Abstract
The lactating mammary gland is composed of multiple cell types that tightly coordinate the accumulation, production, and secretion of milk components, including essential metals such as zinc (Zn). Our previous studies in animal and cell models implicated the Zn transporter Zip3 (Slc39a3) in mammary gland Zn acquisition. Herein, we investigated this hypothesis directly by utilizing Zip3-null mice. Our data verify that Zip3 is expressed in secretory mammary cells; however, Zip3 does not play a major role in Zn import from the maternal circulation. Importantly, the primary localization of Zip3 was associated with the luminal membrane of the secretory mammary cells. Consistent with this localization, Zn transfer studies using 65Zn revealed that Zn retention in the secreted milk pool and milk Zn concentration was higher in Zip3-null compared with wild-type mice. Although total mammary gland Zn concentration was not altered, Zip3-null mice also had altered mammary tissue architecture, increased number of apoptotic cells, and reduced mammary gland weight implicating subtle changes in Zip3-mediated intracellular Zn pools in apoptosis regulation. Taken together, our data indicate that Zip3 does not participate in the acquisition of Zn from maternal circulation for secretion into milk but, in contrast, primarily plays a role in the reuptake and cellular retention of Zn in the mammary gland from the previously secreted milk pool, thus regulating cellular function.
Keywords: zinc, lactation, zinc transporter
zinc (Zn) is an essential metal due to its role in numerous biological processes where it participates as a structural or catalytic component in over 300 proteins. Maintaining cellular Zn homeostasis is of critical importance and is achieved through the regulation of mechanisms that modulate Zn import, export, and intracellular compartmentalization. This is achieved through the activities of members of two gene families: Slc30a (ZnT proteins) and Slc39a (Zip proteins). To date, 10 members of the ZnT family have been identified which efflux Zn from the cytoplasm, either across the cell membrane or into intracellular vesicles (20). Fourteen Zip genes have been described (6). In contrast to ZnT function, Zip proteins facilitate Zn import into the cytoplasm. In general, both Zip and ZnT proteins are expressed with tissue-specificity and display unique subcellular localization. Additionally, they often differ in metal specificity, Zn affinity, and regulation (8, 27, 28). These differences necessitate exploration of the role and regulation of Zn transporters in diverse physiological and cellular systems, which will provide important clues as to the function that specific Zn transporters play in biology.
Zip3 is a Zn importer whose expression, as determined by Northern blot analysis, is restricted (5). Recent studies of Zip3-null mice with an EGPF knockin reporter revealed that Zip3 is highly expressed in a cell-type-specific manner in ovaries, testes, and the developing nervous system with limited expression in the small intestinal crypt cells, kidney glomerulus, and discrete regions of the liver (3). Recent studies of Zip3-null mice demonstrated that this gene is not essential but instead plays an important role when dietary Zn becomes limiting (3). Examination of Zip3 subcellular localization revealed that Zip3 is localized to intracellular vesicles under basal culture conditions in HEK 293 and HC11 cells (13, 27). However, Zip3 has been shown to traffic to the plasma membrane in response to rigorous Zn limitation to import Zn from the extracellular space in transfected HEK 293 cells (27), implicating posttranslational regulation of localization as a primary mode of control consistent with acute responsiveness to zinc-limiting conditions.
Zip3 is also expressed in highly specialized, hormonally-responsive secretory tissues, such as the pancreas (3), prostate (1), and mammary gland (13, 14). The lactating mammary gland is a highly specialized secretory tissue that tightly coordinates the accumulation, production, and secretion of milk components, including essential trace minerals such as Zn (19). During lactation, a substantial amount of Zn must be taken up from maternal circulation and transported through the mammary gland and secreted into milk (0.5–1 mg Zn/day) (15). Our previous studies suggested that Zip3 plays a role in this process by mediating Zn uptake in mammary cells in a hormone-dependent manner through the posttranslational relocalization of Zip3 from an intracellular compartment to the cell membrane (13). Curiously, however, we observed no demonstrable phenotype in offspring suckled from Zip3-null mice (3), suggesting that Zip3 may not play a primary role in mediating Zn transfer from maternal circulation into the mammary gland providing Zn for transfer into milk during lactation. To directly explore the role of Zip3 in mammary gland Zn metabolism, we examined effects of a Zip3-null phenotype on mammary gland Zn transport in mice. Herein, we report that Zip3 is primarily localized proximal to the apical (luminal) membrane in the mammary gland of the lactating mouse. Importantly, our data indicate that Zip3 facilitates the reuptake and thus cellular retention of Zn from the secreted milk pool. Taken together, our data suggest that Zn imported via Zip3 may play a role in regulating apoptosis in mammary epithelial cells and demonstrate that Zip3 is not the primary Zn importer responsible for Zn acquisition from maternal circulation into the mammary gland during lactation.
