Abstract
Pituitary adenylate cyclase-activating polypeptide (PACAP) increases excitability of guinea pig cardiac neurons, an effect mediated by PACAP-selective PAC1 receptors. In dissociated guinea pig cardiac neurons, PACAP causes a positive shift of the voltage dependence of activation of the hyperpolarization-activated nonselective cation current (Ih). This observation suggested that an enhancement of Ih contributed to the increase in excitability in neurons within whole-mount cardiac ganglia preparations. To evaluate the role of Ih in the PACAP-induced increase in excitability, we compared the increase in action potentials generated by 10 nM PACAP in control neurons and in neurons treated with ZD7288 (10 or 100 μM) or CsCl (2 or 2.5 mM), drugs known to inhibit Ih. In control cells exposed to PACAP, 1-s depolarizing current pulses elicited multiple action potential firing in 79% of the neurons. In ZD7288- or CsCl-containing solutions, the 10 nM PACAP-induced increase in excitability was markedly suppressed, with 7% and 21% of the neurons generating multiple action potentials, respectively. Prior results indicated that PACAP initiates depolarization by activating an inward current, which is separate from its enhancement of Ih. Here, we show that a PACAP-induced depolarization was comparable in control neurons and neurons bathed in a CsCl-containing solution, an observation indicating that CsCl did not interfere with activation of the PAC1 receptor by PACAP. Additional experiments indicated that pretreatment with the putative M current (IM) inhibitor 1 mM BaCl2, but not 10 μM XE991, initiated multiple firing in a majority of neurons, with resting potentials maintained at approximately −60 mV. Furthermore, in Ba2+-treated cells, 10 nM PACAP increased the number of action potentials generated. Our results indicate that PACAP enhancement of Ih, rather than inhibition of IM and other 1 mM Ba2+-sensitive K+ currents, is a key ionic mechanism contributing to the peptide-induced increase in excitability for neurons within whole-mount cardiac ganglia preparations.
Keywords: hyperpolarization-activated nonselective cation current, neuronal excitability, neuropeptide, parasympathetic neurons
pituitary adenylate cyclase-activating polypeptide (PACAP) is present in virtually all cholinergic parasympathetic preganglionic terminals innervating guinea pig intrinsic cardiac neurons (5). In addition, most guinea pig cardiac neurons express the PACAP-selective PAC1 receptor (2). Neurally released and exogenously applied PACAP can depolarize and increase excitability of the cardiac neurons (2, 19–21). However, the mechanism(s) underlying PACAP actions on guinea pig cardiac neurons are not fully elucidated.
In a recent study, Tompkins and Parsons (21) determined that the PACAP-induced increase in excitability required activation of adenylyl cyclase, rather than phospholipase C. The effect of PACAP on excitability was partially mimicked by the adenylyl cyclase activator forskolin and a membrane-permeable cAMP analog. Furthermore, using dissociated guinea pig cardiac neurons, Merriam et al. (15) determined that PACAP enhanced the hyperpolarization-activated nonselective cation conductance (Ih). Thus, because Ih is enhanced by cAMP (14), it was hypothesized that a shift in the voltage dependence of Ih activation by PACAP might be a mechanism contributing to the peptide-induced increase in cardiac neuron excitability (15, 21).
However, dissociated neurons and neurons within whole-mount cardiac ganglia preparations can have quite different properties. For instance, in intact ganglia, only a subpopulation of the cardiac neurons is reported to express Ih (8), whereas Merriam et al. (15) noted that all the dissociated neurons tested exhibited an Ih. In addition, ∼90% of the neurons are phasic in intact ganglia preparations, whereas within 24–48 h of dissociation, 90% of the isolated neurons exhibit a tonic firing pattern (K. L. Barstow and R. L. Parsons, unpublished observation). Thus it is important to test whether a PACAP-induced enhancement of Ih contributes significantly to the peptide-induced increase in excitability for neurons within whole-mount cardiac ganglia preparations.
Ih is inhibited in cardiac neurons by the drug ZD7288 and by Cs+ (7, 8, 15, 23). We proposed that if a PACAP-induced enhancement of Ih was a key mechanism contributing to the peptide-induced increase in excitability in neurons within intact cardiac ganglia, then pretreatment with ZD7288 or Cs+ should blunt the PACAP effect. Therefore, experiments were undertaken in the present study to test whether pretreatment with ZD7288 (10–100 μM) or Cs+ (2–2.5 mM CsCl) suppressed the PACAP-induced increase in excitability of neurons in whole-mount cardiac ganglia preparations.
Many neuropeptides can increase neuronal excitability by inhibiting the voltage-dependent noninactivating K+ conductance (IM) (4). Ba2+ (1 mM) is a well-established IM inhibitor (4, 7, 23), but it is not selective. In contrast, the compound XE991 (10 μM) is thought to be a more selective inhibitor of IM (22). Thus, to assess whether a PACAP-induced inhibition of IM was a key mechanism underlying the peptide-induced increase in excitability, we also tested whether pretreatment with 10 μM XE991 or 1 mM Ba2+ initiated multiple firing in the guinea pig cardiac neurons and whether pretreatment with an inhibitor of IM suppressed the PACAP-induced increase in excitability.
MATERIALS AND METHODS
General methods.
Experiments were performed in vitro on atrial whole-mount preparations containing the intrinsic cardiac ganglia from Hartley guinea pigs (either sex, 250–350 g), as described previously (2, 19–21). Guinea pigs were killed by isoflurane overdose followed by exsanguination according to animal protocols approved by the University of Vermont Institutional Animal Care and Use Committee and methods described in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals used and their suffering. The heart was quickly removed and placed in cold standard Krebs solution (in mM: 121 NaCl, 5.9 KCl, 2.5 CaCl2, 1.2 MgCl2, 25 NaHCO3, 1.2 NaH2PO4, and 8 glucose), with pH maintained at 7.4 by aeration with 95% O2-5% CO2.
Intracellular recordings from neurons in whole-mount preparations.
