Abstract
Although obesity is associated with multiple features of the metabolic syndrome (insulin resistance, leptin resistance, hepatic steatosis, chronic inflammation, etc.), the molecular changes that promote these conditions are not completely understood. Here, we tested the hypothesis that elevated ceramide biosynthesis contributes to the pathogenesis of obesity and the metabolic syndrome. Chronic treatment for 8 wk of genetically obese (ob/ob), and, high-fat diet-induced obese (DIO) mice with myriocin, an inhibitor of de novo ceramide synthesis, decreased circulating ceramides. Decreased ceramide was associated with reduced weight, enhanced metabolism and energy expenditure, decreased hepatic steatosis, and improved glucose hemostasis via enhancement of insulin signaling in the liver and muscle. Inhibition of de novo ceramide biosynthesis decreased adipose expression of suppressor of cytokine signaling-3 (SOCS-3) and induced adipose uncoupling protein-3 (UCP3). Moreover, ceramide directly induced SOCS-3 and inhibited UCP3 mRNA in cultured adipocytes suggesting a direct role for ceramide in regulation of metabolism and energy expenditure. Inhibition of de novo ceramide synthesis had no effect on adipose tumor necrosis factor-α (TNF-α) expression but dramatically reduced adipose plasminogen activator inhibitor-1 (PAI-1) and monocyte chemoattactant protein-1 (MCP-1). This study highlights a novel role for ceramide biosynthesis in body weight regulation, energy expenditure, and the metabolic syndrome.
Keywords: sphingolipids, insulin resistance, leptin resistance, insulin resistance, suppressor of cytokine signaling-3, tumor necrosis factor-α, plasminogen activator inhibitor-1, monocyte chemoattactant protein-1
excessive consumption of diets high in saturated fats causes obesity and, subsequently or in parallel, leads to chronic inflammation, deranges cellular metabolism, and is a key initiator of the metabolic syndrome.
Although obesity increases the risk for insulin resistance, type 2 diabetes, hepatic steatosis, and cardiovascular disease, the molecular changes that promote these conditions are not completely understood. Increasing evidence suggests that a complex proinflammatory state, together with increased lipid accumulation in extra adipose tissues such as the liver and skeletal muscle, may contribute to the pathogenesis of these obesity-related disorders. A central contributor to the systemic proinflammatory state observed in obesity is adipose tissue, which synthesizes a number of inflammatory adipokines, including tumor necrosis factor-α (TNF-α), monocyte chemoattractant protein-1 (MCP-1), and interleukin-6 (IL-6) (reviewed in Ref. 1), as well as plasminogen activator inhibitor-1 (PAI-1) (19, 29). PAI-1 is the primary inhibitor of plasminogen activation in vivo and an established risk factor for cardiovascular disease, and recent studies suggest that PAI-1 may also contribute to obesity-mediated insulin resistance, diabetes, and weight gain itself (reviewed in Ref. 5). Thus, the dysregulated secretion of adipokines from the adipose tissues in obesity contributes to the insulin resistance in peripheral tissues (muscle, liver) and increased risk for cardiovascular disease. Another mechanism by which obesity leads to insulin resistance in the liver and muscle is via increased intracellular lipid accumulation in these tissues (25, 28, 32, 49, 51). Clearly, a major challenge now is to identify common mechanisms that may link excess nutrients and dysregulated adipokines (e.g., inflammatory cytokines) to the pathogenesis of the metabolic syndrome. Recent evidence by us and others demonstrates that sphingolipid metabolism is altered in obesity and that these lipid mediators may provide a common pathway that link both excess nutrients and inflammation to increased metabolic and cardiovascular risk (41).
Sphingolipids are involved in a number of biological processes, including proliferation, differentiation, apoptosis, and inflammation (14). Ceramide, the backbone of all sphingolipids, is generated in response to a variety of mediators, including proinflammatory cytokines, oxidative stress, and increased levels of free fatty acids (14, 59), conditions that characterize the obese adipose tissue. Emerging evidence suggests that ceramide may be a putative intermediate linking both excess nutrients such as saturated free fatty acids and inflammatory cytokines such as TNF-α to the metabolic syndrome (42, 44, 46, 49). Previous studies have shown modest increases in total ceramide in the skeletal muscle and liver of obese rodents and humans, and this increase correlates negatively with insulin sensitivity (reviewed in Ref. 41). In agreement with these studies, we previously showed that total ceramide levels are also modestly increased in adipose tissues and plasma of diet-induced obese (DIO) mice (46). However, lipidomic analysis revealed specific and dramatic increases in individual ceramide species that were relatively minor constituents in normal lean mice (46). For example, increases of more than 300% were observed for C18 ceramide respectively in adipose tissue and plasma of DIO mice.
In this study, we determined whether inhibiting ceramide synthesis improves features of the metabolic syndrome in genetic and high-fat diet-induced obese mice. We show that administration to obese mice of myriocin, a specific inhibitor of serine palmitoyltransferase (SPT), the enzyme that catalyzes the rate-limiting step (condensation of serine and palmitoyl-CoA) of de novo ceramide synthesis, reduced circulating ceramide and ameliorated multiple aspects of the metabolic syndrome.
MATERIALS AND METHODS
Mice.
Animal studies were approved by our Institutional Animal Care and use Committee. C57BL/6J wild-type (WT) and C57BL/6J ob/ob (8-wk-old males)mice were from the Jackson Laboratory (Bar Harbor, ME). Obese (ob/ob) TNF-α receptor-deficient mice and WT ob/ob controls were kindly provided by Dr. Gokhan S. Hotamisligil (Harvard School of Public Health). The generation and characterization of these mice are described elsewhere (38, 45, 52). Myriocin (0.3 mg/kg, Biomol Research Laboratories) or saline was injected intraperitoneally every other day for 8 wk into 1) genetically obese ob/ob mice; 2) C57BL/6J WT mice placed on a high-fat diet (HFD; no. D12492, 60% kcal from fat; Research Diets, New Brunswick, NJ) at the onset of myriocin treatment (DIO model 1); and 3) C57BL/6J WT mice placed on HFD for 8 wk prior to myriocin treatment, and the HFD continued for an additional 8 wk during the course of myriocin treatment (DIO model 2). Daily food intake was monitored throughout the experimental period by measuring the weight of the food pellets given and what was left at the end of each 24-h period.
In some experiments, saline-injected DIO mice were pair fed to myriocin-treated mice. In this instance,, the pair-fed group was given the same amount of food consumed by the saline-treated DIO the preceding day. This group was added to rule out any effects on food intake on observed outcomes.
RNA analysis.
Real-time RT-PCR was performed in an iCycler (Bio-Rad Laboratories, Hercules, CA) as previously described (42, 46).
Cell culture.
3T3-L1 mouse embryo fibroblasts were grown and differentiated into adipocytes as described previously (42, 43). Adipocytes were treated with ceramide (C6, 25 μM) and harvested 3 h later. Total RNA was isolated and relative gene expression of SOCS-3 and UCP3 determined using real-time RT-PCR as described (42, 46).
Sphingolipid analysis.
Ceramide, sphingosine, and sphingosine 1-phosphate (S1P) were analyzed by high-performance liquid chromatography-tandem mass spectroscopy (HPLC-MS) as previously described (2) (Lipidomics Core, Medical University of South Carolina).
Metabolic parameters.
Insulin was measured with an insulin assay kit (Mercodia Ultrasensitive Insulin ELISA; Alpco Diagnostics, Windham, NH), and glucose was monitored with a Glucometer Elite Blood Glucose Meter (Bayer, Elkhart, IN). For glucose and insulin tolerance tests, mice were injected intraperitoneally with either glucose (2 g/kg body wt) or insulin (Humulin, 0.75 U/kg body wt, Eli Lilly), and blood samples were obtained via tail bleeds at baseline and at 15, 30, 60, 90, and 120 min after glucose or insulin injection and analyzed for glucose levels.