MATERIAL AND METHODS
Generation of Zip3 knockout mice.
Mice with a targeted disruption of the ZIP3 gene were generated by fusing the initiating methionine codon of Zip3 with the open reading frame of the enhanced green fluorescent protein (EGFP) reporter, while deleting the remainder of the ZIP3 open reading frame as previously described (3). Homozygous knockout mice were generated from heterozygous matings and wild-type mice were backcrossed over several generations to obtain wild-type controls for comparison.
PCR-based genotyping.
Genotype was verified by PCR-based genotyping. Genomic DNA (5 μg) was isolated from tail snips using the REDExtract-N-Amp Tissue PCR kit (Sigma-Aldrich). Genomic DNA was amplified by PCR with primers that distinguished Zip3-null from wild-type alleles as previously described (3). Amplification of the Zip3-null allele produced a 326-base pair product, while amplification of the wild-type allele produced a 461 base pair product, permitting verification of genotype.
Animal care.
This study was approved by Animal Research Services at the University of California Davis, CA, which is accredited by the American Association for the Accreditation of Laboratory Animal Care. Homozygous Zip3-null and wild-type mice were generated at the University of Kansas Medical Center as previously described (3) and transferred to the University of California Davis. Mice were fed commercially available rodent chow ad libitum. Mice were bred and litters maintained at 4 pups/dam. Experiments were conducted during early lactation days (LD 4–7) and late lactation days (LD 14–17), as our previous studies indicated that Zip3 expression in the mammary gland declines throughout lactation (14). In experiment 1, basal and stimulated localization of Zip3 in mammary gland from wild-type mice during early lactation was determined by immunostaining. To evaluate basal Zip3 localization, dams (n = 3/group) were removed from pups for 4 h and then killed by decapitation. The mammary gland was dissected and fixed for immunostaining as described below. Alternatively, to evaluate stimulated Zip3 localization, dams (n = 3/group) were removed from pups for 4 h, anesthetized and given a subcutaneous injection of oxytocin to stimulate luminal evacuation of mammary gland alveoli as previously described (12), and the milk was then manually expressed. Dams were killed by decapitation, and the mammary gland was dissected and fixed for immunostaining as described below. In experiment 2, effects of a Zip3-null phenotype on serum, milk, mammary gland, and liver Zn concentration was assessed. Dams (n = 6/group) were removed from pups for 4 h and milk was collected as previously described (12). Dams (n = 5–8/group) were euthanized and the entire left inguinal mammary gland was dissected and weighed. Serum was separated from blood collected via cardiac puncture, and liver was collected from both dams and pups. In experiment 3, effects of a Zip3-null phenotype on mammary gland Zn transfer was assessed. Dams were removed from their pups for 6 h. Pups were returned to dams and allowed to nurse for 30 min to eliminate pooled milk from the mammary gland alveoli. Dams (n = 6/group) were removed from pups, anesthetized as previously described (12), and given an intraperitoneal injection of 0.9% saline containing 25 nmol Zn (as ZnCl2) and 1 μCi 65Zn (Oakridge National Laboratory, Oak Ridge, TN). After 1 h, milk was collected for 10 min following oxytocin injection as previously described (12), and total milk volume was recorded. Each entire mammary gland was dissected and weighed, and the radioactivity in the milk and mammary glands was measured in a gamma scintillation counter. In experiment 4, suckling offspring (n = 6 pups/group) were euthanized, blood was collected by cardiac puncture, and the liver was removed. Plasma and liver Zn concentration was measured by flame atomic absorption spectroscopy.
Zn analysis.
Serum, tissue, and milk Zn concentration was analyzed by flame atomic absorption spectroscopy (model Smith-Heifjie 4000; Thermo Jarrell Ash, Franklin, MA) as previously described (11).
Fluorescent imaging and immunohistochemical staining of mammary gland.
Mammary gland from wild-type and Zip3-null mice was fixed in 4% paraformaldehyde in PBS overnight and then serially dehydrated in ethanol. Tissue was embedded in paraffin, sectioned (4 μm), and mounted on positively charged slides. We capitalized on the fusion of the initiating methionine to EGFP in the Zip3-null mice to explore the expression pattern of Zip3 in the mammary gland, and mammary gland fluorescence was examined. Immunohistochemical staining was performed as described previously (12). Briefly, sections were blocked in 3% H2O2 in MeOH to eliminate endogenous peroxidase activity and permeabilized with trypsin. Nonspecific staining was reduced by sequential incubation with 10% goat serum, followed by avidin and then biotin per manufacturer's instructions (Vector Laboratories, Burlingame, CA). The sections were incubated overnight at 4°C with affinity-purified Zip3 antibody, which has been previously characterized (13), diluted (1:1,000) in PBS containing goat serum (10%), and detected with biotinylated goat anti-rabbit IgG secondary antibody. The antigen-antibody complex was visualized with an avidin-horseradish peroxidase staining kit (ABC Kit; Vector Labs) in the presence of 3–3′-diaminobenzidine and H2O2. Sections were counterstained with toluene blue.
