Abstract
Few studies have examined effects of feeding animals a diet deficient in n-6 polyunsaturated fatty acids (PUFAs) but with an adequate amount of n-3 PUFAs. To do this, we fed post-weaning male rats a control n-6 and n-3 PUFA adequate diet and an n-6 deficient diet for 15 weeks, and measured stable lipid and fatty acid concentrations in different organs. The deficient diet contained nutritionally essential linoleic acid (LA,18:2n-6) as 2.3% of total fatty acids (10% of the recommended minimum LA requirement for rodents) but no arachidonic acid (AA, 20:4n-6), and an adequate amount (4.8% of total fatty acids) of α-linolenic acid (18:3n-3). The deficient compared with adequate diet did not significantly affect body weight, but decreased testis weight by 10%. AA concentration was decreased significantly in serum (−86%), brain (−27%), liver (−68%), heart (−39%), testis (−25%), and epididymal adipose tissue (−77%). Eicosapentaenoic (20:5n-3) and docosahexaenoic acid (22:6n-3) concentrations were increased in all but adipose tissue, and the total monounsaturated fatty acid concentration was increased in all organs. The concentration of 20:3n-9, a marker of LA deficiency, was increased by the deficient diet, and serum concentrations of triacylglycerol, total cholesterol and total phospholipid were reduced. In summary, 15 weeks of dietary n-6 PUFA deficiency with n-3 PUFA adequacy significantly reduced n-6 PUFA concentrations in different organs of male rats, while increasing n-3 PUFA and monounsaturated fatty acid concentrations. This rat model could be used to study metabolic, functional and behavioral effects of dietary n-6 PUFA deficiency.
Keywords: Linoleic acid, Arachidonic acid, Dietary deficiency, PUFA
1. Introduction
In mammals, the nutritionally essential polyunsaturated fatty acids (PUFAs), linoleic acid (LA, 18:2n-6) and α-linolenic acid (α-LNA, 18:3n-3), must be consumed in the diet since neither can be synthesized de novo. Reduced consumption of both leads to marked biochemical and functional consequences [1]. Once consumed, LA and α-LNA are converted to arachidonic acid (AA, 20:4n-6) and docosahexaenoic acid (DHA, 22:6n-3), respectively, by a series of desaturation, elongation and β-oxidation steps in the liver [2–4], or undergo β-oxidation [5]. Alternatively, AA and DHA can be obtained directly in the diet.
Neuronal membranes contain high concentrations of DHA and AA. In brain, AA serves directly as a secondary signaling molecule, or is converted to bioactive eicosanoids. AA and its products have multiple biological effects [6–8]. DHA can participate in signaling or be converted to docosanoids, which have anti-apoptotic, anti-inflammatory, and neuroprotective effects [9–11] . Decreasing concentrations of brain DHA by feeding animals an n-3 PUFA deficient diet causes brain malfunction in experimental animals [12–15].
The cerebral effects of n-3 PUFA dietary deficiency in rodents have been widely reported, but those of dietary n-6 PUFA deprivation have not been as closely studied. Bourre et al. [16] reported that a low LA diet decreased concentrations of LA, AA, docosatetraenoic acid (22:4n-6), and docosapentaenoic acid (DPAn-6, 22:5n-6) in rat brain and other organs and increased concentrations of eicosapentaenoic acid (EPA, 20:5n-3). They did not detect eicosatrienoic acid, 20:3n-9, a marker of LA deficiency [17]. Cunnane et al. [18,19] reported that feeding rats a diet free of LA, which contained unesterified α-LNA and oleic acid (18:1n-9) (artificial oils), reduced whole body concentrations of LA, AA, α-LNA and DPAn-3, and increased 18:1n-9, 20:3n-9 and DHA concentrations. EPA was not detected.
Metabolism and function in brain and other organs depend on maintaining homeostatically balanced concentrations of n-3 and n-6 PUFAs [20–23]. Because the effects of selective dietary n-6 PUFA deprivation on brain and other organs have been addressed to a limited extent in relation to this concept, we though it important to do so in this study. We prepared an n-6 PUFA deficient diet (containing LA at 10% of the daily estimated requirement, 42.8 µmol/g diet [16]) that was n-3 PUFA adequate, and a control diet containing adequate amounts of n-3 and n-6 PUFAs, comparable to one that we used in prior studies [21–26]. Male rats were fed the adequate or deficient diet for 15 weeks after weaning, and concentrations of fatty acids and stable lipids (phospholipids, triacylglycerol, and cholesterol) were measured in brain and other organs. An abstract of part of this work has been published [27].
2. Materials and methods
2.1. Materials
Di-heptadecanoate phosphatidylcholine (di-17:0 PC) and triacylglyceride determination kits were purchased from Sigma-Aldrich (St. Louis, MO, USA). Standards for fatty acid methyl esters (FAMEs) for gas chromatography (GC) were obtained from NuChek Prep (Elysian, MN, USA). Cholesterol quantification kits were purchased from BioVision Inc. (Mountain View, CA, USA). All other chemicals and reagents were purchased from Sigma-Aldrich or Fisher Scientific (Pittsburgh, PA, USA).
2.2. Animals
The protocol was approved by the Animal Care and Use Committee of the Eunice Kennedy Schriver National Institute of Child Health and Human Development and conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23). Fischer-344 (CDF) male rat pups (18 days old) and their surrogate mothers were purchased from Charles River Laboratories (Portage, MI, USA) and were housed in an animal facility with regulated temperature, humidity, and a 12 h light/12 h dark cycle. The pups were allowed to nurse until 21 days old. Lactating rats had free access to water and rodent chow formulation NIH-31 18–4, which contained 4% (wt/wt) crude fat (Zeigler Bros., Gardners, PA, USA) and whose fatty acid composition is reported [28,29]. α-LNA, EPA and DHA contributed 5.1%, 2.0% and 2.3% of total fatty acids, respectively, whereas LA and AA contributed 47.9% and 0.02%, respectively. After weaning, the pups were divided randomly into n-6 PUFA adequate and deficient diet groups. They had free access to food and water, with their food being replaced every 2 or 3 days. Body weight was recorded every week, and blood was collected from the tail vein without an anticoagulant every 2 weeks. After 15 weeks on their diet, rats were asphyxiated by CO2 inhalation and decapitated. The organs were rapidly excised and frozen in 2-methylbutane with dry ice at −50 °C, and stored at −80 °C until assay. The blood was collected from the abdominal aorta without an anticoagulant. The blood was centrifuged at 1500 rpm for 5 min, and the serum was kept at −80 °C until assay.
2.3. n-6 PUFA adequate and deficient diets
The compositions of the n-6 PUFA adequate and deficient diets are given in Table 1. The diets were based on the AIN-93G formulation, and contained 10% fat [23,30,31]. The n-6 PUFA adequate diet contained hydrogenated coconut oil (6 g/100 g diet), safflower oil (3.23 g/100 g) and flaxseed oil (0.77 g/100 g), as in previous studies (Table 1) [21–26]. The n-6 PUFA deficient diet contained hydrogenated coconut oil (8.73 g/100 g), flaxseed oil (0.77 g/100 g), and olive oil (0.5 g/100 g), but not safflower oil, which is a major source of LA (Table 1). The fatty acid concentrations (µmol/g food, and % of total fatty acid) in both diets are shown in Table 2.
Table 1.
Composition of n-6 PUFA adequate and deficient diets
| Component | n-6 PUFA adequate diet |
n-6 PUFA deficient diet |
|---|---|---|
| g/100 g diet | g/100 g diet | |
| Protein (20%) | ||
| Casein | 20 | 20 |
| Carbohydrate (60%) | ||
| Cornstarch | 15 | 15 |
| Sucrose | 10 | 10 |
| Dextrose | 20 | 20 |
| Maltose dextrin | 15 | 15 |
| Fat (10%) | ||
| Hydrogenated coconut oil | 6.00 | 8.73 |
| Safflower oil | 3.23 | 0 |
| Flaxseed oil | 0.77 | 0.77 |
| Olive oil | 0 | 0.5 |
| Additives (10%) | ||
| Cellulose | 4.95 | 4.95 |
| Salts | 3.5 | 3.5 |
| Vitamins | 1.0 | 1.0 |
| L-cystine | 0.3 | 0.3 |
| Choline chloride | 0.25 | 0.25 |
| TBHQ | 0.002 | 0.002 |
TBHQ, tertiary-butylhydroquinone (antioxidant).
