Abstract
Disruptions in hypothalamic-pituitary-adrenal regulation and immunity have been associated with posttraumatic stress disorder (PTSD). We examined the association of PTSD with diurnal rhythms in salivary cortisol in a convenience sample from a population-based study of male and female American Indians. Subjects with and without PTSD were identified from American Indians living on/near a Northern Plains reservation as part of a larger study. Over two days diurnal saliva samples were collected by staff at the University of Colorado Denver Clinical Research Center at waking, 30 minutes after waking, before lunch, and before dinner. Generalized estimating equations linear regression models investigated the influence of PTSD on cortisol over time. The association of a lifetime diagnosis of PTSD with salivary cortisol level was assessed in subjects with complete data (PTSD: n=27; no PTSD n=32) for age, gender, and alcohol consumption in the past month. Subject mean age was 44 years, and 71% were women. When stratified by gender, women with a lifetime diagnosis of PTSD had significantly higher mean cortisol levels throughout the day than women without PTSD (p = 0.01); but there was no significant association between PTSD and cortisol levels in men (p = 0.36). The cortisol awakening response – the difference in cortisol levels from waking to 30 minutes after waking – was not associated with PTSD in men or women. A lifetime diagnosis of PTSD may influence diurnal cortisol among American Indian women. These effects were independent of influences of current alcohol use/abuse. The unexpected elevation in cortisol in American Indian women with a lifetime diagnosis of PTSD may reflect acute anxiety associated with experiencing a number of novel tests in a strange location (e.g., cardiac imaging, medical and dental exams, etc.), or concurrent depression.
Keywords: Health disparities, Northern Plains Indians, cortisol awakening response
1. Introduction
Posttraumatic stress disorder (PTSD) is associated with behavioral and physiological pathology which includes disruption of the hypothalamic pituitary adrenal axis (HPA) (Yehuda, 2001), altered sympathetic activity (Kosten, Mason, Giller, Ostroff, & Harkness, 1987; Murburg, McFall, Lewis, & Veith, 1995), and structural changes in the central nervous system (Bremner, 2003). Adrenal and sympathetic changes may mediate altered immune regulation in PTSD, which has also been noted in several studies (Benight, Harper, Zimmer, Lowery, Sanger, & Laudenslager, 2004; Everson, Kotler, & Blackburn, 1999; Laudenslager, et al., 1998; Watson, Muller, Jones, & Bradley, 1993), as well as poorer overall health in individuals with PTSD (Boscarino, 1997; Kartha, Brower, Saitz, Samel, Keane, Liebschutz et al., 2008; Wolfe, et al., 1999).
There are convincing data indicating that female gender may be an important risk factor for being diagnosed with PTSD. Lifetime prevalence of PTSD in the United States is approximately 5% for men and 10% for women (Kessler, Sonnega, Bromet, Hughes, & Nelson, 1995). Gender differences are widespread in various descriptions of PTSD, and a number of contributing factors have been proposed, ranging from sexual abuse that is experienced more frequently by females to greater overall distress at the time of the precipitating incident (Cortina, Kubiak, Cortina, & Kubiak, 2006; Olff, Langeland, Draijer, & Gersons, 2007).
In addition to gender differences noted in association with PTSD, differences in rates of PTSD have been noted across ethnic groups (Fontana & Rosenheck, 1994; Pole, Best, Weiss, Metzler, Liberman, Fagan, et al., 2001; Ruef, Litz, & Schlenger, 2000). Many earlier studies inadequately represented American Indian populations, and the total number of American Indians represented by studies are quite small, albeit representative of the US population as a whole. As a group, American Indians experience higher rates of trauma exposure than the general population (Beals, Novins, Whitesell, Spicer, Mitchell, & Manson, 2005; Manson, Beals, Klein, Croy, & AISUPERPFP Team, 2005). This elevated trauma frequency is associated with increased risk for alcohol use disorders (Boyd-Ball, Manson, Noonan, & Beals, 2006). Not unexpectedly, PTSD prevalence is high among American Indian populations (Beals, Manson, Shore, Freidman, Ashcraft, Fairbank, et al. 2002; Beals, et al., 2005). American Indian populations are at greater risk for alcohol dependence when compared to Caucasian populations (Gilman et al., 2008), and early trauma, which is elevated in this population, is an important contributing factor in this risk (Boyd-Ball et al., 2006). Current alcohol abuse can disrupt cortisol regulation (Beresford et al, 2006), the current focus of the present observations. Thus it is important to control for alcohol abuse when considering relationships between PTSD and cortisol regulation. Finally, a variety of heath disparities have been noted for American Indian populations (Castor, Smyser, Taualii, Park, Lawson, Forquera, et al., 2006; Galloway & Galloway, 2005; Mensah, Mokodad, Ford, Greenlund, & Croft, 2005) that may be related to earlier trauma and/or health behaviors. .
