Abstract
Compared to young cohorts, old rats drink less water in response to several thirst-inducing stimuli. In these experiments, we characterized water drinking in response to hypotension and cellular dehydration in young (4 mo), middle-aged adult (12 mo) and old (29–30 mo) male Brown Norway rats. We injected the vasodilator, minoxidil as an intravenous bolus in a range of doses (0–20 mg/kg), so that drinking responses could be compared at equivalent reductions of arterial pressure. Old rats had greatly diminished reflex tachycardia and became significantly more hypotensive after minoxidil compared with young and middle-aged rats. When compared at equivalent reductions of arterial pressure, old rats drank one-third as much as middle-aged rats, and one-fifth as much as young rats. In addition, there were age-related deficits in drinking in response to a range of administered loads of sodium (0.15–2 M NaCl, 2 ml/100 g body wt). Urinary excretion of water and sodium in response to the loads was equivalent across ages. Both middle-aged and old rats were less able than young rats to repair their water deficits after sodium loading, attributable almost entirely to their reduced drinking responses compared with young rats. Lastly, age-related declines in drinking appeared to be more severe in response to hypotension than in response to cellular dehydration.
Keywords: aging, drinking, diuresis, natriuresis, dehydration, blood pressure, heart rate
elderly people and old animals have impaired drinking responses to many thirst-inducing challenges (21, 23, 25, 28, 29, 33–35, 40, 41). However, the nature of the underlying causes of reduced drinking that accompanies old age, such as impaired neural or humoral signaling mechanisms, remain largely undetermined. Knowledge of the causes of age-related declines in drinking will increase our understanding of the ways to help the elderly maintain better fluid homeostasis. This is an important consideration for an increasingly aging population and for the relationship between hydrational status and disorders, such as heat-related injury.
Hypotension is a potent stimulus of thirst in rats (e.g., 10, 17, 18, 26, 30, 38). Thirst following hypotension is due mainly to release of renin and formation of ANG II in the circulation (26, 38; for a review, see Ref. 20) and is blocked by interfering with the actions of, or formation of, ANG II (10, 26, 33, 38). There are conflicting reports about whether old rats drink less in response to hypotension. For example, old rats have been found to drink less after subcutaneous injections of isoproterenol compared with young rats (24, 34, 37), although not always (25, 34). These previous studies did not record arterial pressure or control for differences in arterial pressure that may occur with aging (24, 25, 34, 37). This information is important because old rats may have impaired reception or processing of baroreceptor-related information, as suggested by the diminished reflex tachycardia that older rats typically have in response to hypotension (1, 2, 7, 9, 19). Therefore, resulting levels of hypotension and accompanying stimuli to drink, e.g., renin levels, may be different in old rats compared with younger animals for a given dose of hypotensive agent, thereby obscuring age-related differences in drinking. One goal of the present experiments was to measure drinking and blood pressure at the same time in young and old rats in response to the vasodilator minoxidil. In addition, we produced a range of levels of hypotension so that drinking in young and old rats could be assessed at equivalent reductions of arterial pressure.
The presence of osmotic thirst deficits in old animals is also controversial. We (40) and others (25) find that aged rats drink less than young rats to peripherally administered hypertonic NaCl solution, but this finding is also not consistent for all laboratories (25, 34). Our previous work (40) indicated that 20-mo-old Brown Norway rats are refractory to osmotic loads producing 2–4% increases in estimated plasma osmolality, but we have not used smaller loads or older rats. To characterize declines in osmotic thirst with age more thoroughly, a further goal of the present work was to assess osmotically induced drinking over additional doses of NaCl and across a wider range of ages than in our previous tests.
METHODS
Animals.
Male Brown Norway rats aged 3 mo (young), 11 mo (middle-aged adult), and 29 mo (old) were obtained from Harlan (Indianapolis, IN) through services provided by the National Institute on Aging (NIA). They were housed singly in hanging stainless-steel cages in a temperature-controlled room (23°C) on a 12:12-h light-dark cycle. They received ad libitum access to standard Teklad Rodent Diet and tap water. Rats were allowed 1 mo for adaptation and were tested at 4, 12, and 30 mo of age, respectively. All procedures were approved by the University of Iowa Institutional Animal Care and Use Committee.
Drugs.
Minoxidil (Sigma, St. Louis, MO) was dissolved in propylene glycol for a stock solution of 30 mg/ml. It was administered intravenously in a range of doses (0–20 mg/kg body wt).
Catheter surgery.
Rats received femoral venous and arterial catheters under Halothane anesthetic for drug injections and measurement of arterial pressure, respectively. Both catheters were made from polyethylene tubing (PE-50) ∼20 cm in length that was heat welded to a shorter piece of PE-10. The PE-10 was inserted into the vessel and advanced 3.5 cm for arterial lines and 2.5 cm for venous lines. The catheters were brought under the skin and were secured between the scapulae to exit at the base of the neck. When not in use, the catheters were filled with heparinized saline (200 U/ml) and plugged with 23-gauge obturators. Rats were allowed 2–3 days to recover from surgery before testing.
Blood pressure recording.
The analog signal from a Cobe pressure transducer was processed by a CED 1401 laboratory interface (Cambridge Electronics Design, Cambridge, UK) using Spike 2 software (Cambridge Electronics Design) on a Dell Optiplex GX620 Pentium D computer. The data were analyzed by a script file that used the digitized pulsatile pressure to calculate mean arterial pressure (MAP), heart rate (HR), and systolic and diastolic pressures. The data were averaged over 5-min bins. Resting MAP was determined using the last 15 min of baseline, i.e., before injection of vehicle or minoxidil.
Urine analysis.
Urine was collected and the volume (UV) was measured. Urinary sodium concentrations (UNa) and urinary potassium concentrations were determined by ion-specific electrodes (NOVA Biomedical, Waltham, MA) and were used for calculation of urinary sodium excretion (UNaV) and urinary potassium excretion. Relative water balances were calculated by subtracting UV from water intake. Relative sodium balances were calculated by subtracting UNaV from the injected load.
Experiment 1: hypotension-induced thirst.
The test cages were wooden with aluminum-lined interiors (24 × 29 cm) that extended 31 cm above suspended, stainless-steel metabolism cages. On the morning of testing, rats were brought to the experimental room, weighed, and placed in the test cages. The arterial lines were connected to pressure transducers by lengths of PE-50. Venous lines were connected to lengths of PE-10 for injections of vehicle or minoxidil solution. Chemical burettes filled with water were attached to the front of the cages.
Rats were allowed 1 h to become quiescent. Water intakes were determined each 30 min during this time, but were negligible (i.e., average water intakes in 1 h for all animals = 0.2 ± 0.1 ml, means ± SE). Then, the rats received bolus injections over ∼30 s of vehicle (propylene glycol, 100–210 μl) or minoxidil (0–20 mg/kg body wt), and water intakes were recorded every 30 min for 3 h. Arterial blood pressure was recorded continuously. Rats received 2–4 such tests, depending on catheter patency. The tests were separated by 2–5 days.
Experiment 2: osmotically induced thirst.
