SDS-PAGE, it is detectable as a 56-kDa protein. In human macrophages, the 56-kDa protein is present as a minor ACAT protein (96). The physiological function of the 56-kDa ACAT1 is currently unknown.
In macrophage-like cells, the expression of hAcat1 is upregulated by interferon-γ, a cytokine that exerts many proatherosclerotic effects in model animals (25, 95). Urotensin II, the potent vasoconstrictor peptide that can lead to hypertention and atherosclerosis in humans, upregulates hAcat1 expression in human macrophages (89). In addition, the synthetic glucocorticoid dexamethasone, known to promote atherosclerosis in humans (47), upregulates hAcat1 expression in macrophages. Consistent with this observation, a glucocorticoid response element is present within the hAcat1 P1 promoter (97). In contrast, hAcat1 expression is downregulated by adiponectin, an adipocytokine that exerts many antiatherosclerotic effects in cell culture studies (32). Together, these results support the concept that upregulation of Acat1 expression in monocytes and macrophages is closely associated with the initiation and progression of the atherosclerosis in tissue culture and in experimental animal models and perhaps in humans.
Biochemical and cellular properties of ACAT1.
Native ACAT1 is an integral membrane protein present in minute quantities in the endoplasmic reticulum (ER). The recombinant 50-kDa human ACAT1 overexpressed in Chinese hamster ovary (CHO) cells has been purified to homogeneit, with retention of catalytic activity (17). The enzyme responds to cholesterol as its substrate in a sigmoidal manner (17). Additional kinetic evidence has suggested the presence of a sterol substrate site and a sterol activator site in ACAT1. Cholesterol is superior to oxysterols and to any other sterol as an activator for ACAT1. Upon activation by cholesterol, ACAT1 activity is much increased, and the enzyme is promiscuous toward a variety of sterols with a 3-β-OH configuration in the B ring as its substrates (60, 102). These results demonstrate that ACAT1 is allosterically activated by cholesterol.
ACAT1 exists as a homotetrameric enzyme in intact cells and in vitro (99). Deleting the dimer-forming motif near the NH2 terminus converts the enzyme to a fully functionally active dimeric form (101). An additional motif(s) involved in forming the other dimer is located near the COOH terminus of the enzyme (35). ACAT1 contains one disulfide linkage and seven free cysteines (Cys). Guo et al. (36) employed the membrane-impermeable, sulfhydryl-specific reagent polyethylene glycol5000-maleimide (PEG5000-mal) to map the disulfide linkage and to probe the environment of the free sulfhydryls of the enzyme. Their results showed that the first free Cys is located near the NH2 terminus in the cytoplasmic side of the ER membrane. The other six free Cys are buried within several transmembrane domains (TMD) of the protein (36). None of the free Cys is required for the ACAT1 enzyme activity (62); the disulfide linkage is important for structural stability of the enzyme (36). Regarding the ACAT1 topography, initially, Lin et al. (58) used the method of epitope tagging and indirect immunofluorescence in fixed intact cells. Their results suggested that ACAT1 might contain at least 7 TMDs; the region at the COOH-terminal half is very hydrophobic and might contain additional membrane imbedded segment(s). More recently, Guo et al. (37) used PEG5000-mal together with Cys-scanning mutagenesis to further investigate the ACAT1 membrane topography at the COOH-terminal half and proposed a revised nine-TMD model. The active site His460 is located in a hydrophobic environment, within a newly disclosed TMD (TMD no. 7), between R443-Y462 (Fig. 2). In contrast to the nine-TMD model, Joyce et al. (46) had proposed a five-TMD model for ACAT1. The five-TMD model was deduced by monitoring the sidedness of the tag at the end of the COOH terminus of the protein after successive deletions from the ACAT1 COOH terminus to produce various truncated ACAT1s. Similarly to the nine-TMD model, the five-TMD model also proposes the existence of the first four TMDs near the NH2-terminal half and the last TMD near the COOH terminus. However, the five-TMD model predicts that no other TMD exists and that all of the following Cys are located at the cytoplasmic side of the ER membrane: C333, C345, C365, C387, and C467. This prediction is incompatible with the data by Guo et al. (36), who showed that all of these Cys are resistant to modification by the membrane-impermeable reagent PEG5000-mal. To explain the discrepancy in ACAT1 membrane topography determination, a clue has been suggested (37); it is possible that the method of successive truncation(s) from the COOH terminus of ACAT1 employed by Joyce et al. (46) might have created major structural alteration(s) within the ACAT1 polypeptide. In support of this suggestion, Guo et al. (37) have indicated that, in their experience, even the modest truncation(s) from the COOH terminus of ACAT1 led to total inactivation of ACAT1 enzyme activity.
Fig. 2.
Membrane topography of ACAT1, redrawn with modification from Ref. 37. The residues in blue are conserved between ACAT1 and ACAT2; residues in red are conserved between ACAT and diacylglycerol:acyltransferase 1 (DGAT1). Transmembrane domain (TMD)7 (R443–E462) and TMD8 (A463–G483) form part of the subunit interaction surface. The NH2-terminal domain responsible for dimerization of ACAT1 is within residues 43–84 (101). Native cysteines are in gray; the active site His460 within TMD7 is in red. ER, endoplasmic reticulum.
TMD7 (amino acids 443-461) and TMD8 (amino acids 463-483) of ACAT1 are rich in coiled domains and have two distinct functional sides. One side is involved in subunit interaction (to form an ACAT1 dimer); the other side of the coils, including the conserved F453, A457, H460, and F479, is involved in substrate binding and/or in catalysis. Residues F453, A457, and F479 may be part of a lipid-binding tunnel that interacts with the hydrophobic part of cholesterol, whereas residue H460 may interact with the 3-β-OH moiety of the sterol and serve as a general base in catalysis (35). It has been suggested that having the active site of ACAT1 located within the plane of the ER membrane allows the enzyme to serve a dual function; CE generated within the lipid bilayer can leave the cytoplasmic leaflet of the membrane to form lipid droplets or be recruited to the lumen of the membrane for the VLDL assembly process (21). Kinetic evidence from Guo et al. (34) suggests that the highly conserved residue FYXDWWN (403-409 in hACAT1) may be involved in binding fatty acyl-CoA. Recently, Das et al. (23) showed that, in addition to H460, S456 and D400 are also essential for ACAT1 enzyme activity. These authors suggested that ACAT1 may contain thioesterase activity, and this activity may involve the classical Ser/Asp/His catalytic triad, similar to the serine-dependent hydrolases. The results described above were all based on site-specific mutagenesis studies. For a given enzyme, a protein structural model is needed to carry out functional analysis at the refined biochemical level. However, at present, the crystal structure of ACAT1 or any related family member is not available.
Regulation and properties of ACAT2.
