Abstract
Spontaneous clearance of hepatitis C virus (HCV) is rare in immunocompromised patients, such as those who have undergone organ transplantation. It has been recognized that patients receiving liver transplantation for HCV-related disease have decreased graft and patient survival compared with those transplanted for other etiologies. There is a growing trend toward treating HCV recurrence aggressively after liver transplantation. For other organ transplant recipients with concurrent HCV, treatment is not often an option, given the high rates of graft rejection and loss secondary to interferon and its immunomodulatory effects. Although spontaneous clearance of HCV has been reported in recipients of solitary liver and renal transplants, a common factor arising in these cases has been previous exposure to interferon. To date, no reports of spontaneous clearance of HCV RNA have been reported in a multiorgan transplant recipient. A case of spontaneous clearance of HCV RNA in an immunocompromised patient, within five months of simultaneous liver and kidney retransplantation is described. Importantly, this patient had no previous exposure to interferon.
Keywords: Hepatitis C, Interferon, Liver transplantation, Spontaneous clearance
Abstract
L’élimination spontanée du virus de l’hépatite C (VHC) est rare chez les patients immunodéprimés, comme ceux qui doivent, par exemple, subir une greffe d’organe. Il a été démontré que les patients qui reçoivent une greffe de foie pour une maladie liée au VHC présentent une survie du greffon et une espérance de vie diminuées comparativement à ceux qui sont transplantés suite à d’autres étiologies. On observe une tendance nette à traiter les récurrences du VHC de manière énergique après une greffe hépatique. Pour d’autres receveurs de transplantations porteurs du VHC, souvent, le traitement n’est tout simplement pas une option, compte tenu du fort taux de rejet et de perte du greffon secondaires à l’interféron et à ses effets immunomodulateurs. Bien que l’élimination spontanée du VHC ait été signalée chez des receveurs de greffes de foie et des receveurs de greffes de rein, ces sujets auraient en commun d’avoir été exposés à l’interféron. À ce jour, aucun cas d’élimination spontanée de l’ARN du VHC n’a été signalé chez des receveurs de multigreffes. Les auteurs décrivent ici une élimination spontanée de l’ARN du VHC survenue chez un patient immunodéprimé dans les cinq mois suivant une retransplantation foie-rein simultanée. À noter : ce patient n’avait jamais été exposé à l’interféron.
Spontaneous clearance of hepatitis C virus (HCV) occurs in the nontransplant population at a rate of approximately 10% to 50% (1). However, in the immunocompromised post-transplant population, this is rare. Cases of spontaneous clearance have occasionally been reported after solitary liver and renal transplantation, typically well after transplantation and after decreases or withdrawal of immunosuppressive agents have occurred (2–5). Additionally, there are recent reports of spontaneous clearance after liver transplantation in HCV and HIV coinfected recipients after interferon exposure (6). To date, no cases of spontaneous clearance are reported in a multiorgan transplant recipient.
CASE PRESENTATION
A 32-year-old Caucasian woman underwent liver transplantation in 1988 secondary to fulminant hepatic failure from Wilson’s disease. Her immunosuppression consisted of cyclosporine and tapered corticosteroids. Unfortunately, she acquired HCV from contaminated plasma at the time of transplantation. This was documented during national lookback procedures and confirmed serologically. In 1998, she became hemodialysis-dependent secondary to focal glomerulosclerosis and calcineurin inhibitor nephrotoxicity. She subsequently underwent living donor renal transplantation in 2001.
In 2004, she developed an abnormal liver profile and underwent liver biopsy that revealed cirrhosis with typical HCV pathological features. She did not undergo antiviral therapy with interferon given the risk to the renal allograft. In 2005, she developed progressive renal allograft failure and liver decompensation. She was listed for combined liver and renal retransplantation. At that time, she was documented to be genotype 1a with a high viral load (3.21×106 IU/mL).