Immunoblotting.
Crude membrane proteins were isolated from mammary gland by centrifugation of the postnuclear supernatant at 100,000 g for 30 min. Proteins (50–100 μg) were resuspended in sample buffer containing DTT (100 mmol/l) and separated by electrophoresis. Following transfer to nitrocellulose for 60 min at 350 mA, Zip3 was detected by immunoblotting using Zip3 antibody as previously described (13). Equal sample loading was verified by immunoblotting for β-actin. Primary antibodies were detected with horseradish peroxidase-conjugated IgG, and proteins were visualized by enhanced chemiluminescence (SuperSignal Femto; Pierce) followed by exposure to autoradiography film.
Cell death.
The presence of apoptotic cells in the mammary gland was assessed in situ using the TACS-XL Basic In Situ Apoptosis (TUNEL) Detection kit (Trevigen, Gaithersburg, MD) per the manufacturer's instructions.
Statistical analysis.
Results are presented as means ± SD, n = 5–8 dams or 6 pups/lactation day (as noted). Statistical comparisons were made using Student's t-test. Significance was demonstrated at P < 0.05.
RESULTS
Verification of Zip3-null genotype.
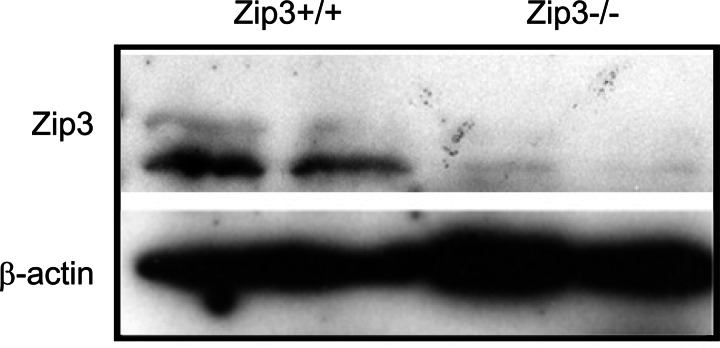
The generation and characterization of Zip3-null mice has been previously described (3). We verified the genotypes of Zip3-null and wild-type mice used in the current studies by PCR-based genotyping (3) (data not shown). Targeted disruption of the Zip3 gene was further verified by immunoblot of membrane proteins isolated from mammary glands taken from Zip3-null and wild-type mice (Fig. 1).
Fig. 1.
Expression of Zip3 protein in the mammary gland is eliminated in Zip3-null mice. Representative immunoblot of crude membrane protein extracts (100 μg) isolated from the mammary glands of wild-type (Zip3+/+) and Zip3-null (Zip3−/−) mice (n = 2 mice/genotype). β-actin was immunoblotted to verify equal protein loading.
Zip3 gene expression in the mammary gland is restricted to epithelial cells, and this protein localizes proximal to the luminal (apical) membrane.
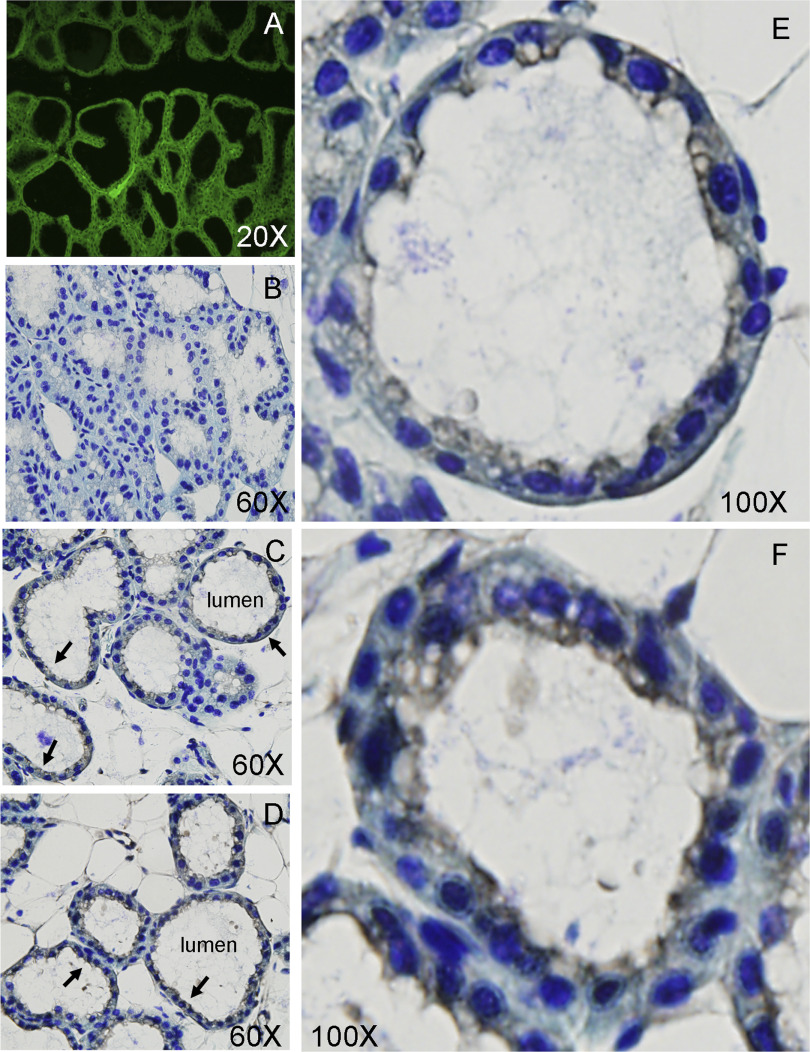
Fusion of the initiating methionine of Zip3 to EGFP permitted the visualization of the expression pattern of Zip3 in the mammary gland (3). While the mammary gland is comprised of numerous cell types, including epithelial, myoepithelial, and endothelial cells, adipocytes, and stromal cells, Zip3 expression was restricted to the secretory epithelial cells that line the lumen of the alveoli (Fig. 2A). Given our previous observation that Zip3 resides in intracellular vesicles under basal culture conditions in vitro, we aimed to determine the localization of Zip3 under basal physiological conditions, in vivo. No staining of Zip3 was detected in Zip3-null mice (Fig. 2B). Immunostaining of Zip3 in mammary gland from lactating mice indicated that most Zip3 protein in secretory epithelial cells was localized intracellularly under basal conditions (Fig. 2, C and E). Interestingly, Zip3 was largely detected proximal to the luminal (apical) membrane, while little Zip3 could be detected at the serosal