The second mammalian ACAT gene, designated as Acat2, was identified through its homology to Acat1 (3, 10, 67) The hAcat2 mRNA encodes a single 46-kDa protein on SDS-PAGE. ACAT2 shares high homology with ACAT1 near the COOH terminus but not near the NH2 terminus. The enzymological properties of the two proteins are similar but not identical (2, 18, 60). hAcat2 promoter contains cis elements for the transcription factors Cdx2 and HNF1α. Both Cdx2 and HNF1α are required to stimulate the expression of hAcat2 (84). HNF1α is functionally expressed in a variety of tissues, including hepatocytes, pancreas, kidney, stomach, and intestinal epithelium, whereas Cdx2 is an intestine-specific transcription factor. Under normal conditions, high expression of Cdx2 is restricted to the differentiated cells of the intestinal villi. In adult livers, in human hepatocytes, and in the human liver cell line L02, the Cdx2 mRNA level is very low, and the amount of ACAT2 mRNA was <10% that of the ACAT1 mRNA. In contrast, various human hepatocellular carcinoma tumor cells contain elevated Cdx2. Notably, in the hepatoma cell line HepG2, due to elevated Cdx2 expression, the amount of ACAT2 mRNA is comparable with that of the ACAT1 mRNA (84).
ACAT2 is also an integral membrane protein. However, its membrane topography significantly differs from that of ACAT1. Regarding the ACAT2 topography, Lin et al. (59) inserted two different tags at various hydrophilic regions and expressed the recombinant proteins in mutant CHO cells lacking endogenous ACAT. Each tagged ACAT2 was expressed in the ER and was shown to be at least partially enzymatically active. They then used double cytoimmunofluorescence and protease protection assays to monitor the sidedness of the tags along the ER membranes (59). Their results suggest that ACAT2 may contain only two detectable TMDs but may also contain several additional membrane domains that are only partially embedded in the lipid bilayer. The conserved H434, equivalent to H460 in hACAT1, may be buried in the cytoplasmic side of the ER membrane (Fig. 3). In contrast to the two-TMD model, Joyce et al. (46) reported that ACAT2 contained five TMDs. They prepared a series of the recombinant ACAT2 proteins successively truncated from the COOH termini after each of the predicted TMDs; the topography was determined by monitoring the membrane sidedness of tags at the COOH termini of each truncated fusion protein. Both the insertion approach used by Lin et al. (59) and the truncation approach used by Joyce et al. (46) run the risk of altering the membrane topography of ACAT2. Lin et al. (59) did report that each tagged ACAT2 employed was at least partially active enzymatically, whereas Joyce et al. (46) did not report the percentage of ACAT activity remaining in any of the truncated proteins employed. In the future, to resolve the discrepancy, other methods for membrane topography determination that produce minimal structural perturbations of ACAT2 will be needed. However, a major discrepancy that cannot be explained by differential structural perturbation(s) of ACAT2 exists between the result of Lin et al. (59) and the result of Joyce et al. (46). Lin et al. (59) showed that the COOH terminus is in the cytoplasmic side of the ER, whereas Joyce et al. (46) showed that the COOH terminus is in the luminal side of the ER. To determine the location of the COOH terminus, both groups used the full-length ACAT2 cDNA construct as the starting material and placed certain tags near or at the COOH terminus of the protein. Both groups expressed the tagged protein by transient transfection in the same host cell line and then used cytoimmunofluorescence to examine the sidedness of the tag after detergent permeabilization. The results obtained sharply contradicted each other. Lin et al. (59) used the double immunostaining procedure for cytoimmunofluorescence and made sure that the two signals colocalized when viewed under microscopy. In contrast, Joyce et al. (46) did not use the double immunostaining procedure for their studies. Lin et al. (59) offered a clue that might explain the result obtained by Joyce et al. (46). The transfection procedure (used by both groups) tends to produce leaky and/or dying cells; such cells can amount to ≤5% of the total cell population and tend to be preferentially stained (nonspecifically) by antibodies. When cytoimmunofluorescence is employed in transiently transfected cells, the use of double immunofluorescence is essential; it enables the investigators to focus on viewing the transfected cells that express the gene product and to avoid the use of leaky/dying cells for data collection. Other explanations are also possible.
Fig. 3.
Membrane topography of ACAT2, redrawn with modification from (59). The topography was determined by employing cytoimmunofluorescence after the protein with the antigenic HA tag was inserted at various positions (indicated by the arrows). The hydrophobic segment that contains the active site His434 (red) may be located within the cytoplasmic side of the membrane and is highlighted in yellow.
The relative expression of ACAT1 and ACAT2 in humans and in animals.
In adult humans, studies carried out by Lee et al. (51) and by Chang et al. (13) using a combination of immunological staining, immunodepletion, and immunoblot analyses showed that ACAT1 is expressed in many different tissues and cell types, including hepatocytes and Kupffer cells of the liver, adrenal glands, neurons, and macrophages, and accounts for >80% of the total ACAT enzyme activity measured in vitro (18, 51, 80). In a separate study, Parini et al. (69) performed similar immunoblot analyses and demonstrated the presence of both ACAT1 and ACAT2 in adult human hepatocytes. These investigators then examined the ACAT enzyme activities in vitro in liver biopsy samples and showed that, among individual samples, the ACAT2 activities were highly variable, whereas the ACAT1 activities were relatively constant. Under the enzyme assay condition employed by these investigators, in three of the four liver biopsy samples examined, ACAT2 provided >50% of the total cholesterol-esterifying activity (69). It should be noted that the biopsy samples employed by these investigators were all collected from patients affected with gallstone disease; none was from a healthy subject. In another study, Smith et al. (83) performed real-time PCR analyses of Acat1 and Acat2 and showed that the ACAT1 mRNAs were on average ninefold (range of 1.7- to 167-fold) more abundant than the ACAT2 mRNAs. Among the 17 individual liver samples examined, the ACAT1 mRNA levels were relatively constant, whereas the ACAT2 mRNA levels were much more heterogeneous. From these studies, it is clear that both ACAT1 and ACAT2 are present in human hepatocytes, although the quantitative aspects of results were different. Together, these studies suggest that the expression of ACAT2 might be inducible under various conditions, whereas the expression of ACAT1 tended to be constitutive. This possibility is supported by the findings that, in activated human macrophages and in advanced atherosclerotic plaques, low but significant amounts of ACAT2 mRNA and protein are detectable (79). In addition, the liver samples of certain patients affected with hepatocellular carcinoma contain highly elevated ACAT2 mRNAs (84). It is also possible that subpopulations of humans may express hepatic ACAT2 at elevated levels constitutively. Further investigations are needed to test this possibility. In intestines, ACAT2 is shown to be the major isoenzyme and is expressed mainly in the apices of the intestinal villi (13, 51); its mRNA in duodenum is approximately threefold more abundant than that of ACAT1 (83).