In July 2006, she underwent a combined liver/renal retransplant. On the day of transplant, the recipient remained viremic (HCV RNA greater than 50 IU/mL). The donor was HCV antibody-negative. The recipient had a complicated postoperative course with multiple intra-abdominal abscesses requiring percutaneous drainage and a prolonged hospital stay. She ultimately recovered and was discharged home. Her immunosuppression consisted of basiliximab induction, followed by delayed tacrolimus, mycophenolate mofetil and tapering doses of prednisone. Cytomegalovirus (CMV) prophylaxis was provided for three months with oral valganciclovir.
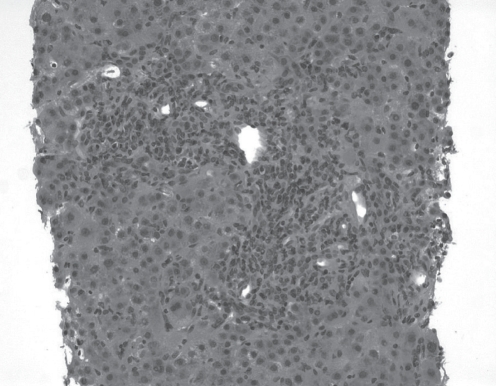
At 11 weeks post-transplant, she developed a significant transaminitis. Her alanine aminotransferase and aspartate aminotransferase levels were 354 U/L and 303 U/L, respectively (normal range lower than 40 U/L), while her bilirubin and alkaline phosphatase levels remained normal. A liver biopsy revealed portal tracts with mild lymphocytic infiltrates; however, no evidence of cellular rejection or fibrosis. At 14 weeks post-transplant, the levels of liver enzymes continued to rise. A second liver biopsy revealed moderate portal-based inflammation with predominant lymphocytic infiltration, without evidence of cellular rejection. Doppler imaging of the hepatic vessels was normal. Twenty weeks postoperatively, the liver enzymes continued to climb (alanine and aspartate aminotransferase 785 U/L and 1169 U/L, respectively). The bilirubin had risen to 29 μmol/L (normal less than 17 μmol/L) and alkaline phosphatase was normal at 81 U/L (normal range less than 113 U/L). A third liver biopsy revealed diffuse moderate lymphocytic infiltration expanding into the lobules with new features of hepatocyte necrosis consistent with severe hepatitis and bridging necrosis (Figure 1). There was no evidence of cellular or ductopenic rejection.
Figure 1).
Hematoxylin and eosin stain from the third liver biopsy, revealing severe hepatitis, with diffuse lymphocytic infiltration and bridging necrosis (original magnification ×40)
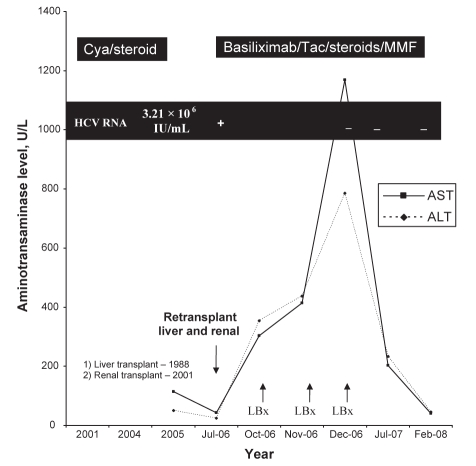
Given the worsening of transaminase levels and histology, the diagnosis was presumed to be recurrent HCV. After ample discussion of the risks and benefits associated with antiviral therapy, the patient consented to antiviral therapy. Pegylated interferon alpha-2a was initiated at 90 μg subcutaneously per week, without ribavirin. On the day before treatment initiation a new viral load was obtained. This specimen was subsequently reported as negative (HCV RNA less than 600 IU/mL), hence the interferon therapy was discontinued. Twenty months after transplant another HCV RNA assessed by polymerase chain reaction remained negative (HCV RNA less than 50 IU/mL), suggesting sustained clearance of the HCV (Figure 2).
Figure 2).
