Abstract
Insulin-induced severe hypoglycemia causes brain damage. The hypothesis to be tested was that diabetes portends to more extensive brain tissue damage following an episode of severe hypoglycemia. Nine-week-old male streptozotocin-diabetic (DIAB; n = 10) or vehicle-injected control (CONT; n = 7) Sprague-Dawley rats were subjected to hyperinsulinemic (0.2 U·kg−1·min−1) severe hypoglycemic (10–15 mg/dl) clamps while awake and unrestrained. Groups were precisely matched for depth and duration (1 h) of severe hypoglycemia (CONT 11 ± 0.5 and DIAB 12 ± 0.2 mg/dl, P = not significant). During severe hypoglycemia, an equal number of episodes of seizure-like activity were noted in both groups. One week later, histological analysis demonstrated extensive neuronal damage in regions of the hippocampus, especially in the dentate gyrus and CA1 regions and less so in the CA3 region (P < 0.05), although total hippocampal damage was not different between groups. However, in the cortex, DIAB rats had significantly (2.3-fold) more dead neurons than CONT rats (P < 0.05). There was a strong correlation between neuronal damage and the occurrence of seizure-like activity (r2 > 0.9). Separate studies conducted in groups of diabetic (n = 5) and nondiabetic (n = 5) rats not exposed to severe hypoglycemia showed no brain damage. In summary, under the conditions studied, severe hypoglycemia causes brain damage in the cortex and regions within the hippocampus, and the extent of damage is closely correlated to the presence of seizure-like activity in nonanesthetized rats. It is concluded that, in response to insulin-induced severe hypoglycemia, diabetes uniquely increases the vulnerability of specific brain areas to neuronal damage.
Keywords: Fluoro-Jade, insulin, seizure, streptozotocin
hypoglycemia is the most prevalent clinical complication in the daily management of insulin-treated people with diabetes, and hypoglycemia continues to be the limiting factor in the glycemic management of diabetes (15). Since severe hypoglycemia affects 40% of insulin-treated people with diabetes (49), concern regarding the hazardous potential for severe hypoglycemia to cause “brain damage” continues to be a very real barrier in striving to fully realize the benefits associated with intensive glycemic control (14). Animal models have unambiguously demonstrated that acute episodes of severe hypoglycemia [blood glucose (BG) <18 mg/dl] reproducibly induce neuronal damage, especially in the vulnerable neurons in the cortex and hippocampus (2, 9, 33, 44, 45, 54). Deficits in learning and memory have been shown to be a direct consequence of this severe hypoglycemia-induced hippocampal neuronal damage (2, 44, 45). However, clincial studies in patients with diabetes have yielded variable results, since episodes of severe hypoglycemia have been shown to alter brain structure (37) and have been reported to cause significant cognitive damage in many (7, 16, 18, 23, 31, 34, 35, 39) but not all (4, 20, 25, 41, 43, 55) studies.
The presence of diabetes and associated hyperglycemia has been shown to worsen the extent of neuronal damage following other forms of central nervous system insults (i.e., stroke) in both preclinical (24, 26, 30) and clinical (5, 11) settings. This study was undertaken to determine whether neuronal damage induced by severe hypoglycemia would be similarly augmented due to the presence of diabetes per se.
RESEARCH DESIGN AND METHODS
Animals.
Nine-week-old male Sprague-Dawley rats (Charles River Laboratories) were housed individually in a temperature- and light-controlled environment, maintaining each animal's diurnal cycle (12:12-h light-dark), and with an ad libitum diet. All studies were done in accordance with and approved by the Animal Studies Committee at the Washington University School of Medicine.
Implantation of arterial and venous catheters. Anesthesia was induced with an intraperitoneal injection of 87 mg/kg ketamine and 2.6 mg/kg xylazine. A Micro-Renathane brand (Braintree Scientific, Boston, MA) catheter was inserted into the left common carotid artery, and two catheters were implanted into the right jugular vein. To prevent clotting, catheters were filled with a 40% polyvinylpyrrolidone (Sigma, St. Louis, MO) in heparin (1,000 USP U/ml) solution (Baxter Healthcare, Deerfield, IL).
Induction of diabetes.
After 3 days of recovery from surgery, rats received intraperitoneal injections of either streptozotocin (STZ; 65 mg/kg, n = 10; Sigma) to induce diabetes (DIAB) or vehicle 0.1 mM sodium citrate (Fisher Scientific) buffer (n = 7) as a control (CONT). One DIAB rat required a second injection of STZ to ensure random BG >300 mg/dl. Following STZ or vehicle injection, measurements of their body weight were made, and random fed BG was measured from the tail vein by Ascensia Contour BG monitors (Bayer HealthCare, Mishawaka, IN).
Hypoglycemic hyperinsulinemic clamp.