In a large scale study of over 3,000 American Indians, the American Indian Services Utilization, Psychiatry Epidemiology, Risk and Protective Factors Project (AISUPERPFP), twice the rate of PTSD was noted among American Indians using culturally appropriate instruments (Manson, Beals, Klein, Croy, & Team, 2005) compared to other reports (Kessler, Sonnega, Bromet, Hughes, & Nelson, 1995). Cardiovascular disease (CVD) is a new and rising health problem for American Indian populations (Galloway & Galloway, 2005). The “Healing Hearts” study reenrolled a follow up group of AI-SUPERPFP subjects with and without a life time history of PTSD to investigate CVD risk factors related to their PTSD status. The goal of Healing Hearts, from which the present convenience sample was drawn, is to examine PTSD as a contributing risk factor for CVD among American Indian populations. With reported links between cortisol, proinflammatory cytokines, stress and CVD (Sorrells and Sapolsky, 2007; Steptoe et al., 2001), we felt that the addition of diurnal salivary cortisol collections might inform the Healing Hearts study.
Healing Hearts subjects came to Denver for an extensive two day cardiovascular assessment consisting of myocardial perfusion imaging using positron emission tomography, ultrasonography of the carotid arteries, ambulatory ECG monitoring, and a comprehensive blood panel reflecting lipids and inflammatory factors. This group is unique and desirable for study because it represents a population based sample, trauma has been assessed on two different occasions several years apart, and it provides the ability to link current salivary cortisol variations throughout the day to a lifetime diagnosis of PTSD with matched controls. Beyond the immediate relevance of cortisol as a general stress marker, the morning rise in salivary cortisol is related to intima media thickness, an atherosclerosis risk marker (Hurwitz, Netterstrom, & Hansen, 2001) and thus of relevance to the increased cardiovascular risk of this cohort. The Healing Hearts study provided an excellent opportunity to assess the morning increase in salivary cortisol as well as the diurnal decline under carefully controlled conditions.
2. Methods
Subjects
Subjects with and without PTSD were identified from the larger AI-SUPERPFP cohort (Beals, et al, 2005) for participation in the Healing Hearts study. These subjects were participants in an earlier population-based sample of male and female American Indians living on/near a Northern Plains reservation who were part of a study of PTSD and trauma among American Indian populations. A lifetime PTSD diagnosis was determined as previously described for this population-based cohort using an adaptation of the University of Michigan Composite International Diagnostic Interview (Beals, et al., 2005), which uses a culturally sensitive approach to assessment of psychiatric diagnoses. Non-PTSD subjects were age and gender matched to those with a PTSD diagnosis.
Saliva Sampling
Saliva was collected using filter paper collection as previously described (Beresford, Arcinegas, Alfers, Clapp, Martin, Beresford, Du, Dengkeng, Davatzikos, & Laudenslager, 2006; Neu, Goldstein, Gao, & Laudenslager, 2007; DiPietro et al., 2008; Kivlighan et al., 2008) over two days while subjects remained at the General Clinical Research Center (GCRC) of the University of Colorado Denver Health Sciences Center.
In brief, the filters were assembled in a small booklet that contained the four collection filters for a single day. The filters were labeled for collection at waking, 30 minutes after waking, before lunch, and before dinner, with a location to enter the exact time of sample collection on the booklet. We anticipated that this sampling schedule would reduce the problems associated with food intake on salivary cortisol levels. Tooth brushing was not allowed prior to sampling and fluids were withheld for at least 20 min prior to sampling. Saliva flow was stimulated using Trident Original Flavor gum only as needed. Filters were separated from each other in the collection booklet by waxed weigh paper to prevent cross contamination. Color tabs on the waxed paper dividers matched color codes on the cover of the booklet and ensured that the correct filter in the booklet was correctly wetted. Saliva collections were monitored by the staff of the GCRC. Subjects remained overnight in the GCRC for the duration of the sampling described herein. As sampling was monitored by the nursing staff, we are certain regarding collection compliance; however, the exact timing varied due to the participation of the subjects in the Healing Hearts project, which required a number of medical procedures as well as dental exams. Missing samples were typically due to inadequate or uneven wetting of the filters which was not noticed at the time by the nursing staff overseeing the collection.