membrane. This staining pattern suggests that Zip3 primarily facilitates the import of Zn from the secreted milk pool, and perhaps from maternal circulation to a much lesser extent. Our previous studies in cultured mammary cells in vitro indicated that Zip3 protein was also localized intracellularly but could be transiently relocalized to the plasma membrane in response to secretory hormones, i.e., prolactin and oxytocin (13). To determine whether physiological stimulation could relocalize Zip3 in vivo, lactating mice were injected with oxytocin and gently milked. As illustrated in Fig. 2, D and F, images of Zip3 in stimulated mammary gland suggests that stimulation results in the relocalization of Zip3 to the plasma membrane in vivo as well.
Fig. 2.
Zip3 gene expression is restricted to mammary epithelial cells, and Zip3 protein localizes proximal to the luminal (apical) membrane. A: detection of Zip3 expression in the epithelial cells of the mammary gland in Zip3-null mice by EGFP fluorescence. B: lack of immunohistological detection of Zip3 in the mammary gland of Zip3-null mice. C and E: wild-type mice under nonstimulated conditions. D and F: wild-type mice stimulated by oxytocin injection and gentle milking. Arrows illustrate Zip3 staining.
Zip3 mediates mammary gland Zn reuptake in vivo.
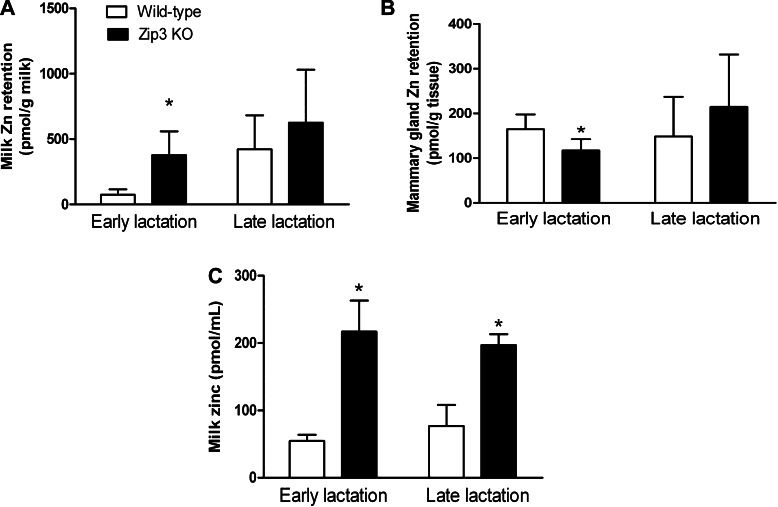
Given the observation that Zip3 localizes proximal to the luminal membrane, we examined the physiological consequence of eliminating its expression with the aim of determining whether a Zip3-null phenotype would significantly affect Zn transport in the mammary gland. We first determined effects on mammary gland Zn transfer from maternal circulation into the mammary gland and subsequently into milk. Recently, suckled dams (30 min postsuckling) were injected with 65Zn, and a complete milk collection was obtained 1 h after 65Zn injection. As illustrated in Fig. 3A, the amount of Zn retained in the secreted milk pool following transfer from maternal circulation in Zip3-null mice was approximately five-fold higher compared with wild-type mice during early lactation. While Zip3-null mice had higher Zn retained in the secreted milk pool than wild-type mice during late lactation, this difference did not reach statistical significance. Conversely, the amount of Zn retained in the mammary gland in Zip3-null mice was ∼40% lower, compared with wild-type mice during early lactation (Fig. 3B). The difference did not reach statistical significance during late lactation. Taken together, our data suggest that Zn transported from maternal circulation into the mammary gland during lactation is directed for secretion into milk after which it is recaptured from the secreted milk pool at least in part due to the activity of Zip3. Consistent with this concept, the Zn concentration of milk collected from Zip3-null mice was significantly higher compared with wild-type mice during both early (∼5-fold) and late (∼2-fold) lactation (Fig. 3C). Together, these observations clearly reflect the primary localization of Zip3 proximal to the luminal membrane.