In mice and in monkeys, the ACAT1/ACAT2 distribution in various tissues is similar to that in humans except for at least one important difference, that ACAT2 is highly expressed in the livers of mice and monkeys under normal conditions (53, 64, 69). Available data show that in rabbits and mice the total ACAT enzyme activities in vitro (ACAT1 and ACAT2) are much higher than those in the human livers (discussed in Ref. 18). In African green monkeys, the hepatic ACAT2 activities are at least fourfold higher than those found in humans, whereas the hepatic ACAT1 activities in these two species are very similar (69). Regarding the roles of ACAT1 and ACAT2 in hepatic lipoprotein synthesis and secretion, in various model systems examined, both ACAT1 and ACAT2 are capable of synthesizing CE that contribute to the lipid core in VLDL (45, 56, 85). In mouse hepatocytes, ACAT2 is the major isoform and plays a key role in the hepatic storage and packaging of CE into apoB-containing lipoproteins (6, 8, 52, 90). In human hepatocytes, both ACAT1 and ACAT2 are present. Whether ACAT1 and/or ACAT2 participate in the biosynthesis of CE in VLDL in intact human livers is yet to be determined. In mouse intestines, ACAT2 plays a key role in providing CE to chylomicrons (8, 72). Whether the same situation holds true in humans has not been determined.
Acat1 knockout and Acat2 knockout mice.
Farese (28) created and characterized both the Acat1−/− and Acat2−/− mice. These mice have served as valuable research tools in lipoprotein and atherosclerosis research (reviewed in Ref. 9).
ACAT and cellular cholesterol trafficking.
Cells acquire cholesterol mainly via two sources, from the low-density lipoprotein (LDL) and from de novo biosynthesis. Cholesterol molecules from both sources move within the cells, eventually reaching the ER; certain portions of them are converted to CE. In most of the cell types examined, ACAT1 plays the major role in catalyzing the reesterification process. The process of cellular cholesterol trafficking has been reviewed in Chang et al. (19), with more recent studies cited in Urano et al. (87).
ACATs as Drug Targets
General properties of the ACAT inhibitors.
During the last several decades, numerous ACAT inhibitors have been synthesized and tested in test tubes, intact cells, animal models, and humans. We refer to two earlier reviews that summarize the structural characteristics and properties of the ACAT inhibitors (48, 82). A few of them are structural analogs of the long-chain fatty acyl-CoA. Almost all of the ACAT inhibitors are very hydrophobic compounds (40). The hydrophobic nature of these compounds may allow them to enter the membrane lipid bilayer such that they can interact with residues that play an essential role(s) in substrate binding and/or in enzyme catalysis. Interestingly, several natural substances present in the ordinary foodstuffs, such as esculeogenin A in tomatoes (31) and piperine in peppers (63), exhibit significant ACAT inhibitory activities. In addition, a peptide segment within the apo-serum amyloid A isoform 2.1 inhibits ACAT activity in macrophages (26).
The isotype-specific ACAT inhibitors.
Most of the ACAT inhibitors developed in the 1990s inhibit both ACAT1 and ACAT2 without much selectivity. After the ACAT genes were identified, investigators began to develop ACAT isotype-selective inhibitors (22, 50, 68). One of the most notable examples is pyripyropene A, which prefers to inhibit ACAT2 over ACAT1 by >1,000-fold (68). Notably, glutamine (Q) 492, valine (V) 493, and serine (S) 494 of ACAT2 were shown to play an important role(s) for mediating the pyripyropene A inhibition (24). (The numberings of these amino acids are according to the monkey ACAT2 sequence.) The QVS sequence is part of the ACAT2 COOH-terminal segment. According to the model by Lin et al. (59), the COOH-terminal segment is located at the cytoplasmic side of the ER membrane; in contrast, according to the model by Joyce et al. (46), the COOH-terminal segment is located at the luminal side of the ER membrane. The QVS sequence is unique in ACAT2; its counterpart cannot be identified in ACAT1. A second notable ACAT isozyme-selective inhibitor called K 604 was developed by Kowa. K 604 prefers to inhibit ACAT1 over ACAT2 by >100-fold (43). The molecular basis that allows the preferential action of K 604 on ACAT1 is yet to be determined.
The pros and cons of the ACAT inhibitors to combat atherosclerosis.
Whether ACAT inhibitors will serve as effective antiatherosclerosis drugs is currently under debate (see Refs. 12, 54, 66, 76, and 77 for different opinions). This review focuses on the effects of inhibiting ACAT in macrophages, aortic smooth muscle cells, and hepatocytes. The formation of CE-laden foam cells in the intimal layer of the arterial wall is an early event in atherogenesis (75). In humans, clinical observations suggest that the lipid-rich plaques are more prone to plaque rupture than the lipid-poor plaques (27, 57). Lipid-rich plaques consist of macrophages and smooth muscle cells (75). In foamy macrophages, ACAT1 is the major isoenzyme (65). Cell culture studies suggest that foam cell formation in smooth muscle cells also depends on ACAT1 (65, 74). In model animals, administration of the isotype-nonselective ACAT inhibitor CI 1011 (5) or F-1394 (49) at low concentration, or the ACAT1-selective inhibitor K 604 (43), reduced the CE content within the atherosclerotic lesions and caused plaque regression without altering the overall serum cholesterol levels. In addition, in a rabbit model for atherosclerosis, the combination of statin and CI 1011 synergistically reduced and stabilized the atherosclerotic plaques (92). However, when tested in human patients, CI 1011 was ineffective in potentiating the effect of statins to decrease the progression of coronary atherosclerosis (66). The latter result cannot be interpreted definitively because it is clouded by drug-drug interaction. CI 1011 has been shown to induce one of the cytochrome P450 enzymes, called CYP 3A4, in human hepatocytes through activation of the pregnane X receptor (78); CYP 3A4 could accelerate the catabolism of statins in vivo. Thus, the coadministration of CI 1011 and statin might allow CI 1011 to diminish the effective concentration of statin in vivo and weaken its effect.
The results of a mouse genetic approach showed that complete deletion of ACAT1 gene in macrophages actually increases the lesion size of the plaques (1, 29, 86). The cellular nature of the lesions of ACAT1-knockout mice is abnormal; these lesions are poor in macrophages and abundant in smooth muscle cells. The outcome of the ACAT1-knockout study implies that an overdose of ACAT1 inhibitor may cause the macrophages to become incompetent in fulfilling their functions as scavenger cells. Other results that disfavor the use of ACAT inhibitor as drug therapy for atherosclerosis include the finding that, when rodent macrophage cell lines were grown under conditions with minimal cellular cholesterol efflux, addition of ACAT inhibitor to the cells caused undesirable buildup of cellular free (unesterified) cholesterol, rendering the cells apoptotic (30, 88). These studies suggest that ACAT inhibitors can be highly toxic to macrophage cells present in the advanced atherosclerotic lesions. It should be pointed out that the cholesterol-dependent apoptosis observed in mouse macrophages cannot be observed in human macrophages (73). In addition, in contrast to the results in rodent macrophages, Rong et al. (74) showed that ACAT1 inhibition in rat and human aortic smooth muscle cells is nontoxic and retards foam cell formation. The discrepancy in results described above might be due to difference in the cell systems employed.