The clamp experiment was performed 1 wk after the date of STZ or citrate buffer injection. After an overnight fast, vascular catheters were externalized and attached to syringes. After a basal period to allow the rats to acclimate, insulin (Humulin R; Eli Lilly, Indianapolis, IN) was administered intravenously with a bolus (6 U/kg) followed by the constant infusion of a lower dose of insulin (0.2 U·kg−1·min−1) into awake, unrestrained rats. At the start of insulin infusion, control rats also received intravenous glucose (50% dextrose; Hospira, Lake Forest, IL) to match the rate of BG decline observed in diabetic rats. Throughout the clamp, BG was measured from the arterial cannula every 15 min using Ascensia Contour BG monitors. On the basis of pilot experiments that determined the nadir hypoglycemia necessary to reproducibly cause neuronal damage, the target for severe hypoglycemia was glucose levels <15 mg/dl. Once the BG dropped to <15 mg/dl, the clock was reset and hypoglycemia maintained at 10–15 mg/dl for 1 h by adjusting the rate of continuous intravenous glucose infusion. This technique allowed both groups to be matched precisely for duration (1 h) and depth of hypoglycemia in unrestrained animals, without the confounding effects of anesthesia. One CONT and one DIAB rat were not included in statistical analysis because they did not reach this level of hypoglycemia. Seizure-like activity was quantified by counting the number of characteristic, brief (<5 s), myoclonic convulsions. After the 1 h of severe hypoglycemia the insulin infusion was discontinued, and the rats were given a 3-ml intraperitoneal injection of glucose in conjunction with increased delivery of glucose through the indwelling catheters to quickly restore normoglycemia. Throughout the 4-h recovery period BG readings were made every 15 min, and the rates of glucose infusion were slowly decreased as the glucose-lowering effects of insulin waned. The rats were euthanized 1 wk following the episode of severe hypoglycemia for histological evaluation (2, 9, 33, 44, 45, 54).
Two more groups of rats that were not subjected to an episode of hypoglycemia served as baseline negative controls for histological purposes. A group of citrate buffer-injected control rats (n = 5) that did not undergo hypoglycemia were euthanized to examine normal histology, whereas a fourth group of STZ-diabetic rats (n = 5) that were not subjected to an episode of hypoglycemia served as a negative control, demonstrating that STZ treatment and diabetes per se do not induce neuronal damage in the absence of severe hypoglycemia.
Histology.
Rats were anesthetized using isoflurane and then transcardially perfused with 100 ml of 0.01 M phosphate-buffered saline (Sigma) and 100 ml of 4% paraformaldehyde (Electron Microscopy Scientific). Perfused brains were left in 4% paraformaldehyde overnight in 4°C and then cryoprotected in 30% sucrose (Sigma). Brains were then set in Tissue-Tek OCT Compound (Sakura Finetek, Torrance, CA) and stored at −20°C. Sections of 20 and 40 microns were cut at −20°C on a Leica CM1850 cryostat (Leica Microsystems, Bannockburn, IL). Perfused frozen coronal brain section (20–40 μm) slides were stained with either Fluoro-Jade B (FJB; Chemicon International) or hematoxylin and eosin (H & E; Sigma) and examined with epifluorescent microscope (Zeiss) using fluorescein isothiocynate (for FJB).
Microscopy and cell counting.
Every 10th section for a total of five coronal sections between 2.8 and 3.3 mm posterior to Bregma were collected and analyzed from each animal. H & E staining was used to help identify the different regions and identify cell morphology. For quantification purposes, the number of FJB-positive (FLB+) cells per high-power field was assessed by an observer blinded to the treatment conditions. The average number of FJB-positive cells per brain section are shown.
Statistical analysis.
All data expressed as means ± SE. P values were obtained by two-tailed Student t-test. Significance was determined by a P value <0.05.
RESULTS
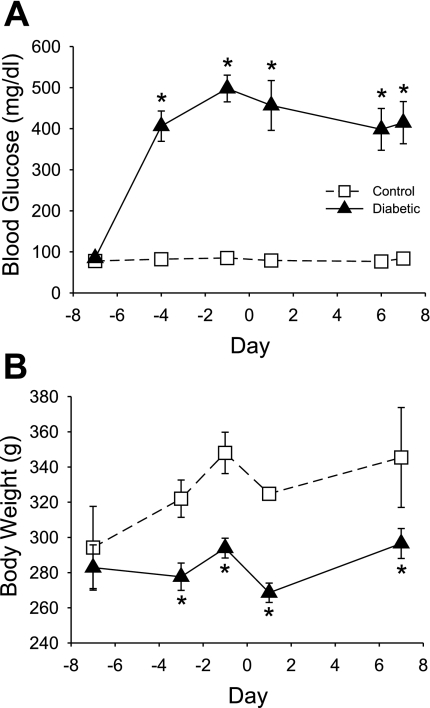
Rats injected with STZ displayed a diabetic phenotype with elevated BG levels from 3 days postinjection until the end of the experiment when compared with control rats injected only with buffer (CONT 79 ± 2 mg/dl vs. DIAB 381 ± 17 mg/dl, P < 0.05; Fig. 1A). Consistent with a diabetic phenotype, DIAB rats failed to gain weight throughout the experiment and had significantly lower body weight than CONT rats throughout the experiment (final body weight: CONT 345 ± 28 g vs. DIAB 297 ± 9 g, P < 0.05; Fig. 1B).
Fig. 1.
Effect of streptozotocin (STZ) on resting blood glucose and body weight. A: following STZ treatment, diabetic rats (n = 9; ▴) had markedly elevated blood glucose levels compared with buffer-treated control rats (n = 6; □). An episode of severe hypoglycemia was induced on day 0. B: diabetic rats failed to gain weight throughout the experiment and had significantly lower body weights than control rats throughout the experiment. *P < 0.05.