Approximately 10 days after the conclusion of the hypotension experiments, rats were tested for osmotically induced drinking. Rats received a series of five tests involving subcutaneous injections of NaCl solution (0.15, 0.5, 1, and 2 M). On test days, rats were weighed and placed in standard metabolism cages. They were injected subcutaneously with isotonic or hypertonic saline (2 ml/kg body wt). Water was provided immediately from glass burettes attached to the front of the cage, and intakes were recorded every 30 min for 3 h. Urine was collected in Nalgene tubes (0.1 ml resolution) located under the funnels. Urine volume was measured at 1.5 and 3 h, and samples were refrigerated for later analysis of sodium and potassium content. To minimize potential discomfort from the subcutaneous injections of hypertonic saline, all solutions were made with 0.2% lidocaine. The animals showed no signs of discomfort. The doses were administered in mixed order with the first and last tests using 0.15 M NaCl. The tests were separated by 4 or 5 days.
The final group numbers between the two experiments are slightly different as data from a few animals were obtained for only one experiment due to miscellaneous problems (e.g., nonpatent catheters) and one death.
Statistical analysis.
Data were analyzed by one-way, univariate, or repeated measures ANOVA. Analysis of the drinking data was conducted on the raw, noncumulative measures at each time point (14). Planned comparisons were made with Fisher's least significant difference tests when the global F ratio was significant. Values are significant at P < 0.05.
RESULTS
Experiment 1: hypotension-induced thirst.
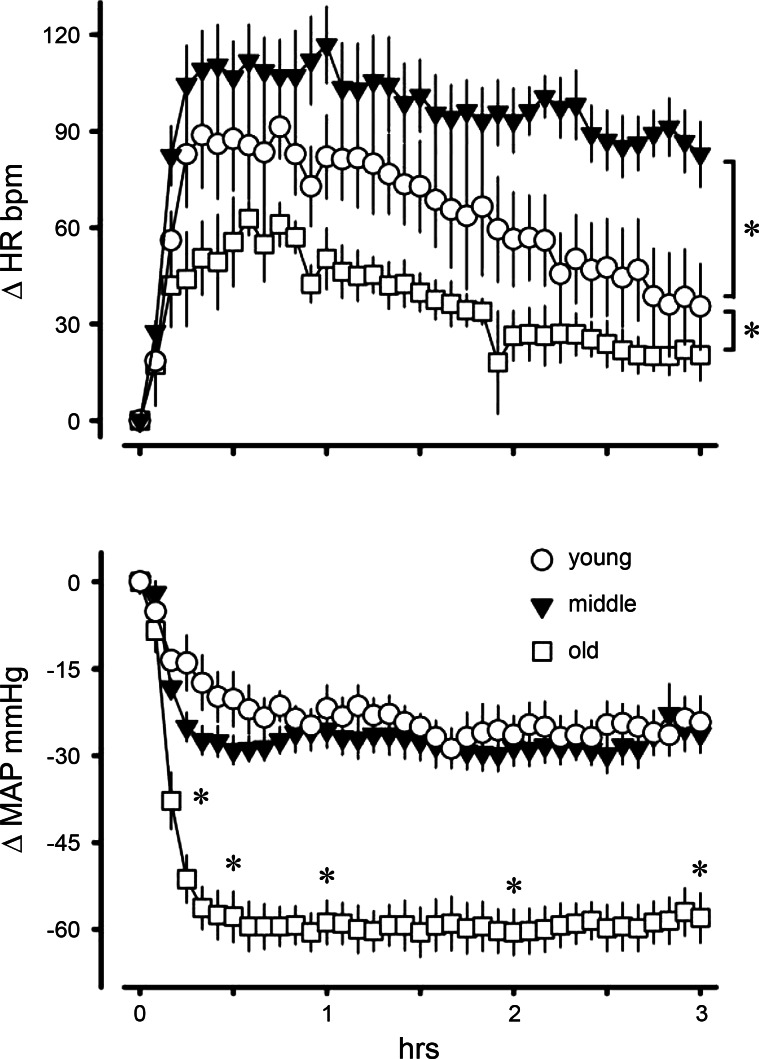
We first determined age-related effects of a moderate dose of minoxidil (5 mg/kg body wt) on MAP and HR in young (n = 5), middle-aged adult (n = 6), and old (n = 4) rats. We used two-way ANOVA to analyze changes in MAP and HR at 10, 30, 60, 120, and 180 min postinjection. Minoxidil caused significantly greater reductions in MAP in old rats compared with either young or middle-aged rats beginning 10 min after injection (time × age interaction: F8,48 = 3.02, P < 0.01; Fig. 1). Minoxidil increased HR significantly more in middle-aged rats, and significantly less in old rats, compared with young cohorts (age main effect: F2,12 = 8.57, P < 0.01). Heart rates for all groups increased significantly in the first hour postinjection before returning toward basal levels (time main effect: F4,48 = 15.61, P < 0.001). We used the ratio of change in HR to change in MAP (i.e., Δbpm/ΔmmHg) as a measure of baroreflex sensitivity. The average ratios over the 3-h test for young, middle-aged, and old rats were 3.6, 4.1, and 0.8, respectively. The ratio in old rats was significantly reduced compared with young and middle-aged rats (age main effect: F2,12 = 6.44, P < 0.05), indicating diminished baroreflex control of HR in the old animals.
Fig. 1.
Changes in heart rate (HR) and mean arterial pressure (MAP) in young (n = 5), middle-aged (n = 6), and old (n = 4) rats after intravenous injection of minoxidil (5 mg/kg body wt). Values are expressed as means ± SE. Old rats had significantly reduced MAP compared with the other groups beginning 10 min postinjection. There was a significant main effect of age on HR. *Significant difference (P < 0.01).
The preliminary results demonstrated age-related effects of minoxidil on MAP and HR. Therefore, in the main experiment, different doses of minoxidil were administered to the different age groups to reduce arterial pressure. The ranges of doses were young, 1–20 mg/kg; middle-aged, 0.5–15 mg/kg; and old, 0.5–3 mg/kg body wt. Initial body weight and resting MAP and HR during the preinjection periods are presented in Table 1. Body weights differed significantly according to age with young rats weighing the least and old rats weighing the most (F2,34 = 70.29, P < 0.001). Resting MAP during the preinjection periods was equivalent between groups. However, a main effect of age (F2,101 = 9.18, P < 0.001) revealed that old rats had significantly slower resting HR compared with young and middle-aged rats. Resting HR of young and middle-aged rats did not differ.
Table 1.
Initial body weight, resting mean arterial pressure and resting heart rate in young, middle-aged adult, and old rats
| Age Group | n | BW, g | MAP, mmHg | HR, bpm | no. obs |
|---|---|---|---|---|---|
| Young | 13 | 297±9 | 109±2 | 366±4 | 38 |
| Middle | 11 | 409±9† | 109±2 | 353±6 | 33 |
| Old | 13 | 447±10* | 107±1 | 335±5* | 33 |
Values are expressed as means ± SE. Body weight (BW) is initial weight. Mean arterial pressure (MAP) and heart rate (HR) are from all observations (obs) during baseline periods before injections of vehicle or minoxidil.
Significantly different from young rats, P < 0.05.
Significantly different from both the other groups, P < 0.05.