For intracellular recordings, the whole-mount preparations were pinned in a 2.5-ml Sylgard-lined chamber and superfused continuously (2–3 ml/min) with a modified Krebs solution containing 10 mM HEPES buffer (2, 10, 11, 19–21). All recordings were obtained with the temperature maintained at 30–32°C. Cardiac ganglia were visualized with an inverted microscope equipped with Hoffman optics, and individual cardiac neurons were impaled using high-impedance 2 M KCl-filled borosilicate microelectrodes (60–100 MΩ). Inasmuch as PACAP increases excitability in virtually all guinea pig cardiac neurons (2, 20), no attempt was made to characterize the cells by subtypes described previously (8). However, inasmuch as ∼90% of the neurons in the guinea pig cardiac ganglia exhibit a phasic firing pattern (10), we can assume that most of the recordings were made from phasic cells, although it is possible that a small percentage of the recordings were made from tonic cells. Membrane potential and action potentials were recorded from the impaled neurons using an Axoclamp-2A amplifier coupled with a Digidata 1322A data acquisition system and pCLAMP 8 (Axon Instruments, Foster City, CA). If required, the resting membrane potential was electrotonically maintained at −55 to −65 mV by injection of hyperpolarizing current through the recording electrode. This ensured that action potential generation was tested at the same potential before and after PACAP application (19–21).
Depolarizing current steps (0.1–0.4 nA, 1 s) were given to characterize changes in neuron excitability before and during drug application. If no more than four action potentials were generated during the supratheshold depolarizing steps, then the cell was classified as “phasic.” However, if at least five action potentials were elicited, then the action potential firing pattern was considered “multiple.” Excitability curves were constructed by plotting the number of action potentials generated by increasing stimulus intensities. Results were averaged from multiple cells under each condition and fit with a straight line. The slope of the regression line was used to compare neuronal excitability between cells studied under different conditions.
Drugs.
PACAP, CsCl, ZD7288 [4-(N-ethyl-N-phenyl-amino)-1, 2-dimethyl-6-(methylamino) pyrimidinium chloride], XE991, and BaCl2 were made up as stock solutions, and aliquots were added directly to the bath solution. When CsCl was added to the bath solution, the concentration was 2 or 2.5 mM. There was no difference in results at either concentration. CsCl and BaCl2 were obtained from Sigma-Aldrich (St. Louis, MO). XE991 and ZD7288 were obtained from Tocris (Ellisville, MO). ZD7288 initially was used at 100 μM, as in our prior study (15), because 100 μM ZD7288 has been shown to effectively inhibit Ih in rat sympathetic and cardiac neurons (12, 13). However, with sustained exposure to 100 μM ZD7288, many guinea pig cardiac neurons were depolarized and exhibited a diminished excitability. Therefore, additional experiments were done with 10 μM ZD7288. Because PACAP-27 was determined previously to be more effective than PACAP-38 on guinea pig cardiac neurons (2, 3), PACAP-27 was used exclusively in this study and is referred to as PACAP. PACAP-27 was obtained from American Peptide (Sunnyvale, CA).
For most experiments, PACAP was added to the bath solution. However, in one series of experiments, PACAP was applied locally by pressure (Picospritzer, General Valve, Fairfield, NJ) via ∼5 μm-diameter “puffer” pipettes that contained 50 μM PACAP and were positioned 50–100 μm from the neuron (19, 20).
Statistical evaluation.
Best-fit linear regression lines were calculated and compared between groups using GraphPad Prism (version 5) statistical software (San Diego, CA). Differences were considered statistically significant if P < 0.05.
RESULTS
Cardiac neuron excitability is enhanced during bath application of PACAP.
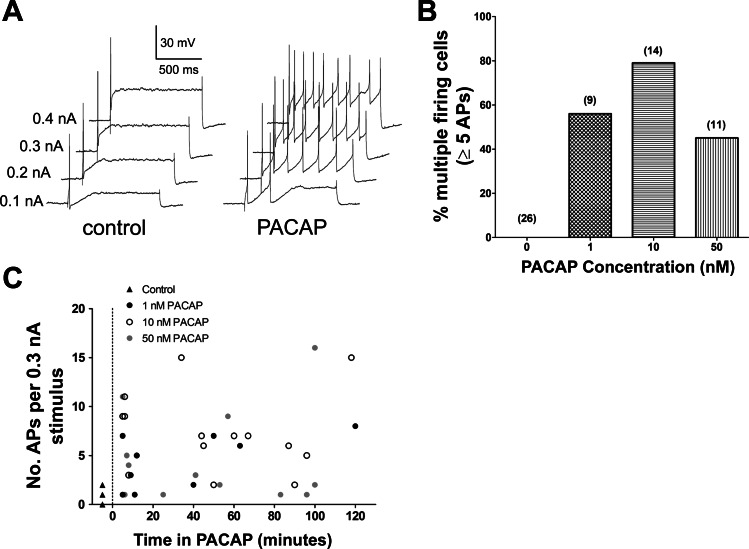
In the initial experiments, we determined the effect on excitability of addition of 1, 10, or 50 nM PACAP directly to the bath. For these experiments, PACAP remained in the bath solution for the entire 120-min recording period, and recordings were made from different cells in the whole-mount preparation at different times over the period of peptide exposure. Excitability was determined from the response to 1-s depolarizing current pulses of increasing amplitude (19–21). Control recordings were made from 29 untreated guinea pig cardiac neurons from 29 whole-mount preparations before exposure to a PACAP-containing solution. Only 1 of the 29 cells was considered to exhibit a multiple firing pattern on the basis of the criteria described in materials and methods; all others were classified as phasic. The maximum number of action potentials elicited by any stimulus was 1 or 2 in 25 cells; 3 cells generated a maximum of 4 action potentials, and 1 cell generated 5 action potentials. In the control cells, action potentials occurred most commonly at the beginning of the depolarization. By contrast, during bath exposure to 1, 10, or 50 nM PACAP, multiple (>5) action potentials often were generated in cells exposed to PACAP by the same depolarizing steps (Fig. 1A). The percentage of cells in PACAP that responded to the depolarizing pulses with multiple action potential firing differed with the different concentrations of PACAP (Fig. 1, B and C). In five cardiac ganglia preparations exposed to 1 nM PACAP continually over a recording period of 120 min, five of nine cells impaled at different times in the PACAP-containing solution produced five or more action potentials in response to suprathreshold voltage steps. In 5 cardiac ganglia preparations exposed to 10 nM PACAP continually over the recording period, 11 of 14 cells tested at different times in the PACAP solution generated 5 or more action potentials in response to the depolarizing steps. During the 120-min exposure to 50 nM PACAP, a peptide-induced increase in action potential generation was noted when recordings were obtained from cells exposed to PACAP for short periods of time. However, in other cells tested in the same whole-mount preparations after longer exposure to the PACAP-containing solution, the peptide-induced increase in excitability appeared to diminish (Fig. 1C). Thus the percentage of cells that exhibited a multiple firing pattern in 50 nM PACAP was less than that seen with prolonged exposure to 1 or 10 nM PACAP (Fig. 1B).