Regarding the effects of inhibiting ACAT in the liver, in animals (rodents, rabbits, and monkeys), ACAT2 is the major isoform. In various animal models tested, the ACAT isotype nonselective inhibitor CI 1011 (alone, without statin) significantly reduced serum cholesterol levels (reviewed in Ref. 61). In addition, deletion of ACAT2 gene in mice results in resistance to diet-induced hypercholesterolemia and to gallstone formation (8, 72), and it also results in protection against atherosclerosis (52, 90). Another study showed that, in a hyperlipidemic mouse model (apoB100 only, LDLr−/−), the in vivo administration of liver-specific antisense oligos against the ACAT2 mRNA reduced ACAT2 protein content only in the liver but not in the intestines; it also successfully reduced serum cholesterol level (4). The latter result suggests that blocking ACAT2 activity in the liver can significantly lower serum cholesterol levels and reduce atherosclerosis in a manner independent of dietary cholesterol absorption. On the other hand, when tested in human trials, administration of the ACAT inhibitor CI 1011 reduced VLDL cholesterol and triglyceride levels but failed to significantly reduce the LDL cholesterol levels (44, 61). These results suggest that the effects of CI 1011 on serum cholesterol levels are species dependent. To provide a possible explanation for these observations, as discussed earlier, the overall ACAT enzyme activity in human livers is much lower than that in rodents, rabbits, and monkeys (20, 69). Thus, hepatic ACAT in humans may not contribute to the CE content present in LDL as much as it does in experimental animal models. The expression of hepatic ACAT2 in humans seems to be highly heterogeneous; inhibiting ACAT2 may effectively reduce the CE content in LDL in subpopulations with elevated hepatic ACAT2. This possibility could be tested in the future.
ACAT inhibitors and Alzheimer's disease.
Alzheimer's disease is the most prevalent neurodegenerative disease in developed countries. It is characterized by massive extracellular accumulation of amyloid plaques, composed mainly of amyloid-β (A-β) peptide aggregates, and intracellular accumulation of hyperphosphorylated tau protein in the cerebellar area. These events lead to widespread neurodegeneration in the brain. Age and genetic variation in apolipoprotein E, a plasma cholesterol transport protein, are two of the most important risk factors for Alzheimer's disease (71, 98). In addition, studies suggest that cholesterol in membranes impacts on production of A-β (reviewed in Ref. 91). Amyloid precursor protein (APP) can be cleaved via two competing pathways, the α- and the β-secretase pathways, that are distinguished by the subcellular site of proteolysis and the site of cleavage within APP. Under normal conditions, several proteases are capable of catalyzing the α-cleavage, after which the γ-secretase complex, which includes presenilin as a catalytic subunit, further cleaves the APP fragment to produce small nonamyloidogenic fragments. Similarly, but competing with the α-cleavage pathway, the β-secretase pathway involves sequential cleavages by the β-secretase and γ-secretase complexes, which generates A-β. A-β is secreted from the cell to the extracellular space, where it aggregates over time. In the central nervous system, neurons are the major sources of A-β production. Evidence suggests that the activities of α-secretase, β-secretase, and γ-secretase are dependent on cholesterol metabolism. In CHO cells and various neuron-like cells grown in culture, reduction of CE either by genetic inactivation of Acat1 or by pharmacological inhibition of ACAT decreases A-β secretion (70). In addition, in a mouse model of Alzheimer's disease, ACAT inhibitors CP-113,818 and CI 1011 substantially diminishes amyloid plaque density (41, 42). Although the exact mechanism by which ACAT inhibitors reduce amyloid plaques remains to be explored, these exciting findings suggest that ACAT inhibitors may serve as effective therapeutic or chemopreventive agents for a certain form(s) of Alzheimer's disease.
Summary
ACAT1 and ACAT2 play key roles in cellular cholesterol metabolism. Understanding the functions of the ACAT enzymes has greatly increased the knowledge pertaining to cellular cholesterol homeostasis. Abnormalities in lipoprotein/cholesterol metabolism may lead to atherosclerosis. Although the statin drugs have significantly reduced the mortality of patients suffering from coronary heart disease, the risk of death among those with coronary heart disease remains high. As discussed in this chapter, inhibiting ACAT1 and ACAT2 may complement the action of statins by directly or indirectly diminishing foamy macrophage formation, thus further reducing the incidence of atherosclerotic cardiovascular disease. More recent evidence suggests that inhibiting ACAT enzyme activity may also ameliorate the symptoms of Alzheimer's disease. Further development of ACAT inhibitors with better pharmacological properties (such as those that do not cause drug-drug interactions with statins) may prove fruitful for the better treatment of cardiovascular disease and neurodegenerative diseases.
GRANTS
This work was supported by grants from the National Heart, Lung, and Blood Institute (HL-60306 and HL-36705 to T. Y. Chang) and from Ministry of Science and Technology of China (No. 2006CB910600 to B. L. Li).
Acknowledgments
We thank Stephanie Murphy for editing the manuscript.
REFERENCES