Table 2.
Fatty acid composition of n-6 PUFA adequate and deficient diets
| Fatty acid | n-6 PUFA adequate diet |
n-6 PUFA deficient diet |
||
|---|---|---|---|---|
| µmol/g food | % of total fatty acids |
µmol/g food | % of total fatty acids |
|
| 12:0 | 54.6 ± 3.3 | 29.0 | 81.1 ± 20.7 | 43.8 |
| 14:0 | 23.5 ± 1.4 | 12.5 | 34.6 ± 9.0 | 18.7 |
| 14:1n-5 | 0.06 ± 0.01 | 0.03 | 0.06 ± 0.02 | 0.03 |
| 16:0 | 18.2 ± 1.0 | 9.7 | 20.6 ± 5.3 | 11.1 |
| 16:1n-7 | 0.08 ± 0.01 | 0.04 | 0.10 ± 0.03 | 0.1 |
| 18:0 | 17.1 ± 1.0 | 9.0 | 22.0 ± 5.8 | 11.9 |
| 18:1n-9 | 14.4 ± 0.8 | 7.7 | 13.6 ± 3.5 | 7.3 |
| 18:2n-6 | 52.1 ± 7.6 | 27.6 | 4.2 ± 1.1 | 2.3 |
| 18:3n-3 | 8.5 ± 0.5 | 4.5 | 8.9 ± 2.4 | 4.8 |
| 20:4n-6 | N.D | N.D | ||
| 20:5n-3 | N.D | N.D | ||
| 22:6n-3 | N.D | N.D | ||
| Saturated | 113.5 ± 6.6 | 60.1 | 158.2 ± 40.8 | 85.5 |
| Monounsaturated | 14.6 ± 0.8 | 7.7 | 13.7 ± 3.5 | 7.4 |
| n-6 PUFA | 52.1 ± 7.6 | 27.6 | 4.2 ± 1.1 | 2.3 |
| n-3 PUFA | 8.5 ± 0.5 | 4.5 | 8.9 ± 2.4 | 4.8 |
| n-6/n-3 | 6.1 | 0.5 | ||
Values are mean ± SD (n=3).
To analyze each diet, total lipids were extracted from random ∼0.6 g samples (n=3) and were methylated. The resulting FAMEs were separated by GC as described below. The n-6 PUFA adequate diet contained LA at 52.1 µmol/g (27.6% of total fatty acids), whereas the deficient diet contained LA at 4.2 µmol/g (2.3% of total fatty acids), which is 10% of the minimum requirement for rodents (42.8 µmol/g) (Table 2) [16]. Both diets contained α-LNA at 8.5–8.9 µmol/g (4.5–4.8% of total fatty acids), which is the minimum level for n-3 PUFA adequacy in rodents [13,32], and oleic acid (18:1n-9) at 13.6–14.4 µmol/g (7.3–7.7 % of total fatty acids). Other n-3 and n-6 PUFAs were absent in both diets. The diets were prepared by Dyets Inc. (Bethlehem, PA).
2.4. Lipid extraction and methylation
Total lipid from organs was extracted by the Folch procedure [33]. An aliquot of total lipid extract was methylated with 1% H2SO4-methanol for 3 h at 70 °C (16, 36). Before the sample was methylated for GC analysis, di-17:0 PC was added as an internal standard.
2.5. Gas chromatography analysis
FAMEs (nmol/g tissue wet wt or nmol/ml plasma) in total lipids were determined using a GC (6890N, Agilent Technologies, Palo Alto, CA, USA) equipped with an SP-2330 fused silica capillary column (30 m × 0.25 mm i.d., 0.25 µm film thickness) (Supelco, Bellefonte, PA, USA) and a flame ionization detector [26]. Concentrations were calculated by proportional comparison of peak areas to the area of the 17:0 internal standard.
2.6. Chemical analysis
To quantify total cholesterol and triacylglycerol, the lipid extract was dried in a SpeedVac (Model AES 1010, Savant, Holbrook, NY, USA) and the residue was dissolved in 0.1% Triton X-100. Total cholesterol and triacylglycerol concentrations were determined with commercial kits. Phospholipid concentrations were determined as reported by Rouser et al. [34]. An aliquot of total lipids was added to the tube and dried in a SpeedVac. Water (0.5 ml) and 0.65 ml of perchloric acid (70%) were added to the dried extract, followed by digestion at 180 °C for 1 h. The sample was cooled to room temperature and 0.5 ml of ascorbic acid (10%, w/v), 0.5 ml of ammonium molybdate (2.5%, w/v), and 3.0 ml of water were added. The mixture was boiled for 5 min at 100°C to develop color, and its absorbance was read at 797 nm when it had cooled to room temperature. Standards for this assay were purchased from Sigma, and phospholipid concentrations were determined using standard curves.
2.7. Statistical analysis
Data are expressed as means ± SD. An unpaired Student’s t-test was used to compare means in 2 groups having possibly equal variances with an F-test, and the Aspin–Welch test was used to compare means in 2 groups having unequal variances. Statistical significance was taken at p≤0.05.
3. Results
3.1. Growth and tissue weight
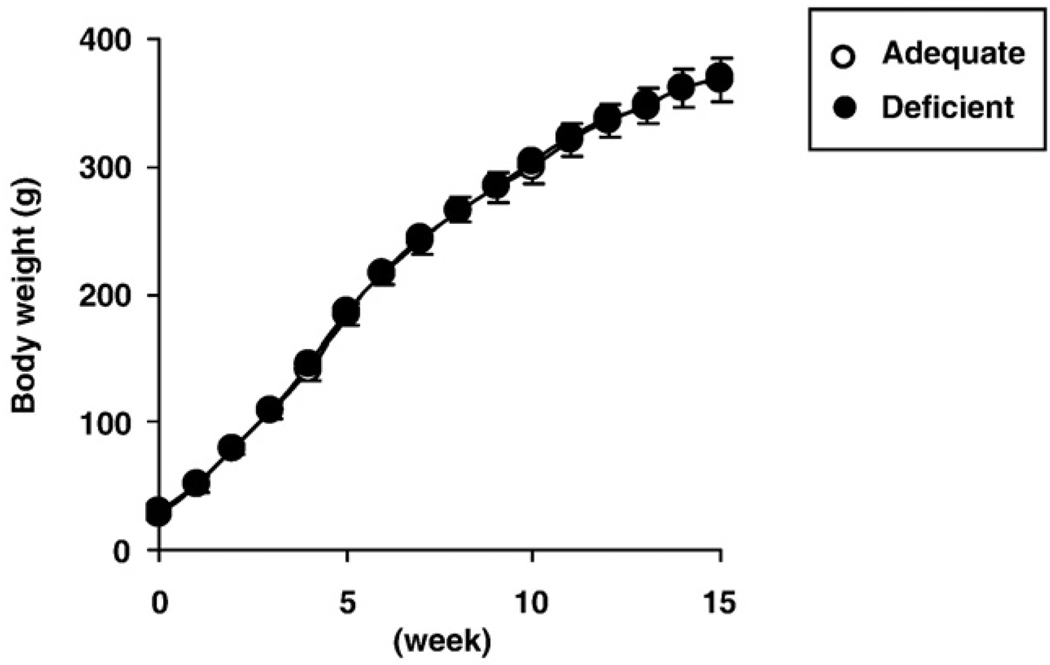
Rat body weight was measured every week over the 15-week feeding period. There was no difference in weight gain between rats fed the two diets over the entire period (Fig. 1). Initial body weight after weaning and final body weight are presented in Table 1. The deficient diet did not cause evident skin problems or hair loss.