Fig. 1.
Bath application of nanomolar concentrations of pituitary adenylate cyclase-activating polyeptide (PACAP) increases neuronal excitability. A: responses to depolarizing current pulses (1 s) of increasing intensities (0.1–0.4 nA) in the same cell before (control) and 5 min after application of 10 nM PACAP (PACAP). Multiple action potentials were generated during exposure to PACAP. B: percentage of neurons firing ≥5 action potentials (APs) in response to suprathreshold depolarizing current pulses in control preparations and ganglia preparations exposed to 1, 10, or 50 nM PACAP for up to 120 min. Total number of cells exposed to peptide at each concentration is given in parentheses. C: number of action potentials produced by a 0.3-nA current pulse in different cells impaled at different times during 120-min exposure to 1, 10, or 50 nM PACAP.
These results indicate that, with 10 nM PACAP, excitability remained increased in a high percentage of the different cardiac neurons that were impaled and tested at different times over 120 min of continual drug exposure. Thus 10 nM PACAP was used in the remaining experiments designed to test the potential involvement of Ih or IM in the peptide-induced increase in excitability.
Pretreatment with ZD7288 or Cs+ suppressed PACAP-induced increase in cardiac neuron excitability.
Merriam et al. (15) demonstrated that PACAP enhanced activation of Ih. To test a potential role of a PACAP-induced enhancement of Ih in the peptide-induced increase in cardiac neuron excitability, experiments were completed to determine whether the addition of ZD7288 or CsCl to the bath solution suppressed the effect of PACAP on excitability.
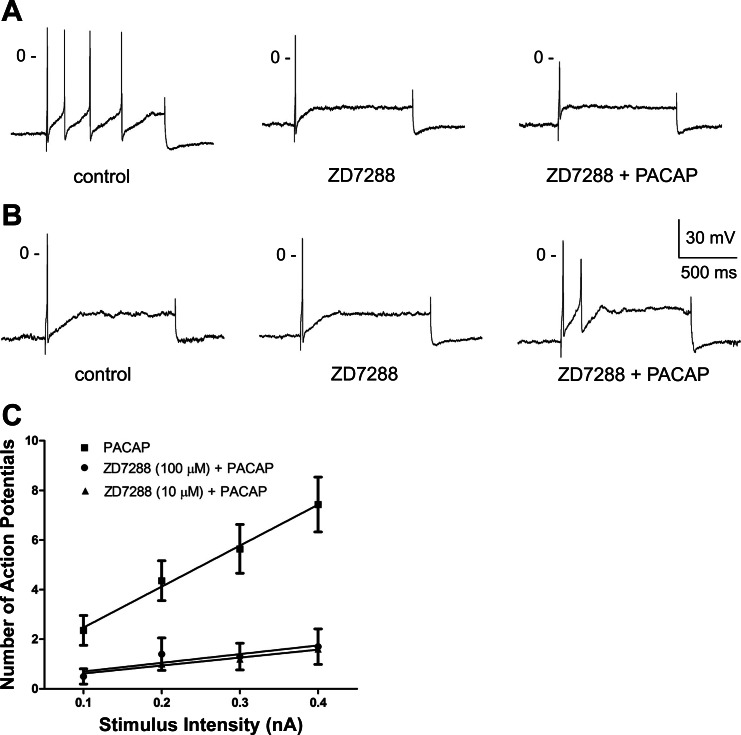
The ability of 100 μM ZD7288 to suppress the 10 nM PACAP-induced increase in excitability was tested in five cardiac ganglia preparations. In ZD7288-treated neurons, there was no inward rectification during hyperpolarizations produced by long current injections (data not shown). In addition, over time in ZD7288, some neurons appeared to have less-negative resting membrane potentials and a diminished excitability compared with untreated cells. Nevertheless, action potential recordings were obtained from 12 neurons in the ZD7288-treated cardiac ganglia preparations.
In four of the five whole-mount ganglia preparations, recordings were obtained from a cell in control solution and then in the same neuron after ∼5 min of exposure to 100 μM ZD7288. One of the four cells generated four action potentials in control solution but only generated one action potential when the same stimulus was applied in the presence of 100 μM ZD7288 (Fig. 2A ). In another cell, the firing pattern was phasic in control solution, but no action potentials could be elicited by the same depolarizing current steps during exposure to ZD7288 (data not shown). In three of the four cells, PACAP was added along with ZD7288 after the initial exposure to ZD7288. In two cells, addition of PACAP did not cause the cells to generate multiple action potential activity (Fig. 2, A and B). In the third cell, no action potentials were elicited during exposure to ZD7288 + PACAP. Overall, in these 5 preparations, action potential recordings were obtained from 10 neurons at different times during the 120-min exposure to ZD7288 + PACAP. Only 1 of the 10 cells exposed to PACAP in the presence of ZD7288 exhibited a multiple firing pattern. The average excitability curve for these 10 cells is shown in Fig. 2C.
Fig. 2.
