Abstract
BACKGROUND:
Several studies have suggested that proton pump inhibitors are efficacious in preventing rebleeding when administered immediately after endoscopic treatments. However, there are limited clinical outcome data on the use of intravenous pantoprazole.
OBJECTIVE:
To evaluate the efficacy of intravenous pantoprazole after successful endoscopic treatment for peptic ulcer bleeding using evidence from randomized controlled trials (RCTs).
METHODS:
The Cochrane Library, MEDLINE, EMBASE and several Chinese databases up to July 2008 were searched. RCTs that compared the relative effectiveness of intravenous pantoprazole with placebo, H2 receptor antagonist or other agents for patients with peptic ulcer bleeding who were pretreated with successful endoscopic therapies were retrieved.
RESULTS:
Five RCTs comprising a total of 821 participants were included in the final meta-analysis. Overall, there were significant differences in ulcer rebleeding (RR 0.31; 95% CI 0.18 to 0.53; pooled rates were 4.7% for pantoprazole and 15.0% for control), surgical intervention (RR 0.28, 95% CI 0.09 to 0.83; pooled rates were 1.4% in pantoprazole group versus 6.5% in control) and total length of hospital stay (weighted mean difference −1.53; 95% CI −1.91 to −1.16), but not on mortality (RR 0.72, 95% CI 0.29 to 1.81; pooled mortality rates were 1.9% for pantoprazole versus 2.8% for control) and blood transfusion requirements (weighted mean difference −0.53; 95% CI for random effects −1.04 to −0.02) when compared with control treatments. A series of subgroup analyses supported the results from the main analysis.
CONCLUSIONS:
Intravenous administration of pantoprazole after endoscopic therapy for peptic ulcer bleeding reduces rates of ulcer rebleeding, surgical intervention and overall duration of hospital stay, but not mortality and blood transfusion requirements compared with placebo, H2 receptor antagonist or somatostatin.
Keywords: Endoscopic treatment, Pantoprazole, Peptic ulcer bleeding
Abstract
HISTORIQUE :
Plusieurs études laissent supposer que les inhibiteurs de la pompe à protons sont efficaces pour prévenir des récidives d’hémorragie s’ils sont administrés immédiatement après les traitements endoscopiques. Cependant, on possède peu de données d’issues cliniques sur l’utilisation du pantoprazole par intraveineuse (IV).
OBJECTIF :
Évaluer l’efficacité du pantoprazole IV après le traitement endoscopique réussi de l’hémorragie d’un ulcère gastroduodénal au moyen de données probantes tirées d’essais aléatoires et contrôlés (EAC).
MÉTHODOLOGIE :
Les auteurs ont fait des recherches dans la Bibliothèque Cochrane, MEDLINE, EMBASE et plusieurs bases de données chinoises jusqu’à juillet 2008. Ils ont extrait les EAC dans lesquels on comparait l’efficacité relative du pantoprazole IV à un placebo, à un antagoniste des récepteurs H2 ou à d’autres agents chez des patients souffrant d’hémorragie attribuable à un ulcère gastroduodénal ayant déjà été traités avec succès par endoscopie.
RÉSULTATS :
La méta-analyse finale incluait cinq EAC, pour un total de 821 participants. Dans l’ensemble, par rapport aux sujets témoins, on constatait une différence significative pour ce qui est des récidives d’hémorragie de l’ulcère (RRR 0,31; 95 % IC 0,18 à 0,53; les taux regroupés s’élevaient à 4,7 % dans le groupe prenant du pantoprazole et à 15,0 % dans le groupe témoin), des interventions chirurgicales (RRR 0,28; 95 % IC 0,09 à 0,83; les taux regroupés s’élevaient à 1,4 % dans le groupe prenant du pantoprazole et à 6,5 % dans le groupe témoin) et de l’hospitalisation totale (différence moyenne pondérée −1,53; 95 % IC −1,91 à −1,16), mais non pour ce qui est de la mortalité (RRR 0,72; 95 % IC 0,29 à 1,81; les taux de mortalité regroupés s’élevaient à 1,9 % dans le groupe prenant du pantoprazole et à 2,8 % dans le groupe témoin) et des besoins de transfusion sanguine (différence moyenne pondérée −0,53; 95 % IC pour les effets aléatoires −1,04 à −0,02). Une série d’analyses de sous-groupes a corroboré les résultats de l’analyse principale.
CONCLUSIONS :
L’administration de pantoprazole IV après le traitement endoscopique de l’hémorragie d’un ulcère gastroduodénal réduit les taux de récidive d’hémorragie de l’ulcère, d’intervention chirurgicale et la durée des hospitalisations, mais non la mortalité et les besoins de transfusion sanguine par rapport à un placebo, à un antagoniste des récepteurs H2 ou à la somostatine.
Bleeding is a common and potentially life-threatening complication of peptic ulcer disease and is associated with a mortality rate of approximately 3% to 10%, although it is reported that approximately 80% of peptic ulcer bleeding (PUB) episodes resolve spontaneously (1,2). In recent years, epidemiological studies have shown a decreasing trend in the occurrence of upper gastrointestinal hemorrhage related to peptic ulcers, but without a significant decrease in rebleeding or mortality rates. This could be explained by the significantly increased incidence of comorbid illnesses and the increased use of nonsteroidal anti-inflammatory drugs (NSAIDs), especially in elderly patients (3–5).
In vitro studies (6–9) have identified that clotting proceeds more efficiently and that the dissolution of clots by proteolytic enzymes occurs more slowly at high pH levels, while the function of platelets is severely impaired at low pH. Pepsin can digest blood clots overlying ulcer craters and its activity is pH-related. These data suggest that low intragastric pH may promote the recurrence of PUB.
Endoscopic hemostasis has been the first choice of treatment for PUB, providing better outcomes compared with medical and surgical therapies (10–13). Nevertheless, 15% to 20% of patients treated endoscopically experienced rebleeding, with a mortality rate of 6% to 7% (14). High intragastric acid secretion was observed in these patients. Thus, adjuvant medications that promote acid suppression, thereby supporting and maintaining hemostasis, seem to be beneficial. Although several studies (15–18) have demonstrated that proton pump inhibitors (PPIs) are efficacious in preventing rebleeding when administered immediately after endoscopic treatments, there are limited clinical outcome data on pantoprazole. Indeed, most of the data supporting the use of PPIs for PUB involved omeprazole rather than pantoprazole mainly due to its availability and cost-effectiveness. However, omeprazole is not available as an intravenous (IV) formulation in many countries, including those in North America. Furthermore, evidence indicates that pantoprazole, when infused continuously at the same dose as omeprazole in healthy volunteers and PUB patients, is highly effective in suppressing gastric acid by raising the intragastric pH to higher than 6 (19,20). Additionally, IV pantoprazole infusion is especially distinguished by the lack of clinically relevant drug interactions, no dosage adjustment for patients with renal insufficiency or with mild to moderate hepatic dysfunction.