Cortisol Assay
Filters were carefully cut and extracted in 0.5 ml of assay buffer as previously described in detail (Beresford, et al., 2006; DiPietro, Costigan, Nelson, Gurewitsch, & Laudenslager, 2008; Neu, Goldstein, Gao, & Laudenslager, 2007). In brief, the buffer containing the cut filters was shaken overnight in microcentrifuge tubes. The 25 µl of extraction buffer was added in duplicate to the appropriate wells of the assay plate. Extraction dilutes the saliva to approximately 1:5. Salivary cortisol concentration in the extraction buffer was determined using a commercial expanded range high sensitivity EIA kit (No. 1–3002/1–3012, Salimetrics, LLC) that detects cortisol levels in the range of 0.003 – 3.0 µg/dl (0.083 – 82.77 nmol/L). Standard curves were fit by a weighted regression analysis using commercial software (Revelation 3.2) for the ELISA plate reader (Dynex MRX). After taking dilution into consideration, the detection limit is 0.018 µg/dl (0.50 nmol/L) for cortisol. This kit shows minimal cross reactivity (4% or less) with other steroids present in the saliva. Controls run on every plate for determination of inter-assay coefficients variability are less than 7.5% for high and low control levels. Intra-assay coefficients of variation for duplicate determinations are less than 3%. This procedure has been cross validated with outside laboratories with excellent correspondence to both DELFIA and Mass spectrographic procedures (r2 = 0.85) and levels within 10–15% reflecting the losses associated with extraction.
Current Alcohol use
Alcohol use was assessed by asking participants in one of their surveys “In the past month, how many days did you drink alcohol?” and “On those days during the past month when you drank alcohol, about how many drinks did you normally have each day?” Total alcohol consumption in the past month was computed as the product of the responses to these questions. Alcohol consumption was categorized into 5 groups as 0, 1–2, 3–10, 11–32, and 33+ drinks per month. Alcohol use was adjusted in the model as indicated below.
Statistical analysis
Descriptive statistics were presented as mean and standard deviation for continuous variables and percents for categorical variables. We used t-tests and chisquare tests to examine differences in demographic variables according to PTSD status. We used generalized estimating equations linear regression models (Diggle, Heagerty, Liang, & Zeger, 2002) to evaluate the association of PTSD and the salivary cortisol profile. This method allowed full use of the data while accounting for the correlation of repeated measures over time. Models were constructed with an independent correlation structure and robust standard errors. The four collection times each day were included in the regression models as dummy variables. PTSD was fit using an indicator variable. All regressions were adjusted for continuous age, collection day (day 1 or 2), and alcohol consumption in the past month. We included product terms in the regression models to assess the interaction of PTSD with gender, time, and age. To normalize the distribution (Kertes & Gunnar, 2004; Watamura, Sebanc, & Gunnar, 2002), we used the natural logarithm of cortisol as the dependent variable in regression models. We also used general estimating equations regression models to assess the association of PTSD and the initial cortisol response upon awakening. For these models, the dependent variable was the natural logarithm of the difference in cortisol measurements from awakening to 30 minutes after awakening, with up to two observations per participant (days 1 and 2). Models were adjusted for age, collection day, and alcohol consumption in the past month.
Statistical analyses were conducted using Stata 10.0 for Windows (StataCorp LP, College Station TX, 2008). Participants were required to have complete covariate data to be included in analyses. The threshold for significance was set at 0.05. Wald tests were used to make statistical inference from the regression models. Although statistical testing was performed on log-transformed cortisol values, for ease of interpretation all means are reported on the original cortisol scale (µg/dL, where 1 µg/dl = 27.59 nmol/L).
3. Results
Participant characteristics
Salivary cortisol measurements were collected from 66 participants. One participant was excluded because cortisol levels were out of the range of the assay, one was excluded due to day 2 measurements being 10 times higher than day 1 measurements, and five additional participants (8%) were excluded due to missing alcohol use data. Among the remaining 59 participants included in the analyses, the majority were female (71%), the average age was 44 years (SD: 10, range: 22 – 61 years), and slightly less than half (46%) had lifetime PTSD. Alcohol consumption characteristics of both groups are given in Table 1. Gender, age, and alcohol consumption did not differ by PTSD status (p = 0.65, 0.85, 0.96, respectively).
Table 1.
Characteristics of participants included in the analysis according to posttraumatic stress disorder status
| Characteristic | PTSDa (n=27) |
No PTSD (n=32) |
|---|---|---|
| Age, mean years (SD) | 44 (10) | 44 (10) |
| Sex, % | ||
| Female | 74 | 69 |
| Male | 26 | 31 |
| Alcohol consumption in past month, % | ||
| 0 drinks | 33 | 41 |
| 1–2 drinks | 15 | 16 |
| 3–10 drinks | 19 | 13 |
| 11–32 drinks | 15 | 16 |
| ≥ 33 drinks | 19 | 16 |
PTSD = posttraumatic stress disorder.