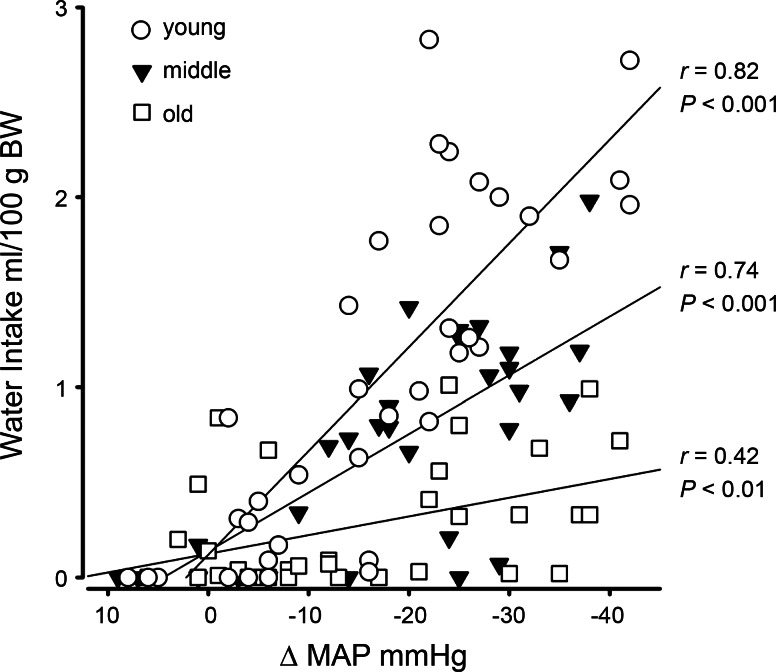
Linear regression analysis was performed on 3-h water intakes, both as absolute and as body weight-adjusted (i.e., ml/100 g body wt) measures, plotted against changes in MAP after injections of vehicle or drug. All correlation coefficients and slope comparisons were significant using either analysis, although the magnitude of the age-related differences was greater with the body weight-adjusted measures. The results for body weight-adjusted intakes are presented in Fig. 2. The correlation coefficients indicated statistically significant relationships between the change in MAP and water intake for all ages. The correlation coefficients were significantly different from each other, confirming that the samples were independent (χ2 = 8.28, df = 2, P < 0.05). The slope of the regression line for young rats was significantly different from that of middle-aged rats (t67 = 4.71, P < 0.01) which, in turn, was significantly different from that of old rats (t62 = 5.12, P < 0.01). The slopes of the regression equations were young, y = 0.11 − 0.055 x; middle-aged, y = 0.14 − 0.031 x; and old, y = 0.12 − 0.01 x. The differences in slopes showed that young rats drank more than five times as much per unit reduction in MAP as old rats and drank nearly double that of middle-aged rats. Middle-aged rats drank three times as much per unit reduction in MAP as old rats.
Fig. 2.
Regression analysis of total amount of water ingested in 3 h postinjection of vehicle or minoxidil plotted against average change in mean arterial pressure (ΔMAP) postinjection for young, middle-aged, and old rats. Intakes are adjusted for body weight. All correlations were statistically significant. The slopes of the regression lines were significantly different (P <0.01). Regression equations: young, y = 0.11–0.055x; middle-aged, y = 0.14–0.031x; old, y = 0.12–0.01x.
One goal of this experiment was to evaluate water drinking across ages at equivalent reductions in MAP. For this analysis, the water-drinking data were grouped according to the respective changes in arterial pressure into four levels ranging from: +8 to −5 mmHg, −6 to −16 mmHg, −17 to −30 mmHg, and −31 to −45 mmHg. We used the average change in arterial pressure over the last 2.5 h of testing, when the pressures appeared stable, for this grouping. We obtained two to four observations from each rat. Thus, the cardiovascular data were analyzed with univariate ANOVA with age and MAP level as fixed factors. The resulting four levels of changes in MAP were significantly different from each other (F3,92 = 31.60, P < 0.001; Table 2) and equivalent across ages. The HR responses of old rats were blunted and significantly reduced compared with young and middle-aged rats, both early in the experimental session (t30 min; age main effect: F2,92 = 8.10, P < 0.001), i.e., after a period of rapid changes in MAP and over the remaining 2.5 h of testing when arterial pressure was stable (age main effect: F2,92 = 3.47, P < 0.05).
Table 2.
Changes in mean arterial pressure grouped into four levels after injections of vehicle or minoxidil and corresponding heart rates in young, middle-aged adult, and old rats
| Age Group | MAP Level | 30 min post |
Remaining 2.5 h | |||
|---|---|---|---|---|---|---|
| ΔMAP, mmHg | Δ HR, bpm | ΔMAP, mmHg | ΔHR, bpm | no. obs | ||
| Young | 1 | 1±1 | 0±8 | 0±1 | −1±7 | 10 |
| 2 | −10±1 | 52±18 | −12±1 | 43±17 | 9 | |
| 3 | −20±1 | 96±10 | −23±1 | 70±9 | 14 | |
| 4 | −32±2 | 96±13 | −38±2 | 61±11 | 5 | |
| Middle-Aged | 1 | 7±3 | −2±10 | 4±2 | 5±4 | 7 |
| 2 | −10±4 | 58±15 | −12±2 | 37±14 | 6 | |
| 3 | −23±2 | 80±11 | −24±1 | 68±10 | 15 | |
| 4 | −32±1 | 124±18 | −35±1 | 95±18 | 5 | |
| Old | 1 | 2±1 | −4±3* | 0±1 | 2±2* | 10 |
| 2 | −6±1 | 26±10* | −9±1 | 25±7* | 8 | |
| 3 | −21±2 | 51±13* | −23±1 | 48±12* | 8 | |
| 4 | −31±2 | 53±9* | −36±2 | 45±9* | 7 | |
Values are expressed as means ± SE. Change in mean arterial pressure (ΔMAP) and change in heart rate (ΔHR) from observations (obs) taken 30 min postinjection of vehicle or minoxidil, and averaged over the remaining 2.5 h of testing. Old rats had significantly reduced ΔHR at both times.
Significantly different from both the other groups, P < 0.05.
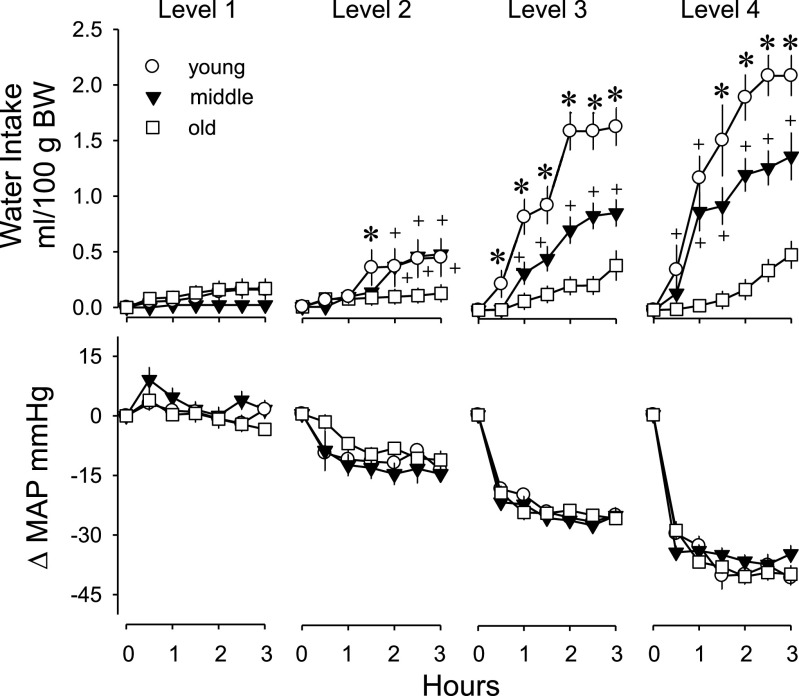
Water intake was analyzed as rate of intake, i.e., ml/30 min of testing, both as absolute and body weight-adjusted measures. The intake data were analyzed using a two-way mixed model ANOVA with time as the within-subjects repeated measure and age as a between-subjects variable. Because we were unable to obtain observations at all pressure levels for most rats, the level of MAP was treated as a separate between-subjects variable. For presentation, the drinking data are shown in Fig. 3 as cumulative intakes at equivalent reductions in MAP. For body weight-adjusted intakes, all main effects and interactions were significant, including the three-way interaction of time × MAP × age (F30,460 = 2.32, P < 0.001). The groups drank small amounts of water at basal levels of MAP (level 1). At all levels of reduced MAP (levels 2, 3, and 4), young and middle-aged rats drank significantly more water compared with old rats and compared with intakes at basal MAP. In addition, young rats drank more than middle-aged rats at MAP levels 3 and 4. Old rats did not drink significantly more water at any level of reduced MAP compared with intakes at basal MAP.