A Cochrane systematic review (21) concluded that PPIs effectively reduced ulcer rebleeding and surgical intervention when compared with placebo or H2 receptor antagonists (H2RAs). However, the study included few randomized controlled trials (RCTs) that investigated pantoprazole. Conversely, somatostatin, which is conventionally administered to PUB patients in clinical practice, is less evaluated than PPIs, although the drug has been demonstrated more effective at inhibiting gastric acid secretion than pantoprazole (14). Only a few high-quality studies concerning IV pantoprazole have been published (22–26). Among them, one RCT, presented as an abstract (27), revealed that the efficacy of high-dose infusion of ranitidine to prevent recurrent ulcer bleeding was similar to that of pantoprazole infusion. Another study (26) that compared pantoprazole with somatostatin reported no difference in the outcome of surgical intervention. To further explore the role of pantoprazole as an adjuvant therapy to endoscopic treatment for PUB patients, we conducted a meta-analysis to evaluate its efficacy by comparing pantoprazole with either placebo, H2RAs or other agents.
METHODS
Literature search
Articles selected for analysis were reviewed separately by two of the authors (JT, ZB) and studies fulfilling the inclusion criteria underwent further analysis. Relevant papers were identified by searching the CENTRAL (The Cochrane Library), MEDLINE, and EMBASE databases up to July 2008 by using the search terms “peptic ulcer*” or “gastrointestinal ulcer*” or “stomach ulcer*” or “gastric ulcer*” or “GU” or “duodenal ulcer*” or “DU” and “pantoprazole”. The search strategy was designed using a combination of subject and text words. The Chinese biomedicine literature database, Chinese technological periodical full-text database and the Chinese periodical full-text database were also searched. In addition, relevant articles published in journals were manually searched and companies and researchers in the field were contacted to identify any ongoing or unpublished studies.
Review methods
Three reviewers (JW, BM, YL) independently reviewed trials and abstracts identified in the search for fulfillment of predefined inclusion criteria. The full text of all relevant studies was obtained whenever possible. If it was not clear whether the trial met the inclusion criteria, further information was sought from the original authors. The inclusion of trials was determined independently by two reviewers (LJ, SS) and any disagreements were resolved by consensus.
The inclusion criteria were as follows:
RCTs that compared the relative effectiveness of IV pantoprazole with placebo, H2RA or other medication after endoscopic hemostasis for PUB;
Patients with endoscopically confirmed active bleeding or with major stigmata of recent bleeding from peptic ulcers were included if they were initially successfully treated with endoscopic therapies, regardless of hemostatic approaches. The major stigmata of ulcer hemorrhage were defined as arterial spurting, oozing bleeding, nonbleeding visible vessel and adherent clot according to endoscopic appearance. Patients with other causes of gastrointestinal hemorrhage were excluded; and
Regarding interventions, the treatment group must have received pantoprazole and the control group must have received either placebo, H2RAs or another agent that was conventionally used for controlling bleeding. The route of administration was restricted to IV only and concomitant therapy must have been applied equally to both intervention arms.
DEFINITION OF OUTCOME MEASURES
Primary outcomes measure
Ulcer rebleeding:
Most trials were defined by the number of patients with endoscopically confirmed rebleeding within three, seven or 30 days after randomization.
Secondary outcome measures
Mortality:
Because PUB is a potentially fatal complication – especially in elderly patients with concomitant disease – data were not separated according to cause of death. Thus, 30-day mortality or in-hospital mortality was used.
Need for surgery:
Failure of endoscopic retreatment or occurrence of a second rebleeding constituted indications for surgery.
Blood transfusion requirements:
When available, blood transfusion requirements were expressed as mean (± SD) units transfused per patient for patients who needed blood transfusion only after endoscopic treatment.
Total duration of hospital stay:
The duration of hospital stay was expressed as mean (± SD) days in hospital.
Statistics
The methodological quality of the studies was independently assessed by three reviewers (JL, RL, XH), based on three criteria: randomization, double-blinding, and description of withdrawals and dropouts. The Cochrane Collaboration’s RevMan 4.2 software was used for statistical analysis. Statistical heterogeneity was measured by graphic examination of forest plots and statistically through a homogeneity test based on the χ2 test in which P<0.10 was considered to be significant for heterogeneity. The I2 statistic was calculated to assess the impact of heterogeneity on the results. A value of greater than 50% was considered substantial heterogeneity. When heterogeneity was detected, clinical parameters were used to explore the heterogeneity. Dichotomous data were analyzed using relative risk (RR) and were reported with 95% CIs. Continuous data were analyzed using mean differences with their corresponding SEs and were reported with 95% CI. Weighted mean differences (WMD) were used for outcomes measured on different scales that measured the same outcome. A fixed effects model was used unless there was significant heterogeneity, in which case a random effects model was applied.
RESULTS
The preliminary search identified 1243 potentially relevant papers. A total of 1237 articles were excluded from further analysis because they were not RCTs (1120 articles); involved monotherapy with endoscopy (54 articles) or pantoprazole (32 articles); articles that compared two different oral regimens of pantoprazole (13 articles); and duplications (18 articles). One RCT was excluded (27) because it was only presented as an abstract from which no raw data could be extracted. Five trials (22–26) comprising 821 patients were included in the final meta-analysis. Data regarding characteristics of the studies, patients’ baseline characteristics and quality assessment of the studies are summarized in Table 1, Table 2 and Table 3, respectively. Apart from the main analysis, several subgroup analyses were undertaken according to the type of control group, the initial dose of pantoprazole (high dose versus low dose) and the method of IV pantoprazole administration (ie, bolus infusion versus continuous infusion). Also, studies included in the present meta-analysis were divided into three subsets in terms of timing for the primary outcome assessment (ulcer rebleeding rate) for the first three days, days 4 to 7, and 30 days. Details are as follows:
Subgroup A: Ulcer rebleeding within three days (A1), four to seven days (A2) and 30 days (A3).
Subgroup B: Pantoprazole versus placebo (B1), pantoprazole versus ranitidine (B2) and pantoprazole versus somatostatin (B3).
Subgroup C: Pantoprazole bolus infusion (C1) and pantoprazole continuous infusion (C2).
Subgroup D: Initial high-dose (80 mg) pantoprazole (D1) and low-dose (40 mg) pantoprazole (D2).
TABLE 1.
Clinic characteristics of studies included in the meta-analysis
| Author (Reference) | Country | Treatment/Control, n |
Intervention |
Outcomes | ||
|---|---|---|---|---|---|---|
| Pantoprazole | Control | |||||
| Jensen et al (25) | USA | 72/77 | Pantoprazole 80 mg IV bolus followed by 8 mg/h continuous infusion for the first 3 days | Ranitidine 50 mg IV bolus followed by 6.25 mg/h continuous infusion for the first 3 days | Ulcer rebleeding rate for the first three days, days 4–7 and 30 days, 30-day mortality, mean hospital stay, mean number of transfusions | |
| Hsu et al (24) | China | 52/50 | Pantoprazole 40 mg IV bolus followed by 40 mg/12 h for the first 3 days | Ranitidine 50 mg IV bolus followed by 50 mg/12 h for the first 3 days | Ulcer rebleeding rate for 8 weeks, 8-week mortality, the need for surgery, mean hospital stay, mean number of transfusions | |
| Tsibouris et al (26) | Greece | 82/82 | Pantoprazole 40 mg IV bolus followed by 8 mg/h continuous infusion for the first 2 days | Somatostatin 250 μg IV bolus followed by 250 μg/h continuous infusion for the first 2 days | Ulcer rebleeding rate for the first 3 days and 30 days, 30-day mortality, mean hospital stay, mean number of transfusions | |
| Hung et al (23) | China | 54/49/50* | Pantoprazole 80 mg IV bolus followed by 8 mg/h continuous infusion for the first 3 days | Pantoprazole 80 mg IV bolus followed by 40 mg/12 h for the first 3 days | No acid suppression for the first 3 days | Ulcer rebleeding rate for 30 days, 30-day mortality, the need for surgery, mean number of transfusions, mean hospital stay |
| Zargar et al (22) | India | 102/101 | Pantoprazole 80 mg IV bolus followed by 8 mg/h continuous infusion for the first 3 days | Placebo 80 mg IV bolus followed by 8 mg/h continuous infusion for the first 3 days | Ulcer rebleeding for the first 3 days and days 4–7, mortality, the need for surgery, mean hospital stay, mean number of transfusions | |
Continuous infusion n=54, bolus infusion n=49, no treatment n=50. IV Intravenous
TABLE 2.