- 1.Accad M, Smith S, Newland DL, Sanan DA, King LE Jr, Linton MF, Fazio S, Farese RV Jr. Massive xanthomatosis and altered composition of atherosclerotic lesions in hyperlipidemic mice lacking acyl CoA:cholesterol acyltransferase 1. J Clin Invest 105: 711–719, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.An S, Cho KH, Lee WS, Lee JO, Paik YK, Jeong TS. A critical role for the histidine residues in the catalytic function of acyl-CoA:cholesterol acyltransferase catalysis: evidence for catalytic difference between ACAT1 and ACAT2. FEBS Lett 580: 2741–2749, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Anderson RA, Joyce C, Davis M, Reagan JW, Clark M, Shelness GS, Rudel LL. Identification of a form of acyl-CoA:cholesterol acyltransferase specific to liver and intestine in nonhuman primates. J Biol Chem 273: 26747–26754, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Bell TA 3rd, Brown JM, Graham MJ, Lemonidis KM, Crooke RM, Rudel LL. Liver-specific inhibition of acyl-coenzyme a:cholesterol acyltransferase 2 with antisense oligonucleotides limits atherosclerosis development in apolipoprotein B100-only low-density lipoprotein receptor−/− mice. Arterioscler Thromb Vasc Biol 26: 1814–1820, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Bocan TM, Krause BR, Rosebury WS, Mueller SB, Lu X, Dagle C, Major T, Lathia C, Lee H. The ACAT inhibitor avasimibe reduces macrophages and matrix metalloproteinase expression in atherosclerotic lesions of hypercholesterolemic rabbits. Arterioscler Thromb Vasc Biol 20: 70–79, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Brown JM, Bell TA 3rd, Alger HM, Sawyer JK, Smith TL, Kelley K, Shah R, Wilson MD, Davis MA, Lee RG, Graham MJ, Crooke RM, Rudel LL. Targeted depletion of hepatic ACAT2-driven cholesterol esterification reveals a non-biliary route for fecal neutral sterol loss. J Biol Chem 283: 10522–10534, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buhman KF, Accada M, Farese RV Jr. Mammalian acyl-CoA:cholesterol acyltransferases. Biochim Biophys Acta 1529: 142–154, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Buhman KK, Accad M, Novak S, Choi RS, Wong JS, Hamilton RL, Turley S, Farese RV Jr. Resistance to diet-induced hypercholesterolemia and gallstone formation in ACAT2-deficient mice. Nat Med 6: 1341–1347, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Buhman KK, Chen HC, Farese RV Jr. The enzymes of neutral lipid synthesis. J Biol Chem 276: 40369–40372, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Cases S, Novak S, Zheng YW, Myers H, Lear SR, Sande E, Welch CB, Lusis AJ, Spencer TA, Krause BR, Erickson SK, Farese RV Jr. ACAT-2, a second mammalian acyl-CoA:cholesterol acyltransferase. Its cloning, expression, and characterization. J Biol Chem 273: 26755–26764, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Cases S, Smith SJ, Zheng YW, Myers HM, Lear SR, Sande E, Novak S, Collins C, Welch CB, Lusis AJ, Erickson SK, Farese RV Jr. Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc Natl Acad Sci USA 95: 13018–13023, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang C, Dong R, Miyazaki A, Sakashita N, Zhang Y, Liu J, Guo M, Li BL, Chang TY. Human acyl-CoA:cholesterol acyltransferase (ACAT) and its potential as a target for pharmaceutical intervention against atherosclerosis. Acta Biochim Biophys Sin (Shanghai) 38: 151–156, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Chang CC, Sakashita N, Ornvold K, Lee O, Chang ET, Dong R, Lin S, Lee CY, Strom SC, Kashyap R, Fung JJ, Farese RV Jr, Patoiseau JF, Delhon A, Chang TY. Immunological quantitation and localization of ACAT-1 and ACAT-2 in human liver and small intestine. J Biol Chem 275: 28083–28092, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Chang CC, Chen J, Thomas MA, Cheng D, Del Priore VA, Newton RS, Pape ME, Chang TY. Regulation and immunolocalization of acyl-coenzyme A: cholesterol acyltransferase in mammalian cells as studied with specific antibodies. J Biol Chem 270: 29532–29540, 1995. [DOI] [PubMed] [Google Scholar]
- 15.Chang CC, Doolittle GM, Chang TY. Cycloheximide sensitivity in regulation of acyl coenzyme A:cholesterol acyltransferase activity in Chinese hamster ovary cells. 1. Effect of exogenous sterols. Biochemistry 25: 1693–1699, 1986. [DOI] [PubMed] [Google Scholar]
- 16.Chang CC, Huh HY, Cadigan KM, Chang TY. Molecular cloning and functional expression of human acyl-coenzyme A:cholesterol acyltransferase cDNA in mutant Chinese hamster ovary cells. J Biol Chem 268: 20747–20755, 1993. [PubMed] [Google Scholar]
- 17.Chang CC, Lee CY, Chang ET, Cruz JC, Levesque MC, Chang TY. Recombinant human acyl-CoA:cholesterol acyltransferase 1 (ACAT1) purified to essential homogeneity utilizes cholesterol in mixed micelles or vesicles in a highly cooperative manner. J Biol Chem 273: 35132–35141, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Chang CC, Sakashita N, Ornvold K, Lee O, Chang ET, Dong R, Lin S, Lee CY, Strom SC, Kashyap R, Fung JJ, Farese RV Jr, Patoiseau JF, Delhon A, and Chang TY. Immunological quantitation and localization of ACAT-1 and ACAT-2 in human liver and small intestine. J Biol Chem 275: 28083–28092, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Chang TY, Chang CC, Ohgami N, Yamauchi Y. Cholesterol sensing, trafficking, and esterification. Annu Rev Cell Dev Biol 22: 129–157, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Chang TY, Chang CC, Lin S, Yu C, Li BL, Miyazaki A. Roles of acyl-coenzyme A:cholesterol acyltransferase-1 and -2. Curr Opin Lipidol 12: 289–296, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Chang TY, Chang CC, Lu XH, Lin S. ACAT catalysis may be completed within the plane of the membrane: a working hypothesis. J Lipid Res 42: 1933–1938, 2001. [PubMed] [Google Scholar]
- 22.Cho KH, An S, Lee WS, Paik YK, Kim YK, Jeong TS. Mass-production of human ACAT-1 and ACAT-2 to screen isoform-specific inhibitor: a different substrate specificity and inhibitory regulation. Biochem Biophys Res Commun 309: 864–872, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Das A, Davis MA, Rudel LL. Identification of putative active site residues of ACAT enzymes. J Lipid Res 49: 1770–1781, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das A, Davis MA, Tomoda H, Omura S, Rudel LL. Identification of the interaction site within acyl-CoA:cholesterol acyltransferase 2 for the isoform-specific inhibitor pyripyropene A. J Biol Chem 283: 10453–10460, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daugherty A, Webb NR, Rateri DL, King VL. Thematic review series: The immune system and atherogenesis. Cytokine regulation of macrophage functions in atherogenesis. J Lipid Res 46: 1812–1822, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Ely S, Bonatesta R, Ancsin JB, Kindy M, Kisilevsky R. The in-vitro influence of serum amyloid A isoforms on enzymes that regulate the balance between esterified and un-esterified cholesterol. Amyloid 8: 169–181, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Falk E, Shah P, Fuster V. Coronary plaque disruption. Circulation 92: 657–671, 1995. [DOI] [PubMed] [Google Scholar]