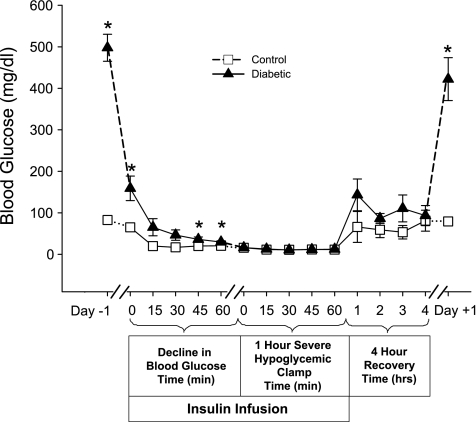
During the hypoglycemic hyperinsulinemic clamp, both groups experienced the same degree of hypoglycemia for 1 h [CONT 12 ± 0.2 mg/dl, DIAB 11 ± 0.5 mg/dl, P = not significant (NS); Fig. 2]. During the 1 h of severe hypoglycemia, rats became unresponsive, lost their righting reflexes, and displayed brief episodes of seizure-like activity. Immediately following this period of severe hypoglycemia, glucose was infused so that BGs were rapidly (within 15 min) returned to euglycemic levels (Fig. 2). During the recovery period, rats regained consciousness and their righting reflexes, and their episodes of seizure-like activity ceased. Exogenous glucose was infused for ∼4 h until insulin's effects wore off and glycemia could be maintained in the absence of glucose infusion. After 1 h of recovery, there was a trend for DIAB rats to have a higher BG than CONT rats (143 ± 38 vs. 67 ± 38 mg/dl, P = NS). At the end of recovery, both groups of rats reached similar levels of glycemia (DIAB 93 ± 24, CONT 67 ± 38, P = NS; Fig. 2). During recovery, each group received similar amounts of glucose (DIAB 3.2 ± 0.2 g, CONT 3.5 ± 0.3 g, P = NS). One day following recovery, BGs were higher in the DIAB (422 ± 52 mg/dl) than in the CONT (79 ± 5 mg/dl) rats and remained elevated until the rats were euthanized (final glucose: CONT 84 ± 14 mg/dl vs. DIAB 414 ± 145 mg/dl, P < 0.05). Animals regained their previous weight 1 wk after the episode of severe hypoglycemia. Note that the negative control group of STZ-diabetic rats (n = 5) that were not subjected to an episode of severe hypoglycemia had a random glucose (479 ± 48 mg/dl) on the day of their euthanization not different from the DIAB rats that were exposed to severe hypoglycemia.
Fig. 2.
Blood glucose of rats before, during, and after severe hypoglycemia. Blood glucose levels of the control (n = 6; □) and diabetic (n = 9; ▴) rats are shown during the hyperinsulinemic severe hypoglycemic clamp as well as 1 day prior to and 1 day after the clamp. On the day of the clamp, insulin was infused intravenously at a constant rate (0.2 U·kg−1·min−1) to lower blood glucose. Once the blood glucose was ≤15 mg/dl, the clock was reset and glucose was infused to maintain a level of severe hypoglycemia between 10 and 15 mg/dl for 1 h. Following the 1 h of severe hypoglycemia, the insulin infusion was stopped and the rate of glucose infusion increased to restore euglycemia for 4 h. *P < 0.05.
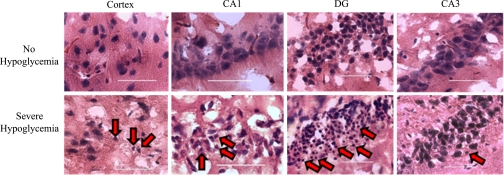
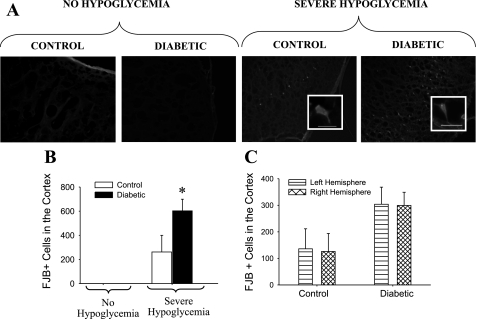
Rats that underwent a single episode of severe hypoglycemia had condensed morphology and pyknotic nuclei in the cortex and hippocampus (CA1 region, dentate gyrus, CA3 region) compared with rats that did not undergo severe hypoglycemia as identified by the nuclear H & E staining (Fig. 3). Brain sections were also stained for FJB, a specific marker for neuronal degeneration (40), which stained positively in cells of the cortex and hippocampus. FJB-positive (FJB+) cells were counted and used as a marker to quantify the extent of brain cell damage (Figs. 4 and 5). It is important to note that, in the absence of severe hypoglycemia, the induction of diabetes with STZ itself did not result in the appearance of FJB+ cells in the cortex, hippocampus, or hypothalamus (Figs. 4A and 5A). In the rats that underwent severe hypoglycemia, particularly noteworthy was the significantly more (P < 0.05) FJB+ cells seen throughout the cortex of DIAB vs. CONT rats (Fig. 4B). DIAB rats had a 2.3-fold increase in the amount of FJB+ cells found in the cortex (CONT 262 ± 138 FJB+ cells vs. DIAB 603 ± 96 FJB+ cells, P < 0.05). The amount of FJB+ cells was assessed in both hemispheres of each section. There was no difference in the amount of cortical FJB+ cells in the left vs. the right lobe of the brain in either group (CONT 136 ± 75, 126 ± 68; DIAB 304 ± 65, 299 ± 50 FJB+ cells, left and right hemispheresm respectively; Fig. 4C).
Fig. 3.