Fig. 1.
Body weights of rats fed n-6 PUFA adequate (○) and deficient diets (•) over 15 weeks after weaning. Values are mean ± SD (n=10 for both dietary groups).
Feeding the n-6 PUFA deficient compared to adequate diet did not significantly affect the wet weight of brain, liver, heart, epididymal adipose tissue, lung, kidney, or spleen, but it reduced the weight of the testis by 10% (Table 3).
Table 3.
Body and tissue weights of n-6 PUFA adequate and deficient diets at 15 weeks
| Tissue | Dietary groups |
|
|---|---|---|
| Adequate |
Deficient |
|
| weight (g) | weight (g) | |
| Initial body weight | 29 ± 2 | 30 ± 2 |
| Final body weight | 379 ± 20 | 380 ± 16 |
| Brain | 1.9 ± 0.1 | 1.9 ± 0.1 |
| Liver | 10.4 ± 1.2 | 10.2 ± 1.3 |
| Heart | 0.9 ± 0.1 | 0.9 ± 0.1 |
| Testis | 3.0 ± 0.1 | 2.7 ±0.2** |
| Epididymal adipose tissue | 12.4 ± 1.4 | 11.8 ± 1.3 |
| Lung | 1.8 ± 0.3 | 1.8 ± 0.3 |
| Kidney | 2.3 ± 0.2 | 2.4 ± 0.2 |
| Spleen | 0.7 ± 0.1 | 0.8 ± 0.1 |
Values are mean±SD (n = 10 for both groups).
p<0.01.
3.2. Stable lipid concentrations in serum and body organs
Feeding the n-6 PUFA deficient diet compared to adequate diet decreased serum triacylglycerol, total phospholipid, and total cholesterol concentrations. These concentrations in brain, liver, heart, testis and epididymal adipose tissue were not significantly changed (Table 4).
Table 4.
Concentrations of stable lipids in serum, brain, liver and heart from n-6 PUFA adequate and deficient rats
| Lipid | Triacylglycerol |
Total cholesterol |
Total phospholipid |
|||
|---|---|---|---|---|---|---|
| Adequate | Deficient | Adequate | Deficient | Adequate | Deficient | |
| Serum (µmol/ml serum) | 2.2 ± 0.8 | 1.5 ± 0.3 * | 1.7 ± 0.4 | 1.0 ± 0.2 *** | 2.6 ± 0.4 | 1.8 ± 0.2*** |
| Brain (µmol/g brain) | 0.57 ± 0.22 | 0.53 ± 0.27 | 84.1 ± 15.7 | 97.2 ± 16.4 | 57.4 ± 5.2 | 61.1 ± 3.7 |
| Liver (µmol/g liver) | 20.6 ± 3.9 | 20.6 ± 2.9 | 4.3 ± 1.6 | 5.8 ± 2.3 | 30.4 ± 8.0 | 29.4 ± 4.5 |
| Heart (µmol/g heart) | 10.1 ± 3.6 | 11.5 ± 6.2 | 3.7 ± 1.3 | 3.3 ± 1.1 | 34.7 ± 5.0 | 4.0 ± 3.1 |
| Testis (µmol/g testis) | 1.8 ± 0.5 | 1.7 ± 0.6 | 6.3 ± 1.0 | 5.1 ± 1.6 | 15.0 ± 1.5 | 15.1 ± 1.3 |
| Epididymal adipose tissue (µmol/g adipose tissue) |
74.9 ± 18.9 | 70.6 ± 9.3 | 2.6 ± 0.7 | 2.6 ± 0.3 | 1.5 ± 0.6 | 1.5 ± 0.3 |
Values are mean ± SD (n=10 for both groups).
p<0.05
p<0.001, differs significantly from mean in adequate group.
3.3. Fatty acid concentrations in serum over 15 weeks
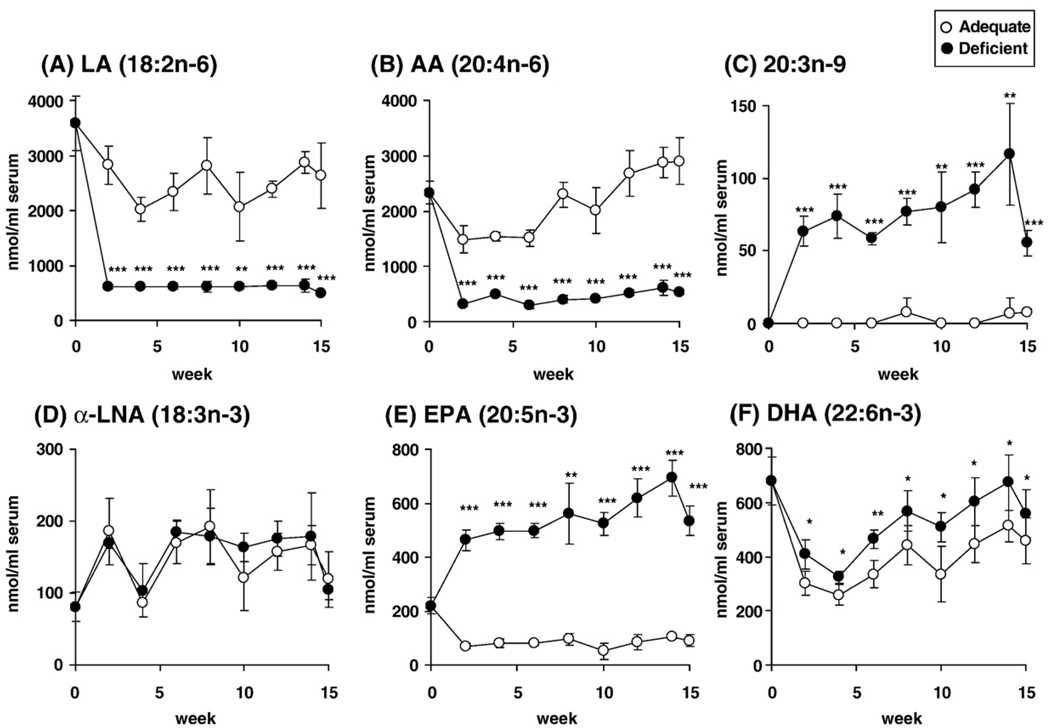
Serum fatty acid concentrations were determined every 2 weeks for up to 15 weeks. After 2 weeks, the n-6 PUFA deficient diet compared with adequate diet decreased the LA concentration by 78% (Fig. 2A) and the AA concentration by 79% (Fig. 2B). EPA and DHA concentrations were increased by 560% (Fig. 2E) and 35% (Fig. 2F), respectively, but the α-LNA concentration was not significantly affected (Fig. 2D). The concentration of 20:3n-9 was increased by the deficient diet at 2 weeks (Fig. 2C). The changes in the concentrations of LA, AA, EPA, DHA, and 20:3n-9 remained significant throughout the 15-week feeding period.
Fig. 2.
Fatty acid concentrations in serum total lipids of rat fed n-6 PUFA adequate (○) and deficient (•) diets over 15 weeks. (A) LA, (B) AA, (C) 20:3n-9, (D) α-LNA, (E) EPA, (F) DHA. Values are mean ± SD (n=4–5 for both dietary groups). * p<0.05, **p<0.01, and ***p<0.001 differs significantly from mean in the adequate group.
n-6 PUFA deprivation for 15 weeks decreased the total fatty acid concentration in serum by 37% (Table 5), in line with it reducing the concentrations of the stable lipids in which the fatty acids were esterified (Table 4). Serum LA, 20:3n-6 and AA concentrations were decreased by 37–81%, and 22:4n-6 and 22:5n-6 were not detected. Total n-3 PUFA, EPA, and DHA concentrations were increased by 59%, 488%, and 22%, respectively. The concentration of 20:3n-9 was increased by 686%.