Salivary cortisol profile
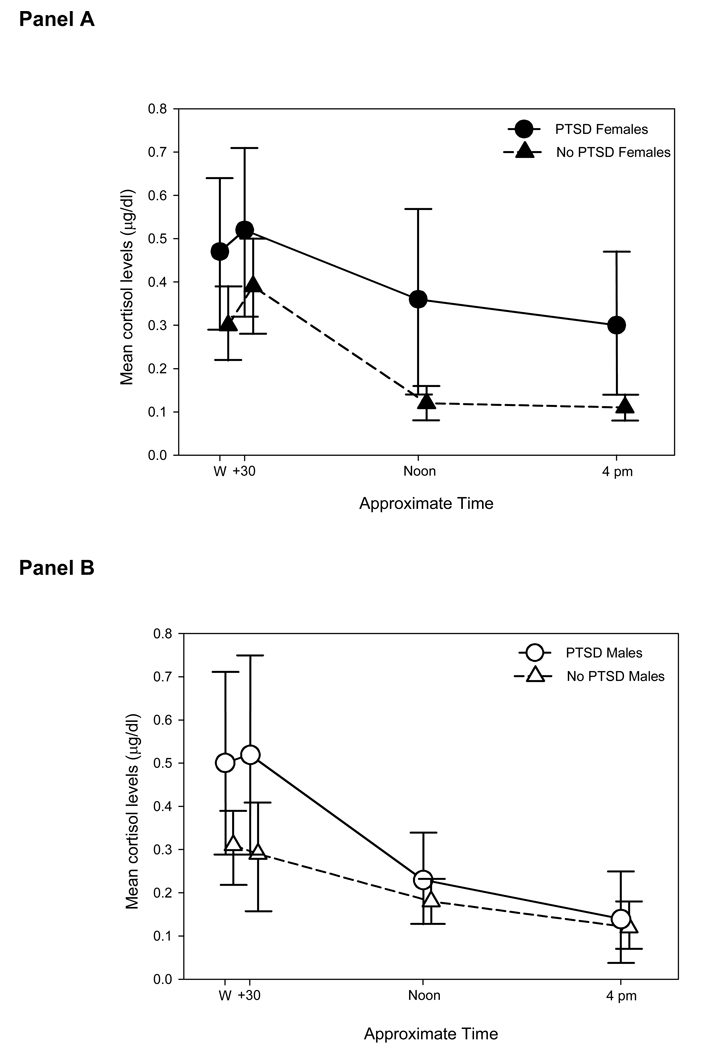
Initial analysis of the cortisol measurements revealed a marginally significant interaction between PTSD status, gender, and time point (χ2(2) = 5.99, p = 0.05); therefore, all further analyses were stratified by gender. Mean cortisol levels with 95% confidence intervals for females (Panel A) and males (Panel B) with and without PTSD are shown in Figure 1. In females, PTSD was associated with significantly elevated cortisol levels (χ2(1) = 6.32, p = 0.01). Compared to their counterparts with no PTSD, female participants with PTSD had a higher baseline cortisol level immediately after awakening (collection time 1) and maintained higher levels throughout the day. The overall association of PTSD with cortisol levels was not significant in males (χ2(1) = 0.85, p = 0.36). Compared to their counterparts with no PTSD, male participants with PTSD had higher mean cortisol levels immediately after awakening and 30 minutes later (collection times 1 and 2), but had similar mean levels at the pre-lunch and pre-evening meal collection points (collection times 3 and 4). The main effect for collection day was not statistically significant for either gender.
Figure 1.
Mean salivary cortisol (µg/dl) profile in female (Panel A, open symbols) and male (Panel B, filled symbols) participants with (circles) and without (triangles) posttraumatic stress disorder (PTSD) collapsed across the first and second day of collection. There was no difference between the levels noted on the first and the second sampling days. Location of means along the x-axis is offset and approximate to permit the visualization of the 95% confidence intervals for each mean. Note that 1 µg/dl = 27.56 nmol/L and that all analyses were performed on natural logarithm transferred data.