ZD7288 suppresses PACAP-induced increase in excitability. A and B: recordings from 2 cells that were initially bathed in control solution, then in a solution containing 100 μM ZD7288, and finally in a solution containing 100 μM ZD7288 + 10 nM PACAP. Exposure to ZD7288 reduced excitability in one cell (A), and PACAP did not increase excitability in either of the ZD7288-treated cells. C: excitability curves summarizing the effect of 10 nM PACAP on excitability for 14 cells kept in control solution, 5 cells bathed in a solution containing 10 μM ZD7288, and 10 cells bathed in a solution containing 100 μM ZD7288. Values are means ± SE of number of action potentials generated by a 1-s depolarizing current pulse at multiple stimulus intensities, with lines fit with a linear regression. Both concentrations of ZD7288 significantly reduced slope of regression line: 16.50 ± 0.92 for PACAP alone, 3.2 ± 0.28 (P < 0.001) for PACAP + 10 μM ZD7288, and 3.5 ± 1.32 (P < 0.001) for PACAP + 100 μM ZD7288.
Because many neurons were depolarized and appeared to have a diminished excitability during prolonged exposure to 100 μM ZD7288, additional experiments were done with 10 μM ZD7288. Two preparations were pretreated with 10 μM ZD7288 for ∼10 min and then bathed in a solution containing 10 μM ZD7288 + 10 nM PACAP for 120 min. Although some neurons in these two ganglia preparations exposed to 10 μM ZD7288 + 10 nM PACAP had resting potentials in the range seen in untreated preparations and generated robust action potentials, other neurons had depolarized resting membrane potentials, and in these cells it was difficult to elicit action potentials, even with hyperpolarization to about −60 mV. Action potentials were successfully recorded from five different cells from these two whole-mount ganglia preparations during the 120-min period. All the neurons retained a phasic firing pattern in the presence of 10 μM ZD7288 + 10 nM PACAP. The excitability curve for these five neurons also is shown in Fig. 2C.
The suppression of the PACAP-induced increase in action potential generation by ZD7288 is consistent with the prior suggestion that a PACAP-induced enhancement of Ih contributes to the peptide-induced increase in cardiac neuron excitability (15). However, because the neurons appeared to be considerably less excitable in the presence of 10 or 100 μM ZD7288 than in untreated cells, we were concerned that prolonged exposure at 10 or 100 μM ZD7288 might have had nonspecific effects on excitability. Thus we confirmed these results using CsCl, another established inhibitor of Ih in cardiac neurons (7, 8, 15, 23).
Recordings were made from 14 neurons in 4 cardiac ganglia preparations treated with 2.5 mM CsCl. As with ZD7288, the Cs+-treated cells did not exhibit a gradual decline in the hyperpolarization produced by long current injection, a characteristic sign of Ih activation in cells not treated with Cs+ or ZD7288 (data not shown).
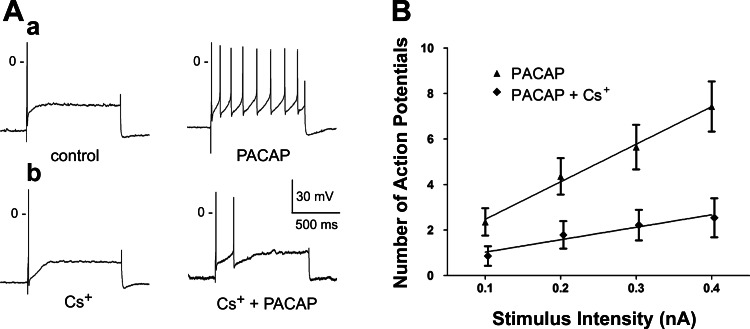
In each preparation, excitability was first determined in one cell treated for ∼20 min with a Cs+-containing solution, and then the same cell was tested after exposure for 5–10 min to a solution containing Cs+ + 10 nM PACAP. All four cells exposed to Cs+ alone exhibited a phasic firing pattern. During exposure to PACAP + Cs+, three of the four cells retained a phasic firing pattern, whereas in one of the four cells the firing pattern shifted from phasic to multiple. Figure 3A shows a typical PACAP-induced increase in excitability of cells in control solution and the lack of a similar effect when Cs+ was included in the bath solution.
Fig. 3.
Cs+ suppresses PACAP-induced increase in excitability. A: in a control cell, response to a 1-s, 0.3-nA depolarizing current pulse was enhanced during exposure to 10 nM PACAP, as evidenced by increased number of action potentials elicited (a); in a second cell bathed in a solution containing 2.5 mM Cs+, only 2 action potentials were elicited by depolarizing current pulse during exposure to 10 nM PACAP (b). B: excitability curves summarizing the effect of 10 nM PACAP on excitability for 14 cells kept in control solution and 14 cells bathed in a solution containing 2.5 mM Cs+. Values are means ± SE of number of action potentials generated by a 1-s depolarizing current pulse at multiple stimulus intensities, with lines fit with a linear regression. Slopes were significantly different: 16.50 ± 0.92 for PACAP alone and 5.35 ± 0.92 for PACAP + Cs+ (P = 0.001).
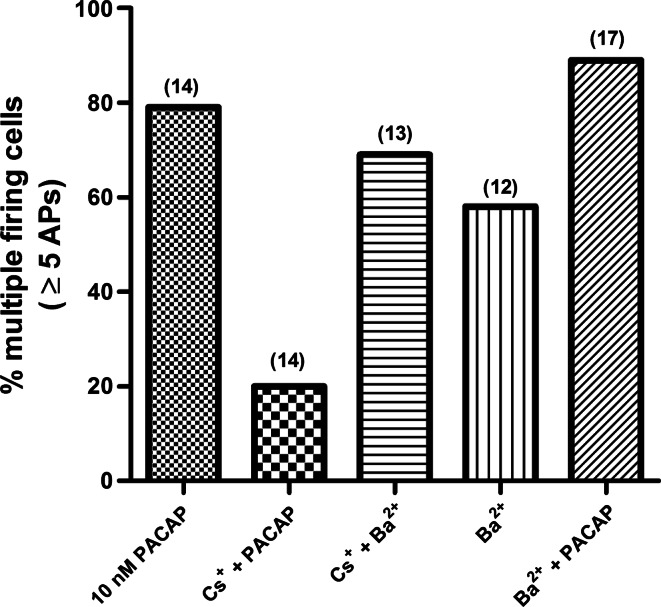
Ten other neurons in these four preparations were then sampled at different times over the 120-min exposure to Cs+ + PACAP. Only 3 of the 14 neurons sampled exhibited the multiple firing pattern. This represents a considerably smaller percentage of neurons exhibiting the multiple firing pattern than seen with 10 nM PACAP alone. We also determined the excitability curve for these 14 cells in Cs+ + PACAP (Fig. 4; slope = 5.4 ± 0.92). The slope was significantly less than that determined for 14 cells exposed PACAP alone (slope = 16.50 ± 0.92, P = 0.001; Fig. 3B). These observations indicate that the presence of Cs+ greatly diminished the ability of PACAP to increase cardiac neuron excitability.