Fig. 3.
Zinc retention is higher in the milk of Zip3-null mice. Wild-type and Zip3-null mice [Zip3 KO (knockout)] were removed from their pups for 6 h and were then returned and allowed to nurse for 30 min to eliminate pooled milk from the mammary gland alveoli. Anesthetized dams were given an injection of 0.9% saline ip containing 25 nmol Zn (as ZnCl2) and 1 μCi 65Zn, and milk was then collected 1 h later (10 min collection). The entire mammary glands were dissected and weighed, and the radioactivity in the milk (A) and mammary glands (B) was measured in a gamma scintillation counter. Data represent mean ± SD Zn retained/g (n = 5 or 6 dams/group). *Significant effect of genotype, P < 0.05. Milk was gently collected from wild-type and Zip3-null mice after oxytocin injection, and the Zn concentration was measured by atomic absorption spectroscopy (C). Data represent mean ± SD Zn concentration, n = 6 dams/group. *Significant effect of genotype, P < 0.05.
The higher milk Zn concentration in Zip3-null mice did not appear to be a consequence of greater Zn availability from maternal circulation or greater Zn concentration in the mammary gland itself (Table 1). In fact, Zip3-null dams were marginally Zn-deficient by late lactation. Despite the fact that maternal liver (P = 0.09) and serum (P = 0.07) Zn concentration in the Zip3-null mice during late lactation was lower, mammary gland Zn concentration was maintained, and milk Zn concentration remained significantly higher than in wild-type dams. These results clearly demonstrate that the mammary gland can tightly regulate Zn uptake from the maternal circulation, despite a reduction in serum Zn concentration.
Table 1.
Serum and tissue Zn concentrations in lactating Zip3-null and wild-type dams and their offspring
| Genotype |
||
|---|---|---|
| Wild-Type | Zip3-null | |
| Dams | ||
| Serum, μg Zn/mL | ||
| Early | 0.46±0.22 | 0.55±0.12 |
| Late | 1.01±0.46 | 0.58±0.25 (P = 0.07) |
| Liver, μg Zn/g | ||
| Early | 5.56±2.29 | 5.34±1.11 |
| Late | 6.64±0.79 | 5.98±0.65 (P = 0.09) |
| Mammary gland, μg Zn/g | ||
| Early | 9.35±3.94 | 11.94±1.3 |
| Late | 11.95±0.5 | 12.4±0.72 |
| Offspring | ||
| Serum, μg Zn/mL | ||
| Early | 0.44±0.18 | 0.51±0.11 |
| Late | 0.85±0.29 | 0.70±0.29 |
| Liver, μg Zn/g | ||
| Early | 30.86±2.46 | 27.79±2.05* |
| Late | 30.36±3.04 | 27.11±3.18 |
Data represent means ± SD; n = 6–8 dams or 6 pups/day.
Significant effect of genotype; P < 0.05.
A Zip3-null phenotype results in impaired Zn status of offspring.
We next explored effects of increased Zn concentration in the milk of Zip3-null dams on the Zn status of the suckling offspring. We observed no significant effect of a Zip3-null phenotype on serum Zn concentration during the suckling period. However, the liver Zn content of Zip3-null pups was significantly lower compared with wild-type pups during early lactation (Table 1).
A Zip3-null phenotype decreases milk volume and disrupts mammary gland morphology.