Fig. 3.
Cumulative water intake and change in mean arterial pressure (ΔMAP) after injections of vehicle or minoxidil in young, middle-aged, and old rats. Measures were grouped according to changes in arterial pressure into four levels (1, 2, 3, and 4) ranging from +8 to −5 mmHg, −6 to −16 mmHg, −17 to −30 mmHg, and −31 to −45 mmHg. The average change in arterial pressure over the last 2.5 h of testing was used for grouping the data. Values are expressed as means ± SE. +Significantly different from old rats; P < 0.05. *Significantly different from both the other groups; P < 0.05.
Most of the drinking occurred in the first 90 min. Young rats drank significantly more than basal amounts of water within 30 min in response to the two greatest reductions in MAP (levels 3 and 4) and within 90 min in response to the mildest reduction in MAP (level 2). Middle-aged adult rats drank significantly more than basal levels of intakes within 60 min at MAP levels 3 and 4 and did not drink to the mildest reductions in MAP (level 2) until 120 min. Old rats never drank significantly more than basal amounts of water during any 30-min period.
Experiment 2: osmotically induced thirst.
In this experiment, each rat received all doses of NaCl solution. The sodium loads were administered based on body weight, and all intake and excretion data were analyzed as measures adjusted for body weight. The results were analyzed using a mixed-model ANOVA with time and dose as within-subjects repeated measures and age as the between-subjects measure. The water intakes were analyzed as rate of intake (i.e., ml/30 min). Intakes during the two 0.15 M tests were small and consistent, so the average of the two tests was used to simplify the analysis and presentation.
Young rats weighed significantly less than both middle-aged and old rats, and the latter two groups did not differ in body weight (age main effect: F2,33 = 20.54, P < 0.001; Table 3). The groups were significantly heavier during testing with 2 M NaCl compared with the other doses (dose main effect: F3,99 = 9.12, P < 0.001.)
Table 3.
Body weight, Na load, water intake, and both water and Na excretions and balances 3 h after injections of four different concentrations of NaCl in young, middle-aged adult, and old rats
| Dose NaCl, M | BW, g | Na Load, μmol | Na Load, μmol/100 g | Water Intake, ml/100 g | UV, ml/100 g | Water Balance, ml/100 g | UNa, μmol/ml | UNaV, μmol/100 g | % Na Load Excreted | Na Balance, μmol/100 g | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Young (n = 10) | 0.15 | 340±13* | 102±4* | 30±1 | 0.4±0.1* | 0.4±0.1 | 0.0±0.1 | 128±13 | 45±11 | 152±38 | −15±11 |
| 0.50 | 339±13* | 338±14* | 100±1 | 0.5±0.1* | 0.4±0.1 | 0.1±0.2 | 161±12 | 64±17 | 65±18 | 35±17 | |
| 1.00 | 338±14* | 676±28* | 200±1 | 1.0±0.2* | 0.4±0.1 | 0.6±0.2* | 170±17 | 62±20 | 31±10 | 138±20 | |
| 2.00 | 350±13* | 1384±46* | 396±2 | 1.8±0.2* | 0.9±0.2 | 0.9±0.2* | 199±23 | 162±22 | 41±6 | 234±22 | |
| Middle-aged (n = 14) | 0.15 | 411±10 | 123±1 | 30±1 | 0.2±0.1 | 0.3±0.1 | −0.1±0.1 | 133±10 | 33±9 | 108±30 | –3±9 |
| 0.50 | 410±9 | 410±9 | 100±1 | 0.3±0.1 | 0.3±0.1 | 0.0±0.2 | 187±14 | 46±15 | 46±15 | 54±15 | |
| 1.00 | 404±9 | 811±20 | 199±1 | 0.5±0.1 | 0.5±0.1 | 0.0±0.2 | 180±12 | 75±11 | 38±5 | 123±11 | |
| 2.00 | 416±9 | 1659±34 | 399±1 | 0.9±0.2 | 1.1±0.1 | −0.1±0.2 | 209±15 | 210±21 | 53±5 | 189±21 | |
| Old (n = 12) | 0.15 | 430±10 | 129±3 | 30±1 | 0.1±0.1 | 0.4±0.1 | −0.3±0.1 | 64±7* | 27±6 | 90±20 | 3±6 |
| 0.50 | 432±7 | 433±7 | 100±1 | 0.3±0.1 | 0.5±0.1 | −0.2±0.1 | 109±8* | 53±14 | 53±14 | 47±14 | |
| 1.00 | 430±8 | 859±17 | 200±1 | 0.6±0.1 | 0.8±0.1 | −0.3±0.1 | 113±11* | 91±9 | 45±5 | 109±9 | |
| 2.00 | 433±7 | 1737±27 | 402±1 | 1.0±0.1 | 1.1±0.2 | −0.1±0.2 | 163±6* | 175±28 | 43±7 | 227±27 |
Values are means ± SE. BW, body weight. Na load, amount of injected sodium. UV, urine volume. UNa, urinary sodium concentration. UNaV, urinary sodium excretion. Na load excreted, UNaV/Na load × 100. There were significant dose-related increases in Na load, water intake, UV, UNa, UNaV, and Na Balance. There were significant dose × age interactions for water intake and water balances.
Significantly different from both the other groups, P < 0.01.
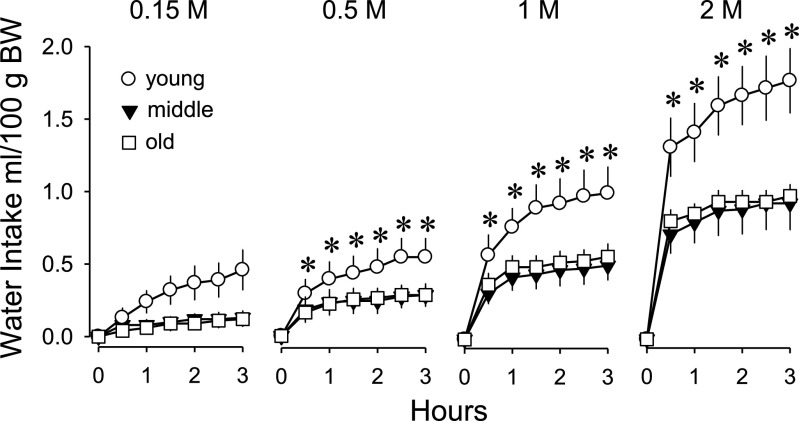
The groups received significantly different absolute amounts of sodium for a given dose because of the age-related differences in body weight (age main effect: F3,33 = 22.48, P < 0.001; Table 3). However, the loads were equivalent on a body weight basis as intended. All main effects and interactions involving water intake were significant, including the three-way interaction of time × dose × age (F30,495 = 1.66, P < 0.05). Most drinking occurred in the first 30 min. At this time, the groups drank equivalent amounts in response to subcutaneous injections of isotonic saline and drank significantly more with each increase in dose. Additionally, young rats drank significantly more than old rats at all hypertonic doses and significantly more than middle-aged rats at the 1 and 2 M doses. There were no differences between middle-aged and old rats in the amount consumed during the first 30 min at any dose. The rate at which rats drank in the first 30 min was dose dependent and proportional to the loads, although young rats drank approximately twice as much as the older groups. Drinking over the entire 3-h test is presented in Fig. 4 as cumulative intakes. Over 3 h, young rats drank significantly more than middle-aged and old rats at all doses, including after isotonic saline. Drinking by middle-aged and old rats did not differ at any dose. Over the entire test, no group drank more in response to 0.5 M NaCl than to isotonic saline. Thus, all ages had significantly increased drinking responses to 0.5 M NaCl compared with isotonic saline in the first 30 min but not cumulatively over 3 h. All groups had significantly increased water intakes at 1 and 2 M doses of NaCl over 3 h compared with water intakes after isotonic saline.