Fig. 4.
Cardiac neuron excitability is modified by different experimental conditions. Percentage of neurons firing ≥5 action potentials in response to suprathreshold depolarizing current pulses in preparations exposed to 10 nM PACAP alone (11 of 14 cells), 2.5 mM Cs+ + 10 nM PACAP (3 of 14 cells), 1 mM Ba2+ + 2.5 mM Cs+ (9 of 13 cells), 1 mM Ba2+ (7 of 12 cells), and 1 mM Ba2+ + 10 nM PACAP (15 of 17 cells) is shown. For each condition, recordings were obtained from different cells at different times over a 120-min period. Number of cells tested in each condition is given in parentheses.
We considered that exposure to Cs+ might have somehow interfered with the activation of the PAC1 receptor by PACAP. When PACAP is applied locally by pressure, a transient depolarization and a long-lasting increase in excitability are produced (19). However, with bath application, only the change in excitability can be quantified. Merriam et al. (15) reported that PACAP elicited a small inward current in the presence of 100 μM ZD7288, an observation indicating that conductances other than Ih, or along with Ih, contribute to generation of the PACAP-induced depolarization. We took advantage of this information to ensure that Cs+ did not suppress the PACAP effect on excitability by altering the ability of PACAP to activate the PAC1 receptor, rather than by inhibiting Ih. Thus, in 4 cells bathed in a solution containing 2 mM CsCl, we tested whether a peptide-induced depolarization could be elicited after pressure application of PACAP. In three of the four cells bathed in the Cs+-containing solution, brief local pressure application of PACAP initiated a depolarization. The averaged depolarization for these 4 cells (9.1 ± 2.2 mV) was similar to the PACAP-induced depolarization in 10 other cells recorded when Cs+ was not included in the bath solution (9.0 ± 3.1 mV). In these 10 control cells, PACAP elicited a depolarization in 8 of the 10 cells, so that even in the absence of Cs+, PACAP did not elicit a depolarization in all cells. It should be noted also that although PACAP elicited a depolarization in three of the four Cs+-treated cells, an increase in excitability was produced in only one of the four cells. Overall, the slope of the excitability curve after PACAP application for the 4 Cs+-treated cells was significantly less (P = 0. 002) than that determined for 10 cells not exposed to Cs+ (data not shown). Thus Cs+ depressed the PACAP-induced increase in excitability produced after local pressure application but did not change the magnitude of the peptide-induced depolarization. Therefore, we conclude that Cs+ does not alter the PACAP activation of the PAC1 receptor, nor does it significantly affect the ionic mechanism(s) underlying the depolarization.
Ba2+ increases action potential firing in Cs+-treated cardiac neurons.
Because prolonged exposure to 10 or 100 μM ZD7288 apparently depressed excitability, we wanted to ensure that the prolonged exposure to Cs+ did not nonspecifically depress cardiac neuron excitability. Thus we determined whether an increase in excitability would be produced in Cs+-treated preparations that were exposed to inhibitors of the depolarization-activated noninactivating K+ current (IM). IM is present in mammalian cardiac neurons and acts to limit action potential firing (7, 23). Inhibition of IM increases excitability, leading to multiple action potential generation. Consequently, we initially tested whether treatment with two established IM blockers, 10 μM XE991 and 1 mM Ba2+, decreased spike adaptation, producing a shift from the phasic to the multiple firing pattern in the guinea pig cardiac neurons.
The effect of XE991 was examined first, because it is suggested to be a more specific inhibitor of IM than Ba2+ (22). Recordings were made from 10 cells in 3 whole-mount preparations exposed to 10 μM XE991 for up to 120 min. As in the prior experiments, action potentials were elicited by l-s depolarizing steps of increasing magnitude, with the resting potential maintained at about −60 mV. In these 3 XE991-treated preparations, none of the 10 neurons tested exhibited a multiple firing pattern (data not shown).
We then tested the effect of 1 mM Ba2+. During exposure to 1 mM Ba2+, ∼60% of the cardiac neurons in six whole-mount ganglia preparations exhibited a multiple firing pattern (Fig. 4), an observation consistent with many cardiac neurons expressing IM.
We suggest that XE991 did not shift the firing pattern from phasic to multiple, because the cells were maintained electrotonically at approximately −60 mV, a membrane potential where the effect of XE991 on spike adaptation can be limited (18). Thus, for the remaining studies done to test whether Cs+ nonspecifically depressed excitability, 1 mM Ba2+ was used to inhibit IM.
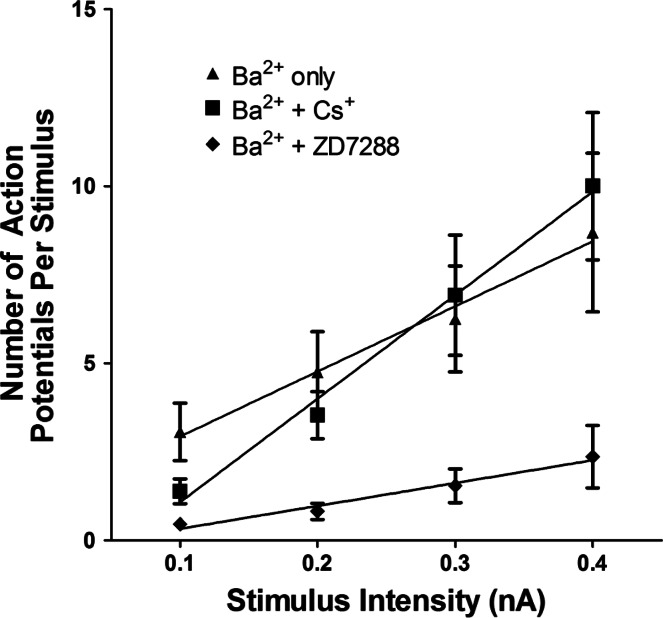
In these experiments, the change in excitability was compared in different cells, which were tested at different times over the 120-min exposure to 1 mM BaCl2 in the control solution (12 cells in 6 preparations) or in a solution containing 2.5 mM CsCl (13 cells in 3 preparations). In the absence of Cs+, 7 of the 12 cells exhibited a multiple firing pattern when exposed to Ba2+, and in the Cs+-treated preparations, Ba2+ induced a multiple firing pattern in 9 of 13 cells (Fig. 4). The excitability curves determined for these two groups of cells are shown in Fig. 5. Cs+ did not produce a general suppression of cardiac neuron excitability, inasmuch as the slope of the excitability curve for cells treated with Ba2+ + Cs+ was significantly greater than that determined for cells treated with Ba2+ alone (18.38 ± 1.43 vs. 29.23 ± 1.82, P = 0.01).