Baseline comparison of patient demographics and ulcer characteristics
| Baseline characteristics |
Author (reference) |
||||
|---|---|---|---|---|---|
| Hung et al (23) | Zargar et al (22) | Hsu et al (24) | Jensen et al (25) | Tsibouris et al (26) | |
| Cases, n (T/C) | 54/49/50* | 102/101 | 52/50 | 72/77 | 82/82 |
| Age, years, mean (SD) (T/C, n) | 63.7 (NA)/57.8 (NA)/62.5 (NA) | 55.3 (9.2)/52.4 (8.8) | 63.2 (18.2)/64.7 (13.8) | 59.6 (16.1)/55.6 (16.8) | 67.8 (13.1)/66.4 (13) |
| NSAID use, n (T/C) | 15/11/10 | 18/15 | 14/16 | 69/71 | 57/57 |
| HP-positive, n (T/C) | 33/30/26 | 62/58 | NA | 41/27 | 60/60 |
| History of ulcer bleeding, n (T/C) | NA | 21/18 | 14/9 | NA | NA |
| GU, n (T/C) | 19/14/20 | 18/16 | 25/25 | 34/31 | 44/44 |
| DU, n (T/C) | NA | 84/85 | 27/25 | 37/46 | 38/38 |
| Ulcer size, cm (SD) (T/C) | 1.2 (NA)/1.1 (NA)/1.0 (NA) | 1.2 (0.8)/1.3 (0.8) | 1.2 (0.8)/1.2 (0.6) | 1.1 (0.5)/1.2 (0.5) | 1.3 (0.6)/1.2 (0.6) |
| Inpatient bleeding, n (T/C) | NA | 12/14 | NA | NA | 5/5 |
| Hemoglobin on admission, (g/L) (SD) (T/C) | 94 (NA)/101 (NA)/98 (NA) | 94 (1.8)/91 (21) | 103 (30)/100 (28) | NA | NA |
| Shock on admission, n (T/C) | 1/1/1 | 28/24 | 3/3 | NA | 36/36 |
| Comorbid diseases, n (T/C) | NA | 25/22 | NA | NA | 46/46 |
| Endoscopic appearance, n (T/C) | |||||
| Spurting bleeding | 6/5/4 | 12/14 | 4/2 | 35/37 | 16/16 |
| Oozing bleeding | 28/24/29 | 25/31 | 18/17 | 33/36 | 36/36 |
| Nonbleeding visible vessel | 5/8/3 | 65/56 | 18/21 | 4/4 | 30/30 |
| Adherent clot | 33/31/28 | –/– | 12/12 | –/– | –/– |
Continuous infusion n=54, bolus infusion n=49, no treatment n=50. C Control subjects; DU Duodenal ulcer; GU Gastric ulcer; HP Helicobacter pylori; NA Not applicable; NSAID Nonsteroidal anti-inflammatory drug; T Treated subjects
TABLE 3.
Quality assessments of studies included in the meta-analysis
| Author (Reference) | Randomization | Allocated concealment | Blinding | Follow-up (weeks) | Loss of follow-up | Dropout (T/C) | Quality grade* |
|---|---|---|---|---|---|---|---|
| Hung et al (23) | Adequate | Not used | Unclear | 8 | Not described | 11/4 | C |
| Zargar et al (22) | Adequate | Adequate | Adequate (double-blind) | 6 | Not described | 0/0 | A |
| Hsu et al (24) | Adequate | Adequate | Unclear | 8 | Not described | 0/0 | B |
| Jensen et al (25) | Adequate | Adequate | Adequate (double-blind) | 4 | Not described | 10/13 | A |
| Tsibouris et al (26) | Adequate | Adequate | Adequate (double-blind) | 4 | Not described | 0/0 | A |
Based on reference 21; C Number of patients in control group; T Number of patients in treatment group
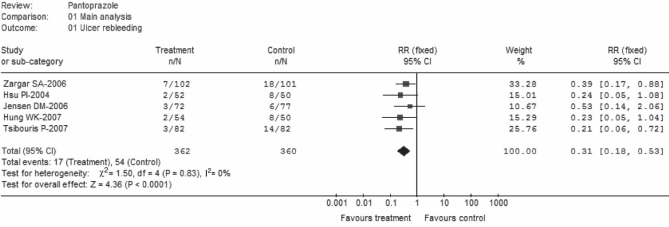
Ulcer rebleeding
Five trials (22–26) comprising a total of 362 patients in the pantoprazole group and 360 patients in the control group reported a rebleeding rate (Figure 1). The pooled effect favoured pantoprazole treatment, with an RR of 0.31 (95% CI 0.18 to 0.53). No significant heterogeneity was observed among the five trials (P=0.83; I2=0%). Pooled rebleeding rates were 4.7% for pantoprazole and 15.0% for control. Of the five trials, two (23,24) did not report rebleeding rates for the first three days, but reported 30-day and eight-week rebleeding rates, respectively. Because there was a concern that this could bias the results of the present analysis, a post hoc sensitivity analysis was performed with the exclusion of these two trials. The result remained statistically significant in favour of pantoprazole treatment (RR 0.34; 95% CI 0.19 to 0.63).
Figure 1).