Hepatic triglyceride.
Hepatic triglyceride was measured as previously described (22). Briefly, liver samples (20–30 mg) were homogenized in isopropanol and centrifuged (2000 g, 10 min), and 100 μl of supernatant was dried (Speedvac), dissolved in isopropanol, and assayed for triglyceride content with a Triglyceride E test (Wako).
Insulin signaling: western blot analysis.
Mice were injected either with human insulin (Humulin, 0.75 U/kg body wt) or an equal volume of saline through the tail vein and killed 10 min later, and the liver, epididymal adipose tissues, and muscle were collected. Tissues were homogenized and centrifuged (15, 000 g, 30 min) in ice-cold homogenization buffer (26), and the supernatants (30 μg protein, BCA Protein Assay) were subjected to 10% SDS-PAGE followed by Western blot analysis with antibodies to Akt and phospho-Akt (Cell Signaling Technology, Beverly, MA).
Indirect calorimetry.
Indirect calorimetry was performed using a computer-controlled, open-circuit system (Oxymax System; The Scripps Research Institute, La Jolla, CA) that is a part of an integrated Comprehensive Lab Animal Monitoring System (CLAMS; Columbus Instruments, Columbus, OH). Testing occurred in respiratory chambers equipped with a food tray connected to a balance and 16 photo beams to detect motor activity. Exhaust air from each chamber was sampled at 15-min intervals for 1 min. Sample air was sequentially passed through O2 and CO2 sensors (Columbus Instruments) for determination of O2 and CO2 content, from which measures of oxygen consumption (V̇o2) and carbon dioxide production (V̇co2) were calculated. Respiratory exchange ratio (RER) was calculated as the ratio of V̇co2 to V̇o2.
Histological analysis.
Formalin-fixed, paraffin-embedded sections (6 μm) of adipose and liver tissues were stained with hematoxylin and eosin. Average adipocyte diameters were calculated by measuring the diameters from six random microscopic fields from each mouse by use of the image analysis SPOT software (Diagnostic Instruments).
Statistical analysis.
Statistical comparison was performed using the unpaired Student's t-test.
RESULTS
Effect of myriocin on plasma sphingolipid metabolism in obese mice.
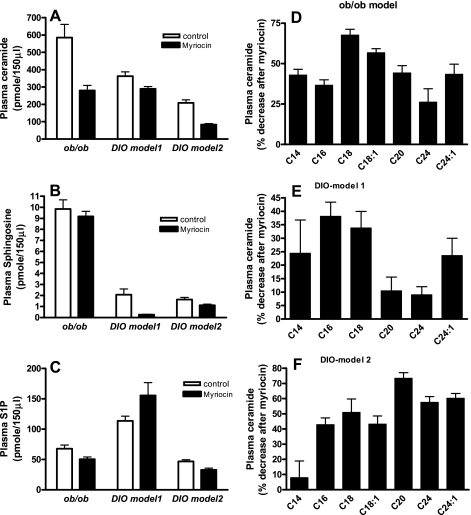
We previously showed that plasma ceramide levels were increased in genetically obese ob/ob mice and in C57BL/6J mice placed on an HFD (60% kcal from fat) for 16 wk, with the largest increases observed for C16 and C18 ceramide species (42, 46). De novo ceramide synthesis was inhibited by treating obese mice with myriocin, a specific inhibitor of SPT, the enzyme that catalyzes the initial rate-limiting step (condensation of serine and palmitoyl-CoA) of in vivo ceramide synthesis (14, 59). Myriocin treatment of ob/ob mice significantly decreased (49%, P < 0.001) total plasma ceramide (Fig. 1A) and modestly decreased (24%, P < 0.05) the downstream metabolite S1P (Fig. 1C). In the DIO model 1, myriocin modestly yet significantly decreased (20%, P < 0.05) total plasma ceramide (Fig. 1A) and dramatically decreased (90%, P < 0.01) the downstream ceramide metabolite sphingosine (Fig. 1B) but had no significant effect on S1P (Fig. 1C). In DIO model 2, myriocin treatment decreased total ceramide by 60% (P < 0.001; Fig. 1A), sphingosine by 31% (P < 0.05; Fig. 1B), and S1P by 30% (P < 0.05; Fig. 1C). Lipidomic profiling of individual ceramide species indicates that, whereas there is a general decrease in all ceramide species in response to inhibition of de novo ceramide synthesis in all models (Fig. 1, D, E, and F), the magnitude and specificity differed. In the ob/ob mice, the largest decrease was observed for C18 (70%, P < 0.001) and C18:1 (52%, P < 0.001) ceramide (Fig. 1D). When mice were treated with myriocin at the onset of the HFD (DIO model 1), the largest decrease was observed for C16 (40%, P < 0.05) and C18 (36%, P < 0.05) ceramide (Fig. 1E). Conversely, when myriocin treatment was initiated in DIO mice (DIO model 2) decreases (42–76%) were observed for all ceramide species except for C14; the largest decrease (76%, P < 0.01) was observed for C20 ceramide (Fig. 1F). Thus, although inhibition of de novo ceramide synthesis results in overall inhibition of total ceramide synthesis, the magnitude of inhibition of specific ceramide species and ceramide metabolites (e.g., sphingosine and S1P) differed for each model. These model-specific differences may translate into the magnitude of downstream effects (e.g., effects on weight gain, metabolism, gene expression, insulin signaling, etc.) observed in these mice.
Fig. 1.
Plasma sphingolipid profiles in response to myriocin treatment in obese mice. Levels of ceramide, sphingosine, and sphingosine 1-phosphate (S1P) were determined by HPLC-TMS after myriocin treatment. A: total ceramide (sum of individual ceramide species) in control and myriocin-treated obese mice. B and C: sphingosine and S1P, respectively, in control and myriocin-treated mice. D, E, and F: ceramide species in control and myriocin-treated ob/ob, diet-induced obese (DIO) model 1, and DIO model 2 mice, respectively. Data are means ± SD (n = 8).
Inhibiting ceramide synthesis reduces body weight and fat mass.
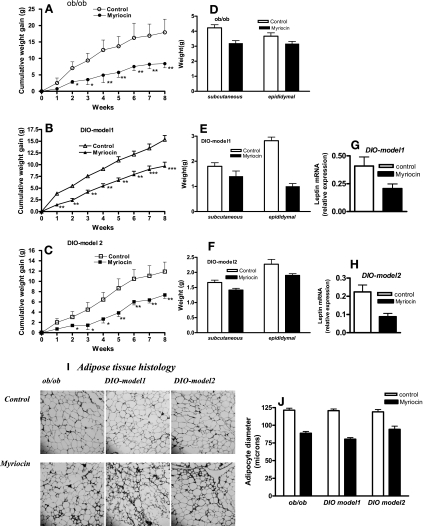
To determine the effect of ceramide synthesis on body weight regulation, the growth of myriocin-treated and control mice was analyzed. Although the body weights of vehicle-treated mice increased rapidly, myriocin-treated mice gained significantly less weight (Fig. 2, A, B, and C). Abdominal subcutaneous and epididymal fat pad weights were also significantly (P < 0.05) reduced in mice on the HFD when de novo ceramide synthesis was inhibited (Fig. 2, D, E, and F). Parallel to the reduction in fat pad weights, leptin gene expression was similarly reduced in myriocin-treated DIO mice (Fig. 2, G and H). Histological analysis of adipose tissue sections from mice treated with myriocin indicated the presence of pockets of smaller adipocytes (Fig. 2I), which was also reflected in the smaller adipocyte diameters (Fig. 2J).