Table 5.
Fatty acid concentrations in serum, brain, and liver
| Fatty acid | Serum |
Brain |
Liver |
|||
|---|---|---|---|---|---|---|
| Adequate |
Deficient |
Adequate |
Deficient |
Adequate |
Deficient |
|
| nmol/ml serum | nmol/ml serum | nmol/g brain | nmol/g brain | nmol/g liver | nmol/g liver | |
| n-6 PUFA | ||||||
| 18:2 | 2627 ± 593 | 492±26*** (−81%) | 477 ± 28 | 261±19*** (−45%) | 18829 ± 4051 | 5593±867*** (−70%) |
| 18:3 | 26 ± 7 | 6±1*** | ND | ND | 392 ± 108 | 99 ± 19*** |
| 20:3 | 92 ± 19 | 58 ± 6*** | 307 ± 17 | 466 ± 29*** | 1135 ± 179 | 1276 ± 200 |
| 20:4 | 2906 ± 415 | 531 ±45*** (−81%) | 12350 ± 397 | 8927±723*** (−28%) | 19496 ± 2742 | 3157 ± 919*** (84%) |
| 22:4 | 69 ± 12 | ND | 2855 ± 107 | 1827 ± 127*** | 500 ± 202 | 102±21*** |
| 22:5 | 21 ± 6 | ND | 96 ± 29 | ND | 320 ± 59 | 68 ± 9*** |
| Total | 5741 ± 1022 | 1088 ± 67*** (−81%) | 16085 ± 454 | 11480 ± 844*** (−29%) | 40672 ± 7146 | 13295 ± 1950*** (67%) |
| n-3 PUFA | ||||||
| 18:3 | 119 ± 39 | 105 ± 14 | ND | ND | 896 ± 285 | 970 ± 250 |
| 20:5 | 91 ± 24 | 535 ± 53*** (+488%) | ND | 236 ± 30 | 512 ± 67 | 5359 ± 732*** (+947%) |
| 22:5 | 171 ± 45 | 138 ± 40 | 207 ± 75 | 537 ± 125*** | 1140 ± 289 | 1774 ± 380 *** |
| 22:6 | 459 ± 84 | 558 ± 89* (+22%) | 15879 ± 546 | 17692 ± 1246** (+11%) | 6459 ± 922 | 11125 ± 1602** * (+72%) |
| Total | 840 ± 169 | 1335 ± 118*** (+59%) | 16086 ± 549 | 18465 ± 1307*** (+15%) | 9008 ± 1485 | 19229 ± 2641*** (+113%) |
| Others | ||||||
| 16:0 | 3854 ± 1075 | 2849 ± 492* | 21906 ± 1821 | 21953 ± 1262 | 47398 ± 11456 | 57438 ± 10838 |
| 16:1n-7 | 671 ± 273 | 548 ± 128 | 331 ± 291 | 388 ± 211 | 9497 ± 3247 | 12575 ± 3070* |
| 18:0 | 1684 ± 244 | 1209 ± 115*** | 24660 ± 223 | 25413 ± 1500 | 20188 ± 2630 | 20693 ± 4601 |
| 18:1n-9 | 1772 ± 578 | 1727 ± 356 | 14691 ± 1673 | 17054 ± 1542** | 19408 ± 5138 | 32175 ± 5567 *** |
| 18:1n-7 | 1093 ± 315 | 1055 ± 165 | 9783 ± 836 | 10368 ± 413 | 12174 ± 2186 | 16982 ± 2229 *** |
| 20:3n-9 | 7 ± 2 | 55 ± 9*** | 30 ± 6 | 253 ± 19** | 116 ± 27 | 991 ± 144*** |
| Total saturated | 5537 ± 1301 | 4058 ± 601** | 46566 ± 2384 | 47366 ± 1746 | 67586 ± 12561 | 78131 ± 11514 |
| Total mono | 3537 ± 1132 | 3330 ± 609 | 24805 ± 2177 | 27810 ± 1586** (+12%) | 41079 ± 10211 | 61731 ± 10247*** (+50%) |
| Total | 15663 ± 3471 | 9867 ± 1292** (−37%) | 103572 ± 4017 | 105374 ± 3573 | 158460 ± 25238 | 173376 ± 22684 |
| Ratio of n-6/n-3 | 6.9 | 0.8 | 1.0 | 0.6 | 4.5 | 0.7 |
| FA composition (% of Total FA) | ||||||
| Total n-6 PUFA | 37% | 11% | 16% | 11% | 26% | 7.7% |
| Total n-3 PUFA | 5.3% | 14% | 16% | 18% | 5.7% | 11% |
| Total saturated | 35% | 41% | 45% | 45% | 43% | 45% |
| Total monounsaturated | 23% | 34% | 24% | 26% | 26% | 36% |
| 20:3n-9 | 0.04% | 0.6% | 0.03% | 0.2% | 0.07% | 0.6% |
Values are mean ± SD (n=10 for both groups).
p<0.05
p<0.01
p<0.001, differs significantly from mean in adequate group.
ND=not detected.
3.4. n-6 PUFA concentrations in brain and other organs
The n-6 PUFA deficient compared with adequate diet reduced the total n-6 PUFA concentration in brain (−29%), liver (−67%), heart (−42%), testis (−40%), and adipose tissue (−90%) (Table 5 and Table 6). The AA concentration was decreased in brain by 28%, liver by 84%, heart by 39%, testis by 25%, and epididymal adipose tissue by 79%, whereas the concentration of LA was decreased in brain by 45%, liver by 70%, heart by 44%, testis by 71%, and adipose tissue by 91%.
Table 6.