Ulcer rebleeding (main analysis). Intravenous pantoprazole as an adjuvant therapy following successful endoscopic treatment for peptic ulcer bleeding. df Degrees of freedom
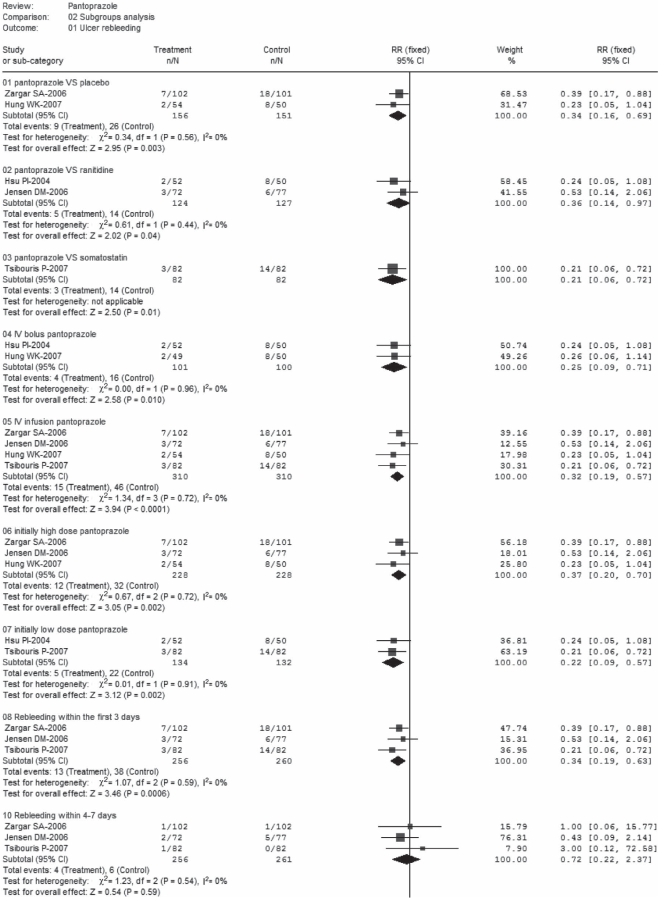
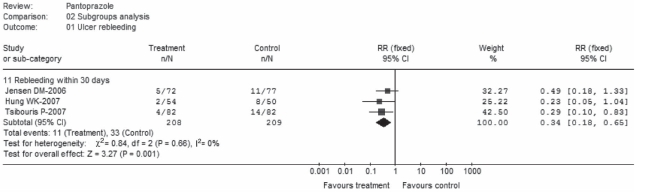
Three trials (22,25,26) separately reported results for ulcer three-day rebleeding rates, and three trials (23,25,26) reported 30-day rebleeding rates (Figure 2). There was no significant heterogeneity in each subset; pooled rates for three-day rebleeding rates were 5.1% in the pantoprazole group versus 14.6% in control, while 30-day rebleeding rates were 5.3% versus 15.8%, respectively. Significant differences in favour of pantoprazole treatment were observed in subgroups A1 (RR 0.34; 95% CI 0.19 to 0.63) and A2 (RR 0.34; 95% CI 0.18 to 0.65). Furthermore, the day 4 to 7 rebleeding rates were reported in three trials (22,25,26); data were homogeneous but nonsignificant (RR 0.72; 95% CI 0.22 to 2.37). Ulcer rebleeding rates were 1.6% in the pantoprazole group compared with 2.3% in the control group.
Figure 2).
Ulcer rebleeding (subgroup analysis). df Degrees of freedom.
Ulcer rebleeding (subgroup analysis). df Degrees of freedom; IV Intravenous; VS Versus
Subgroup analyses that compared pantoprazole with three different agents in the five trials (22–26) were performed. The results were homogeneous and significant, with respective RRs of 0.34 (95% CI 0.16 to 0.69), pooled rates of 5.8% for pantoprazole versus 17.2% for control in subgroup B1 (22,23), 0.36 (95% CI 0.14 to 0.97) and 4.0% versus 11.0% in subgroup B2 (24,25) and 0.21 (95% CI 0.06 to 0.72) and 3.7% versus 17.1% in subgroup B3. Pantoprazole was prescribed as an IV bolus infusion in four studies (22,23,25,26) and an IV continuous infusion in two (23,24). No significant heterogeneity was found in each subset, and the results were significant in favour of pantoprazole treatment (RR 0.32, 95% CI 0.19 to 0.57; RR 0.25; 95% CI 0.09 to 0.71, respectively). Pooled rates were 4.0% versus 16% in subgroup C1 and 4.8% versus 14.8% in subgroup C2, which compared pantoprazole treatment to the control. Furthermore, pantoprazole was initially administered at high dose (80 mg) in three trials (22,23,25) and at low dose (40 mg) in the other two (24,26). Data for rebleeding were homogeneous and significant, with RRs of 0.37 (95% CI 0.20 to 0.70) and 0.22 (95% CI 0.09 to 0.57), respectively. Pooled rates were 5.3% versus 14.0% in subgroup D1 and 3.7% versus 16.7% in subgroup D2.
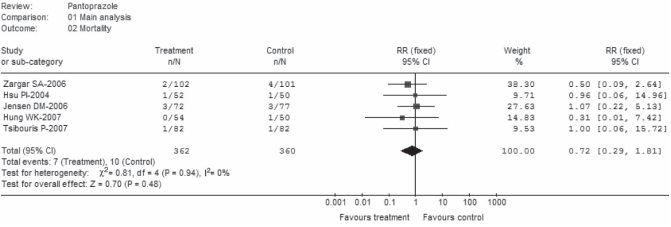
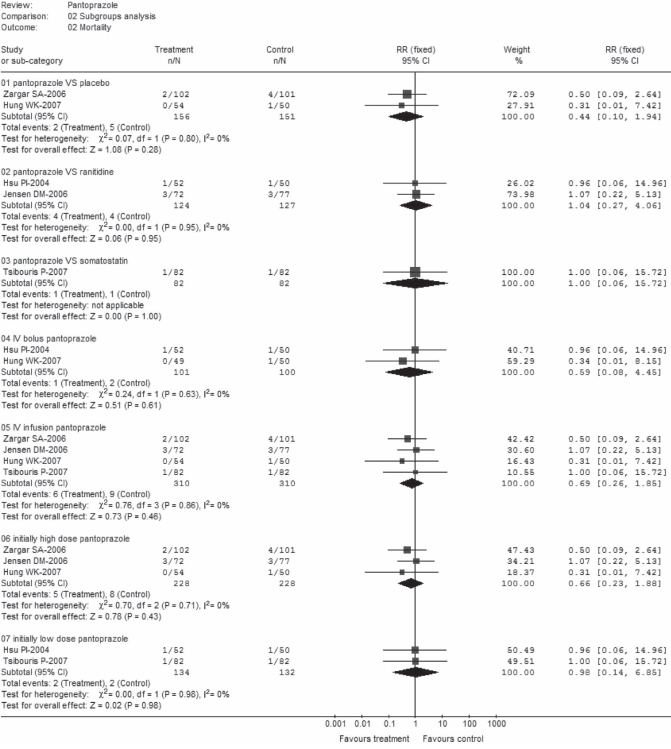
Mortality
Overall, mortality was not significantly affected by pantoprazole treatment (RR 0.72; 95% CI 0.29 to 1.81) (Figure 3). There was no heterogeneity among the studies (P=0.94, I2=0%); pooled rates in the pantoprazole group were 1.9% and 2.8% in control. Of the five trials, three (23, 25,26) stated all-cause mortality within 30 days of random assignment, one (24) reported the eight-week mortality rate and the other (22) did not specify the time for mortality assessment. When these two trials were removed for sensitivity analysis, the overall effect did not change (RR 0.84; 95% CI 0.25 to 2.85).
Figure 3).
Mortality (main analysis). df Degrees of freedom
There was no statistically significant effect of pantoprazole treatment on pooled mortality rates when pantoprazole was compared with placebo (RR 0.44; 95% CI 0.10 to 1.94), ranitidine (RR 1.04; 95% CI 0.27 to 4.06) or somatostatin (RR 1.00; 95% CI 0.06 to 15.72) (Figure 4). Respective pooled rates were 1.3% versus 3.3% for subgroup B1, 3.2% versus 3.1% in subgroup B2 and 1.2% for each treatment group in subgroup B3. Similar effects were found in subgroups C1 (RR 0.59; 95% CI 0.08 to 4.45), C2 (RR 0.69; 95% CI 0.26 to 1.85), D1 (RR 0.66; 95% CI 0.23 to 1.88) and D2 (RR 0.98; 95% CI 0.14 to 6.85), with pooled mortality rates of 1% versus 2%, 1.9% versus 2.9%, 2.2% versus 3.8% and 1.5% versus 1.5%, respectively. Again, no heterogeneity was observed in each subset.
Figure 4).