Neuronal damage as assessed by hematoxylin and eosinophilic staining. Compared with rats that did not undergo hypoglycemia, neuronal damage induced following 1 h of severe hypoglycemia was evidenced by hematoxylin and eosinophilic staining. The rats that underwent severe hypoglycemia show brain cells characterized by condensed shrunken morphology and pyknotic nuclei (red arrows) with eosinophilic staining compared with the nuclei of rats that did not undergo hypoglycemia. This brain damage morphology is most evident in the cortex and specific regions of the hippocampus [CA1 and dentate gyrus (DG)]. ×400 Magnification with the scale bar indicating 50 microns.
Fig. 4.
Neuronal damage in the cortex. A: Fluoro-Jade B-positive (FJB+) staining in representative images taken at 3.1 mm posterior to Bregma in the lateral ectorhinal cortex superior to the rhinal fissure and viewed at ×100 magnification. A, insets: FJB+ cells viewed at ×400 magnification, with the bar indicating 100 microns. The 4 groups shown are nondiabetic control and STZ-diabetic rats that did not undergo hypoglycemia as well as nondiabetic control rats and STZ-diabetic rats that were subjected to severe hypoglycemia. B: there were no cortical FJB+ cells seen in rats that did not undergo hypoglycemia, whereas, in response to severe hypoglycemia, STZ-induced diabetes caused a 2.3-fold increase in the number of cortical FJB+ cells per brain section compared with nondiabetic controls. *P < 0.05. C: there was no difference in the amount of FJB+ cells in the left (bars with horizontal lines) vs. the right (cross-hatched bars) hemisphere of the brain in either control (n = 6) or diabetic (n = 9) rats.
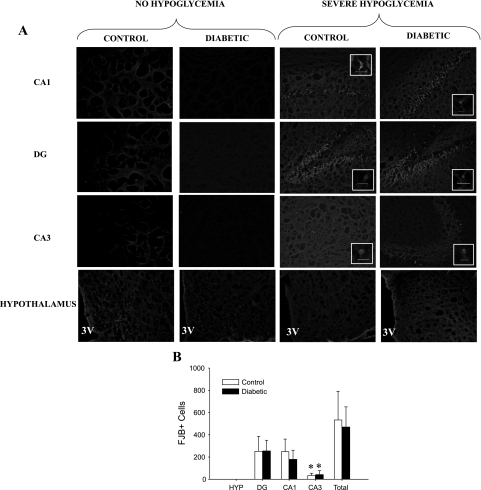
Fig. 5.
Hippocampal and hypothalamic neuronal damage. A: FJB+ staining in the CA1, DG, and CA3 regions of the hippocampus and the hypothalamus viewed at ×100 magnification. A, insets: FJB+ cells viewed at ×400 magnification, with the bars indicating 100 microns. The pictures of the hypothalamus (HYP) were taken lateral to the 3rd ventricle (3V). The 4 groups shown are nondiabetic control and STZ-diabetic rats that did not undergo hypoglycemia, nondiabetic control rats that were subjected to severe hypoglycemia, and STZ-diabetic rats that were subjected to severe hypoglycemia. B: in response to severe hypoglycemia, both control (n = 5) and diabetic (n = 8) rats demonstrated no neuronal damage in the hypothalamus, but hippocampal neuronal damage was most evident in the DG and CA1 regions, with significantly less damage in the CA3 region. *P < 0.05.
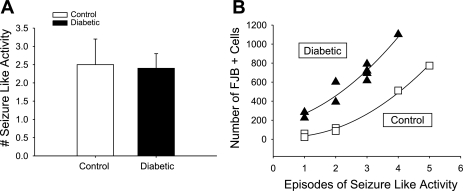
With regard to other areas of the brain, no appreciable Fluoro-Jade staining was noted in the glucose-sensing regions of the hypothalamus (Fig. 5A). However, there was significant FJB staining in the different regions of the hippocampus [dentate gyrus (DG), CA1, CA3] in the rats that underwent severe hypoglycemia (Fig. 5A). In both CONT and DIAB rats that underwent severe hypoglycemia, the number of cells staining positive for FJB were most notable in the DG and CA1 regions, with significantly less damage in the CA3 region (Fig. 5B). There was not a significant difference in the amount of total hippocampal FJB+ cells in DIAB (470 ± 181 cells) vs. CONT (532 ± 259 cells). Given that all blood sugars during the period of severe hypoglycemia were maintained in a narrow range (10–15 mg/dl), the amount of FJB staining was not correlated to glycemia (P = NS). There was no correlation between the length of time it took for the glucose levels to drop to a value <15 mg/dl and the amount of FJB+ cells in the cortex or hippocampus (P = NS). There was no significant difference in the amount of visible seizure-like activity between groups during the 1 h of severe hypoglycemia (CONT 2.5 ± 0.7 seizures, DIAB 2.4 ± 0.4 seizures; Fig. 6A). However, there was a strong correlation between the amount of FJB+ cells in the cortex and the number of seizure-like episodes (CONT r2 = 0.995, DIAB r2 = 0.924; Fig. 6B).
Fig. 6.
A: correlation between seizure-like activity and neuronal damage. Both groups experienced the same number of seizure-like events (control 2.5 ± 0.7, diabetic 2.4 ± 0.4, P = NS). B: the amount of damage incurred in the cortex strongly correlates with the amount of seizure-like activity occurring during the 1 h of severe hypoglycemia [control r2 = 0.996 (□), n = 6; diabetic r2 = 0.945 (▴), n = 9].
DISCUSSION
Severe hypoglycemia reproducibly causes brain damage in animals. In the setting of other causes of brain damage (i.e., ischemia, stroke, traumatic brain injury), it has been observed that the presence of diabetes and hyperglycemia leads to more extensive neuronal damage (5, 11, 24, 26, 30). In this study, severe hypoglycemia was shown to cause brain damage in the cortex and the hippocampus, and the extent of damage was closely correlated to the presence of seizure-like activity. Under conditions studied, the results indicate that diabetes per se increases the sensitivity of the cortex to the damaging effects resulting from a single episode of severe hypoglycemia.