Fig. 5.
Cs+ does not suppress increase in excitability initiated by 1 mM Ba2+. Excitability curves summarize the effect of Ba2+ on excitability for 12 cells kept in control solution, 13 cells bathed in a solution containing 2.5 mM Cs+, and 11 cells bathed in a solution containing 10 μM ZD7288 (7 cells) or 100 μM ZD7288 (4 cells). Values are means ± SE of number of action potentials generated by a 1-s depolarizing current pulse at multiple stimulus intensities, with lines fit with a linear regression. Slopes were significantly greater in cells exposed to Ba2+ alone and Ba2+ + Cs+ (18.38 ± 1.4 vs. 29.23 ± 1.82) than in cells exposed to Ba2+ + ZD7288 (6.45 ± 0.74, P < 0.001).
We also determined whether Ba2+ could increase excitability in ZD7288-treated preparations. Experiments were completed in preparations exposed to 10 or 100 μM ZD7288. ZD7288 at 10 or 100 μM suppressed the ability of 1 mM Ba2+ to increase action potential firing (Fig. 5). These results further support our concern that, under the conditions of the present experiments, ZD7288 suppressed excitability by mechanisms other than simply inhibiting Ih.
PACAP can increase excitability in Ba2+-treated cardiac neurons.
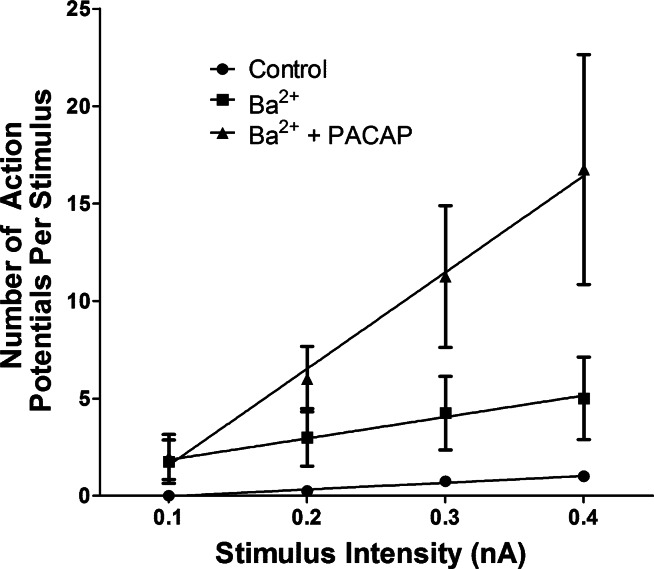
Because neuropeptides commonly increase neuronal excitability by inhibiting IM, we next tested whether 1 mM Ba2+ would occlude the 10 nM PACAP-induced increase in excitability. In the first series of experiments, recordings initially were obtained from cells bathed in control solution and then ≥5 min after the bath was switched to a solution containing 1 mM BaCl2. Recordings were obtained from the same cells 5–10 min after the bath was switched to a solution containing 1 mM BaCl2 + 10 nM PACAP. This recording protocol was successfully completed in four cells. In three of the four cells, exposure to Ba2+ did not initiate a shift from the phasic to the multiple firing pattern; in the fourth cell, however, after exposure to Ba2+, there was a dramatic increase in the number of action potentials produced during the depolarizing steps. In all four cells after exposure to PACAP + Ba2+, action potential firing was markedly enhanced. Thus the three cells that did not exhibit a multiple firing pattern in Ba2+ did so during exposure to Ba2+ + PACAP. Overall, the number of action potentials produced by each depolarizing current pulse was greater in the presence of Ba2+ + PACAP than Ba2+ alone. The excitability curve in Ba2+ and Ba2+ + PACAP determined for these four cells is shown in Fig. 6.
Fig. 6.
PACAP increases excitability in Ba2+-treated cells. Excitability curves are summarized for 4 cells bathed first in control solution, then exposed to a solution containing 1 mM Ba2+, and finally bathed in a solution containing 1 mM Ba2+ + 10 nM PACAP. Values are means ± SE of number of action potentials generated by a 1-s depolarizing current pulse at multiple stimulus intensities, with lines fit with a linear regression. Averaged slopes were 3.50 ± 0.74 in control solution, 11.00 ± 7.02 during exposure to 1 mM Ba2+ alone, and 49.50 ± 14.98 during exposure to 1 mM Ba2+ + 10 nM PACAP. Slopes were significantly greater for cells exposed to Ba2+ alone and Ba2+ + PACAP than for the same cells exposed only to control solution. Slope was significantly greater for Ba2+ + PACAP than Ba2+ alone.