Salivary cortisol awakening response
To evaluate the salivary cortisol awakening response, we examined the difference in cortisol levels between saliva collection at awakening (collection time 1) and 30 minutes later (collection time 2). Table 2 presents, separately for females and males, the mean cortisol awakening response in participants with and without PTSD. The awakening response was not significantly associated with PTSD in either females or males in GEE regression models adjusting for age, day of collection, and alcohol consumption in the past month. In addition, there was no significant increase in mean cortisol levels 30 minutes after awakening -- the 95% confidence intervals around the awakening response point estimates for all four gender/PTSD groups included 0 µg/dL (no difference). The small sample size as well as variability in the levels may have masked any statistically significant association.
Table 2.
Mean salivary cortisol awakening responsea in female and male participants according to posttraumatic stress disorder status
| Sex: | PTSDb Mean µg/dL (95% CIc) |
No PTSD Mean µg/dL (95% CI) |
P-valued |
|---|---|---|---|
| Female | 0.03 (−0.03, 0.09) | 0.07 (−0.01, 0.14) | 0.43 |
| Male | 0.01 (−0.11, 0.12) | −0.02 (−0.12, 0.08) | 0.79 |
Difference in cortisol level between salivary collection time 1 (awakening) and 2 (awakening + 30min), with 2 observations per person (collection day 1 and collection day 2)
PTSD = posttraumatic stress disorder
CI = confidence interval
P-value from regression model of ln(cortisol difference) and PTSD adjusting for age, collection day, and alcohol consumption in the past month.
4. Discussion
The present study provides information regarding salivary cortisol assessed at four times during the day on two sequential days, during which time the subjects underwent a variety of health assessments in a novel environment. This study is one of the first explorations into ethnic differences in physiological regulation as it relates to PTSD among American Indian populations. The differences noted are not as typically described in other studies of HPA regulation following trauma, where subjects with PTSD typically have lower levels of salivary cortisol throughout the day compared to normal populations (Heim, Ehlert, & Hellhammer, 2000; Kellner, Yehuda, Arlt, & Wiedemann, 2002; Neylan, et al., 2005). A flattening of the diurnal decline in salivary cortisol has also been noted one year following a natural disaster among individuals reporting greater repression regarding discussing the events of the disaster (Benight, Harper, Zimmer, Lowery, Sanger, & Laudenslager, 2004). In the present study, we found that American Indian women presenting with a lifetime diagnosis of PTSD showed an elevated level of salivary cortisol with a diurnal pattern otherwise very similar to matched female controls. This association was not statistically significant in males, although the overall mean cortisol levels were higher in males with PTSD compared to their counterparts without PTSD. Although this is certainly not the first report of male-female differences with respect to biomarkers and stress, the small sample of males with a lifetime diagnosis of PTSD (n = 7) available to this study may have yielded insufficient power to detect a PTSD/cortisol association, and the lack of gender difference in this study should be interpreted with caution.
With these precautions in mind, perhaps women with a lifetime history of PTSD felt more threatened by the two days of testing than the men. Previous research has suggested that women perceive higher levels of threat than men to similar events (Olff, et al., 2007), and a model of perceived threat to the social self might be operating in the present testing situation. In response to experiential conflict situations in which each partner discussed and attempted to resolve three marriage conflicts, women were more responsive than men in their neuroendocrine response to a challenge (Kiecolt-Glaser, et al., 1996). Women may be more responsive than men to social evaluative challenges or stressors (Dickerson & Kemeny, 2004), and female HPA responsivity has been suggested to be greater among clinical populations (Kudielka & Kirschbaum, 2005). These observations from recent comprehensive reviews are consistent with the data noted herein. That is, if we assume the transportation to a novel site and the unusual medical testing they experienced (evaluative in many respects) were viewed as threats by these women, the overall higher diurnal levels noted in the PTSD group fit nicely within this conceptualization.
As this was a convenience sample from a study focused on cardiovascular function, a few comments should address the significance of the cortisol observation on CVD. The parent study assessed plasma levels of proinflammatory cytokines such as IL-6, as well as c-reactive protein, which are frequent measures in studies of CVD risk (Suarez, 2003; Welsh et al., 2008) and are important CVD risk predictors. These markers are the focus of a larger analysis to be reported elsewhere for the full parent study population.
Limitations of the present study include a lack of assessment of the subject’s perceptions of threat during the testing period, a lack of information regarding the initial response to the critical incident, no information regarding current PTSD status or depression for the cohort or reproductive status for the females, and lack of access to diurnal salivary patterns collected in the home environment. It is very likely that we have measured salivary cortisol in response to an acute stressor or challenge (coming to Denver for medical and dental assessments) for these subjects. Most of these subjects had never left their home state, much less traveled by van to a testing site about 500 miles away. In addition, the process of recruiting these subjects may have reactivated the affective aspects of the PTSD, creating additional trauma intrusions. However this was not the focus of the parent study and we unfortunately do not have data bearing on this important issue. Finally, alcohol use was self-reported, and we have no way to verify whether it was an accurate representation of actual alcohol intake. As the present study was attached to an ongoing project, we could not address these limitations, however, these issues should be addressed in follow up studies of this population.