Fig. 4.
Cumulative water intake after subcutaneous injection of NaCl solutions in young, middle-aged, and old rats. Values are expressed as means ± SE. There were significant dose-related increases in drinking for all ages. Young rats drank significantly more than the other ages at all hypertonic concentrations of load. *Significantly different from both the other groups; P < 0.05.
There were no effects of time on UV; therefore, the results for the two 90-min collection periods were combined (Table 3). There were dose-related increases in UV at the 1 and 2 M doses (dose main effect: F3,99 = 25.59, P < 0.001), but no effects of age. All main effects and interactions for water balance were significant, including the three-way interaction of dose × time × age (F6,99 = 3.54, P < 0.01). In the first 90 min of testing, water balances were predominantly positive, as water intakes generally outpaced urinary excretion. In the second 90 min of testing, water balances were uniformly negative as rate of ingestion slowed but rate of excretion did not (data not shown). Overall, there were dose-related increases in cumulative water balance, weighted heavily by the significantly increased water balances of young animals at 1 and 2 M doses. In addition, water balances of young rats after 1 and 2 M NaCl were significantly increased compared with those of middle-aged and old rats. Water balances of middle-aged and old rats were never significantly different.
Analysis of UNa was performed on the total urine collected in 3 h. Some animals did not urinate during testing, so the analysis is based on fewer observations. There were main effects of age (F2,104 = 24.81, P < 0.001) and dose (F3,104 = 17.40, P < 0.001). Urinary sodium concentration was significantly reduced in old rats compared with middle-aged and young rats, and it did not differ between middle-aged and young rats. All doses above isotonic significantly increased UNa, with the 2 M dose producing significantly greater UNa than the other doses. Urinary sodium excretion was significantly increased after 1 and 2 M NaCl (dose main effect: F3,99 = 59.60, P < 0.001), but there were no effects of age. The fraction of the sodium load that was excreted over 3 h was affected by dose, but not by age. After isotonic saline, about as much sodium was excreted as was injected. At all hypertonic doses, the fraction of the load excreted was significantly reduced, and was about half of the injected load compared with excretion after isotonic saline (dose main effect: F3,99 = 15.07, P < 0.001). The resulting sodium balances increased significantly with dose (main effect: F3,99 = 130.05; P < 0.001) but were not affected by age.
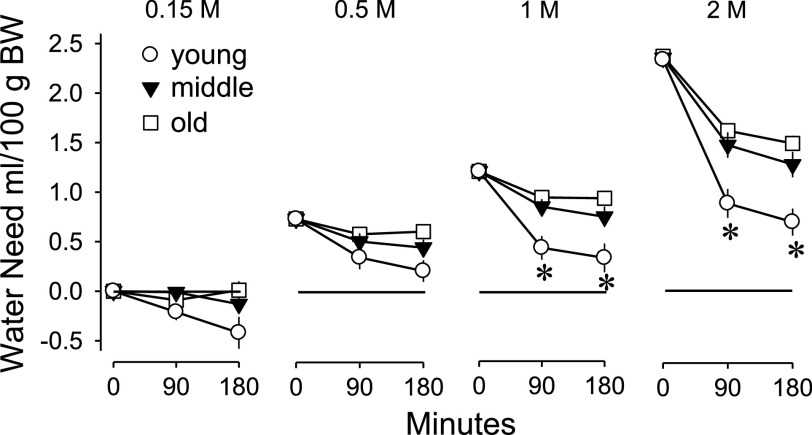
The loads of NaCl were based on body weight, so the water needed to dilute the loads to isotonicity with body fluids is readily estimated (42). The estimates of cumulative “water need” are shown in Fig. 5. The amount of water needed immediately after injection is shown at 0 min. The reduced measures at 90 and 180 min are accomplished both by ingesting water and by excreting sodium. There were significant time × age (F2,33 = 5.50, P < 0.05) and dose × time (F3,99 = 31.53, P < 0.001) interactions for estimated water need. All ages repaired their water deficits significantly more in the first 90 min of testing than in the second 90 min. Additionally, young rats repaired their water deficits significantly more than middle-aged and old rats in the first 90 min of testing at the two highest doses. Middle-aged and old rats never consumed sufficient water to repair more than half of the estimated water need.
Fig. 5.
Cumulative amount of water needed to dilute the administered sodium loads to isotonicity with body fluids in young, middle-aged, and old rats. Values are expressed as means ± SE. Young rats repaired significantly more of their water deficits at the two greatest loads compared with the other ages. *Significantly different from both of the other groups; P < 0.05.
DISCUSSION
The main findings of the present study were 1) there were age-related declines in drinking in response to hypotension. With advancing age, rats drank less and were slower to respond, after reductions in arterial pressure. Importantly, age-related differences in drinking were evident at equivalent reductions in arterial pressure. 2) There were age-related declines in drinking in response to osmotic challenge. As a result, middle-aged and old rats were less able than young rats to repair estimated water needs following administration of NaCl loads. 3) Age-related declines in drinking were relatively more severe following hypotension than osmotic challenge. Compared with young rats, old rats were virtually unresponsive in terms of hypotension-related drinking yet retained about half the osmotic drinking response at all levels of dehydration.
In the first experiment, we characterized water drinking in response to hypotension between young (4 mo), middle-aged adult (12 mo), and old (30 mo) male rats of the Brown Norway strain. We injected the vasodilator minoxidil in an intravenous bolus across a range of doses (0–20 mg/kg) so that age-related drinking could be compared at different levels of MAP. At baseline, MAP was equivalent across ages, but old rats had significantly reduced resting HR. Old rats became significantly more hypotensive in response to minoxidil, possibly from diminished reflex tachycardia compared with young and middle-aged rats. Over a range of reductions in MAP, old rats drank only one-third as much as middle-aged adult rats, and only one-fifth as much as young rats. Regression analysis showed a strong relationship between reductions in arterial pressure and drinking for young (r = 0.82) and middle-aged (r = 0.74) rats but not for old (r = 0.42) rats.