Fatty acid concentrations in heart, testis, and adipose at 15 week deprivation
| Fatty acid | Heart |
Testis |
Epididymal adipose tissue |
|||
|---|---|---|---|---|---|---|
| Adequate |
Deficient |
Adequate |
Deficient |
Adequate |
Deficient |
|
| nmol/g heart | nmol/g heart | nmol/g testis | nmol/g testis | nmol/g adipose tissue | nmol/g adipose tissue | |
| n-6 PUFA | ||||||
| 18:2 | 16632 ± 999 | 9249 ± 1274*** (−44%) | 6102 ± 1868 | 1763 ± 734 *** (−71%) | 56143 ± 16376 | 5265 ± 907*** (−91%) |
| 18:3 | 102 ± 45 | 58 ± 6* | 51 ± 9 | 37 ± 10** | 299 ± 87 | 100 ± 39*** |
| 20:3 | 373 ± 49 | 770 ± 49*** | 426 ± 41 | 500 ± 73 ** | 388 ± 107 | 81 ± 38 *** |
| 20:4 | 13434 ± 503 | 8208 ± 715*** (−39%) | 5481 ± 606 | 4117 ± 571 *** (−25%) | 1709 ± 399 | 365 ± 63 *** (−79%) |
| 22:4 | 717 ± 70 | 102 ± 34*** | 843 ± 82 | 493±78 *** (−42%) | 515 ± 117 | 314 ± 130 *** |
| 22:5 | 478 ± 63 | 55 ± 22*** | 5899 ± 804 | 4320 ± 616 *** | 74 ± 14 | ND |
| Total | 31737 ± 867 | 18442 ± 2039*** (−42%) | 18802 ± 1719 | 11231 ± 1591 *** (−40%) | 59127 ± 16920 | 6124 ± 1025*** (−90%) |
| n-3 PUFA | ||||||
| 18:3 | 307 ± 55 | 647 ± 82*** | 528 ± 218 | 498 ± 247 | 6619 ± 1844 | 5272 ± 892 |
| 20:5 | 122 ± 80 | 1486 ± 202*** (+1118%) | 57 ± 14 | 291 ± 43 *** (+411%) | 285 ± 257 | 253 ± 256 |
| 22:5 | 1779 ± 177 | 2308 ± 150*** | 133 ± 37 | 219 ± 55 *** | 374 ± 92 | 276 ± 55* |
| 22:6 | 7555 ± 446 | 9337 ± 337*** (+24%) | 496 ± 49 | 1299 ± 163 *** (+162%) | 518 ± 169 | 634 ± 296 |
| Total | 9762 ± 643 | 13778 ± 482*** (+41%) | 1213 ± 306 | 2307 ± 373*** (+90%) | 7795 ± 1947 | 6435 ± 1213 |
| Others | ||||||
| 16:0 | 9702 ± 572 | 10235 ± 2025 | 14999 ± 1619 | 15380 ± 2458 | 62611 ± 14275 | 66700 ± 10380 |
| 16:1n-7 | 777 ± 176 | 2142 ± 510*** | 2795 ± 928 | 3588 ± 1778 | 19703 ± 4206 | 30617 ± 4409 *** |
| 18:0 | 16406 ± 624 | 15841 ± 1022 | 3111 ± 287 | 3035 ± 360 | 7769 ± 1964 | 6507 ± 1115 |
| 18:1n-9 | 3897 ± 611 | 8329 ± 2843*** | 7863 ± 1705 | 10250 ± 2929 * | 40315 ± 9406 | 54807 ± 9115** |
| 18:1n-7 | 2868 ± 152 | 3321 ± 204*** | 1533 ± 278 | 1853 ± 188 ** | 12384 ± 2860 | 15010 ± 2603 * |
| 20:3n-9 | 58 ± 23 | 524 ± 39*** | 51 ± 9 | 234 ± 30 *** | 167 ± 47 | 185 ± 37 |
| Total saturated | 26107 ± 974 | 26076 ± 2090 | 18109 ± 1825 | 18414 ± 2739 | 70380 ± 16210 | 73207 ± 11476 |
| Total mono | 5133 ± 1205 | 12962 ± 3042*** (+153%) | 12191 ± 2428 | 15691 ± 4814 * (+29%) | 72401 ± 16337 | 100435 ± 15866** (+39%) |
| Total | 73539 ± 4129 | 70257 ± 6461 | 50366 ± 5955 | 47876 ± 8072 | 209872 ± 50977 | 186386 ± 29260 |
| Ratio of n-6/n-3 | 3.3 | 1.3 | 16 | 4.8 | 7.5 | 1.0 |
| FA composition (% of Total FA) | ||||||
| n-6 PUFA | 43% | 26% | 37% | 23% | 28% | 3.2% |
| n-3 PUFA | 13% | 20% | 2.4% | 4.8% | 3.7% | 3.5% |
| Total saturated | 36% | 37% | 36% | 38% | 34% | 39% |
| Total monounsaturated | 8% | 18% | 24% | 33% | 34% | 54% |
| 20:3n-9 | 0.08% | 0.7% | 0.1% | 0.5% | 0.08% | 0.1% |
Values are mean ± SD (n=10 for both groups).
p<0.05
p<0.01
p<0.001, differs significantly from mean in adequate group.
ND, not detected.
3.5. n-3 PUFA concentrations in brain and other organs
The n-6 PUFA deficient diet increased total n-3 PUFA concentrations in brain (+15%), liver (+113%), heart (+41%), and testis (+90%), but not in adipose tissue (Table 5 and Table 6). The DHA concentration was increased in brain by 11%, in liver by 72%, in heart by 24%, and in testis by 162%, and the EPA concentration was increased in liver by 947%, in heart by 1118%, and in testis by 411%. EPA was detected in the brain of the n-6 PUFA deprived rats, but not of the rats fed the adequate diet. The concentration of α-LNA was not changed significantly in liver, testis, or epididymal adipose tissue by the n-6 PUFA deficient diet, but was increased in the heart by 111%. α-LNA was not detected in the brain of either dietary group.
3.6. Eicosatrienoic acid (20:3n-9) concentrations in brain and other organs
Eicosatrienoic acid (20:3n-9), a marker of LA deficiency [17], is synthesized from 18:1n-9 by elongases and desaturases. Its concentration was increased in brain by 743%, in liver by 754%, in heart by 803%, and in testis by 359%, but not in epididymal adipose tissue, of rats fed the n-6 PUFA deficient compared with adequate diet (Table 5 and Table 6). The concentration of 20:3n-9 remained less than 1% of total fatty acids in each organ of rats fed the n-6 PUFA deficient diet.
3.7. Saturated and monounsaturated fatty acid concentrations in the brain and other organs
n-6 PUFA deprivation did not affect concentrations of saturated fatty acids 16:0 and 18:0 in any of the organs analyzed. Total monounsaturated fatty acid concentrations were increased by 12– 153% in the brain and other organs (Table 5 and Table 6).
4. Discussion
In this study, we prepared an n-6 PUFA deficient diet (containing 10% of the recommended LA requirement of 42.8 µmol/g [16]) that contained a nutritionally adequate amount of n-3 PUFAs, and examined the effects of feeding this diet for 15 weeks in just-weaned male rats. Feeding the deficient compared with adequate diet decreased the total n-6 PUFA concentration (29–90%) in brain and other organs, and reciprocally increased concentrations of total n-3 PUFAs (15–113%) and of total monounsaturated fatty acids (12–153%). The concentration of 20:3n-9 was markedly increased (39–754%) in each organ, but remained less than 1% of total fatty acids. The deficient diet did not retard body growth or cause evident skin problems or hair loss, but reduced testis weight by 10%.
In addition to differing in their PUFA concentrations, the two diets differed in their saturated fatty acid concentration (Table 2). The n-6 PUFA adequate diet was approximately comparable to the n-3 PUFA adequate diet (Table 1 and Table 2) that we had used as a control diet in n-3 PUFA deprivation studies [21–26].
Several studies have used an n-6 PUFA deficient diet having an adequate n-3 PUFA supply [16,18,35]. In one comparable study, a complete dietary LA deficiency was maintained for 14 weeks from 35 days of age in Sprague–Dawley rats. The changes produced were greater than in this paper. Body weight was reduced by 15%, and there was mild scaling and some hair loss and a 19% reduction in testis weight [18,35].
In rats fed the n-6 PUFA deficient compared with adequate diet, the AA concentration was reduced in total lipids of serum (−81%), brain (−28%), liver (−84%), heart (−39%), testis (−25%) and adipose tissue (−79%) (Table 5 and Table 6). The different percent reductions may be related to differences in AA turnover rates and/or stable lipid concentrations among the organs (Table 4) and lead to different organ functional changes.
In male post-weaning rats fed an n-3 PUFA deficient compared with adequate diet for 15 weeks, the DHA concentration in brain, liver and heart was decreased by 42%, 89% and 93%, respectively [21,22,24]. DHA half-lives were prolonged in brain phospholipids, and Ca2+-independent phospholipase A2 (iPLA2) mRNA and activity in brain were decreased, which may have helped to slow DHA loss [9,26,36,37]. On the other hand, mRNA levels and activities of AA selective cytosolic cPLA2, secretory sPLA2, [37,38] and cyclooxygenase-2 were increased in the n-3 PUFA deprived rats. It thus would be of interest to see if opposite directional changes in brain enzyme activities occur and if DHA turnover is increased in the brain of rats fed the n-6 PUFA deprived diet in this study. If so, this would imply that these enzymatic and kinetic parameters are closely tied to the balance of brain n-6 and n-3 PUFA concentrations.
Bourre et al. [16] reported a normal AA concentration in the brain of pups killed at weaning (21 days of age, Wister), whose mothers had been fed a low LA-containing diet (150 mg LA/100 g diet, 5.3 µmol/g diet) starting 3 weeks before mating. A maintained AA level in the fetus is consistent with upregulated conversion from LA in the maternal, fetal and newborn liver, and with regulated transport of AA across the placenta [39,40]. The ability of liver microsomes to desaturate 18:2n-6 to 18:3n-6 is higher in fetal and pregnant rats than in male adult rats [41], and Δ6 desaturase activity of the mouse liver increases after birth [42]. Based on animal and clinical studies, recommended minimal dietary requirements for LA are 1200 mg/100 g diet for rodents, and 2–3% of energy (1000–1500 mg/100 g food) for humans [16,43–45].