Fig. 2.
Body weights, adiposity, and adipose leptin expression in response to myriocin treatment in obese mice. Body weights (A, B, C) and fat pad weights (D, E, F) of control, and myriocin-treated obese (ob/ob, DIO) mice. Leptin mRNA expression in epididymal fat pads of control and myriocin-treated DIO model 1 (G) and DIO model 2 (H) mice were determined by real time RT-PCR. I and J: hematoxylin & eosin-stained histological sections of epididymal fat pads and adipocyte diameters, respectively, from control and myriocin-treated obese mice. For A–H and J, data are means ± SD (n = 12). *P < 0.05; **P < 0.01; ***P < 0.001.
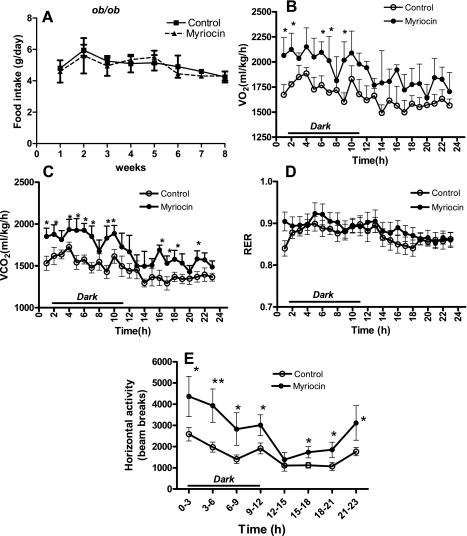
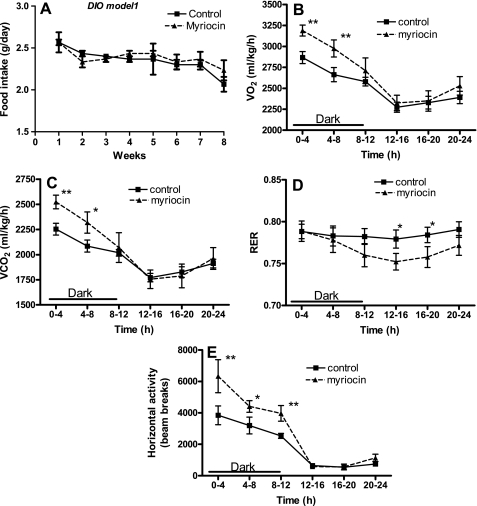
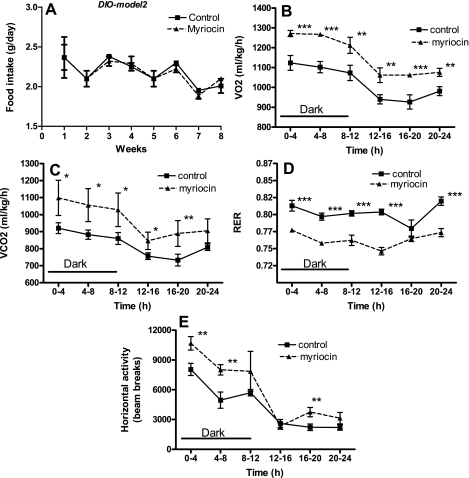
We next determined whether the mechanism of reduction in body weights of myriocin-treated mice was due to decreased food intake and/or increased metabolism/energy expenditure. Average daily food intake was unchanged in myriocin-treated ob/ob mice compared with controls throughout the duration of the experiment (Fig. 3A). Myriocin-treated mice showed significantly higher rates of V̇o2 (Fig. 3B) and CO2 release (Fig. 3C), indicative of increased metabolism. Consistent with their elevated metabolic rates, ambulatory activity also was significantly increased (P < 0.01; Fig. 3E). The RER, a ratio of V̇co2 to V̇o2, was unchanged in myriocin-treated ob/ob mice (Fig. 3D). In C57BL/6J DIO mice also, myriocin treatment did not result in any significant changes in food intake (Figs. 4A and 5A). Moreover, after 8 wk of myriocin treatment, body weights of pair-fed saline-injected DIO control mice were similar to those of normal saline-injected controls (controls: 51.7 ± 1.3 g; pair fed: 50.6 ± 2.9 g), and higher than in myriocin-treated mice (46 ± 1.7 g). These results further support our observations that the reduction in body weights of the myriocin-treated mice is not a result of any changes in food intake but rather via an alternate mechanism such as an increase in metabolism. Indeed, similar to the ob/ob mice, inhibiting de novo ceramide synthesis in DIO mice also resulted in increased V̇o2 (Figs. 4B and 5B), CO2 output (Figs. 4C and 5C), and increased activity (Figs. 4E and 5E), all indicative of increased metabolism and energy expenditure. In these DIO models, myriocin treatment resulted in a significantly lower RER during the light phase in DIO model 1 (Fig. 4D, P < 0.05) and during both the light and dark cycles in DIO model 2 (Fig. 5D, P < 0.001), suggesting a shift toward fat utilization as their fuel source.
Fig. 3.
Metabolic function in response to myriocin in genetically obese ob/ob mice. A: food intake throughout the experimental period. Whole body O2 consumption (V̇o2; B), CO2 release (V̇co2; C), respiratory exchange ratio (RER; D), and ambulatory activity (E) were measured as described (CLAMS; Columbus Instruments, Columbus, OH) in control and myriocin-treated mice at the end of the 8-wk treatment regimen. For A, data are means ± SD (n = 12). For B–E, data are means ± SD (n = 6). *P < 0.05; **P < 0.01.
Fig. 4.
Metabolic function in response to myriocin in DIO model 1 mice. A: food intake throughout the experimental period. Whole body V̇o2 (B), V̇co2 (C), RER (D), and activity (E) were measured as described (CLAMS) in control and myriocin-treated mice at the end of the 8-wk treatment regimen. For A, data are means ± SD (n = 12). For B–E, data are means ± SD (n = 6). *P < 0.05; **P < 0.01.
Fig. 5.
Metabolic function in response to myriocin in DIO-model 2 mice. A: food intake throughout the experimental period. Whole body V̇o2 (B), V̇co2 (C), RER (D), and activity (E) were measured as described (CLAMS) in control and myriocin-treated mice at the end of the 8-wk treatment regimen. For A, data are means ± SD (n = 12). For B–E, data are means ± SD (n = 6). *P < 0.05; **P < 0.01; ***P < 0.001.
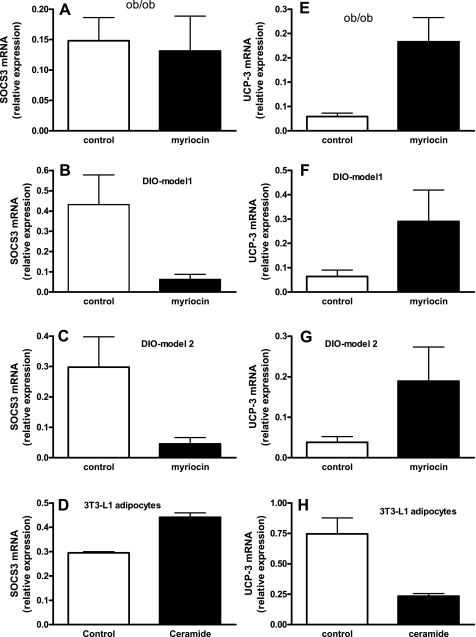
Inhibition of de novo ceramide synthesis is associated with decreased adipose expression of SOCS-3 and increased UCP3.