Since severe hypoglycemia is not defined clinically by a specific value, but rather as a low-blood sugar event that requires the assistance of another person, a marked variability exists in the severity of hypoglycemia in clinical reports and the observation that cognitive damage is seen in some (7, 16, 18, 23, 31, 34, 35, 39) but not all (4, 20, 25, 41, 43, 55) studies. Unlike retrospective clinical studies, severe hypoglycemia induced in prospective studies with laboratory rats has reproducibly caused brain damage (2, 9, 33, 44, 45, 54). Consistent with the notion that hypoglycemic seizures are a marker of severe hypoglycemia, the extent of neuronal damage experienced by each rat was strongly correlated (r2 > 0.9) to the number of events of seizure-like activity. These correlations do not necessarily imply causality, since in this setting of profound hypoglycemia, vulnerable brain regions may be susceptible to both damage and seizure activity (2, 9, 33, 44, 45, 54). As in the real-world setting, seizures in these experiments were defined clinically. In the absence of EEG monitoring, we cannot rule out the possible presence (or effect) of subclinical seizures (i.e., not associated with any obvious motor activity) to the observed brain damage. Clinical studies corroborate this finding since neurocognitive deficits are not necessarily associated with severe hypoglycemia but are associated with witnessed hypoglycemic seizures (23, 39). Our novel findings indicate that seizure-like activity may serve as a clinical biomarker to identify those subjects who are at the highest risk for brain damage and those who would most likely benefit from neuroprotective therapy (2, 9, 33, 44, 45, 54).
With the clamp technique, all groups were precisely matched for duration and nadir of hypoglycemia (10–15 mg/dl). Given this very narrow range, seizures and neuronal damage were not correlated with blood glucose levels. Thus, increased seizure-like activity and more extensive brain damage were due not to more profound hypoglycemia but rather biological variability in being able to cope with (or respond to) the hypoglycemic episode. Although there is some evidence that diabetes per se can alter the glycemic thresholds for hypoglycemic comas (48), the current results do not indicate whether diabetes alters thresholds for hypoglycemic seizures because all blood sugars were lowered to the same degree. However, the difference in the regression curves demonstrates that although diabetic rats did not have more seizure-like activity, they experienced greater neuronal damage per seizure episode compared with control rats (Fig. 6B). These findings indicate that diabetes does not alter susceptibility to hypoglycemia-induced seizure-like activity but does render brain regions, specifically the cortex, more vulnerable to damage.
Consistent with previous reports, it was noted that hypoglycemic brain damage preferentially occurs in the CA1 and dentate gyrus regions of the hippocampus as well as the cortex (2, 9, 33, 44, 45, 54). Although hypoglycemia-induced neuronal injury to the hypothalamus has been reported as present (50), absent (51), or variable (3), in the current experiments, no hypothalamic brain damage was noted (Fig. 5). Hippocampal damage due to severe hypoglycemia has been associated with learning and memory deficits (2, 44, 45). The cortex, however, encompasses a diverse region responsible for auditory, olfactory, vision, sensory, and motor pathways. It remains unclear why diabetes uniquely increases the cortex's susceptibility to hypoglycemic damage. Previous studies have indicated that levels of chronic hyperglycemia similar to those achieved in our model cause morphological/biochemical changes that appear to differentially affect the cortex compared with the hippocampus. Specifically, STZ diabetes preferentially decreases dendritic neuronal length in the cortex compared with the hippocampus (28). Additionally, the induction of STZ diabetes alters neuronal cell density differently in the hippocampus compared with the cortex (27). Also, the cortex of STZ-diabetic rats experiences a preferential downregulation of N-methyl-d-aspartate receptors (6), which may be particularly important given the putative role of excess glutamate in mediating hypoglycemia-induced neuronal damage (2, 47). Taken together, this differential regional effect of diabetes on cortical vs. hippocampal brain morphology and biochemistry may have contributed to the enhanced susceptibility of the cortex to damage induced by hypoglycemia. Consistent with these findings of preferential damage to the cortex are clinical observations that severe hypoglycemia in people with diabetes leads to cortex-dependent deficits in motor speed, psychomotor efficiency, verbalization, and visual reasoning (7, 16, 39).
For patients with diabetes, severe hypoglycemia usually occurs following a high-dose, subcutaneous, single injection of insulin. However, in the laboratory setting, hyperinsulinemic hypoglycemic clamp methods were employed to control the absolute nadir and duration of hypoglycemia to reproducibly induce neuronal damage. A major confounding factor in laboratory severe hypoglycemia experiments is the use of anesthetic agents, which differs from real-world hypoglycemia because anesthesia 1) markedly reduces brain glucose uptake, which may increase brain damage, 2) abrogates seizure activity often associated with severe hypoglycemia, 3) induces hypothermia, 4) impairs the increased blood pressure (Cushing) response to hypoglycemia, and 5) has been shown to be neurotoxic (1, 10, 21, 22, 32). To circumvent these technical problems and consistently recreate real-world hypoglycemic conditions, our experiments were conducted in awake, freely mobile rodents without the confounding effects of anesthesia. Previous studies have identified an EEG pattern of isoelectricity as a marker for severe hypoglycemia (2, 9, 44, 45, 54). Since brain glucose metabolism can be markedly altered by diabetes (8, 17, 36, 42, 52), and the glycemic threshold for hypoglycemic coma may be altered by diabetes (48), it was speculated that the glycemic threshold for eliciting an isoelectric EEG recording could be altered in diabetic rats. Thus, for protocol standardization, it was thought to be critically important to match groups for the degree and duration of hypoglycemia rather than a specific EEG pattern.