The n-6 PUFA deficient diet increased concentrations of total n-3 PUFAs, EPA, and DHA in brain and organs other than adipose tissue (Table 5 and Table 6). The α-LNA concentration was increased significantly only in the heart. These results suggest that the n-6 PUFA deficiency stimulated conversion of α-LNA to EPA and DHA and suppressed β-oxidation of α-LNA, leading to accumulation of long chain n-3 PUFAs in the organs. In rats, a totally LA deficient diet increased α-LNA disappearance by 14% and the conversion of α-LNA to longer-chain n-3 PUFAs by 25%, but reduced whole body n-3 PUFA concentration by 21% [19].
Synthesis of DHA from α-LNA and of AA from LA is catalyzed by common hepatic desaturase and elongase enzymes [46,47]. Expression of these enzymes can be altered by multiple physiological and nutritional conditions. For example, liver Δ5 and Δ6 desaturases and elongases 2 and 5 were transcriptionally upregulated in rats fed an n-3 PUFA deficient compared with adequate diet, in association with increased liver conversion of α-LNA to DHA [25]. The enzymes also may be upregulated by the n-6 PUFA deficient diet [48–50], but this has to be experimentally confirmed.
The n-6 PUFA deficient diet increased monounsaturated fatty acid concentrations in the brain and other organs (Table 5 and Table 6). It also promoted accumulation of 20:3n-9, which is converted from 18:1n-9 by desaturation and elongation and is a marker of LA deficiency [17]. The increased levels of unsaturated fatty acids (n-3 PUFAs, monounsaturated fatty acids, and 20:3n-9) were not accompanied by increased saturated fatty acid concentrations in the organs (Table 5 and Table 6). On the other hand, an n-3 PUFA deficient diet reciprocally increased concentrations of n-6 PUFAs in brain and liver but did not change monounsaturated fatty acid concentrations [21,22,24]. Taken together, the results suggest that n-6 PUFA deprivation can increase desaturation and elongation of n-3 PUFAs and concentrations of saturated and monounsaturated fatty acids, whereas n-3 PUFA deprivation can increase desaturation and elongation of n-6 PUFAs [3,21,25,50].
While dietary AA supplementation has been shown to improve membrane fluidity, synaptic plasticity, and spatial cognition in aged rats [51–53], the effects of dietary n-6 PUFA deprivation on brain function and behavior in rodents have not been thoroughly studied. Knowing these effects may be clinically relevant, as AA concentrations were reported to be decreased in postmortem brain tissue from Alzheimer disease and schizophrenic patients [54–57]. Additionally, enzymes that regulate AA metabolism, including cPLA2, sPLA2 and cyclooxygenase-2, were transcriptionally upregulated in postmortem brain from bipolar disorder patients, and drugs that are used to treat the disease downregulate these brain enzymes as well as AA turnover in brain phospholipids when given chronically to rats [58,59]. This suggests a potential therapeutic role for reducing the body’s n-6 PUFA stores under some conditions.
The LA deficient diet decreased serum triacylglycerol, total cholesterol and total phospholipid concentrations, but did not affect these stable lipid concentrations in brain, liver, heart, testis, or adipose tissue (Table 4). Non-specific essential fatty acid deficiency in rats also has been reported to decrease concentrations of these lipids in plasma, while increasing them in liver [60,61].
The rat testis contains relatively high concentrations of LA, AA and DPAn-6 (Table 6) [62,63], and the n-6 PUFA deficient diet decreased testis weight by 10% (Table 3). Non-specific essential fatty acid deficiency also has been reported to cause testicular atrophy. Supplementation with LA methyl ester but not with α-LNA methyl ester prevented this atrophy, arguing for a critical role of n-6 PUFAs in maintaining testicular integrity [63]. DPAn-6 may play such a role, since the other organs examined had relatively high DPAn-6 concentrations in the rats fed the n-6 PUFA adequate diet (Table 5 and Table 6). DPAn-6 can be converted to AA in rat testis, and it is metabolized to thromboxane and hydroxyl fatty acids by platelet cyclooxygenase and lipoxygenase [64–66].
In summary, an n-6 PUFA deficient diet fed to post-weaning rats for 15 weeks profoundly affected fatty acid and lipid composition in multiple organs. This model could be used for further studies of physiological and metabolic effects of n-6 PUFA deficiency.
Acknowledgements
This research was supported entirely by the Intramural Research Program of the National Institute on Aging, NIH. The authors thank the NIH Fellows Editorial Board and Ms. Kathy Benjamin for editorial assistance.
Abbreviations
- AA
arachidonic acid
- DPA
docosapentaenoic acid
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- FAME
fatty acid methyl ester
- GC
gas chromatography
- LA
linoleic acid
- α-LNA
α-linolenic acid
- PLA2
phospholipase A2
- PL
phospholipids
- TG
triacylglycerol
References
- 1.Holman RT. Essential fatty acid deficiency. Progr. Chem. Fats Other Lipids. 1969;9:275–348. [Google Scholar]
- 2.Jump DB, Botolin D, Wang Y, Xu J, Christian B, Demeure O. Fatty acid regulation of hepatic gene transcription. J. Nutr. 2005;135:2503–2506. doi: 10.1093/jn/135.11.2503. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura MT, Nara TY. Essential fatty acid synthesis and its regulation in mammals. Prostaglandins Leukot. Essent. Fatty Acids. 2003;68:145–150. doi: 10.1016/s0952-3278(02)00264-8. [DOI] [PubMed] [Google Scholar]
- 4.Sprecher H. Metabolism of highly unsaturated n-3 and n-6 fatty acids. Biochim. Biophys. Acta. 2000;1486:219–231. doi: 10.1016/s1388-1981(00)00077-9. [DOI] [PubMed] [Google Scholar]
- 5.Gavino GR, Gavino VC. Rat liver outer mitochondrial carnitine palmitoyltransferase activity towards long-chain polyunsaturated fatty acids and their CoA esters. Lipids. 1991;26:266–270. doi: 10.1007/BF02537135. [DOI] [PubMed] [Google Scholar]
- 6.Uauy R, Dangour AD. Nutrition in brain development and aging: role of essential fatty acids. Nutr. Rev. 2006;64:S24–S33. doi: 10.1301/nr.2006.may.s24-s33. discussion S72–91. [DOI] [PubMed] [Google Scholar]
- 7.Farooqui AA, Horrocks LA, Farooqui T. Glycerophospholipids in brain: their metabolism, incorporation into membranes, functions, and involvement in neurological disorders. Chem. Phys. Lipids. 2000;106:1–29. doi: 10.1016/s0009-3084(00)00128-6. [DOI] [PubMed] [Google Scholar]
- 8.Youdim KA, Martin A, Joseph JA. Essential fatty acids and the brain: possible health implications. Int. J. Dev. Neurosci. 2000;18:383–399. doi: 10.1016/s0736-5748(00)00013-7. [DOI] [PubMed] [Google Scholar]