Human obesity is associated with leptin resistance, and recent studies have shown that SOCS-3 is a postreceptor inhibitor of leptin signaling, important in the development of leptin resistance (3, 17, 48, 54). Thus, high-fat feeding leads to a large increase in SOCS-3 expression and to the development of leptin resistance and weight gain (21, 53). Although inhibition of de novo ceramide synthesis had no effect on adipose SOCS-3 expression in the leptin-deficient genetically obese ob/ob mice (Fig. 6A), it dramatically and significantly reduced adipose tissue SOCS-3 gene expression in HFD-induced obese mice (Fig. 6, B and C, P < 0.01). Moreover, direct treatment of 3T3-L1 adipocytes with the short-chain ceramide analog C6 (25 μm, 3 h) significantly (P < 0.001) increased SOCS-3 mRNA in these cells (Fig. 6D). These studies suggest that aberrant ceramide biosynthesis in response to an HFD contributes directly to the expression of the leptin resistance factor SOCS-3 in adipocytes and thereby perhaps contributes to leptin resistance associated with HFD induced obesity.
Fig. 6.
Regulation of supressor of cytokine signaling 3 (SOCS-3) and UCP3 expression. A, B, and C: SOCS-3 gene expression in epididymal fat pads of control and myriocin-treated ob/ob, DIO model 1 and DIO model 2 mice, respectively. D: SOCS-3 mRNA expression in cultured 3T3-L1 adipocytes in response to C6 ceramide (25 μM, 3 h). E, F, and G: UCP3 gene expression in epididymal fat pads of control and myriocin-treated ob/ob, DIO model 1 and DIO model 2 mice, respectively. H: UCP3 mRNA expression in cultured 3T3-L1 adipocytes in response to C6 ceramide (25 μM, 3 h). Data are means ± SD (n = 6).
To begin to understand the mechanism(s) by which inhibition of de novo ceramide synthesis leads to increased metabolism, we determined the expression of UCP3 in white adipose tissues. UCPs are mitochondrial inner-membrane proteins that promote mitochondrial energy expenditure and play important roles in whole body energy expenditure (35). Inhibition of de novo ceramide synthesis significantly increased the expression of UCP3 in adipose tissues of both ob/ob (Fig. 6E, P < 0.01) and DIO mice (Fig. 6, F and G, P < 0.05). These studies suggest that the mechanisms by which decreased ceramide leads to increase in metabolism may be related to the induction of UCP3. To further confirm this hypothesis, we determined whether ceramide directly regulates the expression of UCP3 in cultured 3T3-L1 adipocytes. Indeed, UCP3 gene expression was dramatically reduced (Fig. 6H, P < 0.01) in cultured adipocytes after 3 h of ceramide treatment.
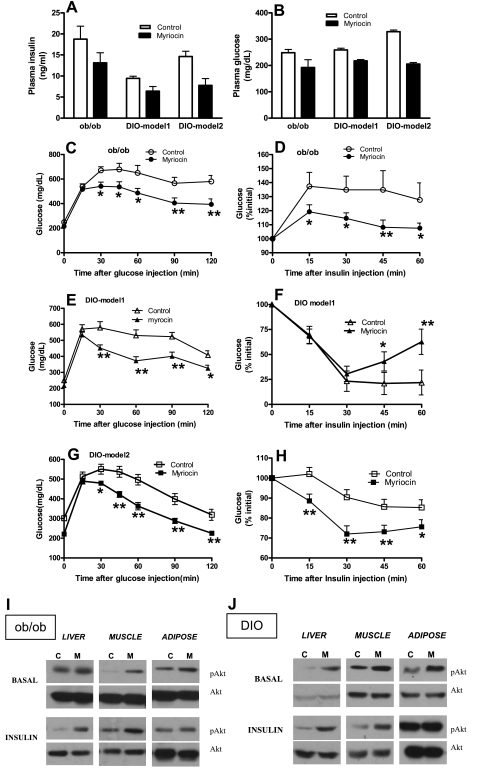
Inhibiting ceramide synthesis improves glucose tolerance and insulin sensitivity primarily through restoration of insulin signaling in liver and muscle.
We next investigated whether inhibition of de novo ceramide synthesis in obese mice leads to improved glucose tolerance and insulin sensitivity. Both plasma insulin (Fig. 7A) and glucose levels (Fig. 7B) were significantly (P < 0.05) reduced in myriocin-treated ob/ob and DIO mice. Moreover, plasma glucose levels were also similar in control saline- and pair-fed saline-treated DIO mice (control: 207 ± 28 mg/dl; pair-fed control: 212 ± 28 mg/dl), whereas it was significantly lower in myriocin-treated DIO mice (182.8 ± 17.5 mg/dl, P < 0.01).
Fig. 7.
Glucose homeostasis in response to myriocin treatment in obese mice. Plasma insulin (A) and plasma glucose (B) were measured after 8 wk of myriocin treatment in ob/ob and DIO mice. C, E, and G: glucose tolerance test. Mice fasted for ∼6 h were injected with a bolus of glucose, and blood samples were obtained at indicated times and analyzed for glucose levels. D, F, and H: insulin tolerance test. Mice were injected with insulin (0.75 U/kg body wt), blood samples obtained at indicated times, and glucose levels were monitored. For A–H, data are means ± SD (n = 8). *P < 0.05; **P < 0.01. Representative Western blots show insulin signaling in response to myriocin treatment. Control (C) and myriocin-treated (M) ob/ob (I) and DIO mice (J) were fasted for ∼6 h before receiving a 0.75 U/kg insulin injection in the tail vein. Animals were killed 10 min later, and tissues were collected for Western blotting using antibodies to phosphorylated Ser473 of Akt and Akt. Data are representative of 3 different independent experiments (n = 6).
Inhibition of de novo ceramide synthesis improved glucose tolerance as indicated by the glucose tolerance test. Thus, myriocin-treated obese mice were more efficient in their ability to clear an intraperitoneally injected bolus of glucose than controls (Fig. 7, C, E, and G). Insulin tolerance tests indicated that myriocin-treated obese mice were more efficient at insulin-mediated suppression of plasma glucose than untreated controls (Fig. 7, D, F, and H), indicative of improved insulin sensitivity. Interestingly, in DIO model 1, injection with insulin resulted in a steep decrease in plasma glucose at a similar rate in both myriocin-treated and untreated mice during the initial 30 min (Fig. 7F). After the initial 30 min, myriocin-treated mice were able to prevent hypoglycemia and rapidly restore glucose hemostasis, while glucose levels remained low in untreated controls. Collectively, these data suggest that inhibition of de novo ceramide synthesis improves glucose intolerance and insulin resistance associated with obesity and leads to effective maintenance of glucose hemostasis.
Since an obligatory step in the insulin signaling pathway leading to increased glucose uptake by the muscle and adipose and decreased glucose output by the liver is phosphorylation and activation of the kinase Akt (4), we analyzed Akt phosphorylation in these tissues. In response to an intravenous injection of insulin, myriocin-treated ob/ob mice showed a marked increase in Akt phosphorylation in the liver compared with untreated mice, without alteration in total Akt (Fig. 7Iob/ob). In the muscle, inhibition of de novo ceramide enhanced both basal and insulin-mediated Akt phosphorylation (Fig. 7I, ob/ob), while no effect was observed in adipose tissues. In DIO mice, myriocin treatment increased both basal and insulin-mediated Akt phosphorylation in the liver and in the muscle (Fig. 7J, DIO). In adipose tissue, basal Akt phosphorylation was moderately but significantly induced by myriocin treatment; however, no difference was observed between myriocin-treated and untreated mice in response to insulin (Fig. 7J, DIO). Collectively, the data suggest that amelioration of whole body insulin resistance in myriocin-treated mice can be attributed largely to restoration of both hepatic and muscle insulin sensitivity.