Mortality (subgroup analysis). df Degrees of freedom; IV Intravenous; VS Versus
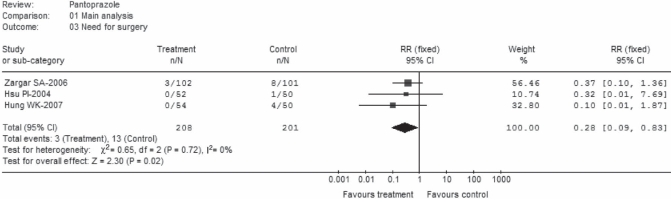
Need for surgery
Among the five RCTs, four (22–24,26) provided rate data for surgery intervention. Unfortunately, data from one trial (26) could not be extracted the due to mixed results in this outcome. Thus, the remaining three trials (22–24) comprising 208 patients in the pantoprazole arm and 201 patients in the control group were assessed. There was no significant heterogeneity among the trials (P=0.72; I2=0%) (Figure 5). Pooled rates were 1.4% in the pantoprazole group versus 6.5% in control. There was a highly significant difference favouring pantoprazole treatment over control (RR 0.28; 95% CI 0.09 to 0.83).
Figure 5).
Need for surgery (main analysis). df Degrees of freedom
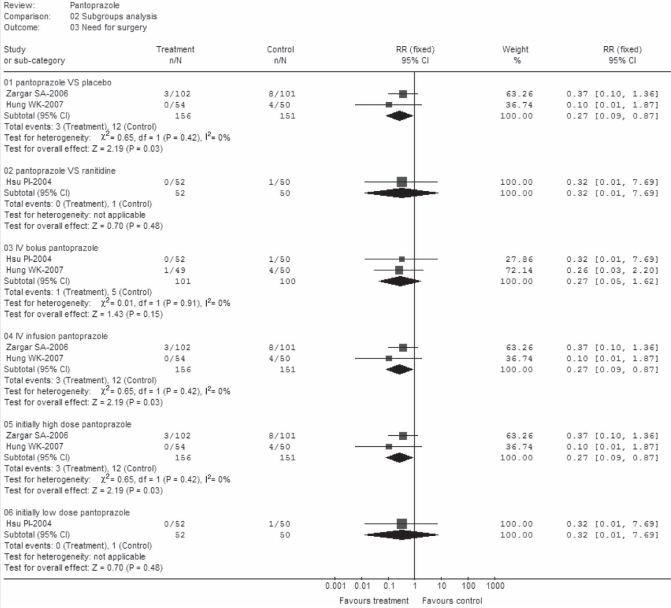
Significant differences in favour of pantoprazole were observed in all subsets except for subgroups B3 and subgroup D2 (Figure 6). However, there was only one trial involved in each subset (24) and this may not represent a robust result (RR 0.32; 95% CI 0.01 to 7.69).
Figure 6).
Need for surgery (subgroup analysis). df Degress of freedom; IV Intravenous; VS Versus
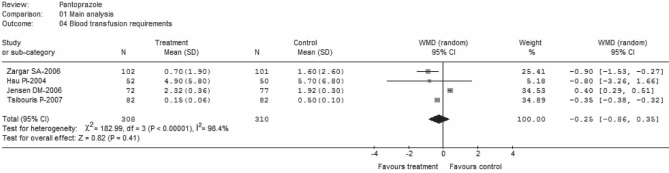
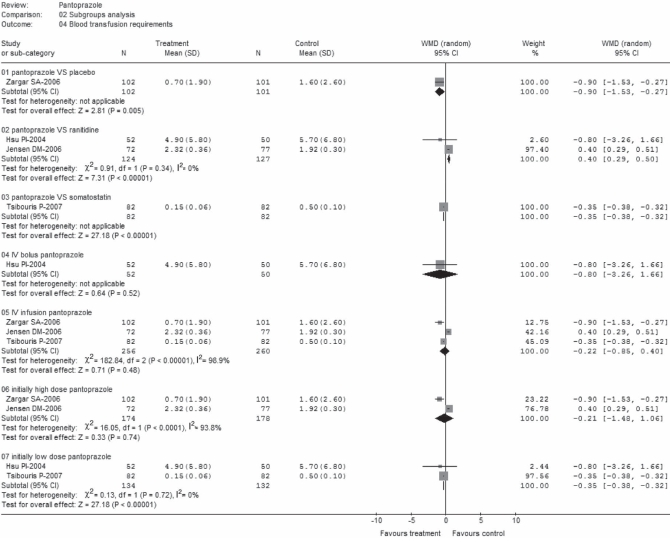
Blood transfusion requirements
Data regarding mean (± SD) of units of blood transfused per treatment group were extracted from four trials (22,24–26). These trials comprised a total of 618 patients; 308 on pantoprazole treatment and 310 as control (Figure 7). There was statistically significant heterogeneity among the trials (P<0.00001; I2=98.4%). No statistically significant difference (WMD −0.25; 95% CI for random effects −0.86 to 0.35) was found between groups, with transfusion requirements in mean units of blood ranging from 0.15±0.06 units to 4.9±5.8 units for pantoprazole treatment; and from 0.5±0.1 units to 5.7±6.8 units for control treatment (24,26). Categories of blood products were specified, but the total units were not reported in one study (25). Data abstracted from this study refer only to red blood cells, which is the most often used clinical practice in such circumstances. Conversely, although one trial (24) provided the total units of blood products, it was unclear from the data whether it was presented in units transfused after endoscopic hemostasis or total duration of hemostasis. However, when these two studies (24,25) were excluded in a sensitivity analysis, the result became marginally significant (WMD −0.53, 95% CI random effects − 0.04 to −0.02).
Figure 7).
Blood transfusion requirements (main analysis). df Degrees of freedom; WMD Weighted mean difference
Incompatible results were also observed in subgroup analyses (Figure 8) in which data were heterogeneous and nonsignificant in groups C2 and D1, and homogeneous and significant in groups B2 and D2. The other subsets (B1, B3 and C1) included one trial in each group, and only group C1 (24), demonstrated an insignificant effect (WMD −0.80; 95% CI random effects −3.26 to −1.66).
Figure 8).
Blood transfusion requirements (subgroup analysis). df Degrees of freedom; IV Intravenous; VS Versus; WMD Weighted mean difference
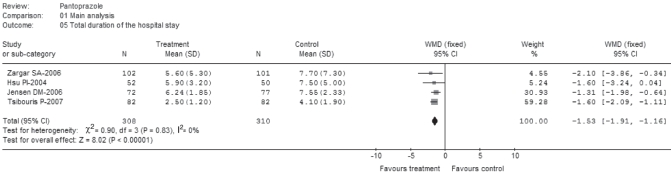
Total duration of hospital stay
Four trials (22,24–26) reported a mean (± SD) of hospitalization days comprising a total of 308 patients on pantoprazole treatment and 310 patients as controls (Figure 9). No statistically significant heterogeneity was observed among the trials (P=0.83; I2=0%). There was a statistically significant difference between the two groups (WMD −1.53; 95% CI −1.91 to−1.16). Among the four trials, the mean duration of hospital stay ranged from 2.5±1.2 days to 6.24±1.85 days for pantoprazole treatment and from 4.1±1.9 days to 7.55±2.33 days for the control treatment (25,26).
Figure 9).