Recordings were then obtained from 13 other cells at different times in these 4 preparations maintained for up to 120 min in a solution containing 1 mM BaCl2 + 10 nM PACAP. In all, 15 of 17 cells exposed to BaCl2 + PACAP exhibited multiple action potential firing (Fig. 4). We also determined the averaged excitability curves for all cells treated with Ba2+ and Ba2+ + PACAP. For cells treated with 1 mM Ba2+ + 10 nM PACAP, the slope of the averaged excitability curve (36.39 ± 3.26, n = 17) was significantly greater (P = 0.01) than that determined for cells exposed to 1 mM Ba2+ (18.38 ± 1.42, n = 12) and greater than the slope of the averaged excitability curve for cells exposed to PACAP (Fig. 2; 16.50 ± 0.92). Thus, although both Ba2+ and PACAP cause an increase in excitability, the presence of Ba2+ does not occlude a further increase in excitability by PACAP.
DISCUSSION
In the present study, we determined the percentage of PACAP-stimulated cells that exhibit phasic or multiple action potential firing when bathed in a control solution vs. a ZD7288- or Cs+-containing solution to assess the potential role of Ih in the PACAP-induced increase in excitability for neurons in intact cardiac ganglia preparations. Inhibition of Ih by exposure to ZD7288 or Cs+ significantly suppressed the ability of PACAP to increase cardiac neuron excitability. In contrast, pretreatment with Ba2+ to inhibit IM did not block the ability of PACAP to increase cardiac neuron excitability. Taken together, our results support the hypothesis that a PACAP-induced enhancement of Ih, but not an inhibition of IM, is a key ionic mechanism underlying the peptide-induced modulation of excitability in the majority of guinea pig cardiac neurons.
Our initial experiments demonstrated that, with bath application of PACAP, the peptide-induced increase in excitability was concentration dependent. With 10 nM PACAP, the peptide-induced increase in excitability remained over the 120-min recording period, whereas during bath exposure to 50 nM PACAP, an increase in excitability occurred initially but, then, appeared to decline with continued exposure. Because peptide receptors often become desensitized with prolonged exposure to an agonist, it was important to use a concentration of PACAP that caused a long-lasting increase in excitability of a majority of the cardiac neurons. Thus 10 nM PACAP was used for the experiments in the present study.
Using dissociated guineas pig cardiac neurons, Merriam et al. (15) demonstrated that PACAP enhanced the hyperpolarization-activated cyclic nucleotide-gated nonselective cation conductance Ih. Because Ih is known to modulate neuronal excitability (17), it was suggested that an enhanced activation of Ih could be a key ionic mechanism underlying the PACAP-induced increase in excitability. This hypothesis is supported by the results from the present study demonstrating that the ability of PACAP to increase neuronal excitability is significantly suppressed in ganglia preparations exposed to ZD7288 or Cs+, treatments well known to suppress Ih.
However, prolonged exposure to 10 or 100 μM ZD7288 by itself appeared to depress excitability, and the fact that Ba2+ did not increase excitability in preparations treated with ZD7288 reinforced this conclusion. In contrast, Cs+ did not diminish the ability of Ba2+ to increase action potential firing, indicating that Cs+ did not nonspecifically depress neuronal excitability.
Pressure application of PACAP depolarized the cardiac neurons to a comparable extent in the presence and absence of Cs+. Merriam et al. (15) noted that PACAP elicited a small inward current in ZD7288-treated dissociated cardiac neurons. They concluded that the PACAP-induced inward current was due to modulation of membrane ionic conductances in addition to Ih. The observation that the PACAP-induced depolarization was similar in untreated and Cs+-treated cells is consistent with this conclusion. The similarity in amplitude of the PACAP-induced depolarization in untreated and Cs+-treated cells also indicated that prolonged exposure to Cs+ did not block activation of the PAC1 receptor by PACAP.
PACAP did increase excitability in a small subset of the Cs+-treated cells. This result was not unexpected given two prior observations. 1) Edwards et al. (8) reported that Ih is not expressed in all guinea pig cardiac neurons. Consequently, ionic mechanisms other than a PACAP-enhanced Ih must also contribute to the enhanced excitability in cells not expressing Ih. 2) A small percentage of the guinea pig cardiac neurons in untreated preparations are tonic, so that multiple action potentials are elicited during long depolarizing pulses (10, 11). Therefore, it is very feasible that there could have been a tonic cell in the Cs+-treated group that would exhibit a multiple firing pattern, even if the action of PACAP were blocked.
The specific inhibitor of IM, XE991, did not affect spike adaptation of the cardiac neurons under the conditions of the present study. We suggest that, for guinea pig cardiac neurons, similar to cultured mouse sympathetic neurons, the action of XE991 is affected by the value of the resting membrane potential (18). Because we maintained the resting potential of the cardiac neurons at about −60 mV, the effect of XE991 was diminished. In contrast, the ability of Ba2+ to inhibit IM is less dependent on the value of the resting membrane potential (18) and initiated multiple action potential firing in ∼60% of the cardiac neurons under the same recording conditions.
Because many neuropeptides can increase neuronal excitability by inhibiting IM (4), we determined whether PACAP still modulated neuronal excitability when ganglia preparations were bathed in a solution containing 1 mM Ba2+. At this concentration, Ba2+ has been reported to effectively inhibit IM in rat cardiac neurons (7, 23). However, we realize that Ba2+ is not selective for IM and can block other K+ conductances, which must temper our interpretations. At 1 mM, Ba2+ significantly enhanced excitability of many guinea pig cardiac neurons. PACAP could increase action potential firing in Ba2+-treated cells, suggesting that a prior suppression of IM or other 1 mM Ba2+-sensitive K+ conductances did not block the ability of PACAP to increase excitability. Furthermore, PACAP could increase excitability in cells that did not change their firing pattern when initially exposed to Ba2+. Together, these observations indicate that a PACAP-induced inhibition of IM was not a crucial mechanism contributing to the peptide-induced increase in guinea pig cardiac neuron excitability. However, our present observations do not exclude the possibility that PACAP might affect IM, because this was not tested directly in the present study.