The separate group of STZ-diabetic rats that did not undergo an episode of severe hypoglycemia served as negative controls, and they did not have any observable brain damage. Thus, the observed brain damage was specific to hypoglycemia per se and not due to STZ, hyperglycemia, or hypoinsulinemia. Cannulation of the left common carotid artery did not appear to have any significant effects because there was equal damage in both left and right hemispheres in response to global severe hypoglycemia (Fig. 4C).
Consistent with other studies using an STZ-diabetic rat model, our rats were diabetic for a total of 14 days (12, 13). The STZ-diabetic rat is a model of poorly controlled diabetes characterized by hyperglycemia, relative insulin deficiency, and hyperlipidemia. One or all of these factors may have played a contributing role by which diabetes leads to increased neuronal damage in response to an episode of severe hypoglycemia, but the exact mechanism was not directly assessed in these studies. There are many potential mechanisms for increased neuronal damage due to diabetes. The greater net drop in glycemia experienced in the diabetic rats (i.e., from 381 to 11 mg/dl) may have exacerbated the brain damage. Diabetes is well known to decrease brain glucose transporters (52) and alter glucose transport and metabolism (17), which may have led to the increased susceptibility to brain damage in DIAB rats despite equivalent systemic hypoglycemia. Following the episode of hypoglycemia, the effects of chronic hyperglycemia may have been toxic to vulnerable neurons, causing increased brain damage (26, 30). Since insulin has been shown to be neuroprotective in other models of brain damage, the chronic relative insulin deficiency in STZ rats may have played a role in enhanced brain damage (19, 29, 38, 53). There is evidence that neuronal damage induced from an episode of severe hypoglycemia is actually initiated when high concentrations of glucose are infused during the recovery period (46). Although reintroduction of glucose may contribute to neuronal damage, on the basis of the fact that equal amounts of glucose were given to both groups (DIAB 3.2 ± 0.2 g, CONT 3.5 ± 0.3 g, P = NS), it is unlikely that the reintroduction of glucose contributed to the differential amount of hypoglycemia-induced cortical damage observed in our STZ-diabetic rats.
In summary, severe hypoglycemia in nonanesthetized rats causes brain damage in the cortex and regions within the hippocampus, and the extent of damage is closely correlated to the presence of seizure like activity. In conclusion, under our experimental conditions, diabetes uniquely increases the susceptibility of specific brain regions to brain damage induced by severe hypoglycemia.
GRANTS
We gratefully acknowledge research support from the National Institute of Diabetes and Digestive and Kidney Diseases (DK-073683) and Juvenile Diabetes Research Foundation. A. J. Bree was supported by the Howard Hughes Medical Institute as part of a Summer Undergraduate Research Fellowship.
Acknowledgments
We thank Ronaldo Perez for technical support. We thank Drs. Owen Chan and Philip Cryer for valuable discussions.
REFERENCES
- 1.Alkire MT, Pomfrett CJ, Haier RJ, Gianzero MV, Chan CM, Jacobsen BP, Fallon JH. Functional brain imaging during anesthesia in humans: effects of halothane on global and regional cerebral glucose metabolism. Anesthesiology 90: 701–709, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Auer RN Hypoglycemic brain damage. Metab Brain Dis 19: 169–175, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Auer RN, Hugh J, Cosgrove E, Curry B. Neuropathologic findings in three cases of profound hypoglycemia. Clin Neuropathol 8: 63–68, 1989. [PubMed] [Google Scholar]
- 4.Austin EJ, Deary IJ. Effects of repeated hypoglycemia on cognitive function: a psychometrically validated reanalysis of the Diabetes Control and Complications Trial data. Diabetes Care 22: 1273–1277, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Baird TA, Parsons MW, Phanh T, Butcher KS, Desmond PM, Tress BM, Colman PG, Chambers BR, Davis SM. Persistent poststroke hyperglycemia is independently associated with infarct expansion and worse clinical outcome. Stroke 34: 2208–2214, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Bean L, Zheng H, Patel KP, Monaghan DT. Regional variations in NMDA receptor downregulation in streptozotocin-diabetic rat brain. Brain Res 1115: 217–222, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Bjorgaas M, Gimse R, Vik T, Sand T. Cognitive function in type 1 diabetic children with and without episodes of severe hypoglycaemia. Acta Paediatr 86: 148–153, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Boyle PJ, Kempers SF, O'Connor AM, Nagy RJ. Brain glucose uptake and unawareness of hypoglycemia in patients with insulin-dependent diabetes mellitus. N Engl J Med 333: 1726–1731, 1995. [DOI] [PubMed] [Google Scholar]
- 9.Butcher SP, Sandberg M, Hagberg H, Hamberger A. Cellular origins of endogenous amino acids released into the extracellular fluid of the rat striatum during severe insulin-induced hypoglycemia. J Neurochem 48: 722–728, 1987. [DOI] [PubMed] [Google Scholar]