The observation that liver Zn concentration in the Zip3-null pups was lower despite the fact they suckled milk containing approximately two- to five-fold greater Zn concentrations was surprising. An interesting observation that we made was that we collected significantly less milk from Zip3-null compared with wild-type mice during early lactation (Table 2, P < 0.01). On the basis of this, we estimated that Zn intake of offspring from Zip3-null dams (when normalized to the total volume collected in 10 min) may have actually been ∼2.5-fold less (mean = 0.13 μg total Zn available to offspring) compared with wild-type offspring (mean = 0.30 μg total Zn available to offspring). We speculate that this helps to explain the lower Zn concentration detected in the liver of Zip3-null offspring. Curiously, no difference in milk volume between Zip3-null and wild-type dams was observed during late lactation, which potentially resulted in a mean total Zn intake of 0.18 μg Zn and 0.43 μg Zn in wild-type and Zip3-null offspring, respectively.
Table 2.
Mammary gland weight and milk volume in Zip3-null and wild-type dams
| Genotype |
||
|---|---|---|
| Wild-Type | Zip3-null | |
| Mammary gland, g | ||
| Early | 2.52±0.35 | 2.24±0.54 |
| Late | 2.90±0.77 | 1.94±0.57* |
| Milk, μl | ||
| Early | 85±28 | 9±12* |
| Late | 34±37 | 36±65 |
Data represent means ± SD (n = 6/d).
Significant effect of genotype; P < 0.01.
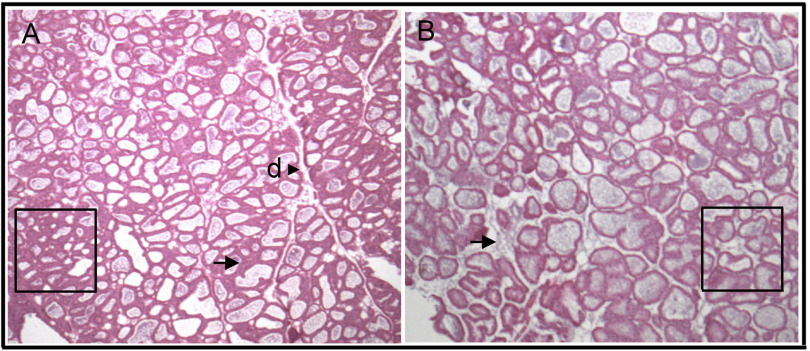
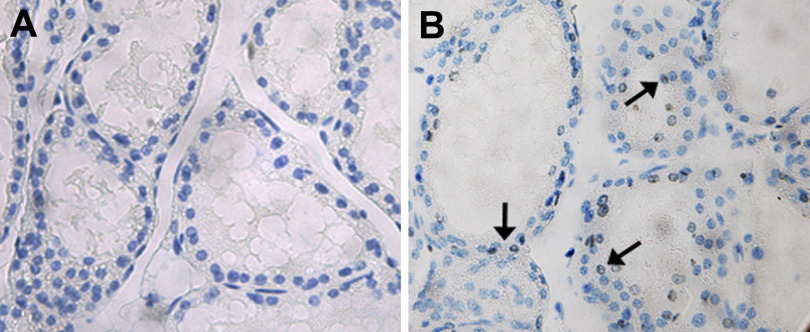
These results caused us to wonder why milk volume was compromised during early lactation. Total mammary gland weight did not differ between Zip3-null and wild-type mice during early lactation (Table 2). Interestingly, analysis of the gross mammary gland morphology revealed that mammary glands from Zip3-null mice had less dense tissue structure and loose alveolar organization compared with the mammary glands from wild-type mice (Fig. 4). To determine whether the loss of tissue integrity was due to cell death we assessed DNA fragmentation as an indicator of apoptosis utilizing an immunohistochemical TUNEL assay. As illustrated in Fig. 5, we detected fragmented DNA in the mammary gland of Zip3-null mice during early lactation, which was subsequently associated with a ∼35% reduction mammary gland weight in Zip3-null mice compared with wild-type mice by late lactation (Table 2, P < 0.05). Together, our data suggest that one function of Zn reuptake from the secreted milk pool via Zip3 is to regulate mammary cell apoptosis.
Fig. 4.
Overall mammary gland morphology is compromised in Zip3-null mice. Representative images of mammary gland from wild-type (A) and Zip3-null (B) mice during early lactation stained with hematoxylin and eosin are shown. Magnification, ×20. Four points to note are the greater overall density (boxes), structured morphometric organization, and the well-defined ductal branching (d) observed in mammary gland from wild-type mice. In contrast, note the distinct and loose association of alveoli structures (arrows) in mammary gland from Zip3-null mice.
Fig. 5.
Zip3-null mice have enhanced apoptosis in the mammary gland. Representative images of cells containing fragmented DNA (hallmark of apoptosis) in mammary gland from wild-type (A) and Zip3-null (B) mice during early lactation are shown. Fragmented DNA was detected by TUNEL staining and tissue sections were counterstained with toluene blue; magnification, ×40. Note the complete lack of TUNEL staining in the mammary gland from wild-type mice and the detection of apoptotic nuclei (arrows) in the mammary gland form the Zip3-null mice.