Older animals, like older humans, have impaired baroreflexes (12, 13, 16). For example, reflex tachycardia following injections of isoproterenol or nitroprusside is greatly reduced in old rats (1, 9, 19, 37; but see Ref. 8). In our preliminary experiment assessing HR and MAP after a single dose of minoxidil (5 mg/kg body weight), reflex tachycardia was substantially reduced in old rats compared with either young or middle-aged animals. On the basis of the ratio of Δbpm/ΔmmHg, the baroreflex response of old rats was only one-fourth that of young and middle-aged rats. In the main experiment, old rats also had diminished reflex tachycardia at all levels of hypotension compared with middle-aged and young rats. This diminished baroreflex buffering probably accounts for much of the significantly greater arterial pressure changes found with older rats following minoxidil and why older rats required lower doses of minoxidil to reduce arterial pressure to targeted levels compared with younger animals. Nitroprusside also produces exaggerated depressor responses with attenuated tachycardia in aged vs young rats (9). Interestingly, Table 2 shows that by 30 min after administration of minoxidil—after a significant drop in arterial pressure—HR for young and middle-aged rats increased substantially, then decreased as arterial pressure stabilized for the next 2.5 h. However, the HR of old rats increased by 30 min after administration of minoxidil but did not decline as arterial pressure stabilized. This may indicate that old rats are less able to adjust to the stress of the testing situation than young and middle-aged rats. Others (36) have noted increased variability in the responses of old rats due to increased “handling stress” during testing. The variability in responding by older rats, and presumably the stress involved in testing, can be reduced by extensive preexperimental handling (36). Similarly, we have observed highly variable daily water intakes in old rats compared with young rats upon initial receipt from the supplier, which we attributed to shipping stress or a response to the novel laboratory environment (40). For this reason, we allow 3–4 wk for adaptation to laboratory conditions before beginning aging studies rather than the 1–2 wk that we typically use in studies unrelated to aging. Extra handling of old rats and additional adaptation time before testing are likely to be beneficial to older animals by reducing the effects of stress.
The present study clearly shows age-related declines in drinking in response to hypotension caused by minoxidil. The most pronounced finding was the near absence of hypotension-related drinking by old rats. Drinking caused by minoxidil is largely renin dependent. As with drinking in response to diazoxide (10, 31), drinking after minoxidil is blocked by treatment with an angiotensin-converting enzyme inhibitor (e.g., captopril; 31, 39). Minoxidil stimulates renin secretion by baroreflex activation of renal sympathetic (β-adrenergic receptor-mediated) nerves (27). Under unstressed, basal conditions plasma renin activity is similar in young and aged rats (3, 4, 5). However, old rats secrete less renin in response to sympathetic β-adrenergic receptor activation compared with young rats (3, 4). Old rats have reduced renal sympathetic nerve activity in response to hypotension (e.g., after nitroprusside; Ref. 19); therefore, they probably have reduced renin secretion during hypotension as well. We postulate then that old rats drink less in response to hypotension because of reduced baroreflex-mediated renin secretion. The almost complete lack of hypotension-related drinking in old rats is not likely to result from reduced sensitivity to ANG II or to impaired conversion of ANG I to ANG II. First, old rats drink as much as young rats in response to subcutaneous injections of ANG II in the Sprague-Dawley, Fischer 344, and Munich Wistar strains (25, 33, 34), so the sensitivity of central ANG II receptors, and the functional integrity of neural pathways “downstream” from the receptors, appear normal in old rats. Second, old rats drink as much as young rats in response to subcutaneous injections of ANG I in both the Sprague-Dawley and Fischer strains (34, 37), so conversion of ANG I to ANG II is apparently normal in old rats as well. Results from similar experiments in Brown Norway rats are not available. However, Silver et al. (35) report that old rats from the cross of female Fischer and male Brown Norway strains (F334 × BN) drink less than young rats in response to subcutaneous ANG II on a body weight-adjusted basis but equivalently on an absolute basis. At present, then, the most parsimonious explanation for age-related declines in drinking to hypotension is simply that old rats secrete less renin in response to hypotension than young rats and do not produce equivalently dipsogenic levels of circulating ANG II.
Work investigating the effects of age on renin-dependent drinking typically has used the hypotensive agent, isoproterenol, with mixed results (24, 25, 34, 37). Isoproterenol releases renin reflexively due to the hypotension it produces but also by directly activating β-adrenoceptors on juxtaglomerular cells (22, 26). Age-related declines in drinking after isoproterenol seem to depend on sex, strain of rat, and other factors (24, 25, 34, 37). Notably, these studies do not report levels of arterial pressure during testing. Since older rats have diminished tachycardia following isoproterenol compared with young rats (37), it is possible that arterial pressure was further reduced in older animals compared with younger animals in those studies. Therefore, the comparable drinking responses of old rats vs. young rats in some studies could reflect diminished drinking by older animals relative to the degree of hypotension that was produced. By measuring MAP in our studies, we quantified the changes in arterial pressure produced by minoxidil and compared drinking at equivalent levels of hypotension. At equivalent reductions in MAP, old rats drank significantly less than young and middle-aged rats. Indeed, at equivalent reductions in arterial pressure, hypotension-related drinking was nearly absent in older rats compared with young rats.
In the second experiment, we examined age-related water drinking and urinary excretion in response to loads of NaCl solution. Consistent with our previous work (40), young rats drank about twice as much as middle-aged adult and old rats in response to hypertonic loads of NaCl. Middle-aged adult and old rats never differed in their drinking responses. Most drinking occurred in the first 30 min of testing for all ages and, at each hypertonic concentration of NaCl, drinking in the first 30 min was proportional to the load. Together with previous work (40), the present results indicate that age-related reductions in osmotic drinking begin at least by 12 mo of age in the Brown Norway strain and are apparent using a dose (2 ml/kg body wt of 0.5 M) of NaCl solution calculated to produce a 1% increase in plasma osmolality. Notably, however, in the first 30 min of testing, all ages drank significantly more water in response to all hypertonic loads compared with basal intakes observed after isotonic saline. This includes the group of very old (30 mo) rats, whose drinking responses were indistinguishable from the much younger (12 mo) middle-aged adult rats. Thus, peak osmotic drinking is observed in young rats (range 4–6 mo) and is significantly diminished by middle age (12 mo). However, we have no evidence of further impairments or reductions in osmotic drinking beyond middle age, even up to 30 mo. Martin et al. (24) report a similar lack of difference in osmotic drinking between adult (5–10 mo) and senescent (>31 mo) rats. The present findings also agree with those of McKinley et al. (25), who observed that young (3 mo) rats drink the most in response to osmotic challenge. However, those authors noted a significant reduction in osmotic drinking by 6 mo of age and continued, gradual reductions in osmotic drinking through 24 mo. The discrepancy in results may reflect significant methodological differences between studies. McKinley et al. (25) administered a single intraperitoneal injection of 0.4 M NaCl in a volume of 20 ml/kg body wt to each rat. We administered a range of doses (0.15–2 M) of NaCl injected subcutaneously in one-tenth the volume, i.e., 2 ml/kg body wt. The total amount of sodium delivered in the study by McKinley et al. was more than our highest dose and in a larger volume. Absorption and excretion of these divergent loads, administered in different volumes by alternative routes, and the number of tests involved all could contribute to the different findings of the studies. Thus, although there is some agreement concerning age-related differences in osmotic drinking between young and middle-aged adult rats, it is less certain whether osmotic drinking is further impaired beyond middle age in rats.