In summary, the present observations underscore the importance of both gender and ethnic factors in considering physiological aspects of PTSD. Knowledge that American Indian females with PTSD may be at greater risk for pathophysiological responses (e.g., elevated HPA activity) in the context of PTSD might alert the clinician to earlier intervention strategies. Future research should include larger samples of American Indians, particularly males, to facilitate more in-depth analysis of the association between PTSD and cortisol levels in these populations.
Regarding the study of American Indian populations, Beals et al. (2002) wrote, “…Race and ethnicity are, and will remain, important indicator variables for furthering our understanding of psychiatric disorder; this effort reaffirms the importance of moving beyond a simple notation of differences and the need to “unpack” the social constructions that race and ethnicity represent…” This special issue of Brain Behavior and Immunity moves in that direction by addressing physiological underpinnings of gender and ethnic health disparities.
Acknowledgements
The authors are very grateful to the study participants for their willingness to share their experiences and time with the project scientists and research assistants. The authors would also like to thank Mark Goldstein, MA and Kendra Sherwood, MA and the staff of the General Clinical Research Center for their expert assistance in coordinating and processing the samples collected as part of this study. This project was supported in by funds from National Institutes of Health grants R01 AA013973 (MLL), R01 HL073824 (SMM), MH056120 (JDB), K24 MH076955 (JDB), K24 HL077506 (VV), MO1 #RR000051 (General Clinical Research Center) and the Behavioral Immunology and Endocrinology Laboratory, University of Colorado Denver School of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beals J, Manson SM, Shore JH, Friedman M, Ashcraft M, Fairbank JA, et al. The prevalence of posttraumatic stress disorder among American Indian Vietnam veterans: disparities and context. Journal of Traumatic Stress. 2002;15(2):89–97. doi: 10.1023/A:1014894506325. [DOI] [PubMed] [Google Scholar]
- Beals J, Novins DK, Whitesell NR, Spicer P, Mitchell CM, Manson SM. Prevalence of mental disorders and utilization of mental health services in two American Indian reservation populations: mental health disparities in a national context. American Journal of Psychiatry. 2005;162(9):1723–1732. doi: 10.1176/appi.ajp.162.9.1723. [DOI] [PubMed] [Google Scholar]
- Benight C, Harper M, Zimmer D, Lowery M, Sanger J, Laudenslager M. Repression following a series of natural disasters: Immune and neuroendocrine correlates. Psychology & Health. 2004;19:337–352. [Google Scholar]
- Beresford TP, Arciniegas DB, Alfers J, Clapp L, Martin B, Beresford HF, et al. Hypercortisolism in alcohol dependence and its relation to hippocampal volume loss. Journal of Studies on Alcohol. 2006;67:861–867. doi: 10.15288/jsa.2006.67.861. [DOI] [PubMed] [Google Scholar]
- Boscarino JA. Diseases among men 20 years after exposure to severe stress: Implications for clinical research and medical care. Psychosomatic Medicine. 1997;59(6):605–614. doi: 10.1097/00006842-199711000-00008. [DOI] [PubMed] [Google Scholar]
- Boyd-Ball AJ, Manson SM, Noonan C, Beals J. Traumatic events and alcohol use disorders among American Indian adolescents and young adults. Journal of Traumatic Stress. 2006:937–947. doi: 10.1002/jts.20176. [DOI] [PubMed] [Google Scholar]
- Bremner JD. Functional neuroanatomical correlates of traumatic stress revisited 7 years later, this time with data. Psychopharmacology Bulletin. 2003;37(2):6–25. [PubMed] [Google Scholar]
- Castor ML, Smyser MS, Taualii MM, Park AN, Lawson SA, Forquera RA, et al. A nationwide population-based study identifying health disparities between American Indians/Alaska Natives and the general populations living in select urban counties. American Journal of Public Health. 2006;96(8):1478–1484. doi: 10.2105/AJPH.2004.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clow A, Thorn L, Evans P, Hucklebridge F. The awakening cortisol response: methodological issues and significance. Stress. 2004;7(1):29–37. doi: 10.1080/10253890410001667205. [DOI] [PubMed] [Google Scholar]