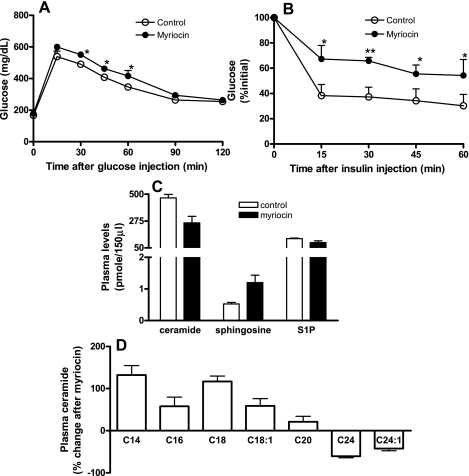
Interestingly, we observed that inhibition of de novo ceramide synthesis in lean C57BL/6J mice on a normal chow diet also led to modest glucose intolerance and insulin resistance (Fig. 8, A and B). This may suggest that normal physiological levels of ceramide are necessary to maintain glucose homeostasis and that either increasing (as in obesity) or decreasing ceramide biosynthesis from this physiological threshold may impair glucose homeostasis. Interestingly, in myriocin-treated lean mice on the chow diet, whereas total plasma ceramide levels were decreased, plasma sphingosine was significantly increased (Fig. 8C). Moreover, the decrease in total ceramide levels observed in myriocin-treated lean mice was due primarily to a decrease in C24 and C24:1 ceramide, ceramide species that are the most abundant and practically determine total ceramide levels in the plasma of normal mice (46). Alternatively, other species of ceramide, including C14, C16, C18, and C18:1, which were relatively minor components in normal plasma (46), were in fact increased to varying extents after myriocin treatment (Fig. 8D). These changes in ceramide profiles, together with increased sphingosine in myriocin-treated lean mice, appear to be associated with glucose intolerance and insulin resistance in this model. These data are novel, as they suggest for the first time that physiological levels of specific ceramide species and/or sphingosine are important to maintain normal glucose homeostasis and that changes and/or remodeling of these levels may result in glucose intolerance and insulin resistance.
Fig. 8.
Glucose homeostasis and plasma sphingolipid profiles in response to myriocin treatment in lean chow-fed C57BL/6J mice. A and B: glucose and insulin tolerance tests, respectively, of control and myriocin-treated lean mice. Data are means ± SD (n = 8). C: plasma ceramide (total), sphingosine, and S1P in control and myriocin-treated mice. D: %change after myriocin treatment in specific ceramide species in plasma. For C and D, data are means ± SD (n = 6). *P < 0.05; **P < 0.01.
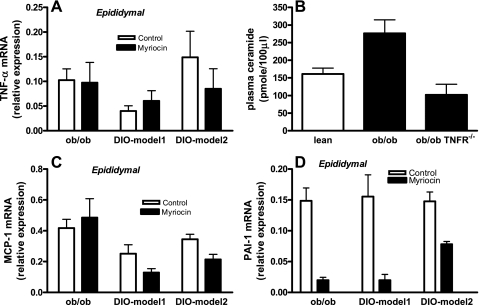
Effect of inhibition of ceramide synthesis on adipose expression of TNF-α, MCP-1, and PAI-1.
Adipose inflammation is a hallmark of obesity, and the expression of cytokines and chemokines such as TNF-α and MCP-1 are increased in adipose tissues of obese mice. Mice lacking these molecules or their receptors showed reduced weight and/or improved insulin sensitivity in various models of rodent obesity (16, 22, 52, 55). Myriocin treatment, while improving insulin sensitivity in obese mice (Fig. 7), did not decrease the expression of adipose TNF-α (Fig. 9A). These results are consistent with a pathway in which changes in adipose cytokines such as TNF-α may act upstream of ceramide formation. Indeed, plasma ceramide levels were significantly reduced in obese (ob/ob) mice that lack both TNF receptors (p55, p75; Fig. 9B), directly confirming that TNF-α is upstream of a pathway that contributes to elevated ceramide biosynthesis in obesity. On the other hand, the expression of MCP-1 mRNA was significantly reduced in adipose tissues of myriocin-treated DIO mice (Fig. 9C, P < 0.01), suggesting that ceramide directly contributes to obesity-associated increase in adipose MCP-1. We also determined the contribution of ceramide to PAI-1, a prothrombotic/proinflammatory adipokine that is consistently elevated in obesity. Increased levels of PAI-1 is one of the biochemical hallmarks of obesity, and recent data suggest that PAI-1 can influence weight and adipose tissue accumulation and may also directly contribute to obesity-mediated insulin resistance and type 2 diabetes (5). PAI-1 mRNA expression was dramatically and significantly reduced in adipose tissues of myriocin-treated obese mice (Fig. 9D, P < 0.01). Together, these results suggest that elevated TNF-α in obesity may lead to the upregulation of ceramide biosynthesis, which in turn may contribute directly to increased MCP-1 and PAI-1 associated with obesity and the metabolic syndrome.
Fig. 9.
Adipose expression of proinflammatory and prothrombotic genes in response to myriocin. TNF-α (A), monocyte chemoattractant protein-1 (MCP-1) (C), and plasminogen activator inhibitor 1 (PAI-1) (D) gene expressions were measured in adipose tissues of control and myriocin-treated ob/ob and DIO mice by real-time RT-PCR. B: plasma ceramide levels in obese ob/ob and TNF-α receptor-deficient (ob/ob TNF-αR−/−) mice. Data are means ± SD (n = 8).
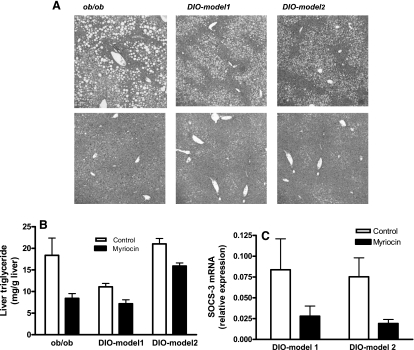
Inhibition of de novo ceramide biosynthesis improves hepatic steatosis.
Hepatic steatosis (nonalcoholic fatty liver), an important component of the metabolic syndrome, is the most common liver abnormality in the US. It not only contributes to hepatic insulin resistance, but may also lead to hepatic fibrosis and cirrhosis (37). Hematoxylin and eosin staining showed pronounced steatosis with macrovesicular fat accumulation in obese mice (Fig. 10A, top), which was significantly reduced after myriocin treatment (Fig. 10A, bottom). Hepatic triglycerides, one of the major storage forms of lipids in the liver, were also reduced in myriocin-treated obese mice (Fig. 10B; for all obese models, P < 0.01). Recent studies suggest a central role for hepatic SOCS-3 expression in the pathology of hepatic steatosis (50). Our data show that, although myriocin treatment resulted in significant reductions in hepatic SOCS-3 expression in the DIO models (Fig. 10C, DIO model 1, P < 0.05; DIO model 2, P < 0.01), it had no effect on hepatic SOCS-3 gene expression in ob/ob mice (data not shown). These data suggest that decreased SOCS-3 expression in response to decreased ceramide synthesis may, at least in part, have contributed to improved hepatic steatosis and the subsequent improvement of hepatic insulin resistance observed in myriocin-treated mice.
Fig. 10.