Perspectives and Significance
The cardiac neurons are the last neurons in the pathway that provides the parasympathetic inhibitory influence on cardiac tissues. Although the cardiac ganglia were initially thought to be simple relay stations, it is now recognized that these ganglia collectively form an intrinsic cardiac nervous system in which information from multiple inputs is integrated (1, 16). Excitability of guinea pig cardiac neurons can be enhanced by a number of bioactive substances, including substance P, histamine, and PACAP (2, 9–11, 20). Comparison of results obtained in the present with results from prior studies indicates that the mechanisms underlying the modulation of cardiac neuron excitability produced by different agonists can be quite different. Furthermore, results from this study provide strong support for the hypothesis that, in the case of PACAP, an enhancement of Ih, rather than an inhibition of IM or other 1 mM Ba2+-sensitive K+ currents, is a key ionic mechanism underlying the peptide-induced increase in guinea pig cardiac neuron excitability. We propose that the effect of PACAP on Ih is mediated through activation of adenylyl cyclase and generation of cAMP. Because Ih is a pacemaker current known to facilitate multiple action potential firing, it is very likely that a PACAP-induced shift in the voltage dependence of activation of Ih to more positive potentials, an effect mediated by an increase in cAMP, is a critical mechanism contributing to the peptide-induced increase in the excitability of guinea pig cardiac neurons.
GRANTS
J. D. Tompkins was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant K01 DK-081444, and Y. T. Lawrence was supported by National Center for Research Resources Grant P20 RR-16435.
Acknowledgments
We thank Beth Young for expert technical assistance with some of the experiments.
REFERENCES
- 1.Adams DJ, Cuevas J. Electrophysiological properties of intrinsic cardiac neurons. In: Basic and Clinical Neurocardiology, edited by JA Armour and JL Ardell. Oxford, UK: Oxford University Press, 2004.
- 2.Braas KM, May V, Harakall SA, Hardwick JC, Parsons RL. Pituitary adenylate cyclase-activating polypeptide expression and modulation of neuronal excitability in guinea pig cardiac ganglia. J Neurosci 18: 9766–9779, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braas KM, Rossignol TM, Girard BM, May V, Parsons RL. Pituitary adenylate cyclase activating polypeptide (PACAP) decreases neuronal somatostatin immunoreactivity in cultured guinea-pig parasympathetic cardiac ganglia. Neuroscience 126: 335–346, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Brown D M currents: an update. Trends Neurosci 11: 294–299, 1988. [DOI] [PubMed] [Google Scholar]
- 5.Calupca MA, Vizzard MA, Parsons RL. Origin of pituitary adenylate cyclase-activating polypeptide (PACAP)-immunoreactive fibers innervating guinea pig parasympathetic cardiac ganglia. J Comp Neurol 423: 26–39, 2000. [PubMed] [Google Scholar]
- 6.Chevaleyre V, Castillo PE. Assessing the role of Ih channels in synaptic transmission and mossy fiber LTP. Proc Natl Acad Sci USA 99: 9538–9543, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuevas J, Harper AA, Trequattrini C, Adams DJ. Passive and active properties of isolated rat intracardiac neurons: regulation by H- and M-currents. J Neurophysiol 78: 1890–1902, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Edwards FR, Hirst GD, Klemm MF, Steele PA. Different types of ganglion cell in the cardiac plexus of guinea-pigs. J Physiol 486: 453–471, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hardwick JC, Kotarski AF, Powers MJ. Ionic mechanisms of histamine-induced responses in guinea pig intracardiac neurons. Am J Physiol Regul Integr Comp Physiol 290: R241–R250, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Hardwick JC, Mawe GM, Parsons RL. Evidence for afferent fiber innervation of parasympathetic neurons of the guinea-pig cardiac ganglion. J Auton Nerv Syst 53: 166–174, 1995. [DOI] [PubMed] [Google Scholar]
- 11.Hardwick JC, Mawe GM, Parsons RL. Tachykinin-induced activation of non-specific cation conductance via NK3 neurokinin receptors in guinea-pig intracardiac neurones. J Physiol 504: 65–74, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris NC, Constanti A. Mechanism of block by ZD 7288 of the hyperpolarization-activated inward rectifying current in guinea pig substantia nigra neurons in vitro. J Neurophysiol 74: 2366–2378, 1995. [DOI] [PubMed] [Google Scholar]
- 13.Hogg RC, Harper AA, Adams DJ. Developmental changes in hyperpolarization-activated currents Ih and IK(IR) in isolated rat intracardiac neurons. J Neurophysiol 86: 312–320, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Luthi A, McCormick DA. Modulation of pacemaker current through Ca2+-induced stimulation of cAMP production. Nat Neurosci 2: 634–641, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Merriam LA, Barstow KL, Parsons RL. Pituitary adenylate cyclase-activating polypeptide enhances the hyperpolarization-activated nonselective cationic conductance, Ih, in dissociated guinea pig intracardiac neurons. Regul Pept 123: 123–133, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Parsons RL Mammalian cardiac ganglia as local integration centers: histochemical and electrophysiological evidence. In: Neural Mechanisms of Cardiovascular Regulation, edited by NJ Dun, BH Machado, and PM Pilowsky. Boston, MA: Kluwer Academic, 2004.
- 17.Robinson RB, Sigelbaum SA. Hyperpolarization-activated cation currents: from molecules to physiological function. Annu Rev Physiol 65: 453–480, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Romero M, Reboreda A, Sanchez E, Lamas JA. Newly developed blockers of the M-current do not reduce spike frequency adaptation in cultured mouse sympathetic neurons. Eur J Neurosci 19: 2693–2702, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Tompkins JD, Ardell JL, Hoover DB, Parsons RL. Neurally released pituitary adenylate cyclase-activating polypeptide enhances guinea pig intrinsic cardiac neurone excitability. J Physiol 582: 87–93, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tompkins JD, Hardwick JC, Locknar SA, Merriam LA, Parsons RL. Ca2+ influx, but not Ca2+ release from internal stores, is required for the PACAP-induced increase in excitability in guinea pig intracardiac neurons. J Neurophysiol 95: 2134–2142, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Tompkins JD, Parsons RL. Identification of intracellular signaling cascades mediating the PACAP-induced increase in guinea pig cardiac neuron excitability. J Mol Neurosci 36: 292–298, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang HS, Pan Z, Shi W, Brown BS, Wymore RS, Cohen IS, Dixon JE, McKinnon D. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science 282: 1890–1893, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Xi-Moy SX, Dun NJ. Potassium currents in adult rat intracardiac neurones. J Physiol 486: 15–31, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]