DISCUSSION
Expression of the mouse Zip3 gene is cell-type specific and appears to be particularly active in some highly specialized secretory tissues, such as the prostate and mammary gland. This likely reflects the fact that these tissues are morphologically and functionally quite similar in that they consist of multiple cell types that support the development of alveolar structures that function to produce, sequester and secrete Zn-rich biological fluids, such as seminal fluid and milk, respectively. It is therefore important to explore the role of Zip3 in these complex, secretory tissues. We previously detected Zip3 expression in cultured mammary epithelial cells (13, 14), which is the cell-type that lines the alveolar lumen and is responsible for the uptake, production, and secretion of milk components. In this study, we capitalized on our fusion of EGFP immediately downstream of the initiating methionine of Zip3 and demonstrated that in mouse mammary gland, Zip3 distribution is exclusively restricted to the mammary epithelial cell, suggesting that Zip3 plays a specific role in this unique cell type. Our previous study in lactating rats detected Zip3 at or proximal to the serosal membrane in mammary epithelial cells during early lactation after which we were unable to detect expression during late lactation (14). This decline in Zip3 expression was associated with the decline in milk Zn concentration that occurs during lactation (14). As a result, we postulated that one role for Zip3 in the mammary epithelial cell was to import Zn from maternal circulation. Results from our current study further these observations and, importantly, provide direct functional evidence to the contrary. Similar to our previous results in lactating rats, we were able to detect a minimal amount of Zip3 associated with the serosal membrane during early lactation in mice. Thus, we reasoned that if Zip3 plays a major role in Zn uptake from maternal circulation, Zip3-null mice injected with 65Zn would have less Zn taken up into the mammary gland and less Zn transported into milk. Surprisingly, while Zip3-null mice had less 65Zn retained in the mammary gland, they also had greater 65Zn retention in the milk pool and greater total milk Zn concentration compared with wild-type mice. This directly reflects the fact that the majority of Zip3 was located proximal to the luminal (or apical) membrane and likely traffics to the luminal membrane in response to cellular cues in vivo as well as in vitro (13). Taken together, our data provide functional evidence that Zip3 plays a role in the reuptake of Zn from the secreted milk pool, similar to what has been postulated for the function of Zip3 in prostate (1). It is interesting to note that initial studies of Zip3-null mice lead to the conclusion that this protein plays a key physiological role in the homeostasis of Zn when this essential metal becomes limiting, consistent with a role in the reuptake of this metal.
Importantly, association of endogenous Zip3 with the apical membrane and thus lack of Zn reuptake from the luminal milk pool in Zip3-null mice likely explains the lack of overt Zn-deficient phenotype in the offspring suckled from Zip3-null mice that we previously observed (3). In this study, we directly measured Zn status of the offspring and noted that plasma Zn concentration was not significantly affected. However, liver Zn concentration was significantly lower in the offspring of Zip3-null mice by postnatal day 4. Whether this reflects reduced placental Zn transfer during pregnancy or effects of decreased Zn intake due to lower milk intake during early lactation is not understood at this time. Our previous studies do indicate that a Zip3-null phenotype exacerbates effects of Zn deficiency during pregnancy (2, 3), but to our knowledge, there is no evidence that Zip3 is actively expressed in placenta. Alternatively, reduced delivery of Zn from the maternal circulation to the fetus during pregnancy may play a role, as results from this study showed that while plasma Zn concentration normally increases following parturition, this physiological readjustment was absent in Zip3-null dams. This suggests that marginal Zn deficiency may underlie the Zip3-null phenotype, which becomes apparent and exacerbated by restricting Zn intake (2, 3). The lack of a robust Zn-deficient phenotype in Zip3-null mice has been postulated to reflect redundancy of Zn import protein function; however, the contribution of specific Zn transporting mechanism(s) responsible for redundancy is likely quite complex and has not been elucidated. Our data suggest that functional redundancy may not be as likely as has been postulated. Our recent studies of mice with targeted deletions of Zips1, -2, and -3, all of the three closely related Slc39a members of subfamily II, suggest that these proteins do not exert compensatory effects, but instead have cell-type-specific functions, especially when Zn is deficient (10).