- 9.Strokin M, Sergeeva M, Reiser G. Docosahexaenoic acid and arachidonic acid release in rat brain astrocytes is mediated by two separate isoforms of phospholipase A2 and is differently regulated by cyclic AMP and Ca2+ Br. J . Pharmacol. 2003;139:1014–1022. doi: 10.1038/sj.bjp.0705326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bazan NG. Omega-3 fatty acids, pro-inflammatory signaling and neuroprotection. Curr. Opin. Clin. Nutr. Metab. Care. 2007;10:136–141. doi: 10.1097/MCO.0b013e32802b7030. [DOI] [PubMed] [Google Scholar]
- 11.Bazan NG. The onset of brain injury and neurodegeneration triggers the synthesis of docosanoid neuroprotective signaling. Cell. Mol. Neurobiol. 2006;26:901–913. doi: 10.1007/s10571-006-9064-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fedorova I, Salem N., Jr Omega-3 fatty acids and rodent behavior. Prostaglandins Leukot. Essent. Fatty Acids. 2006;75:271–289. doi: 10.1016/j.plefa.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Bourre JM, Francois M, Youyou A, Dumont O, Piciotti M, Pascal G, Durand G. The effects of dietary alpha-linolenic acid on the composition of nerve membranes, enzymatic activity, amplitude of electrophysiological parameters, resistance to poisons and performance of learning tasks in rats. J. Nutr. 1989;119:1880–1892. doi: 10.1093/jn/119.12.1880. [DOI] [PubMed] [Google Scholar]
- 14.Salem N, Jr, Litman B, Kim HY, Gawrisch K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids. 2001;36:945–959. doi: 10.1007/s11745-001-0805-6. [DOI] [PubMed] [Google Scholar]
- 15.Moriguchi T, Greiner RS, Salem N., Jr Behavioral deficits associated with dietary induction of decreased brain docosahexaenoic acid concentration. J. Neurochem. 2000;75:2563–2573. doi: 10.1046/j.1471-4159.2000.0752563.x. [DOI] [PubMed] [Google Scholar]
- 16.Bourre JM, Piciotti M, Dumont O, Pascal G, Durand G. Dietary linoleic acid and polyunsaturated fatty acids in rat brain and other organs. Minimal requirements of linoleic acid. Lipids. 1990;25:465–472. doi: 10.1007/BF02538090. [DOI] [PubMed] [Google Scholar]
- 17.Lundberg WO. The significance of cis, cis, cis 5, 8, 11 eicosatrienoic acid in essential fatty acid deficiency. Nutr. Rev. 1980;38:233–235. doi: 10.1111/j.1753-4887.1980.tb05910.x. [DOI] [PubMed] [Google Scholar]
- 18.Cunnane SC, Anderson MJ. Pure linoleate deficiency in the rat: influence on growth, accumulation of n-6 polyunsaturates, and [1–14C]linoleate oxidation. J. Lipid Res. 1997;38:805–812. [PubMed] [Google Scholar]
- 19.Bazinet RP, Douglas H, Cunnane SC. Whole-body utilization of n-3 PUFA in n-6 PUFA-deficient rats. Lipids. 2003;38:187–189. doi: 10.1007/s11745-003-1050-8. [DOI] [PubMed] [Google Scholar]
- 20.Contreras MA, Rapoport SI. Recent studies on interactions between n-3 and n-6 polyunsaturated fatty acids in brain and other tissues. Curr. Opin. Lipidol. 2002;13:267–272. doi: 10.1097/00041433-200206000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Igarashi M, DeMar JC, Jr, Ma K, Chang L, Bell JM, Rapoport SI. Upregulated liver conversion of a-linolenic acid to docosahexaenoic acid in rats on a 15 week n-3 PUFA-deficient diet. J. Lipid Res. 2007;48:152–164. doi: 10.1194/jlr.M600396-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.Igarashi M, Ma K, Chang L, Bell JM, Rapoport SI. Rat heart cannot synthesize docosahexaenoic acid from circulating alpha -linolenic acid because it lacks elongase-2. J. Lipid Res. 2008;49:1735–1745. doi: 10.1194/jlr.M800093-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeMar JC, Jr, Ma K, Bell JM, Igarashi M, Greenstein D, Rapoport SI. One generation of n-3 polyunsaturated fatty acid deprivation increases depression and aggression test scores in rats. J. Lipid Res. 2006;47:172–180. doi: 10.1194/jlr.M500362-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Igarashi M, DeMar JC, Jr, Ma K, Chang L, Bell JM, Rapoport SI. Docosahexaenoic acid synthesis from a-linolenic acid by rat brain is unaffected by dietary n-3 PUFA deprivation. J. Lipid Res. 2007;48:1150–1158. doi: 10.1194/jlr.M600549-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Igarashi M, Ma K, Chang L, Bell JM, Rapoport SI. Dietary n-3 PUFA deprivation for 15 weeks upregulates elongase and desaturase expression in rat liver but not brain. J. Lipid Res. 2007;48:2463–2470. doi: 10.1194/jlr.M700315-JLR200. [DOI] [PubMed] [Google Scholar]
- 26.DeMar JC, Jr, Ma K, Bell JM, Rapoport SI. Half-lives of docosahexaenoic acid in rat brain phospholipids are prolonged by 15 weeks of nutritional deprivation of n-3 polyunsaturated fatty acids. J. Neurochem. 2004;91:1125–1137. doi: 10.1111/j.1471-4159.2004.02789.x. [DOI] [PubMed] [Google Scholar]
- 27.Igarashi M, Gao F, Ma K, Bell JM, Rapoport SI. AOCS Annual Meeting&Expo. vol. 99. Seattle: AOCS Press; 2008. p. 77. [Google Scholar]
- 28.Igarashi M, Ma K, Chang L, Bell JM, Rapoport SI, Demar JC., Jr Low liver conversion rate of a-linolenic to docosahexaenoic acid in awake rats on a high-docosahexaenoate-containing diet. J. Lipid Res. 2006;47:1812–1822. doi: 10.1194/jlr.M600030-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.DeMar JC, Jr, Ma K, Chang L, Bell JM, Rapoport SI. alpha-Linolenic acid does not contribute appreciably to docosahexaenoic acid within brain phospholipids of adult rats fed a diet enriched in docosahexaenoic acid. J. Neurochem. 2005;94:1063–1076. doi: 10.1111/j.1471-4159.2005.03258.x. [DOI] [PubMed] [Google Scholar]
- 30.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 31.Reeves PG, Rossow KL, Lindlauf J. Development and testing of the AIN-93 purified diets for rodents: results on growth, kidney calcification and bone mineralization in rats and mice. J. Nutr. 1993;123:1923–1931. doi: 10.1093/jn/123.11.1923. [DOI] [PubMed] [Google Scholar]
- 32.Bourre JM, Durand G, Pascal G, Youyou A. Brain cell and tissue recovery in rats made deficient in n-3 fatty acids by alteration of dietary fat. J. Nutr. 1989;119:15–22. doi: 10.1093/jn/119.1.15. [DOI] [PubMed] [Google Scholar]
- 33.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 34.Rouser G, Fkeischer S, Yamamoto A. Two dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970;5:494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- 35.Cunnane SC, Trotti D, Ryan MA. Specific linoleate deficiency in the rat does not prevent substantial carbon recycling from [(14)C]linoleate into sterols. J. Lipid Res. 2000;41:1808–1811. [PubMed] [Google Scholar]
- 36.Rao JS, Ertley RN, DeMar JC, Jr, Rapoport SI, Bazinet RP, Lee HJ. Dietary n-3 PUFA deprivation alters expression of enzymes of the arachidonic and docosahexaenoic acid cascades in rat frontal cortex. Mol. Psychiatry. 2007;12:151–157. doi: 10.1038/sj.mp.4001887. [DOI] [PubMed] [Google Scholar]
- 37.Dennis EA. Diversity of group types, regulation, and function of phospholipase A2. J. Biol. Chem. 1994;269:13057–13060. [PubMed] [Google Scholar]