- 10.Canal CE, McNay EC, Gold PE. Increases in extracellular fluid glucose levels in the rat hippocampus following an anesthetic dose of pentobarbital or ketamine-xylazine: an in vivo microdialysis study. Physiol Behav 84: 245–250, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke 32: 2426–2432, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Chan O, Inouye K, Akirav EM, Park E, Riddell MC, Matthews SG, Vranic M. Hyperglycemia does not increase basal hypothalamo-pituitary-adrenal activity in diabetes but it does impair the HPA response to insulin-induced hypoglycemia. Am J Physiol Regul Integr Comp Physiol 289: R235–R246, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Chan O, Inouye K, Vranic M, Matthews SG. Hyperactivation of the hypothalamo-pituitary-adrenocortical axis in streptozotocin-diabetes is associated with reduced stress responsiveness and decreased pituitary and adrenal sensitivity. Endocrinology 143: 1761–1768, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Cox DJ, Irvine A, Gonder-Frederick L, Nowacek G, Butterfield J. Fear of hypoglycemia: quantification, validation, and utilization. Diabetes Care 10: 617–621, 1987. [DOI] [PubMed] [Google Scholar]
- 15.Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care 26: 1902–1912, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Golden MP, Ingersoll GM, Brack CJ, Russell BA, Wright JC, Huberty TJ. Longitudinal relationship of asymptomatic hypoglycemia to cognitive function in IDDM. Diabetes Care 12: 89–93, 1989. [DOI] [PubMed] [Google Scholar]
- 17.Gutniak M, Blomqvist G, Widén L, Stone-Elander S, Hamberger B, Grill V. d-[U-11C]glucose uptake and metabolism in the brain of insulin-dependent diabetic subjects. Am J Physiol Endocrinol Metab 258: E805–E812, 1990. [DOI] [PubMed] [Google Scholar]
- 18.Hershey T, Lillie R, Sadler M, White NH. Severe hypoglycemia and long-term spatial memory in children with type 1 diabetes mellitus: a retrospective study. J Int Neuropsychol Soc 9: 740–750, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Hui L, Pei DS, Zhang QG, Guan QH, Zhang GY. The neuroprotection of insulin on ischemic brain injury in rat hippocampus through negative regulation of JNK signaling pathway by PI3K/Akt activation. Brain Res 1052: 1–9, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Jacobson AM, Musen G, Ryan CM, Silvers N, Cleary P, Waberski B, Burwood A, Weinger K, Bayless M, Dahms W, Harth J. Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med 356: 1842–1852, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeong YB, Kim JS, Jeong SM, Park JW, Choi IC. Comparison of the effects of sevoflurane and propofol anaesthesia on regional cerebral glucose metabolism in humans using positron emission tomography. J Int Med Res 34: 374–384, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Johnson SA, Young C, Olney JW. Isoflurane-induced neuroapoptosis in the developing brain of nonhypoglycemic mice. J Neurosurg Anesthesiol 20: 21–28, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Kaufman FR, Epport K, Engilman R, Halvorson M. Neurocognitive functioning in children diagnosed with diabetes before age 10 years. J Diabetes Complications 13: 31–38, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Kondo F, Asanuma M, Miyazaki I, Kondo Y, Tanaka K, Makino H, Ogawa N. Progressive cortical atrophy after forebrain ischemia in diabetic rats. Neurosci Res 39: 339–346, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Kramer L, Fasching P, Madl C, Schneider B, Damjancic P, Waldhausl W, Irsigler K, Grimm G. Previous episodes of hypoglycemic coma are not associated with permanent cognitive brain dysfunction in IDDM patients on intensive insulin treatment. Diabetes 47: 1909–1914, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Lin B, Ginsberg MD, Busto R. Hyperglycemic exacerbation of neuronal damage following forebrain ischemia: microglial, astrocytic and endothelial alterations. Acta Neuropathol 96: 610–620, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Malone JI, Hanna SK, Saporta S. Hyperglycemic brain injury in the rat. Brain Res 1076: 9–15, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Martinez-Tellez R, Gomez-Villalobos MJ, Flores G. Alteration in dendritic morphology of cortical neurons in rats with diabetes mellitus induced by streptozotocin. Brain Res 1048: 108–115, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Mielke JG, Taghibiglou C, Wang YT. Endogenous insulin signaling protects cultured neurons from oxygen-glucose deprivation-induced cell death. Neuroscience 143: 165–173, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Moreira T, Cebers G, Pickering C, Ostenson CG, Efendic S, Liljequist S. Diabetic Goto-Kakizaki rats display pronounced hyperglycemia and longer-lasting cognitive impairments following ischemia induced by cortical compression. Neuroscience 144: 1169–1185, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Musen G, Lyoo IK, Sparks CR, Weinger K, Hwang J, Ryan CM, Jimerson DC, Hennen J, Renshaw PF, Jacobson AM. Effects of type 1 diabetes on gray matter density as measured by voxel-based morphometry. Diabetes 55: 326–333, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Nakao Y, Itoh Y, Kuang TY, Cook M, Jehle J, Sokoloff L. Effects of anesthesia on functional activation of cerebral blood flow and metabolism. Proc Natl Acad Sci USA 98: 7593–7598, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nellgård B, Wieloch T. Cerebral protection by AMPA- and NMDA-receptor antagonists administered after severe insulin-induced hypoglycemia. Exp Brain Res 92: 259–266, 1992. [DOI] [PubMed] [Google Scholar]