0: Amelioration of hepatic steatosis in myriocin-treated DIO mice. A: liver histology of control (top) and myriocin-treated (bottom) obese (ob/ob, DIO) mice. Sections obtained from 6 different animals were stained with hematoxylin & eosin. Original magnification, ×200. B: liver triglyceride content. C: SOCS-3 gene expression in liver from control and myriocin-treated mice. Data are means ± SD (n = 8).
DISCUSSION
Given the disease burden and health costs associated with the increased prevalence of obesity and the metabolic syndrome, identifying mechanisms in the pathogenesis of these disorders is crucial for the development of rational therapeutic options. Our studies support a novel role for ceramide biosynthesis in the onset of multiple features of the metabolic syndrome, including weight gain, hepatic steatosis, insulin resistance, and the induction of inflammatory adipokines that increase metabolic and cardiovascular risk (PAI-1, MCP-1). Our studies also suggest that SOCS-3 and UCP3 may play a central mechanistic role in ceramide-mediated pathways that contribute to the metabolic syndrome.
We previously demonstrated that ceramide levels were increased in the adipose tissues and plasma of obese mice (42, 46), with the largest increase observed for C16 and C18 ceramide. Inhibition of de novo ceramide generation with myriocin, a specific inhibitor of SPT, decreased total plasma ceramide. Interestingly, 16 wk on the HFD (DIO model 2) decreased plasma ceramide by almost 50% in response to myriocin. This reflects the large decreases observed for all individual ceramide species, specifically C20, C24, and C24:1, which we have previously shown are major constituents of plasma ceramide (46). In contrast, the largest decreases in plasma ceramide in myriocin-treated DIO model 1 (8 wk on the HFD) were observed for C16 and C18 ceramide, which represent minor constituents in the plasma ceramide profile (46). Although the reason for this is unclear, these selective changes in specific plasma ceramides in response to myriocin in the various treatment regimens and obese models may be related to the expression of ceramide synthase/longevity assurance genes, a family of genes that regulate the fatty acid composition of ceramide (39). At present, however, it is also not known which tissues contribute to plasma ceramide levels, although the adipose tissues may be a candidate (F. Samad, unpublished observations).
Inhibition of de novo ceramide synthesis in obese mice resulted in decreased weight. This weight loss occurred despite similar food intake, indicating a shift in total energy balance toward increased metabolism, which was manifested by increased V̇o2, increased V̇co2, and increased activity. Regulation of body weight is governed by multiple pathways, including leptin signaling and the expression of UCPs. Leptin, a hormone secreted primarily by adipocytes, regulates central and peripheral signaling pathways leading to decreased food intake and/or increased metabolism/energy expenditure (10, 11, 58). Although obesity in the ob/ob mice is due to leptin deficiency, DIO rodents and most obese humans are resistant to the effects of leptin on the regulation of body weight. Thus, a major hallmark of leptin resistance is hyperleptinemia, increased food intake, and decreased metabolism (10). Our studies suggest that inhibition of de novo ceramide synthesis in DIO mice may improve leptin signaling, as indicated by weight reduction, decreased adipocyte size, decreased adipose tissue leptin expression, and increased metabolism, in myriocin-treated mice. However, weight reduction in myriocin-treated ob/ob mice that lack leptin is probably mediated by mechanisms unrelated to leptin signaling (e.g., UCP expression as discussed below). Although the proposition that ceramide synthesis may be involved in the pathogenesis of obesity-mediated leptin resistance is intriguing, further studies are needed to definitively prove this hypothesis. The mechanism of increased metabolism observed in myriocin-treated mice may also be mediated by the induction of UCPs, which are mitochondrial inner-membrane proteins that promote mitochondrial energy expenditure and thermogenesis and thereby play important roles in whole body energy expenditure (24). Inhibition of de novo ceramide biosynthesis increased adipose UCP3 gene expression, and treatment of 3T3-L1 adipocytes with the short-chain ceramide analog C6 directly reduced UCP3 expression. These studies suggest for the first time that aberrant ceramide accumulation may contribute to weight gain via direct effects on genes (e.g., UCP3) involved in energy metabolism and expenditure.
We show that improvements in insulin sensitivity in myriocin-treated mice are mediated primarily at the level of insulin signaling in the liver and muscle, with a lesser and/or an indirect contribution by the adipose tissues. In addition to insulin-mediated increase in Akt phosphorylation, we also observed a sustained increase in basal Akt phosphorylation in the liver and muscle in myriocin-treated mice. These results are similar to the sustained increase in hepatic Akt phosphorylation observed in the liver specific c-Jun NH2-terminal kinase-1 (JNK1)-deficient mice, which also resulted in improved insulin sensitivity (56). Importantly, ceramide has been shown to activate JNK (40). An interesting question is whether inhibition of de novo ceramide synthesis in vivo also leads to the downregulation of JNK signaling pathways, and these studies are currently in progress. In our studies, we observed that inhibition of de novo ceramide synthesis of normal lean mice on a chow diet led to modest glucose intolerance and insulin resistance. These studies may suggest that physiological levels of ceramide/ceramide subspecies profiles and/or its downstream metabolites (e.g., sphingosine, S1P, etc.) plays a role in maintaining glucose hemostasis. Indeed, such a concept is not unlikely, given that sphingolipids are components of lipid rafts/caveoli, important in various signaling pathways including insulin signaling (8, 18, 36).
The improvements in hepatic insulin signaling in myriocin-treated obese mice also paralleled a significant reduction in hepatic steatosis. Fatty liver is associated with obesity, insulin resistance, and type 2 diabetes (37), and removal of liver fat improves hepatic insulin sensitivity (9). Importantly, increased adipose ceramide characterized subjects with fatty liver independently of obesity (23), suggesting that the decrease in fatty liver in myriocin-treated mice may also be indirectly related to inhibition of ceramide production from adipose tissues. Dexamathasone treatment induced a dramatic increase in ceramide within the portal vein, suggesting that ceramide is secreted from adipose tissues and may be an important modulator of insulin sensitivity in the liver (15). Thus, the reduction in plasma ceramide in myriocin-treated obese mice in this study may also reflect decreased secretion of ceramide from adipose tissue stores. Moreover, the reduced adipose expression of molecules such as PAI-1 and MCP-1 observed in this study may also contribute to the amelioration of insulin resistance in peripheral tissues.
Myriocin failed to reduce adipose TNF-α expression in obese mice, suggesting that ceramide works independently or is downstream of this inflammatory mediator. Obese (ob/ob) mice deficient in both TNF-α receptors (p55, p75) have significantly lower levels of plasma ceramide compared with wild-type ob/ob mice, indicating that TNF-α is upstream of the pathway leading to elevated ceramide in the setting of obesity. In contrast to TNF-α, inhibition of de novo ceramide biosynthesis decreased both PAI-1 and MCP-1 expression in adipose tissue of obese mice. Elevated PAI-1 is one of the biochemical hallmarks of obesity (6) and, may contribute directly to insulin resistance, type 2 diabetes, and cardiovascular complications and may even influence adipose tissue accumulation (5). Ceramide can induce the expression of PAI-1 from adipocytes (42) and endothelial cells (47). Conversely, mice deficient for PAI-1 were protected from HFD-induced increase in adipose and plasma ceramide (46), suggesting an unexpected link between sphingolipid metabolism and PAI-1. MCP-1 is also elevated in adipose tissues in obesity and implicated to play a role in adipose tissue macrophage recruitment and insulin resistance (20, 22, 30, 31) and accelerated atherosclerosis (12, 13, 34). We previously showed that ceramide directly induces MCP-1 gene expression in adipocytes (42), and here we provide evidence that ceramide contributes to adipose tissue MCP-1 expression in vivo in DIO mice.