Total duration of hospital stay (main analysis). df Degrees of freedom; WMD Weighted mean difference
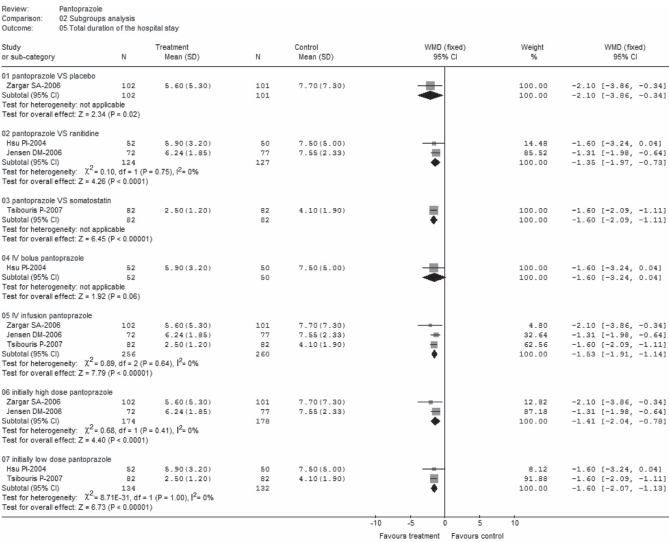
Data from each subgroup (Figure 10) was homogenous and significant in favour of pantoprazole, with the exception of subgroup C1, for which only one study was included (24) and was nonsignificant (WMD −1.60; 95% CI −3.24 to 0.04).
Figure 10).
Total duration of hospital stay (subgroup analysis). df Degrees of freedom; IV Intravenous; VS Versus; WMD Weighted mean difference
DISCUSSION
The results demonstrated that IV pantoprazole given after initial successful endoscopic control of PUB produced a statistically significant beneficial effect in terms of rebleeding, need for surgery and total duration of hospital stay. Mortality and blood transfusion requirements showed a trend toward better results in the pantoprazole group, although these differences were not statistically significant. Because the present study was limited in terms of inclusion, the conclusion reached may not be reliable due to the small number of trials analyzed. Furthermore, one RCT (27) that compared the treatment effect of pantoprazole with ranitidine was published only as an abstract, which constrained our ability to completely evaluate and generalize the reported outcomes. On the other hand, all of the included study data were analyzed after randomization of endoscopic treatments. Because variations exist in the methods of endoscopic treatment and technical knowledge of the endoscopists in each trial, outcomes may be biased.
It was not the purpose of the present review to examine the evidence concerning the timing of initiation of IV infusion of pantoprazole for PUB patients. The analysis of the studies was confined to RCTs conducted with patients who had undergone endoscopic treatment of PUB. We acknowledge, however, that pantoprazole treatment was often initiated before endoscopic hemostasis in clinical practice.
For ulcer bleeding, only the data regarding days 4 to 7 were nonsignificant. The result may be affected by administration of oral PPIs or H2RAs after three-day IV treatment as stated in the studies. However, this finding also suggests that PUB patients may require treatment for longer than 72 h with either high-dose IV or oral pantoprazole to reduce early rebleeding.
There was no evidence to indicate that pantoprazole produced a reduction in mortality following PUB, although there was a substantial and statistically significant reduction in rebleeding rates associated with pantoprazole treatment. This suggests that much of the mortality following an episode of PUB may be unrelated to continued or recurrent bleeding but is more likely to be the result of comorbid disease being exacerbated by the bleeding episode. Although some studies specified that the cause of death was related to associated diseases, most of the deaths were not related to rebleeding. Caution is recommended when interpreting the results of blood transfusion requirements because of the increase in substantially significant heterogeneity when the studies are collectively taken into consideration. Heterogeneity would have originated from discrepancies in the criteria for the need of blood transfusion; and the mixed outcomes in individual studies that did not directly report data regarding categories of blood products.
Of the five RCTs, only one (23) assigned patients to two treatment groups (bolus infusion and continuous infusion), and these groups were compared with a nontreatment group labelled as the control. This study only provided outcomes for bolus or continuous infusion compared with the control, but also reported comparative outcomes of these two treatment groups. Data in each arm for subgroup analysis were separately included (bolus and infusion, respectively). In the main analysis, however, we only included the results of continuous infusion versus no treatment because the regimen was used more often in clinical practice than bolus infusion. Moreover, observation from healthy volunteers further confirmed that continuous IV pantoprazole was superior to intermittent bolus injection for increasing intragastric acidity to higher than pH 6 (19). In the subgroup analyses, bolus infusion and continuous infusion had similar treatment effects on the outcomes of ulcer rebleeding, mortality and blood transfusion requirements, but not for surgical intervention and the overall duration of hospital stay. As shown in the forest plot, continuous infusion seemed be more efficacious than bolus infusion on the outcomes of surgical intervention and hospital stay. However, these results should be interpreted with caution due to the low recruitment number involved in each subgroup and the low quality of study methodology (23). Furthermore, the study authors provided bolus infusion versus continuous infusion data for the all of the outcomes mentioned above. The difference in each end point was minor and nonsignificant. However, to date, there is insufficient evidence and no consensus on the optimal dose of pantoprazole for the treatment of PUB. In the subgroup analyses for initial dose of pantoprazole, we determined that 80 mg and 40 mg had similar superiority over control on ulcer rebleeding and total duration of hospital stay, but not on mortality and blood transfusion requirements. Regarding the results of surgical intervention, high doses of pantoprazole seemed to be more effective. However, in the subset of patients who were on low-dose pantoprazole, there was only one study (24) involved. Therefore, these results were not robust.
One of the five trials (22) used a placebo as the control treatment, whereas in another (23), the randomly assigned patients were not prescribed any antacid treatment for the first three days (ie, no treatment was considered as placebo during the analysis). In fact, the treatment effect between placebo and no treatment are not the same (28). We acknowledge, however, that this assumption could bias the outcomes and should be undertaken with caution. Furthermore, it is unethical to withhold treatment from patients with ulcer complications such as PUB. Therefore, future studies should investigate the use of other agents such as H2RAs as the comparator rather than placebo. On the other hand, it was demonstrated that somatostatin could significantly reduce splanchnic blood flow in patients with variceal and nonvariceal bleeding (29,30). In addition to its vasoactive effect, somatostatin appeared to act as a potent inhibitor of gastric acid secretion through the inhibition of gastrin (31,32). This additional advantage of somatostatin may be of importance for the early control of PUB. Furthermore, because the drug is conventionally used for prevention of ulcer rebleeding, studies comparing the treatment effect of pantoprazole with somatostatin are expected in the future.
Two trials (23,26) separately provided outcomes of 24 h pH monitoring during the first day of drug infusion. One study (26) that compared pantoprazole with somatostatin reported that both medications were equally effective in achieving acid suppression of more than pH 6, although in that trial, pH monitoring was performed in only some of the patients. The other trial (23) demonstrated the difference between continuous and bolus infusions of pantoprazole in the effect on intragastric pH by means of mean pH value and duration of pH greater than 6. This study showed that pantoprazole bolus infusion or continuous infusion both had excellent outcomes over the control. However, continuous infusion achieved a higher mean pH value and a longer duration at higher than pH 6 than bolus infusion, although the difference was not significant (P=0.069 and P=0.182, respectively). In one study (23), the trend of less rebleeding associated with higher gastric pH was noted. This finding further confirms the fact that hemostasis works best at near neutral pH. Therefore, intragastric pH studies after IV PPI infusion would also be of significant clinical interest to document proof-of-concept of the treatment effect (intragastric pH 6 or higher), and future studies should be coupled with gastric pH measurements to achieve the optimal level of acid suppression in bleeding peptic ulcers. In addition, four trials (22,23,25,26) reported side effects. However, they were minor and the frequencies in the two groups were similar.