Trend of hepatitis C RNA, aminotransaminase profile and immunosuppression from time of renal transplant until 16 months after combined liver and renal retransplant. ALT Alanine aminotransferase; AST Aspartate aminotransferase; Cya Cyclosporine; HCV Hepatitis C virus; LBx Liver biopsy; MMF Mycophenolate mofetil; Tac Tacrolimus
DISCUSSION
Recurrence of HCV after liver transplantation is universal and leads to decreased graft and patient survival (7). Antiviral therapy is challenging in this population due to enhanced cytotoxicities, previous treatment failure and the ongoing need for immunosuppression. When a second organ graft is involved, antiviral therapy may be prohibited, given the threat of organ rejection and graft failure secondary to interferon and its immunomodulatory effects (8,9). Spontaneous clearance of HCV after transplant is rare; however, understanding this phenomenon may provide insight into future post-transplant HCV management strategies.
Somsouk et al (2) described the case of a young woman who developed progressive liver disease from HCV after primary renal transplantation. Eleven years after renal transplant, the patient developed allograft failure and immunosuppression was withdrawn. The patient was known to be genotype 1a, and the liver biopsy showed bridging fibrosis. She received one dose of interferon alpha-2b subcutaneously. The patient subsequently cleared the HCV RNA. The authors speculated that with the withdrawal of immunosuppression, the patient was able to mount a CD4 response and clear the HCV RNA. This patient also went on to have regression of hepatic fibrosis. In the present case, this was quite the contrary, given the immediate post-transplant period, our patient’s immunosuppression was actually enhanced with interleukin-2 induction, moderate doses of corticosteroids, tacrolimus and mycophenolate mofetil.
The liver transplant literature reports sporadic cases of spontaneous HCV RNA clearance in the post-transplant period, with each article speculating on different causative factors for the viral clearance. Similar to the previous renal case, Neumann and Neuhaus (3) presented a case of a patient retransplanted for hepatic artery thrombosis, who also had concomitant fibrosing cholestatic HCV and was known to have been viremic with genotype 1b. After retransplant, the patient required decreased immunosuppression secondary to renal failure. The patient subsequently cleared the HCV RNA. Before retransplant, this patient had received interferon monotherapy for three months, with no obvious response. Samonakis et al (4) reported two cases of spontaneous HCV RNA clearance in longer-term post-transplant liver recipients, who were genotype 1 and 4, respectively. Neither of these patients experienced immunosuppression withdrawal; however, both were known to be diabetic with proteinuria and some renal dysfunction. Both patients were converted to sirolimus due to renal dysfunction. One patient’s viral load was noted to be decreasing before the conversion and the patient had pretransplant exposure to interferon monotherapy. The other recipient had previous exposure to interferon and ribavirin, and was considered a treatment failure. The authors speculated that the HCV RNA clearance may be related to renal impairment and proteinuria. However, it is unclear as to whether the changes in immunosuppression such as decreased calcineurin inhibitor, conversion to sirolimus or the previous interferon exposure, may have contributed to the clearance of HCV RNA. In the present case, the patient had normal renal function after the transplant, no decrease or change in immunosuppression, or any exposure to interferon.
Bhagat et al (6) more recently reported two cases of spontaneous clearance of HCV RNA in liver transplant recipients who were coinfected with HIV before transplantation. One patient was multi-infected with hepatitis B (HBV), HCV and HIV, and had received one month of pegylated interferon and ribavirin therapy. However, it was discontinued due to poor tolerability. The HCV RNA level five days before the transplant was 710 IU/mL. One month after liver transplant, the HCV RNA was negative, and at three months the HBV DNA was also negative. The patient’s medication regimen consisted of corticosteroids, tacrolimus and eventually, mycophenolate mofetil in addition to lamivudine/zidovudine and indinavir. The second patient described had undetectable HCV RNA 30 days after transplant and also received six months of standard interferon and ribavirin therapy, followed by three months of pegylated interferon and ribavirin, with pegylated interferon and ribavirin being discontinued six months before transplant. This patient had detectable HCV RNA (3.26×105 IU/mL) two months before transplant. The post-transplant medication regimen included corticosteroids, tacrolimus, muromonab-CD3 for an acute rejection episode, as well as lamivudine, stavvdi and efavirenz. Bhagat et al (6) speculate that the coexistent HBV infection in the first patient may have induced an immune response which led to clearance of the HCV RNA. Interestingly, both patients were receiving antiretroviral therapy which may have enabled clearance by an as yet undefined mechanism. As well, both patients had acute rejection episodes that may have affected immune modulation leading to clearance. Additionally, these individuals had significant interferon exposure. The present case did not include any of these factors to attribute to the clearance of HCV RNA.