- 34.Northam EA, Anderson PJ, Jacobs R, Hughes M, Warne GL, Werther GA. Neuropsychological profiles of children with type 1 diabetes 6 years after disease onset. Diabetes Care 24: 1541–1546, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Northam EA, Anderson PJ, Werther GA, Warne GL, Andrewes D. Predictors of change in the neuropsychological profiles of children with type 1 diabetes 2 years after disease onset. Diabetes Care 22: 1438–1444, 1999. [DOI] [PubMed] [Google Scholar]
- 36.Pelligrino DA, LaManna JC, Duckrow RB, Bryan RM Jr, Harik SI. Hyperglycemia and blood-brain barrier glucose transport. J Cereb Blood Flow Metab 12: 887–899, 1992. [DOI] [PubMed] [Google Scholar]
- 37.Perantie DC, Wu J, Koller JM, Lim A, Warren SL, Black KJ, Sadler M, White NH, Hershey T. Regional brain volume differences associated with hyperglycemia and severe hypoglycemia in youth with type 1 diabetes. Diabetes Care 30: 2331–2337, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Rizk NN, Rafols JA, Dunbar JC. Cerebral ischemia-induced apoptosis and necrosis in normal and diabetic rats: effects of insulin and C-peptide. Brain Res 1096: 204–212, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Rovet JF, Ehrlich RM. The effect of hypoglycemic seizures on cognitive function in children with diabetes: a 7-year prospective study. J Pediatr 134: 503–506, 1999. [DOI] [PubMed] [Google Scholar]
- 40.Schmued LC, Stowers CC, Scallet AC, Xu L. Fluoro-Jade C results in ultra high resolution and contrast labeling of degenerating neurons. Brain Res 1035: 24–31, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Schoenle EJ, Schoenle D, Molinari L, Largo RH. Impaired intellectual development in children with Type I diabetes: association with HbA(1c), age at diagnosis and sex. Diabetologia 45: 108–114, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Simpson IA, Appel NM, Hokari M, Oki J, Holman GD, Maher F, Koehler-Stec EM, Vannucci SJ, Smith QR. Blood-brain barrier glucose transporter: effects of hypo- and hyperglycemia revisited. J Neurochem 72: 238–247, 1999. [DOI] [PubMed] [Google Scholar]
- 43.Strudwick SK, Carne C, Gardiner J, Foster JK, Davis EA, Jones TW. Cognitive functioning in children with early onset type 1 diabetes and severe hypoglycemia. J Pediatr 147: 680–685, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Suh SW, Aoyama K, Chen Y, Garnier P, Matsumori Y, Gum E, Liu J, Swanson RA. Hypoglycemic neuronal death and cognitive impairment are prevented by poly(ADP-ribose) polymerase inhibitors administered after hypoglycemia. J Neurosci 23: 10681–10690, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suh SW, Aoyama K, Matsumori Y, Liu J, Swanson RA. Pyruvate administered after severe hypoglycemia reduces neuronal death and cognitive impairment. Diabetes 54: 1452–1458, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Suh SW, Gum ET, Hamby AM, Chan PH, Swanson RA. Hypoglycemic neuronal death is triggered by glucose reperfusion and activation of neuronal NADPH oxidase. J Clin Invest 117: 910–918, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suh SW, Hamby AM, Swanson RA. Hypoglycemia, brain energetics, and hypoglycemic neuronal death. Glia 55: 1280–1286, 2007. [DOI] [PubMed] [Google Scholar]
- 48.Telushkin PK, Nozdrachev AD, Potapov PP, Medvedeva NB, Stel'makh AY. Glycolysis and oxidation enzyme activity in rat brain during insulin-induced hypoglycemia against the background of alloxan-induced diabetes mellitus. Bull Exp Biol Med 140: 695–697, 2005. [DOI] [PubMed] [Google Scholar]
- 49.ter Braak EW, Appelman AM, van de Laak M, Stolk RP, van Haeften TW, Erkelens DW. Clinical characteristics of type 1 diabetic patients with and without severe hypoglycemia. Diabetes Care 23: 1467–1471, 2000. [DOI] [PubMed] [Google Scholar]
- 50.Tkacs NC, Dunn-Meynell AA, Levin BE. Presumed apoptosis and reduced arcuate nucleus neuropeptide Y and pro-opiomelanocortin mRNA in non-coma hypoglycemia. Diabetes 49: 820–826, 2000. [DOI] [PubMed] [Google Scholar]
- 51.Tkacs NC, Pan Y, Raghupathi R, Dunn-Meynell AA, Levin BE. Cortical Fluoro-Jade staining and blunted adrenomedullary response to hypoglycemia after noncoma hypoglycemia in rats. J Cereb Blood Flow Metab 25: 1645–1655, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Vannucci SJ, Koehler-Stec EM, Li K, Reynolds TH, Clark R, Simpson IA. GLUT4 glucose transporter expression in rodent brain: effect of diabetes. Brain Res 797: 1–11, 1998. [DOI] [PubMed] [Google Scholar]
- 53.Voll CL, Auer RN. Insulin attenuates ischemic brain damage independent of its hypoglycemic effect. J Cereb Blood Flow Metab 11: 1006–1014, 1991. [DOI] [PubMed] [Google Scholar]
- 54.Wieloch T, Engelsen B, Westerberg E, Auer R. Lesions of the glutamatergic cortico-striatal projections in the rat ameliorate hypoglycemic brain damage in the striatum. Neurosci Lett 58: 25–30, 1985. [DOI] [PubMed] [Google Scholar]
- 55.Wysocki T, Harris MA, Mauras N, Fox L, Taylor A, Jackson SC, White NH. Absence of adverse effects of severe hypoglycemia on cognitive function in school-aged children with diabetes over 18 months. Diabetes Care 26: 1100–1105, 2003. [DOI] [PubMed] [Google Scholar]