Overall, the five trials included in the present meta-analysis were almost balanced regarding baseline characteristics of treatment groups. Of note, one trial (22) enrolled more patients with shock on admission in the pantoprazole arm than in the control arm (n=28, n=24, respectively). In another trial (24), fewer patients with a history of ulcer rebleeding in the pantoprazole treatment arm than in the control arm was reported (n=14, n=9, respectively). These differences were not significant; however, they could bias the outcomes, especially on mortality. We could not assess the influence of different stigmata of ulcer hemorrhage on the outcomes in the current meta-analysis because the original studies did not report adequate data for this factor. However, according to the observation from baseline characteristics of patients (Table 2), only one study (22), included fewer patients with oozing bleeding in the pantoprazole group than in control (n=25, n=31, respectively) and more patients with a nonbleeding visible vessel in the pantoprazole treatment group than in control (placebo) (n=65, n=56, respectively). The differences were small and nonsignificant; however, if more patients had oozing bleeding (active bleeding) in the control group, the outcome may have favoured pantoprazole treatment. Notably, the five trials had different definitions for the recruitment of patients with major stigmata of ulcers; two (23,24) enrolled ulcer patients with an endoscopically confirmed adherent clot, whereas the other three trials deliberately excluded these patients. In this case, the outcomes also could be biased. Moreover, one trial (22) involved more patients with duodenal ulcer (84/85) and fewer patients with gastric ulcer (18/16) in the pantoprazole group and in the control group (placebo), respectively (Table 2). Also, in another trial (25), although not statistically significant (P=0.23), there were fewer patients with bleeding from duodenal ulcers in the pantoprazole treatment group (duodenal ulcers to gastric ulcers ratio 37:34) than in the control (ranitidine) treatment group (duodenal ulcers to gastric ulcers ratio 46:31). The imbalance in the ratio of duodenal ulcers to gastric ulcers between the treatment groups could potentially bias the analysis. However, it remains unclear in which direction the imbalance of the ratio of duodenal ulcers to gastric ulcers would bias the outcomes of the trial (7,17). Again, a higher number of patients who were Helicobacter pylori-positive were included in the pantoprazole treatment group than in the control group (41 versus 27) of a trial (25) that also included more patients who were of advanced age in the pantoprazole treatment group than in the control group. It was demonstrated that the difference in H pylori status between the groups was marginally significant (P=0.05). However, we thought this would bias outcomes in favour of pantoprazole treatment on the grounds that PPIs produce a greater degree of suppression of gastric acid secretion in the presence of H pylori infection (33). Conversely, with more elderly patients in the pantoprazole group (31 subjects who were older than 70 years of age) versus 18 subjects who were younger than 70 years of age in the control group, the outcomes could be also biased favouring control treatment (ranitidine).
We did not find any difference in outcomes between the Asian studies and the trials conducted elsewhere in the current meta-analysis mainly due to low recruitment. However, plenty of evidence (21,34,35) has suggested that PPIs were more efficacious for ulcer bleeding among Asian patients than Europeans or North Americans. This could be explained by the lower parietal cell mass and the slower metabolism of PPIs by cytochrome P450 2C19 in the Asian population (36).
Among the five studies, three (22,25,26) were ranked grade A according to the Cochrane quality assessment method (Table 3). In the future, more multicentre, high-quality studies from different countries and regions that compare pantoprazole with other agents rather than placebo are required. Also, results from RCTs investigating dose-effect relationships are expected.
CONCLUSION
In patients with peptic ulcer bleeding, pantoprazole, when administered intravenously after endoscopic therapies, reduces ulcer rebleeding, surgery intervention and the overall duration of hospitalization, but not mortality and blood transfusion requirements compared with placebo, H2RAs or somatostatin.
REFERENCES
- 1.Saltzman JR, Zawacki JK. Therapy for bleeding peptic ulcers. N Engl J Med. 1997;336:1091–3. doi: 10.1056/NEJM199704103361510. [DOI] [PubMed] [Google Scholar]
- 2.Selby NM, Kubba AK, Hawkey CJ. Acid suppression in peptic ulcer haemorrhage: A ‘meta-analysis’. Aliment Pharmacol Ther. 2000;14:1119–26. doi: 10.1046/j.1365-2036.2000.00822.x. [DOI] [PubMed] [Google Scholar]
- 3.Higham J, Kang JY, Majeed A. Recent trends in admissions and mortality due to peptic ulcer in England: Increasing frequency of haemorrhage among older subjects. Gut. 2002;50:460–4. doi: 10.1136/gut.50.4.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paimela H, Paimela L, Myllykangas-Luosujärvi R, et al. Current features of peptic ulcer disease in Finland: Incidence of surgery, hospital admissions and mortality for the disease during the past twenty-five years. Scand J Gastroenterol. 2002;37:399–403. doi: 10.1080/003655202317316015. [DOI] [PubMed] [Google Scholar]
- 5.van Leerdam ME, Vreeburg EM, Rauws EA, et al. Acute upper GI bleeding: Did anything change? Time trend analysis of incidence and outcome of acute upper GI bleeding between 1993/1994 and 2000. Am J Gastroenterol. 2003;98:1494–9. doi: 10.1111/j.1572-0241.2003.07517.x. [DOI] [PubMed] [Google Scholar]
- 6.Patchett SE, O’Donoghue DP. Pharmacological manipulation of gastric juice: Thrombelastographic assessment and implications for treatment of gastrointestinal haemorrhage. Gut. 1995;36:358–62. doi: 10.1136/gut.36.3.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green FW, Jr, Kaplan MM, Curtis LE, et al. Effect of acid and pepsin on blood coagulation and platelet aggregation. A possible contributor prolonged gastroduodenal mucosal hemorrhage. Gastroenterology. 1978;74:38–43. [PubMed] [Google Scholar]
- 8.Patchett SE, Enright H, Afdhal N, et al. Clot lysis by gastric juice: An in vitro study. Gut. 1989;30:1704–7. doi: 10.1136/gut.30.12.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaimoff C, Creter D, Djaldetti M. The effect of pH on platelet and coagulation factor activities. Am J Surg. 1978;136:257–9. doi: 10.1016/0002-9610(78)90241-6. [DOI] [PubMed] [Google Scholar]