Rowland et al. (34) did not find age-related differences in osmotic drinking, but these authors cautioned that their testing conditions were conducted when animals were at peak activity levels shortly after lights-out, which may have somehow benefited the older rats and permitted them a better chance to respond. The beneficial effects of testing under conditions of increased behavioral activation has been noted in rats that had certain brain lesions that failed to drink in response to dipsogenic stimuli when tested during the day, but drank comparably with controls when tested at night or when pretreated with the stimulant caffeine (15, 32). Therefore, there is precedence for the idea that reduced drinking responses of old rats tested during the day could reflect general impairments in behavioral activation in older animals rather than specific impairments in sensing signals related to dehydration. However, the more profound effects of aging on hypotension-related thirst compared with osmotic thirst that we observed in the present work suggests, at minimum, that there is an interaction between specific thirst signals or type of dehydration and level of behavioral activation.
There are age-related differences in renal handling of water and sodium. For example, the aging kidney has reduced concentrating ability (6), and old rats excrete loads of water and hypotonic saline more slowly than young rats (34). Following administration of hypertonic saline loads, old rats ultimately excrete a greater fraction of the injected sodium than young rats (34, 40) by producing a larger volume of dilute urine (40). Unlike our previous work (40), we did not find age-related differences in urine volume or urinary sodium excretion following administration of hypertonic saline. The different outcomes may reflect the experimental histories in the studies. In our earlier report, animals had a series of sodium depletions prior to osmotic challenge, and here the animals had a series of hypotensive episodes prior to osmotic challenge. In other respects, the present results are consistent with our previous work. Old rats had reduced urinary sodium concentration compared with young and middle-aged rats and reduced water balances compared with young rats. Therefore, regardless of experimental history before the osmotic challenge, these new results confirm that young rats are significantly better at repairing estimated water deficits compared with old and middle-aged rats, and this is mainly due to the fact that younger animals drink more in response to osmotic challenge.
The reduced osmotic drinking by older animals could reflect reduced afferent input from osmoreceptors and diminished motivation to drink. Another possibility is that old rats drink less than young rats because they satiate more quickly. It has been shown recently that in response to osmotic loading old human subjects report equivalent thirst, but drink only half as much water, as young subjects (11). This may mean that the satiation of thirst, and not the emergence of thirst, is different in older human subjects (11). The present results in rats could be interpreted similarly. Our old rats drank in response to all levels of osmotic dehydration, and, like young animals, drank in proportion to the loads in the first 30 min, but drank only half as much as young rats. However, differences in satiety cannot explain the diminished drinking of old animals after hypotension as they hardly drank at all in that case.
The results support the suggestion that distinct thirst mechanisms decline at different rates with advancing age (25). In studies using up to 6 cohorts (i.e., 3, 6, 10, 15, 18, and 24 mo), McKinley et al. (25) found that drinking in response to hypovolemia remained fairly intact from ages 6 to 18 mo with an abrupt decline at 24 mo but that osmotic thirst steadily diminished from age 6 mo on. Our data are less able to address the abruptness of age-related declines in drinking as we used fewer cohorts than McKinley et al. (25). However, our results suggest that aging rats have relatively greater reductions in hypotension-related (presumably renin dependent) drinking than in osmotic drinking. Hypotension-related drinking was nearly absent in old rats compared with young rats when assessed at equivalent reductions in arterial pressure. However, even very old rats retained substantial osmotic responsiveness compared with young rats. Additionally, renin-dependent thirst and salt appetite have been found to be greatly diminished with advanced age in rats, while thirst with an osmotic component (i.e., 24-h water deprivation) is less affected by age (33, 34, 40).
Perspectives and Significance
If thirst mechanisms (e.g., renin-angiotensin, osmoreceptors, baroreceptors) decline at different rates with advancing age, then older animals must rely on these mechanisms to different degrees than young rats. For example, older rats are generally deficient in secreting renin (3, 4, 33), and it has been suggested that extrarenal, probably baroreceptor, mechanisms might prove to be more important in stimulating thirst in aging rats in response to drugs like isoproterenol (34). On the other hand, it has been suggested that impairments in baroreceptor mechanisms may be a leading cause of reduced thirst in aging rats (25). Given the possibility that arterial pressure may be reduced further in older rats than in younger rats following administration of hypotensive drugs, baroreflexes and renin-release may be differentially stimulated in younger vs, older rats. In addition, old rats may have relatively greater impairments in reflex, sympathetically mediated renin secretion than in pharmacological β1-adrenoceptor-mediated renin secretion. For example, there is evidence that old rats have reduced baroreflex tachycardia, but normal β1-adrenoceptor-mediated tachycardia (9). Thus, old rats may have greatly reduced drinking after drugs like minoxidil because of impaired baroreflex-mediated renin secretion but have substantial drinking after isoproterenol because of relatively intact renin secretion in response to pharmacological activation of β-adrenoceptors. The relative importance of osmotic, renin-related, and baroreceptor-related mechanisms for the production of thirst requires additional study. Aging rats are proving to be an exceptionally valuable model to investigate diminished responsiveness of thirst mechanisms with advanced age.
GRANTS
This research was supported by National Institutes of Health Grants MH-59239 and AG-25465 to R. L. Thunhurst and HL-57472, DK-66086 and HL-14388 to A. K. Johnson.
REFERENCES
- 1.Barringer DL, Buñag RD. Differential age-dependent attenuation of reflex tachycardia by verapamil in rats. Mech Ageing Dev 58: 111–125, 1991. [DOI] [PubMed] [Google Scholar]
- 2.Barringer DL, Buñag RD. Autonomic regulation of reflex bradycardia in rats declines with age. Exp Gerontol 26: 65–75, 1991. [DOI] [PubMed] [Google Scholar]
- 3.Baylis C, Corman B. The aging kidney: insights from experimental studies. J Am Soc Nephrol 9: 699–709, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Baylis C, Engels K, Beierwaltes WH. Beta-adrenoceptor-stimulated renin release is blunted in old rats. J Am Soc Nephrol 9: 1318–1320, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Baylis C, Engels K, Hymel A, Navar LG. Plasma renin activity and metabolic clearance rate of angiotensin II in the unstressed aging rat. Mech Ageing Dev 97: 163–171, 1997. [DOI] [PubMed] [Google Scholar]