- 28.Farese RV Jr. Acyl-CoA:cholesterol acyltransferase genes and knockout mice. Curr Opin Lipidol 9: 119–124, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Fazio S, Major AS, Swift LL, Gleaves LA, Accad M, Linton MF, Farese RV Jr. Increased atherosclerosis in LDL receptor-null mice lacking ACAT1 in macrophages. J Clin Invest 107: 163–171, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng B, Yao PM, Li Y, Devlin CM, Zhang D, Harding HP, Sweeney M, Rong JX, Kuriakose G, Fisher EA, Marks AR, Ron D, Tabas I. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol 5: 781–792, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Fujiwara Y, Kiyota N, Hori M, Matsushita S, Iijima Y, Aoki K, Shibata D, Takeya M, Ikeda T, Nohara T, Nagai R. Esculeogenin A, a new tomato sapogenol, ameliorates hyperlipidemia and atherosclerosis in ApoE-deficient mice by inhibiting ACAT. Arterioscler Thromb Vasc Biol 27: 2400–2406, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Furukawa K, Hori M, Ouchi N, Kihara S, Funahashi T, Matsuzawa Y, Miyazaki A, Nakayama H, Horiuchi S. Adiponectin down-regulates acyl-coenzyme A:cholesterol acyltransferase-1 in cultured human monocyte-derived macrophages. Biochem Biophys Res Commun 317: 831–836, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Goldstein JL, DeBose-Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell 124: 35–46, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Guo Z, Cromley D, Billheimer JT, Sturley SL. Identification of potential binding sites in yeast and human acyl-CoA sterol acyltransferases by mutagenesis of conserved sequences. J Lipid Res 42: 1282–1291, 2001. [PubMed] [Google Scholar]
- 35.Guo ZY, Chang CC, Chang TY. Functionality of the seventh and eighth transmembrane domains of acyl-coenzyme A:cholesterol acyltransferase 1. Biochemistry 46: 10063–10071, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Guo ZY, Chang CC, Lu X, Chen J, Li BL, Chang TY. The disulfide linkage and the free sulfhydryl accessibility of acyl-coenzyme A:cholesterol acyltransferase 1 as studied by using mPEG5000-maleimide. Biochemistry 44: 6537–6546, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Guo ZY, Lin S, Heinen JA, Chang CC, Chang TY. The active site His-460 of human acyl-coenzyme A:cholesterol acyltransferase 1 resides in a hitherto undisclosed transmembrane domain. J Biol Chem 280: 37814–37826, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Gutierrez JA, Solenberg PJ, Perkins DR, Willency JA, Knierman MD, Jin Z, Witcher DR, Luo S, Onyia JE, Hale JE. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci USA 105: 6320–6325, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hofmann K A superfamily of membrane-bound O-acyltransferases with implications for Wnt signaling. Trends Biochem Sci 25: 111–112, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Homan R, Hamelehle KL. Influence of membrane partitioning on inhibitors of membrane-bound enzymes. J Pharm Sci 90: 1859–1867, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Hutter-Paier B, Huttunen HJ, Puglielli L, Eckman CB, Kim DY, Hofmeister A, Moir RD, Domnitz SB, Frosch MP, Windisch M, Kovacs DM. The ACAT inhibitor CP-113,818 markedly reduces amyloid pathology in a mouse model of Alzheimer's disease. Neuron 44: 227–238, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Huttunen HJ, Kovacs DM. ACAT as a drug target for Alzheimer's disease. Neurodegener Dis 5: 212–214, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ikenoya M, Yoshinaka Y, Kobayashi H, Kawamine K, Shibuya K, Sato F, Sawanobori K, Watanabe T, Miyazaki A. A selective ACAT-1 inhibitor, K-604, suppresses fatty streak lesions in fat-fed hamsters without affecting plasma cholesterol levels. Atherosclerosis 191: 290–297, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Insull W Jr, Koren M, Davignon J, Sprecher D, Schrott H, Keilson LM, Brown AS, Dujovne CA, Davidson MH, McLain R, Heinonen T. Efficacy and short-term safety of a new ACAT inhibitor, avasimibe, on lipids, lipoproteins, and apolipoproteins, in patients with combined hyperlipidemia. Atherosclerosis 157: 137–144, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Iqbal J, Rudel LL, Hussain MM. Microsomal triglyceride transfer protein enhances cellular cholesteryl esterification by relieving product inhibition. J Biol Chem 283: 19967–19980, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joyce CW, Shelness GS, Davis MA, Lee RG, Skinner K, Anderson RA, Rudel LL. ACAT1 and ACAT2 membrane topology segregates a serine residue essential for activity to opposite sides of the endoplasmic reticulum membrane. Mol Biol Cell 11: 3675–3687, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kalbak K Incidence of arteriosclerosis in patients with rheumatoid arthritis receiving long-term corticosteroid therapy. Ann Rheum Dis 31: 196–200, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krause BR, Auerbach BJ, Bocan TM. Direct vascular targets for atherosclerosis prevention. Curr Med Chem 1: 39–46, 2001. [Google Scholar]
- 49.Kusunoki J, Hansoty D, Aragane K, Fallon J, Badimon J, Fisher EA. Acyl-CoA:cholesterol acyltransferase inhibition reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation 103: 2604–2609, 2001. [DOI] [PubMed] [Google Scholar]
- 50.Lada AT, Davis M, Kent C, Chapman J, Tomoda H, Omura S, Rudel LL. Identification of ACAT1- and ACAT2-specific inhibitors using a novel, cell-based fluorescence assay: individual ACAT uniqueness. J Lipid Res 45: 378–386, 2004. [DOI] [PubMed] [Google Scholar]
- 51.Lee O, Chang CC, Lee W, Chang TY. Immunodepletion experiments suggest that acyl-coenzyme A:cholesterol acyltransferase-1 (ACAT-1) protein plays a major catalytic role in adult human liver, adrenal gland, macrophages, and kidney, but not in intestines. J Lipid Res 39: 1722–1727, 1998. [PubMed] [Google Scholar]
- 52.Lee RG, Kelley KL, Sawyer JK, Farese RV Jr, Parks JS, Rudel LL. Plasma cholesteryl esters provided by lecithin:cholesterol acyltransferase and acyl-coenzyme a:cholesterol acyltransferase 2 have opposite atherosclerotic potential. Circ Res 95: 998–1004, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Lee RG, Willingham MC, Davis MA, Skinner KA, Rudel LL. Differential expression of ACAT1 and ACAT2 among cells within liver, intestine, kidney, and adrenal of nonhuman primates. J Lipid Res 41: 1991–2001, 2000. [PubMed] [Google Scholar]