The common factor noted in each previous report is that all patients had previous exposure, albeit sometimes brief, to interferon. Our patient had no previous exposure whatsoever to any interferon product. Her only exposure to an antiviral agent was valganciclovir for CMV prophylaxis during the initial retransplant period.
In the case presented, the patient had a brisk and significant elevation in transaminase levels, peaking at 30 times the upper limit of normal. There was no evidence of vascular abnormalities, exogenous substances or cellular rejection to account for the changes. There were no definite histological or immunological changes to suggest other viral etiologies (eg, CMV, Epstein-Barr virus or HBV) or de novo autoimmune hepatitis. As with previous authors, we can only speculate that the liver enzyme elevation likely represents a ‘clearance phase hepatitis’ perhaps caused by a host immune response or reconstitution, or an as yet undefined immunological phenomenon. The present case demonstrates that despite being significantly immunosuppressed, clearance of HCV RNA can occur spontaneously and is the first reported case of spontaneous clearance of HCV RNA in a multiorgan transplant recipient.
REFERENCES
- 1.Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: A systematic review of longitudinal studies. J Viral Hepat. 2006;13:34–41. doi: 10.1111/j.1365-2893.2005.00651.x. [DOI] [PubMed] [Google Scholar]
- 2.Somsouk MA, Lauer GM, Casson D, et al. Spontaneous resolution of chronic Hepatitis C virus disease after withdrawal of immunosuppression. Gastroenterology. 2003;124:1946–9. doi: 10.1016/s0016-5085(03)00391-3. [DOI] [PubMed] [Google Scholar]
- 3.Neumann UP, Neuhaus P. Discussion on spontaneous resolution of chronic hepatitis C virus after withdrawal of immunosuppression. Gastroenterology. 2004;126:627. doi: 10.1053/j.gastro.2003.12.028. [DOI] [PubMed] [Google Scholar]
- 4.Samonakis DN, Cholongitas E, Triantos CK, et al. Sustained, spontaneous disappearance of serum HCV-RNA under immunosuppression after liver transplantation for HCV cirrhosis. J Hepatol. 2005;43:1091–3. doi: 10.1016/j.jhep.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Doughty AL, Zekry A, Spencer JD, Turhan S, Painter D, McCaughan GW. Spontaneous clearance of hepatitis C infection post-liver transplantation is associated with rapidly changing quasispecies: A single case report. Liver Transpl. 2000;6:648–53. doi: 10.1053/jlts.2000.9740. [DOI] [PubMed] [Google Scholar]
- 6.Bhagat V, Foont JA, Schiff ER, Regev A. Spontaneous clearance of hepatitis C virus after liver transplantation in two patients coinfected with hepatitis C virus and human immunodeficiency virus. Liver Transpl. 2008;14:92–5. doi: 10.1002/lt.21351. [DOI] [PubMed] [Google Scholar]
- 7.Berenguer M. Recurrent hepatitis C: Worse outcomes established, intervention still inadequate. Liver Transpl. 2007;13:641–3. doi: 10.1002/lt.21136. [DOI] [PubMed] [Google Scholar]
- 8.Fabrizi F, Lunghi G, Dixit P, Martin P. Anti-viral therapy of hepatitis C virus-related liver disease in renal transplant patients. Aliment Pharmacol Ther. 2006;24:1413–22. doi: 10.1111/j.1365-2036.2006.03151.x. [DOI] [PubMed] [Google Scholar]
- 9.Wells JT, Lucey MR, Said A. Hepatitis C in transplant recipients of solid organs, other than liver. Clin Liver Dis. 2006;10:901–17. doi: 10.1016/j.cld.2006.08.025. [DOI] [PubMed] [Google Scholar]