- Cortina LM, Kubiak SP, Cortina LM, Kubiak SP. Gender and posttraumatic stress: sexual violence as an explanation for women's increased risk. Journal of Abnormal Psychology. 2006;115(4):753–759. doi: 10.1037/0021-843X.115.4.753. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130(3):355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Diggle PJ, Heagerty P, Liang K-Y, Zeger SL. Analysis of longitudinal data. Oxford; New York: Oxford University Press; 2002. [Google Scholar]
- DiPietro JA, Costigan KA, Nelson P, Gurewitsch ED, Laudenslager ML. Fetal responses to induced maternal relaxation during pregnancy. Biol Psychol. 2008;77(1):11–19. doi: 10.1016/j.biopsycho.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everson MP, Kotler S, Blackburn WD., Jr Stress and immune dysfunction in Gulf War veterans. Annals of the New York Academy of Sciences. 1999;876:413–418. doi: 10.1111/j.1749-6632.1999.tb07665.x. [DOI] [PubMed] [Google Scholar]
- Fontana A, Rosenheck R. Posttraumatic stress disorder among Vietnam theater veterans - A causal model of etiology in a community sample. J Nerv.Ment.Dis. 1994;182:677–684. doi: 10.1097/00005053-199412000-00001. [DOI] [PubMed] [Google Scholar]
- Galloway JM, Galloway JM. Cardiovascular health among American Indians and Alaska Natives: successes, challenges, and potentials. American Journal of Preventive Medicine. 2005;29(5 Suppl 1):11–17. doi: 10.1016/j.amepre.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Gilman SE, Breslau J, Conron KJ, Koenen KC, Subramanian SV, Zaslavsky AM. Education and race-ethnicity differences in the lifetime risk of alcohol dependence. Journal of Epidemiology & Community Health. 2008;62:224–230. doi: 10.1136/jech.2006.059022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25(1):1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Hurwitz EN, Netterstrom B, Hansen AM. Cortisol in urine and saliva: relations to the intima media thickness, IMT. Atherosclerosis. 2001;159(1):175–185. doi: 10.1016/s0021-9150(01)00487-7. [DOI] [PubMed] [Google Scholar]
- Kartha A, Brower V, Saitz R, Samet JH, Keane TM, Liebschutz J, et al. The impact of trauma exposure and post-traumatic stress disorder on healthcare utilization among primary care patients. Medical Care. 2008;46(4):388–393. doi: 10.1097/MLR.0b013e31815dc5d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner M, Yehuda R, Arlt J, Wiedemann K. Longitudinal course of salivary cortisol in post-traumatic stress disorder. Acta Psychiatrica Scandinavica. 2002;105(2):153–155. doi: 10.1034/j.1600-0447.2002.01012.x. [DOI] [PubMed] [Google Scholar]
- Kertes DA, Gunnar MR. Evening activities as a potential confound in research on the adrenocortical system in children. Child Development. 2004;75(1):193–204. doi: 10.1111/j.1467-8624.2004.00663.x. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the national comorbidity survey. Arch.Gen.Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Newton T, Cacioppo JT, MacCallum RC, Glaser R, Malarkey WB. Marital conflict and endocrine function: are men really more physiologically affected than women? Journal of Consulting & Clinical Psychology. 1996;64(2):324–332. doi: 10.1037//0022-006x.64.2.324. [DOI] [PubMed] [Google Scholar]
- Kivlighan KT, DiPietro JA, Costigan KA, Laudenslager ML. Diurnal rhythm of cortisol during late pregnancy: Associations with maternal psychological well-being and fetal growth. Psychoneuroendocrinology. 2008;33:1225–1235. doi: 10.1016/j.psyneuen.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, Mason JW, Giller EL, Ostroff RB, Harkness L. Sustained urinary norepinephrine and epinephrine elevation in post-traumatic stress disorder. Psychoneuroendocrinology. 1987;12:13–20. doi: 10.1016/0306-4530(87)90017-5. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. [Review] [160 refs] Biological Psychology. 2005;69(1):113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Laudenslager ML, Aasal R, Adler L, Berger CL, Montgomery Pt, et al. Elevated cytotoxicity in combat veterans with long-term post-traumatic stress disorder: preliminary observations. Brain, Behavior, & Immunity. 1998;12(1):74–79. doi: 10.1006/brbi.1997.0513. [DOI] [PubMed] [Google Scholar]