- 54.Leon C, Hill JS, Wasan KM. Potential role of acyl-coenzyme A:cholesterol transferase (ACAT) inhibitors as hypolipidemic and antiatherosclerosis drugs. Pharm Res 22: 1578–1588, 2005. [DOI] [PubMed] [Google Scholar]
- 55.Li BL, Li XL, Duan ZJ, Lee O, Lin S, Ma ZM, Chang CC, Yang XY, Park JP, Mohandas TK, Noll W, Chan L, Chang TY. Human acyl-CoA:cholesterol acyltransferase-1 (ACAT-1) gene organization and evidence that the 4.3-kilobase ACAT-1 mRNA is produced from two different chromosomes. J Biol Chem 274: 11060–11071, 1999. [DOI] [PubMed] [Google Scholar]
- 56.Liang JJ, Oelkers P, Guo C, Chu PC, Dixon JL, Ginsberg HN, Sturley SL. Overexpression of human diacylglycerol acyltransferase 1, acyl-coa:cholesterol acyltransferase 1, or acyl-CoA:cholesterol acyltransferase 2 stimulates secretion of apolipoprotein B-containing lipoproteins in McA-RH7777 cells. J Biol Chem 279: 44938–44944, 2004. [DOI] [PubMed] [Google Scholar]
- 57.Libby P, Aikawa M. Stabilization of atherosclerotic plaques: new mechanisms and clinical targets. Nat Med 8: 1257–1262, 2002. [DOI] [PubMed] [Google Scholar]
- 58.Lin S, Cheng D, Liu MS, Chen J, Chang TY. Human acyl-CoA:cholesterol acyltransferase-1 in the endoplasmic reticulum contains seven transmembrane domains. J Biol Chem 274: 23276–23285, 1999. [DOI] [PubMed] [Google Scholar]
- 59.Lin S, Lu X, Chang CC, Chang TY. Human acyl-coenzyme A:cholesterol acyltransferase expressed in Chinese hamster ovary Cells: membrane topology and active site location. Mol Biol Cell 14: 2447–2460, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu J, Chang CC, Westover EJ, Covey DF, Chang TY. Investigating the allosterism of acyl-CoA:cholesterol acyltransferase (ACAT) by using various sterols: in vitro and intact cell studies. Biochem J 391: 389–397, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Llaverias G, Laguna JC, Alegret M. Pharmacology of the ACAT inhibitor avasimibe (CI-1011). Cardiovasc Drug Rev 21: 33–50, 2003. [PubMed] [Google Scholar]
- 62.Lu X, Lin S, Chang CC, Chang TY. Mutant acyl-coenzyme A:cholesterol acyltransferase 1 devoid of cysteine residues remains catalytically active. J Biol Chem 277: 711–718, 2002. [DOI] [PubMed] [Google Scholar]
- 63.Matsuda D, Ohte S, Ohshiro T, Jiang W, Rudel L, Hong B, Si S, Tomoda H. Molecular target of piperine in the inhibition of lipid droplet accumulation in macrophages. Biol Pharm Bull 31: 1063–1066, 2008. [DOI] [PubMed] [Google Scholar]
- 64.Meiner V, Tam C, Gunn MD, Dong LM, Weisgraber KH, Novak S, Myers HM, Erickson SK, Farese RV Jr. Tissue expression studies on the mouse acyl-CoA: cholesterol acyltransferase gene (Acact): findings supporting the existence of multiple cholesterol esterification enzymes in mice. J Lipid Res 38: 1928–1933, 1997. [PubMed] [Google Scholar]
- 65.Miyazaki A, Sakashita N, Lee O, Takahashi K, Horiuchi S, Hakamata H, Morganelli PM, Chang CC, Chang TY. Expression of ACAT1 protein in human atherosclerotic lesions and cultured human monocytes-macrophages. Arterioscler Thromb Vasc Biol 18: 1568–1574, 1998. [DOI] [PubMed] [Google Scholar]
- 66.Nissen SE, Tuzcu EM, Brewer HB, Sipahi I, Nicholls SJ, Ganz P, Schoenhagen P, Waters DD, Pepine CJ, Crowe TD, Davidson MH, Deanfield JE, Wisniewski LM, Hanyok JJ, Kassalow LM. Effect of ACAT inhibition on the progression of coronary atherosclerosis. N Engl J Med 354: 1253–1263, 2006. [DOI] [PubMed] [Google Scholar]
- 67.Oelkers P, Behari A, Cromley D, Billheimer JT, Sturley SL. Characterization of two human genes encoding acyl coenzyme A:cholesterol acyltransferase-related enzymes. J Biol Chem 273: 26765–26771, 1998. [DOI] [PubMed] [Google Scholar]
- 68.Ohshiro T, Rudel LL, Omura S, Tomoda H. Selectivity of microbial acyl-CoA: cholesterol acyltransferase inhibitors toward isozymes. J Antibiot (Tokyo) 60: 43–51, 2007. [DOI] [PubMed] [Google Scholar]
- 69.Parini P, Davis M, Lada AT, Erickson SK, Wright TL, Gustafsson U, Sahlin S, Einarsson C, Eriksson M, Angelin B, Tomoda H, Omura S, Willingham MC, Rudel LL. ACAT2 is localized to hepatocytes and is the major cholesterol-esterifying enzyme in human liver. Circulation 110: 2017–2023, 2004. [DOI] [PubMed] [Google Scholar]
- 70.Puglielli L, Konopka G, Pack-Chung E, Ingano LA, Berezovska O, Hyman BT, Chang TY, Tanzi RE, Kovacs DM. Acyl-coenzyme A: cholesterol acyltransferase modulates the generation of the amyloid beta-peptide. Nat Cell Biol 3: 905–912, 2001. [DOI] [PubMed] [Google Scholar]
- 71.Raffai RL, Weisgraber KH. Cholesterol: from heart attacks to Alzheimer's disease. J Lipid Res 44: 1423–1430, 2003. [DOI] [PubMed] [Google Scholar]
- 72.Repa JJ, Buhman KK, Farese RV Jr, Dietschy JM, Turley SD. ACAT2 deficiency limits cholesterol absorption in the cholesterol-fed mouse: impact on hepatic cholesterol homeostasis. Hepatology 40: 1088–1097, 2004. [DOI] [PubMed] [Google Scholar]
- 73.Rodriguez A, Usher DC. Anti-atherogenic effects of the acyl-CoA:cholesterol acyltransferase inhibitor, avasimibe (CI-1011), in cultured primary human macrophages. Atherosclerosis 161: 45–54, 2002. [DOI] [PubMed] [Google Scholar]
- 74.Rong JX, Kusunoki J, Oelkers P, Sturley SL, Fisher EA. Acyl-coenzymeA (CoA):cholesterol acyltransferase inhibition in rat and human aortic smooth muscle cells is nontoxic and retards foam cell formation. Arterioscler Thromb Vasc Biol 25: 122–127, 2005. [DOI] [PubMed] [Google Scholar]
- 75.Ross R Atherosclerosis—an inflammatory disease. N Engl J Med 340: 115–126, 1999. [DOI] [PubMed] [Google Scholar]
- 76.Rudel LL, Farese RV Jr. ACAT inhibition and the progression of coronary atherosclerosis. N Engl J Med 354: 2616–2617, 2006. [DOI] [PubMed] [Google Scholar]
- 77.Rudel LL, Lee RG, Parini P. ACAT2 is a target for treatment of coronary heart disease associated with hypercholesterolemia. Arterioscler Thromb Vasc Biol 25: 1112–1118, 2005. [DOI] [PubMed] [Google Scholar]
- 78.Sahi J, Milad MA, Zheng X, Rose KA, Wang H, Stilgenbauer L, Gilbert D, Jolley S, Stern RH, LeCluyse EL. Avasimibe induces CYP3A4 and multiple drug resistance protein 1 gene expression through activation of the pregnane X receptor. J Pharmacol Exp Ther 306: 1027–1034, 2003. [DOI] [PubMed] [Google Scholar]