- 10.Laine L. Multipolar electrocoagulation versus injection therapy in the treatment of bleeding peptic ulcers. A prospective, randomized trial. Gastroenterology. 1990;99:1303–6. doi: 10.1016/0016-5085(90)91154-x. [DOI] [PubMed] [Google Scholar]
- 11.Cook DJ, Guyatt GH, Salena BJ, et al. Endoscopic therapy for acute nonvariceal upper gastrointestinal hemorrhage: A meta-analysis. Gastroenterology. 1992;102:139–48. doi: 10.1016/0016-5085(92)91793-4. [DOI] [PubMed] [Google Scholar]
- 12.Gralnek IM, Jensen DM, Gornbein J, et al. Clinical and economic outcomes of individuals with severe peptic ulcer hemorrhage and nonbleeding visible vessel: An analysis of two prospective clinical trials. Am J Gastroenterol. 1998;93:2047–56. doi: 10.1111/j.1572-0241.1998.00590.x. [DOI] [PubMed] [Google Scholar]
- 13.Laine L, Peterson WL. Bleeding peptic ulcer. N Engl J Med. 1994;331:717–27. doi: 10.1056/NEJM199409153311107. [DOI] [PubMed] [Google Scholar]
- 14.Avgerinos A, Sgouros S, Viazis N, et al. Somatostatin inhibits gastric acid secretion more effectively than pantoprazole in patients with peptic ulcer bleeding: A prospective, randomized, placebo-controlled trial. Scand J Gastroenterol. 2005;40:515–22. doi: 10.1080/00365520510015458. [DOI] [PubMed] [Google Scholar]
- 15.Kaviani MJ, Hashemi MR, Kazemifar AR, et al. Effect of oral omeprazole in reducing re-bleeding in bleeding peptic ulcers: A prospective, double-blind, randomized, clinical trial. Aliment Pharmacol Ther. 2003;17:211–6. doi: 10.1046/j.1365-2036.2003.01416.x. [DOI] [PubMed] [Google Scholar]
- 16.Lin HJ, Lo WC, Lee FY, et al. A prospective randomized comparative trial showing that omeprazole prevents rebleeding in patients with bleeding peptic ulcer after successful endoscopic therapy. Arch Intern Med. 1998;158:54–8. doi: 10.1001/archinte.158.1.54. [DOI] [PubMed] [Google Scholar]
- 17.Lau JY, Sung JJ, Lee KK, et al. Effect of intravenous omeprazole on recurrent bleeding after endoscopic treatment of bleeding peptic ulcers. N Engl J Med. 2000;343:310–6. doi: 10.1056/NEJM200008033430501. [DOI] [PubMed] [Google Scholar]
- 18.Javid G, Masoodi I, Zargar SA, et al. Omeprazole as adjuvant therapy to endoscopic combination injection sclerotherapy for treating bleeding peptic ulcer. Am J Med. 2001;111:280–4. doi: 10.1016/s0002-9343(01)00812-9. [DOI] [PubMed] [Google Scholar]
- 19.Brunner G, Luna P, Hartmann M, et al. Optimizing the intragastric pH as a supportive therapy in upper GI bleeding. Yale J Biol Med. 1996;69:225–31. [PMC free article] [PubMed] [Google Scholar]
- 20.van Rensburg CJ, Hartmann M, Thorpe A, et al. Intragastric pH during continuous infusion with pantoprazole in patients with bleeding peptic ulcer. Am J Gastroenterol. 2003;98:2635–41. doi: 10.1111/j.1572-0241.2003.08723.x. [DOI] [PubMed] [Google Scholar]
- 21.Leontiadis GI, Sharma VK, Howden CW. Proton pump inhibitor therapy for peptic ulcer bleeding: Cochrane collaboration meta-analysis of randomized controlled trials. Mayo Clin Proc. 2007;82:286–96. doi: 10.4065/82.3.286. [DOI] [PubMed] [Google Scholar]
- 22.Zargar SA, Javid G, Khan BA, et al. Pantoprazole infusion as adjuvant therapy to endoscopic treatment in patients with peptic ulcer bleeding: Prospective randomized controlled trial. J Gastroenterol Hepatol. 2006;21:716–21. doi: 10.1111/j.1440-1746.2006.04292.x. [DOI] [PubMed] [Google Scholar]
- 23.Hung WK, Li VK, Chung CK, et al. Randomized trial comparing pantoprazole infusion, bolus and no treatment on gastric pH and recurrent bleeding in peptic ulcers. ANZ J Surg. 2007;77:677–81. doi: 10.1111/j.1445-2197.2007.04185.x. [DOI] [PubMed] [Google Scholar]
- 24.Hsu PI, Lo GH, Lo CC, et al. Intravenous pantoprazole versus ranitidine for prevention of rebleeding after endoscopic hemostasis of bleeding peptic ulcers. World J Gastroenterol. 2004;10:3666–9. doi: 10.3748/wjg.v10.i24.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jensen DM, Pace SC, Soffer E, et al. Continuous infusion of pantoprazole versus ranitidine for prevention of ulcer rebleeding: A U.S. multicenter randomized, double-blind study. Am J Gastroenterol. 2006;101:1991–9. doi: 10.1111/j.1572-0241.2006.00773.x. [DOI] [PubMed] [Google Scholar]
- 26.Tsibouris P, Zintzaras E, Lappas C. High-dose pantoprazole continuous infusion is superior to somatostatin after endoscopic hemostasis in patients with peptic ulcer bleeding. Am J Gastroenterol. 2007;102:1192–9. doi: 10.1111/j.1572-0241.2007.01120.x. [DOI] [PubMed] [Google Scholar]
- 27.Fried R, Beglinger C, Meier R, et al. Comparison of intravenous pantoprazole with intravenous ranitidine in peptic ulcer bleeding. Gut. 1999;45 (Abst)A100. [Google Scholar]
- 28.Hróbjartsson A, Gøtzsche PC. Is the placebo powerless? An analysis of clinical trials comparing placebo with no treatment. N Engl J Med. 2001;344:1594–602. doi: 10.1056/NEJM200105243442106. [DOI] [PubMed] [Google Scholar]
- 29.Sonnenberg GE, Keller U, Perruchoud A, et al. Effect of somatostatin on splanchnic hemodynamics in patients with cirrhosis of the liver and in normal subjects. Gastroenterology. 1981;80:526–32. [PubMed] [Google Scholar]
- 30.Saruç M, Can M, Küçükmetin N, et al. Somatostatin infusion and hemodynamic changes in patients with non-variceal upper gastrointestinal bleeding: A pilot study. Med Sci Monit. 2003;9:84–7. [PubMed] [Google Scholar]
- 31.Berstad A, Holm HA, Kittang E. Experience with antipeptic agents. Scand J Gastroenterol Suppl. 1979;55:121–3. [PubMed] [Google Scholar]
- 32.Raptis S, Dollinger HC, von Berger L, et al. Effects of somatostatin on gastric secretion and gastrin release in man. Digestion. 1975;13:15–26. doi: 10.1159/000197691. [DOI] [PubMed] [Google Scholar]
- 33.van Herwaarden MA, Samsom M, van Nispen CH, et al. The effect of Helicobacter pylori eradication on intragastric pH during dosing with lansoprazole or ranitidine. Aliment Pharmacol Ther. 1999;13:731–40. doi: 10.1046/j.1365-2036.1999.00531.x. [DOI] [PubMed] [Google Scholar]
- 34.Lam SK, Hasan M, Sircus W, et al. Comparison of maximal acid output and gastrin response to meals in Chinese and Scottish normal and duodenal ulcer subjects. Gut. 1980;21:324–8. doi: 10.1136/gut.21.4.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caraco Y, Lagerstrom PO, Wood AJ. Ethnic and genetic determinants of omeprazole disposition and effect. Clin Pharmacol Ther. 1996;60:157–67. doi: 10.1016/S0009-9236(96)90131-9. [DOI] [PubMed] [Google Scholar]
- 36.Caraco Y, Wilkinson GR, Wood AJ. Differences between white subjects and Chinese subjects in the in vivo inhibition of cytochrome P450s 2C19, 2D6, and 3A by omeprazole. Clin Pharmacol Ther. 1996;60:396–404. doi: 10.1016/S0009-9236(96)90196-4. [DOI] [PubMed] [Google Scholar]