- 6.Bengele HH, Mathias RS, Perkins JH, Alexander EA. Urinary concentrating defect in the aged rat. Am J Physiol Renal Fluid Electrolyte Physiol 240: F147–F150, 1981. [DOI] [PubMed] [Google Scholar]
- 7.Buñag RD, Teräväinen TL. Waning cardiovascular responses to adrenergic drugs in conscious ageing rats. Mech Ageing Dev 61: 313–326, 1991. [DOI] [PubMed] [Google Scholar]
- 8.Buñag RD, Teräväinen TL, Eriksson L. Enhanced sympathetic mediation of chronotropic baroreflexes in old Sprague-Dawley rats. Mech Ageing Dev 53: 195–208, 1990. [DOI] [PubMed] [Google Scholar]
- 9.Docherty JR, Fitzgerald D, O'Malley K. Age-related reduction of baroreflex tachycardia without loss of β-adrenoceptor-mediated tachycardia in Sprague-Dawley rats. J Cardiovasc Pharmacol 8: 376–380, 1986. [DOI] [PubMed] [Google Scholar]
- 10.Evered MD Relationship between thirst and diazoxide-induced hypotension in rats. Am J Physiol Regul Integr Comp Physiol 259: R362–R370, 1990. [DOI] [PubMed] [Google Scholar]
- 11.Farrell MJ, Zamarripa F, Shade R, Phillips PA, McKinley M, Fox PT, Blair-West J, Denton DA, Egan GF. Effect of aging on regional cerebral blood flow response associated with osmotic thirst and its satiation by water drinking: a PET study. Proc Nat Acad Sci USA 105: 382–387, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrari Au Daffonchio A, Albergati F, Manci G. Differential effects of aging on the heart rate and blood pressure influences of arterial baroreceptors in awake rats. J Hypertens 9: 615–621, 1991. [DOI] [PubMed] [Google Scholar]
- 13.Ferrari AU, Radaelli A, Centola M. Aging and the cardiovascular system. J Appl Physiol 95: 2591–2597, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Fitts DA Misuse of ANOVA with cumulative intakes. Appetite 46: 100–2, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Gardiner TW, Stricker EM. Impaired drinking responses of rats with lesions of nucleus medianus: circadian dependence. Am J Physiol Regul Integr Comp Physiol 248: R224–R230, 1985. [DOI] [PubMed] [Google Scholar]
- 16.Hajduczok G, Chapleau MW, Johnson SL, Abboud FM. Increase in sympathetic activity with age I. Role of impairment of arterial baroreflexes. Am J Physiol Heart Circ Physiol 260: H1113–H1120, 1991. [DOI] [PubMed] [Google Scholar]
- 17.Hosutt JA, Stricker EM. Hypotension and thirst after phentolamine treatment. Physiol Behav 27: 463–468, 1981. [DOI] [PubMed] [Google Scholar]
- 18.Houpt KA, Epstein AN. The complete dependence of beta-adrenergic drinking on the renal dipsogen. Physiol Behav 7: 897–902, 1971. [DOI] [PubMed] [Google Scholar]
- 19.Irigoyen MC, Moreira ED, Werner A, Ida F, Pires MD, Cestari IA, Krieger EM. Aging and baroreflex control of RSNA and heart rate in rats. Am J Physiol Regul Integr Comp Physiol 279: R1865–R1871, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Johnson AK, Thunhorst RL. The neuroendocrinology, neurochemistry and molecular biology of thirst and salt appetite. In: Handbook of Neurochemistry and Molecular Neurobiology: Behavioral Neurochemistry and Neuroendocrinology (3rd ed.), edited by Lajtha A. Berlin: Springer-Verlag, 2007, p. 641–687.
- 21.Kenney WL, Chiu P. Influence of age on thirst and fluid intake. Med Sci Sports Exerc 33: 1524–1532, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Kirby RF, Novak CM, Thunhorst RL, Johnson AK. The role of β1 and β2 adrenoceptors in isoproterenol-induced drinking. Brain Res 656: 79–84, 1994. [DOI] [PubMed] [Google Scholar]
- 23.Mack GW, Weseman CA, Langhans GW, Scherzer H, Gillen CM, Nadel ER. Body fluid balance in dehydrated healthy older men: thirst and renal osmoregulation. J Appl Physiol 76: 1615–1623, 1994. [DOI] [PubMed] [Google Scholar]
- 24.Martin JR, Fuchs A, Harting J. Drinking by senescent and adult rats in response to regulatory challenges. Neurobiol Aging 6: 57–59, 1985. [DOI] [PubMed] [Google Scholar]
- 25.McKinley MJ, Denton DA, Thomas CJ, Woods RL, Mathai ML. Differential effects of aging on fluid intake in response to hypovolemia, hypertonicity, and hormonal stimuli in Munich Wistar rats. Proc Nat Acad Sci USA 103: 3450–3455, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyer DK, Rauscher W, Peskar B, Hertting G. The mechanism of the drinking response to some hypotensive drugs: activation of the renin-angiotensin system by direct or reflex-mediated stimulation of β-receptors. Arch Pharmacol 276: 13–24, 1973. [DOI] [PubMed] [Google Scholar]
- 27.Pettinger WA, Campbell WB, Keeton K. Adrenergic component of renin release induced by vasodilating antihypertensive drugs in the rat. Circ Res 33: 82–86, 1973. [DOI] [PubMed] [Google Scholar]
- 28.Phillips PA, Bretherton M, Johnston CI, Gray L. Reduced osmotic thirst in healthy elderly men. Am J Physiol Regul Integr Comp Physiol 261: R166–R171, 1991. [DOI] [PubMed] [Google Scholar]
- 29.Phillips PA, Rolls BJ, Ledingham JGG, Forsling ML, Morton JJ, Crowe MJ, Wollner L. Reduced thirst after water deprivation in healthy elderly men. N Engl J Med 311: 753–759, 1984. [DOI] [PubMed] [Google Scholar]
- 30.Rettig R, Ganten D, Johnson AK. Isoproterenol-induced thirst: renal and extrarenal mechanisms. Am J Physiol Regul Integr Comp Physiol 241: R152–R157, 1981. [DOI] [PubMed] [Google Scholar]
- 31.Robinson MM, Evered MD. Pressor action of intravenous angiotensin II reduces drinking response in rats. Am J Physiol Regul Integr Comp Physiol 252: R754–R759, 1987. [DOI] [PubMed] [Google Scholar]
- 32.Rowland N Circadian rhythms and partial recovery of regulatory drinking in rats after lateral hypothalamic lesions. J Comp Physiol Psychol 90: 382–393, 1976. [DOI] [PubMed] [Google Scholar]
- 33.Rowland NE, Del Bianco A, Fregly MJ. Age-related decline in thirst and sodium appetite in rats related to kininase II inhibition. Regul Pept 66: 163–167, 1996. [DOI] [PubMed] [Google Scholar]
- 34.Rowland NE, Morien A, Garcea M, Fregly MJ. Aging and fluid homeostasis in rats. Am J Physiol Regul Integr Comp Physiol 273: R1441–R1450, 1997. [DOI] [PubMed] [Google Scholar]
- 35.Silver AJ, Morley JE, Ishimaru-Tseng TV, Morley PMK. Angiotensin II and fluid ingestion in old rats. Neurobiol Age 14: 519–522, 1993. [DOI] [PubMed] [Google Scholar]
- 36.Silverman WF, Aravich PA, Sladek JR Jr, Sladek CD. Physiological and biochemical indices of neurohypophyseal function in the aging Fischer rat. Neuroendocrinology 52: 181–190, 1990. [DOI] [PubMed] [Google Scholar]
- 37.Simpkins JW, Field FP, Ress RJ. Age-related decline in adrenergic responsiveness of the kidney, heart and aorta of male rats. Neurobiol Aging 4: 233–238, 1983. [DOI] [PubMed] [Google Scholar]
- 38.Stocker SD, Sved AF, Stricker EM. Role of renin-angiotensin system in hypotension-evoked thirst: studies with hydralazine. Am J Physiol Regul Integr Comp Physiol 279: R576–R585, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Thunhorst RL, Johnson AK. Effects of arterial pressure on drinking and urinary responses to intracerebroventricular angiotensin II. Am J Physiol Regul Integr Comp Physiol 264: R211–R217, 1993. [DOI] [PubMed] [Google Scholar]
- 40.Thunhorst RL, Johnson AK. Thirst and salt appetite responses in young and old Brown Norway rats. Am J Physiol Regul Integr Comp Physiol 284: R317–R327, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Whyte DG, Thunhorst RL, Johnson AK. Reduced thirst in old, thermally dehydrated rats. Physiol Behav 81: 569–576, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Wolf AV Thirst: Physiology of the Urge to Drink and Problems of Water Lack. Springfield, IL: Thomas, 1958.