While our studies clearly indicate that myriocin treatment improves metabolic and inflammatory parameters in obese mice, they do so concurrent with weight loss. As such, it is possible that the reduced weight may have translated into the downstream improvements in hypoglycemia and inflammation. Conversely, it is also likely that reduced ceramide levels directly influence insulin signaling and inflammatory gene expression in target tissues. In this respect, we previously showed that exogenous ceramide directly upregulates inflammatory gene expression in cultured adipocytes (42), and numerous in vitro studies have demonstrated a direct role for ceramide and/or its metabolites in the development of insulin resistance (41). In more recent, unpublished studies, myriocin treatment of “extremely obese” C57/BL6J mice that were on an HFD for an extensive period of time (20 wk) did not result in weight loss in the first 3 wk, yet hyperglycemia was improved in myriocin-treated mice (F. Samad, unpublished observations). In this model, weight loss was observed only after the third week of myriocin treatment (F. Samad, unpublished observations). It is likely that a combination of weight loss and direct effects of reduced ceramide resulted in the beneficial outcomes observed in this study. Weight loss was not observed in myriocin-treated Zucker and ZDF rats (15). Although this difference is difficult to explain, it may include effects due to different models/genetic backgrounds, diets, duration of experiments, treatment regimens used, and other cofounders in the two studies.
The SOCS family of proteins play central mechanistic roles in the development of both leptin and insulin signaling pathways (17, 27, 50). SOCS-3 expression is high in adipose tissues of various models of murine obesity and appears to contribute to the pathogenesis of the metabolic syndrome (21, 50, 53). SOCS-3 contributes to leptin resistance by binding JAK tyrosine kinase and attenuating its ability to phosphorylate signal transducer and activator of transcription (STAT) proteins (33, 57). SOCS-3 binds insulin receptor and prevents its binding to IRS-1, thus inhibiting IRS-1 phosphorylation and downstream insulin signaling (27). SOCS-3 also inhibits insulin signaling by targeting both IRS-1 and IRS-2 for proteosomal degradation (27). SOCS-3 expression is significantly reduced in TNF receptor-deficient ob/ob mice (7), and we show that inhibition of de novo ceramide synthesis in DIO mice decreases V̇o2 adipose expression of SOCS-3 mRNA. Moreover, ceramide induces SOCS-3 expression in cultured adipocytes. Inhibition of SOCS-3 dramatically ameliorates hepatic steatosis in obese mice (50), suggesting that SOCS-3 also contributes to the pathogenesis of obesity-associated fatty liver. Indeed, our data also show that the significant improvement of hepatic steatosis in myriocin-treated DIO mice is also associated with a concurrent decrease in hepatic SOCS-3 gene expression. Together, these results are indicative of an in vivo mechanism in which chronic increase in TNF-α during weight gain leads to increased ceramide biosynthesis, which in turn directly contributes to induction of SOCS-3, providing a mechanistic link by which ceramide contributes to the pathogenesis of multiple features of the metabolic syndrome. Definitive proof of this hypothesis, however, requires additional experiments, which are currently ongoing.
In conclusion, this in vivo study demonstrates a central role for ceramide biosynthesis in the pathogenesis of obesity and suggests that the ceramide pathway (ceramide and/or its downstream metabolites) may be a novel therapeutic target for inducing weight loss, improving insulin resistance, and specific prothrombotic/proinflammatory profiles in obese patients.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01 HL-071146 to F. Samad and a Blueprint Core grant to A. J. Roberts.
Acknowledgments
We gratefully acknowledge the assistance of Coree Levy (Mouse Behavioral Assessment Core, The Scripps Research Institute).
REFERENCES
- 1.Axelsson J, Heimburger O, Lindholm B, Stenvinkel P. Adipose tissue and its relation to inflammation: the role of adipokines. J Ren Nutr 15: 131–136, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Bielawski J, Szulc ZM, Hannun YA, Bielawska A. Simultaneous quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods 39: 82–91, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Cernkovich ER, Deng J, Bond MC, Combs TP, Harp JB. Adipose-specific disruption of signal transducer and activator of transcription 3 increases body weight and adiposity. Endocrinology 149: 1581–1590, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang L, Chiang SH, Saltiel AR. Insulin signaling and the regulation of glucose transport. Mol Med 10: 65–71, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De TB, Smith LH, Vaughan DE. Plasminogen activator inhibitor-1: a common denominator in obesity, diabetes and cardiovascular disease. Curr Opin Pharmacol 5: 149–154, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Dellas C, Loskutoff DJ. Historical analysis of PAI-1 from its discovery to its potential role in cell motility and disease. Thromb Haemost 93: 631–640, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Emanuelli B, Peraldi P, Filloux C, Chavey C, Freidinger K, Hilton DJ, Hotamisligil GS, Van OE. SOCS-3 inhibits insulin signaling and is up-regulated in response to tumor necrosis factor-alpha in the adipose tissue of obese mice. J Biol Chem 276: 47944–47949, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Foster LJ, Chan QW. Lipid raft proteomics: more than just detergent-resistant membranes. Subcell Biochem 43: 35–47, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Friedman J Fat in all the wrong places. Nature 415: 268–269, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature 395: 763–770, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Friedman JM, Leibel RL. Tackling a weighty problem. Cell 69: 217–220, 1992. [DOI] [PubMed] [Google Scholar]
- 12.Gosling J, Slaymaker S, Gu L, Tseng S, Zlot CH, Young SG, Rollins BJ, Charo IF. MCP-1 deficiency reduces susceptibility to atherosclerosis in mice that overexpress human apolipoprotein B. J Clin Invest 103: 773–778, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, Rollins BJ. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell 2: 275–281, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Hannun YA, Obeid LM. The ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J Biol Chem 277: 25847–25850, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, Nelson DH, Karathanasis SK, Fontenot GK, Birnbaum MJ, Summers SA. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab 5: 167–179, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science 259: 87–91, 1993. [DOI] [PubMed] [Google Scholar]
- 17.Howard JK, Flier JS. Attenuation of leptin and insulin signaling by SOCS proteins. Trends Endocrinol Metab 17: 365–371, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Ikonen E, Vainio S. Lipid microdomains and insulin resistance: is there a connection? Sci STKE 2005: e3, 2005. [DOI] [PubMed]