- Manson SM, Beals J, Klein SA, Croy CD, Team A-S. Social epidemiology of trauma among 2 American Indian reservation populations. American Journal of Public Health. 2005;95(5):851–859. doi: 10.2105/AJPH.2004.054171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB, Mensah GA, et al. State of disparities in cardiovascular health in the United States. Circulation. 2005;111(10):1233–1241. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- Murburg MM, McFall ME, Lewis N, Veith RC. Plasma norepinephrine kinetics in patients with posttraumatic stress disorder. Biol.Psychiatry. 1995;38:819–825. doi: 10.1016/0006-3223(95)00044-5. [DOI] [PubMed] [Google Scholar]
- Neu M, Goldstein M, Gao D, Laudenslager ML. Salivary cortisol in preterm infants: Validation of a simple method for collecting saliva for cortisol determination. Early Human Development. 2007;83:47–54. doi: 10.1016/j.earlhumdev.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Neylan TC, Brunet A, Pole N, Best SR, Metzler TJ, Yehuda R, et al. PTSD symptoms predict waking salivary cortisol levels in police officers. Psychoneuroendocrinology. 2005;30(4):373–381. doi: 10.1016/j.psyneuen.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Olff M, Langeland W, Draijer N, Gersons BP, Olff M, Langeland W, et al. Gender differences in posttraumatic stress disorder. [Review] Psychological Bulletin. 2007;133(2):183–204. doi: 10.1037/0033-2909.133.2.183. [DOI] [PubMed] [Google Scholar]
- Pole N, Best SR, Metzler T, Marmar CR, Pole N, Best SR, et al. Why are hispanics at greater risk for PTSD? Cultural Diversity & Ethnic Minority Psychology. 2005;11(2):144–161. doi: 10.1037/1099-9809.11.2.144. [DOI] [PubMed] [Google Scholar]
- Pole N, Best SR, Weiss DS, Metzler T, Liberman AM, Fagan J, et al. Effects of gender and ethnicity on duty-related posttraumatic stress symptoms among urban police officers. Journal of Nervous & Mental Disease. 2001;189(7):442–448. doi: 10.1097/00005053-200107000-00005. [DOI] [PubMed] [Google Scholar]
- Ruef AM, Litz BT, Schlenger WE. Hispanic ethnicity and risk for combat-related posttraumatic stress disorder. Cultural Diversity & Ethnic Minority Psychology. 2000;6(3):235–251. doi: 10.1037/1099-9809.6.3.235. [DOI] [PubMed] [Google Scholar]
- Sorrells SF, Sapolsky RM. An inflammatory review of glucocorticoid actions in the CNS. Brain, Behavior, and Immunity. 2007;21:259–272. doi: 10.1016/j.bbi.2006.11.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Willemsen G, Owen N, Flower L, Mohamed-Ali V. Acute mental stress elicits delayed increases in circulating inflammatory cytokine levels. [see comments.] Clinical Science. 2001;101:185–192. [PubMed] [Google Scholar]
- Suarez EC. Joint Effect of Hostility and Severity of Depressive Symptoms on Plasma Interleukin-6 Concentration. Psychosomatic Medicine. 2003;65:523–527. doi: 10.1097/01.psy.0000062530.94551.ea. [DOI] [PubMed] [Google Scholar]
- Watamura SE, Sebanc AM, Gunnar MR. Rising cortisol at childcare: relations with nap, rest, and temperament. Developmental Psychobiology. 2002;40(1):33–42. doi: 10.1002/dev.10011. [DOI] [PubMed] [Google Scholar]
- Watson IPB, Muller HK, Jones IH, Bradley AJ. Cell-Mediated Immunity in Combat Veterans with Post- Traumatic Stress Disorder. Med.J.Aust. 1993;159:513–516. doi: 10.5694/j.1326-5377.1993.tb138003.x. [DOI] [PubMed] [Google Scholar]
- Welsh P, Woodward M, Rumley A, Lowe G, Welsh P, Woodward M, Rumley A, Lowe G. Associations of plasma pro-inflammatory cytokines, fibrinogen, viscosity and C-reactive protein with cardiovascular risk factors and social deprivation: the fourth Glasgow MONICA study. British Journal of Haematology. 2008;141:852–861. doi: 10.1111/j.1365-2141.2008.07133.x. [DOI] [PubMed] [Google Scholar]
- Wolfe J, Proctor SP, Erickson DJ, Heeren T, Friedman MJ, Huang MT, et al. Relationship of psychiatric status to Gulf War veterans' health problems. Psychosomatic Medicine. 1999;61(4):532–540. doi: 10.1097/00006842-199907000-00018. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Biology of posttraumatic stress disorder. [Review] [53 refs] Journal of Clinical Psychiatry. 2001;62 Suppl-6 [PubMed] [Google Scholar]