- 38.Strokin M, Sergeeva M, Reiser G. Role of Ca2+-independent phospholipase A2 and n-3 polyunsaturated fatty acid docosahexaenoic acid in prostanoid production in brain: perspectives for protection in neuroinflammation. Int. J. Dev. Neurosci. 2004;22:551–557. doi: 10.1016/j.ijdevneu.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Li Z, Kaplan ML, Hachey DL. Hepatic microsomal and peroxisomal docosahexaenoate biosynthesis during piglet development. Lipids. 2000;35:1325–1333. doi: 10.1007/s11745-000-0649-0. [DOI] [PubMed] [Google Scholar]
- 40.Haggarty P. Placental regulation of fatty acid delivery and its effect on fetal growth—a review. Placenta. 2002;23(Suppl A):S28–S38. doi: 10.1053/plac.2002.0791. [DOI] [PubMed] [Google Scholar]
- 41.Satomi S, Matsuda I. Microsomal desaturation of linoleic into -linolenic acid in livers of fetal, suckling and pregnant rats. Biol. Neonate. 1973;22:1–8. doi: 10.1159/000240535. [DOI] [PubMed] [Google Scholar]
- 42.Bourre JM, Piciotti M, Dumont O. Delta 6 desaturase in brain and liver during development and aging. Lipids. 1990;25:354–356. doi: 10.1007/BF02544347. [DOI] [PubMed] [Google Scholar]
- 43.Collins FD, Sinclair AJ, Royle JP, Coats DA, Maynard AT, Leonard RF. Plasma lipids in human linoleic acid deficiency. Nutr. Metab. 1971;13:150–167. doi: 10.1159/000175332. [DOI] [PubMed] [Google Scholar]
- 44.Wene JD, Connor WE, DenBesten L. The development of essential fatty acid deficiency in healthy men fed fat-free diets intravenously and orally. J. Clin. Invest. 1975;56:127–134. doi: 10.1172/JCI108061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goodgame JT, Lowry SF, Brennan MF. Essential fatty acid deficiency in total parenteral nutrition: time course of development and suggestions for therapy. Surgery. 1978;84:271–277. [PubMed] [Google Scholar]
- 46.Rapoport SI, Rao JS, Igarashi M. Brain metabolism of nutritionally essential polyunsaturated fatty acids depends on both the diet and the liver. Prostaglandins Leukot. Essent. Fatty Acids. 2007;77:251–261. doi: 10.1016/j.plefa.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jump DB. Fatty acid regulation of gene transcription. Crit. Rev. Clin. Lab. Sci. 2004;41:41–78. doi: 10.1080/10408360490278341. [DOI] [PubMed] [Google Scholar]
- 48.Geiger M, Mohammed BS, Sankarappa S, Sprecher H. Studies to determine if rat liver contains chain-length-specific acyl-CoA 6-desaturases. Biochim. Biophys. Acta. 1993;1170:137–142. doi: 10.1016/0005-2760(93)90063-f. [DOI] [PubMed] [Google Scholar]
- 49.Cho HP, Nakamura M, Clarke SD. Cloning, expression, and fatty acid regulation of the human delta-5 desaturase. J. Biol. Chem. 1999;274:37335–37339. doi: 10.1074/jbc.274.52.37335. [DOI] [PubMed] [Google Scholar]
- 50.Jakobsson A, Westerberg R, Jacobsson A. Fatty acid elongases in mammals: their regulation and roles in metabolism. Prog. Lipid. Res. 2006;45:237–249. doi: 10.1016/j.plipres.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 51.Okaichi Y, Ishikura Y, Akimoto K, Kawashima H, Toyoda-Ono Y, Kiso Y, Okaichi H. Arachidonic acid improves aged rats’ spatial cognition. Physiol. Behav. 2005;84:617–623. doi: 10.1016/j.physbeh.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 52.Kotani S, Nakazawa H, Tokimasa T, Akimoto K, Kawashima H, Toyoda-Ono Y, Kiso Y, Okaichi H, Sakakibara M. Synaptic plasticity preserved with arachidonic acid diet in aged rats. Neurosci. Res. 2003;46:453–461. doi: 10.1016/s0168-0102(03)00123-8. [DOI] [PubMed] [Google Scholar]
- 53.Fukaya T, Gondaira T, Kashiyae Y, Kotani S, Ishikura Y, Fujikawa S, Kiso Y, Sakakibara M. Arachidonic acid preserves hippocampal neuron membranefluidity in senescent rats. Neurobiol. Aging. 2007;28:1179–1186. doi: 10.1016/j.neurobiolaging.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 54.Horrobin DF, Manku MS, Hillman H, Iain A, Glen M. Fatty acid levels in the brains of schizophrenics and normal controls. Biol. Psychiatry. 1991;30:795–805. doi: 10.1016/0006-3223(91)90235-e. [DOI] [PubMed] [Google Scholar]
- 55.Soderberg M, Edlund C, Kristensson K, Dallner G. Fatty acid composition of brain phospholipids in aging and in Alzheimer’s disease. Lipids. 1991;26:421–425. doi: 10.1007/BF02536067. [DOI] [PubMed] [Google Scholar]
- 56.McGahon B, Clements MP, Lynch MA. The ability of aged rats to sustain long-term potentiation is restored when the age-related decrease in membrane arachidonic acid concentration is reversed. Neuroscience. 1997;81:9–16. doi: 10.1016/s0306-4522(97)00116-4. [DOI] [PubMed] [Google Scholar]
- 57.Murray CA, Lynch MA. Evidence that increased hippocampal expression of the cytokine interleukin-1 beta is a common trigger for age- and stress-induced impairments in long-term potentiation. J. Neurosci. 1998;18:2974–2981. doi: 10.1523/JNEUROSCI.18-08-02974.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rao JS, Kim HW, Lee HJ, Rapoport SI. Up-regulated arachidonic acid cascade enzymes and their transcription factors in post-mortem frontal cortex from bipolar disorder patients. Society Neurosciences. 2007 Abstracts, November, 2007. [Google Scholar]
- 59.Rao JS, Lee HJ, Rapoport SI, Bazinet RP. Mode of action of mood stabilizers: is the arachidonic acid cascade a common target? Mol. Psychiatry. 2008;13:585–596. doi: 10.1038/mp.2008.31. [DOI] [PubMed] [Google Scholar]
- 60.De Pury GG, Collins FD. Very low density lipoproteins and lipoprotein lipase in serum of rats deficient in essential fatty acids. J. Lipid Res. 1972;13:268–275. [PubMed] [Google Scholar]
- 61.Sinclair AJ, Collins FD. The effect of dietary essential fatty acids on the concentration of serum and liver lipids in the rat. Br. J. Nutr. 1970;24:971–982. doi: 10.1079/bjn19700100. [DOI] [PubMed] [Google Scholar]
- 62.Chanmugam PS, Boudreau MD, Hwang DH. Dietary (n-3) fatty acids alter fatty acid composition and prostaglandin synthesis in rat testis. J. Nutr. 1991;121:1173–1178. doi: 10.1093/jn/121.8.1173. [DOI] [PubMed] [Google Scholar]
- 63.Leat WM, Northrop CA, Harrison FA, Cox RW. Effect of dietary linoleic and linolenic acids on testicular development in the rat. Q. J. Exp. Physiol. 1983;68:221–231. doi: 10.1113/expphysiol.1983.sp002714. [DOI] [PubMed] [Google Scholar]
- 64.Bridges RB, Coniglio JG. The metabolism of 4,7,10,13,16-(5–14C) docosapentaenoic acid in the testis of the rat. Biochim. Biophys. Acta. 1970;218:29–35. doi: 10.1016/0005-2760(70)90089-5. [DOI] [PubMed] [Google Scholar]
- 65.Milks MM, Sprecher H. Metabolism of 4,7,10,13,16-docosapentaenoic acid by human platelet cyclooxygenase and lipoxygenase. Biochim. Biophys. Acta. 1985;835:29–35. doi: 10.1016/0005-2760(85)90026-8. [DOI] [PubMed] [Google Scholar]
- 66.Sprecher H. The metabolism of (n-3) and (n-6) fatty acids and their oxygenation by platelet cyclooxygenase and lipoxygenase. Prog. Lipid Res. 1986;25:19–28. doi: 10.1016/0163-7827(86)90007-x. [DOI] [PubMed] [Google Scholar]