- 19.Juhan-Vague I, Alessi MC, Mavri A, Morange PE. Plasminogen activator inhibitor-1, inflammation, obesity, insulin resistance and vascular risk. J Thromb Haemost 1: 1575–1579, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Kamei N, Tobe K, Suzuki R, Ohsugi M, Watanabe T, Kubota N, Ohtsuka-Kowatari N, Kumagai K, Sakamoto K, Kobayashi M, Yamauchi T, Ueki K, Oishi Y, Nishimura S, Manabe I, Hashimoto H, Ohnishi Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Nagai R, Kadowaki T. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem 281: 26602–26614, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Kanatani Y, Usui I, Ishizuka K, Bukhari A, Fujisaka S, Urakaze M, Haruta T, Kishimoto T, Naka T, Kobayashi M. Effects of pioglitazone on suppressor of cytokine signaling 3 expression: potential mechanisms for its effects on insulin sensitivity and adiponectin expression. Diabetes 56: 795–803, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 116: 1494–1505, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolak M, Westerbacka J, Velagapudi VR, Wagsater D, Yetukuri L, Makkonen J, Rissanen A, Hakkinen AM, Lindell M, Bergholm R, Hamsten A, Eriksson P, Fisher RM, Oresic M, Yki-Jarvinen H. Adipose tissue inflammation and increased ceramide content characterize subjects with high liver fat content independent of obesity. Diabetes 56: 1960–1968, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Kozak LP, Harper ME. Mitochondrial uncoupling proteins in energy expenditure. Annu Rev Nutr 20: 339–363, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Kraegen EW, Cooney GJ, Ye JM, Thompson AL, Furler SM. The role of lipids in the pathogenesis of muscle insulin resistance and beta cell failure in type II diabetes and obesity. Exp Clin Endocrinol Diabetes 109, Suppl 2: S189–S201, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Le BO, Petroulakis E, Paglialunga S, Poulin F, Richard D, Cianflone K, Sonenberg N. Elevated sensitivity to diet-induced obesity and insulin resistance in mice lacking 4E-BP1 and 4E-BP2. J Clin Invest 117: 387–396, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lebrun P, Van OE. SOCS proteins causing trouble in insulin action. Acta Physiol (Oxf) 192: 29–36, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Lelliott C, Vidal-Puig AJ. Lipotoxicity, an imbalance between lipogenesis de novo and fatty acid oxidation. Int J Obes Relat Metab Disord 28, Suppl 4: S22–S28, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Loskutoff DJ, Samad F. The adipocyte and hemostatic balance in obesity: Studies of PAI-1. Arterioscler Thromb Vasc Biol 18: 1–6, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117: 175–184, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes 56: 16–23, 2007. [DOI] [PubMed] [Google Scholar]
- 32.McGarry JD Banting lecture 2001. Dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 51: 7–18, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Narazaki M, Fujimoto M, Matsumoto T, Morita Y, Saito H, Kajita T, Yoshizaki K, Naka T, Kishimoto T. Three distinct domains of SSI-1/SOCS-1/JAB protein are required for its suppression of interleukin 6 signaling. Proc Natl Acad Sci USA 95: 13130–13134, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nelken NA, Coughlin SR, Gordon D, Wilcox JN. Monocyte chemoattractant protein-1 in human atheromatous plaques. J Clin Invest 88: 1121–1127, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nicholls DG, Locke RM. Thermogenic mechanisms in brown fat. Physiol Rev 64: 1–64, 1984. [DOI] [PubMed] [Google Scholar]
- 36.Ohanian J, Ohanian V. Sphingolipids in mammalian cell signalling. Cell Mol Life Sci 58: 2053–2068, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palasciano G, Moschetta A, Palmieri VO, Grattagliano I, Iacobellis G, Portincasa P. Non-alcoholic fatty liver disease in the metabolic syndrome. Curr Pharm Des 13: 2193–2198, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Pandey M, Tuncman G, Hotamisligil GS, Samad F. Divergent roles for p55 and p75 TNF-α receptors in the induction of plasminogen activator inhibitor-1. Am J Pathol 162: 933–941, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pewzner-Jung Y, Ben-Dor S, Futerman AH. When do Lasses (longevity assurance genes) become CerS (ceramide synthases)?: insights into the regulation of ceramide synthesis. J Biol Chem 281: 25001–25005, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Ruvolo PP Intracellular signal transduction pathways activated by ceramide and its metabolites. Pharmacol Res 47: 383–392, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Samad F Contribution of sphingolipids to the pathogenesis of obesity. Future Lipidol 2: 625–639, 2008. [Google Scholar]
- 42.Samad F, Hester KD, Yang G, Hannun YA, Bielawski J. Altered adipose and plasma sphingolipid metabolism in obesity: a potential mechanism for cardiovascular and metabolic risk. Diabetes 55: 2579–2587, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Samad F, Yamamoto K, Loskutoff DJ. Distribution and regulation of plasminogen activator inhibitor-1 in murine adipose tissue in vivo: Induction by tumor necrosis factor-α and lipopolysaccharide. J Clin Invest 97: 37–46, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Samad F Contribution of sphingolipids to the pathogenesis of obesity. Future Lipidology 2: 625–639, 2007. [Google Scholar]
- 45.Sethi JK, Xu H, Uysal KT, Wiesbrock SM, Scheja L, Hotamisligil GS. Characterisation of receptor-specific TNFα functions in adipocyte cell lines lacking type 1 and 2 TNF receptors. FEBS Lett 469: 77–82, 2000. [DOI] [PubMed] [Google Scholar]
- 46.Shah C, Yang G, Lee I, Bielawski J, Hannun YA, Samad F. Protection from high fat diet-induced increase in ceramide in mice lacking plasminogen activator inhibitor 1. J Biol Chem 283: 13538–13548, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soeda S, Honda O, Shimeno H, Nagamatsu A. Sphingomyelinase and cell-permeable ceramide analogs increase the release of plasminogen activator inhibitor-1 from cultured endothelial cells. Thromb Res 80: 509–518, 1995. [DOI] [PubMed] [Google Scholar]
- 48.Steinberg GR, McAinch AJ, Chen MB, O'Brien PE, Dixon JB, Cameron-Smith D, Kemp BE. The suppressor of cytokine signaling 3 inhibits leptin activation of AMP-kinase in cultured skeletal muscle of obese humans. J Clin Endocrinol Metab 91: 3592–3597, 2006. [DOI] [PubMed] [Google Scholar]
- 49.Summers SA Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res 45: 42–72, 2006. [DOI] [PubMed] [Google Scholar]
- 50.Ueki K, Kondo T, Tseng YH, Kahn CR. Central role of suppressors of cytokine signaling proteins in hepatic steatosis, insulin resistance, and the metabolic syndrome in the mouse. Proc Natl Acad Sci USA 101: 10422–10427, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Unger RH Minireview. Weapons of lean body mass destruction: the role of ectopic lipids in the metabolic syndrome. Endocrinology 144: 5159–5165, 2003. [DOI] [PubMed] [Google Scholar]
- 52.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-α function. Nature 389: 610–614, 1997. [DOI] [PubMed] [Google Scholar]
- 53.Wang MY, Orci L, Ravazzola M, Unger RH. Fat storage in adipocytes requires inactivation of leptin's paracrine activity: implications for treatment of human obesity. Proc Natl Acad Sci USA 102: 18011–18016, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang ZW, Pan WT, Lee Y, Kakuma T, Zhou YT, Unger RH. The role of leptin resistance in the lipid abnormalities of aging. FASEB J 15: 108–114, 2001. [DOI] [PubMed] [Google Scholar]
- 55.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest 116: 115–124, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang R, Wilcox DM, Haasch DL, Jung PM, Nguyen PT, Voorbach MJ, Doktor S, Brodjian S, Bush EN, Lin E, Jacobson PB, Collins CA, Landschulz KT, Trevillyan JM, Rondinone CM, Surowy TK. Liver-specific knockdown of JNK1 up-regulates proliferator-activated receptor gamma coactivator 1 beta and increases plasma triglyceride despite reduced glucose and insulin levels in diet-induced obese mice. J Biol Chem 282: 22765–22774, 2007. [DOI] [PubMed] [Google Scholar]
- 57.Yasukawa H, Misawa H, Sakamoto H, Masuhara M, Sasaki A, Wakioka T, Ohtsuka S, Imaizumi T, Matsuda T, Ihle JN, Yoshimura A. The JAK-binding protein JAB inhibits Janus tyrosine kinase activity through binding in the activation loop. EMBO J 18: 1309–1320, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 372: 425–432, 1994. [DOI] [PubMed] [Google Scholar]
- 59.Zheng W, Kollmeyer J, Symolon H, Momin A, Munter E, Wang E, Kelly S, Allegood JC, Liu Y, Peng Q, Ramaraju H, Sullards MC, Cabot M, Merrill AH Jr. Ceramides and other bioactive sphingolipid backbones in health and disease: lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Biochim Biophys Acta 1758: 1864–1884, 2006. [DOI] [PubMed] [Google Scholar]