- 79.Sakashita N, Miyazaki A, Chang CC, Chang TY, Kiyota E, Satoh M, Komohara Y, Morganelli PM, Horiuchi S, Takeya M. Acyl-coenzyme A:cholesterol acyltransferase 2 (ACAT2) is induced in monocyte-derived macrophages: in vivo and in vitro studies. Lab Invest 83: 1569–1581, 2003. [DOI] [PubMed] [Google Scholar]
- 80.Sakashita N, Miyazaki A, Takeya M, Horiuchi S, Chang CC, Chang TY, Takahashi K. Localization of human acyl-coenzyme A: cholesterol acyltransferase-1 (ACAT-1) in macrophages and in various tissues. Am J Pathol 156: 227–236, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shindou H, Shimizu T. Acyl-CoA:lysophospholipid acyltransferases. J Biol Chem 284: 1–5, 2009. [DOI] [PubMed] [Google Scholar]
- 82.Sliskovic DR, Picard JA, Krause BR. ACAT inhibitors: the search for a novel and effective treatment of hypercholesterolemia and atherosclerosis. Prog Med Chem 39: 121–171, 2002. [DOI] [PubMed] [Google Scholar]
- 83.Smith JL, Rangaraj K, Simpson R, Maclean DJ, Nathanson LK, Stuart KA, Scott SP, Ramm GA, de Jersey J. Quantitative analysis of the expression of ACAT genes in human tissues by real-time PCR. J Lipid Res 45: 686–696, 2004. [DOI] [PubMed] [Google Scholar]
- 84.Song BL, Wang CH, Yao XM, Yang L, Zhang WJ, Wang ZZ, Zhao XN, Yang JB, Qi W, Yang XY, Inoue K, Lin ZX, Zhang HZ, Kodama T, Chang CC, Liu YK, Chang TY, Li BL. Human acyl-CoA:cholesterol acyltransferase 2 gene expression in intestinal Caco-2 cells and in hepatocellular carcinoma. Biochem J 394: 617–626, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Spady DK, Willard MN, Meidell RS. Role of acyl-coenzyme A:cholesterol acyltransferase-1 in the control of hepatic very low density lipoprotein secretion and low density lipoprotein receptor expression in the mouse and hamster. J Biol Chem 275: 27005–27012, 2000. [DOI] [PubMed] [Google Scholar]
- 86.Su YR, Dove DE, Major AS, Hasty AH, Boone B, Linton MF, Fazio S. Reduced ABCA1-mediated cholesterol efflux and accelerated atherosclerosis in apolipoprotein E-deficient mice lacking macrophage-derived ACAT1. Circulation 111: 2373–2381, 2005. [DOI] [PubMed] [Google Scholar]
- 87.Urano Y, Watanabe H, Murphy SR, Shibuya Y, Geng Y, Peden AA, Chang CC, Chang TY. Transport of LDL-derived cholesterol from the NPC1 compartment to the ER involves the trans-Golgi network and the SNARE protein complex. Proc Natl Acad Sci USA 105: 16513–16518, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Warner GJ, Stoudt G, Bamberger M, Johnson WJ, Rothblat GH. Cell toxicity induced by inhibition of acyl coenzyme A:cholessterol acyltransferase and accumulation of unesterified cholesterol. J Biol Chem 270: 5772–5778, 1995. [DOI] [PubMed] [Google Scholar]
- 89.Watanabe T, Suguro T, Kanome T, Sakamoto Y, Kodate S, Hagiwara T, Hongo S, Hirano T, Adachi M, Miyazaki A. Human urotensin II accelerates foam cell formation in human monocyte-derived macrophages. Hypertension 46: 738–744, 2005. [DOI] [PubMed] [Google Scholar]
- 90.Willner EL, Tow B, Buhman KK, Wilson M, Sanan DA, Rudel LL, Farese RV Jr. Deficiency of acyl CoA:cholesterol acyltransferase 2 prevents atherosclerosis in apolipoprotein E-deficient mice. Proc Natl Acad Sci USA 100: 1262–1267, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wolozin B Cholesterol, statins and dementia. Curr Opin Lipidol 15: 667–672, 2004. [DOI] [PubMed] [Google Scholar]
- 92.Worthley SG, Helft G, Corti R, Worthley MI, Chew DP, Fayad ZA, Zaman AG, Fallon JT, Fuster V, Badimon JJ. Statin therapy alone and in combination with an acyl-CoA:cholesterol O-acyltransferase inhibitor on experimental atherosclerosis. Pathophysiol Haemost Thromb 36: 9–17, 2007. [DOI] [PubMed] [Google Scholar]
- 93.Yang H, Bard M, Bruner DA, Gleeson A, Deckelbaum RJ, Aljinovic G, Pohl TM, Rothstein R, Sturley SL. Sterol esterification in yeast: a two-gene process. Science 272: 1353–1356, 1996. [DOI] [PubMed] [Google Scholar]
- 94.Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell 132: 387–396, 2008. [DOI] [PubMed] [Google Scholar]
- 95.Yang JB, Duan ZJ, Yao W, Lee O, Yang L, Yang XY, Sun X, Chang CC, Chang TY, Li BL. Synergistic transcriptional activation of human Acyl-coenzyme A: cholesterol acyltransterase-1 gene by interferon-gamma and all-trans-retinoic acid THP-1 cells. J Biol Chem 276: 20989–20998, 2001. [DOI] [PubMed] [Google Scholar]
- 96.Yang L, Lee O, Chen J, Chang CC, Zhou P, Wang ZZ, Ma HH, Sha HF, Feng JX, Wang Y, Yang XY, Wang L, Dong R, Ornvold K, Li BL, Chang TY. Human acyl-coenzyme A:cholesterol acyltransferase 1 (acat1) sequences located in two different chromosomes (7 and 1) are required to produce a novel ACAT1 isoenzyme with additional sequence at the N terminus. J Biol Chem 279: 46253–46262, 2004. [DOI] [PubMed] [Google Scholar]
- 97.Yang L, Yang JB, Chen J, Yu GY, Zhou P, Lei L, Wang ZZ, Cy Chang C, Yang XY, Chang TY, Li BL. Enhancement of human ACAT1 gene expression to promote the macrophage-derived foam cell formation by dexamethasone. Cell Res 14: 315–323, 2004. [DOI] [PubMed] [Google Scholar]
- 98.Ye S, Huang Y, Mullendorff K, Dong L, Giedt G, Meng EC, Cohen FE, Kuntz ID, Weisgraber KH, Mahley RW. Apolipoprotein (apo) E4 enhances amyloid beta peptide production in cultured neuronal cells: apoE structure as a potential therapeutic target. Proc Natl Acad Sci USA 102: 18700–18705, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yu C, Chen J, Lin S, Liu J, Chang CC, Chang TY. Human acyl-CoA:cholesterol acyltransferase 1 is a homotetrameric enzyme in intact cells and in vitro. J Biol Chem 274: 36139–36145, 1999. [DOI] [PubMed] [Google Scholar]
- 100.Yu C, Kennedy NJ, Chang CC, Rothblatt JA. Molecular cloning and characterization of two isoforms of saccharomyces cerevisiae acyl-coA:sterol acyltransferase. J Biol Chem 271: 24157–24163, 1996. [DOI] [PubMed] [Google Scholar]
- 101.Yu C, Zhang Y, Lu X, Chang CC, Chang TY. The role of the N-terminal hydrophilic domain of acyl coenzyme A:cholesterol acyltransferase 1 on the enzyme's quaternary structure and catalytic efficiency. Biochemistry 41: 3762–3769, 2002. [DOI] [PubMed] [Google Scholar]
- 102.Zhang Y, Yu C, Liu J, Spencer TA, Chang CC, Chang TY. Cholesterol is superior to 7-ketocholesterol or 7alpha-hydroxycholesterol as an allosteric activator for acyl-coenzyme A: cholesterol acyltransferase 1. J Biol Chem 278: 11642–11647, 2003. [DOI] [PubMed] [Google Scholar]