The tissue and intracellular distribution pattern of Zip3 suggest that it functions, at least in part, in Zn redistribution and/or retention (3), which is supported by our current data. This begs the question of the functional role of Zn reuptake via Zip3 from the secreted milk (or, in the case of prostate, the prostatic fluid) pool. Specific Zip proteins have recently been associated with distinct cellular functions. For example, alterations in intracellular Zn pools have been associated with changes in cell signaling pathways such that attenuation of the Golgi Zn exporter Zip7 reduces activation of epithelial growth factor receptor/IGF-I receptor/Src signaling (26). Additionally, Zip6 expression is positively associated with E-cadherin expression and has been reported to play a role in modulating cell interactions (21) (V. Lopez and S. L. Kelleher, unpublished observations). Several Zip proteins are associated with modulating cell proliferation or differentiation mechanisms, and Zn has long been known to play a regulatory role in differentiation, cell proliferation, and apoptosis. For example, overexpression of Zip1 in human mesenchymal stem cells resulted in mineralization and increased expression of osteoblast-associated markers, suggesting a role for Zip1-mediated Zn import in osteoblast differentiation (23). Both excess Zn and Zn chelation have been shown to induce apoptosis and inhibit cell proliferation. In fact, reduced expression of Zip1, -2, and -3 is associated with prostate cancer (1, 7), suggesting reduced cellular Zn levels interfere with apoptogenesis resulting in a more proliferative, cancerous cell type. The mammary and prostate glands are similar in morphology and function. Similar to the prostate, the lactating mammary gland also accumulates, sequesters, and secretes a large amount of Zn. However, in contrast to observations in prostate tissue, breast cancer is associated with Zn accumulation and overexpression of Zip proteins in tumor cells (25), suggesting that Zn accumulation inhibits apoptosis and stimulates cell proliferation in mammary cells. In fact, we previously determined that Zip3 attenuation resulted in decreased mammary epithelial cell viability (13), preliminarily illustrating a modulatory role of Zip3-mediated Zn import on cell proliferation and/or apoptotic mechanisms in mammary cells. These in vitro data directly corroborate an interesting observation gleaned from this in vivo study in that we observed reduced mammary gland weight and alterations in tissue architecture in the Zip3-null mice. This might have resulted from reduced cell proliferation and/or increased apoptosis as there was an increase (+0.7 g) in mammary gland weight through lactation in wild-type mice (from 2.2 to 2.9 g), while there was a decrease (−0.6 g) in mammary tissue expansion observed in Zip3-null mice (from 2.5 to 1.9 g). Our data in Zip3-null mice further support our observations in cultured mammary cells implicating a role for Zip3 in regulating apoptosis. While the direct mechanisms responsible for the effects of Zip3-mediated Zn import on apoptosis require further investigation, the physiological implications are interesting. The elevated level of Zip3 expression that occurs in early lactation may function to increase the apoptotic threshold maintaining the highly differentiated secretory phenotype of mammary epithelial cells during lactation. Moreover, the decline in Zip3 expression that occurs as lactation continues may serve to activate apoptogenic mechanisms assisting in the apoptotic clearing of the mammary gland that occurs during involution as offspring are weaned.
An important observation was that morphological alterations in the mammary gland and physiological manifestations, including decreased availability of Zn from maternal circulation and decreased reuptake from the milk Zn pool, occurred without an effect on the total Zn concentration in the mammary gland. One would anticipate that mammary gland Zn concentration would have been compromised; however, this was not observed. This suggests that either compensatory responses in Zn transport mechanisms were activated to maintain total mammary gland Zn levels or that the contribution of Zip3 to the total Zn pool in the mammary gland is quite limited in scope. The former speculation may reflect the partial functional redundancy of Zn importers, the regulation of Zn exporters, or the sequestration of Zn in metallothionein or vesicles. To date, 14 Zn importers have been identified (6) and, to our knowledge, expression of all Zip proteins (with the exception of Zip4, -9, and -11) has been documented in mammary tissue or cultured mammary cells (13, 18, 22, 24–26); thus, functional redundancy is a likely possibility. Our preliminary data suggest that changes in Zip1 expression do not appear to be responsible (S. L. Kelleher, unpublished observations). However, two possible candidates for compensatory Zn acquisition are Zip5 and Zip8, as we have previously localized Zip5 to the serosal membrane of small intestine enterocytes (4) and both Zip8 and Zip5 appear to be associated with the plasma membrane in the mammary gland (S. L. Kelleher, unpublished observations). Alternatively, elimination of Zip3 and decreased Zn reuptake from the secreted milk pool could have reduced Zn exporting mechanisms through alterations in expression of ZnT proteins. Thus far, out of 10 ZnT proteins that have been identified, to our knowledge only expression of ZnT1, -2 and -4 has been detected in mammary tissue (14, 16, 17). Of these three proteins, it seems likely that conservation of tissue Zn levels might occur through decreased expression of the cell membrane-associated Zn exporter ZnT1, as ZnT2 transports Zn into secretory vesicles (V. Lopez and S. L. Kelleher, unpublished observations), and ZnT4 likely transports Zn into the Golgi (9, 17). Alternatively, as total tissue Zn concentration was not affected, our data indicate that subtle changes in specific intracellular Zn pools that are modulated by Zip3 may be responsible for the effects we observed on tissue architecture and cell function. One interesting question that remains is to define the specific intracellular Zn pool(s) in the mammary epithelial cell that is affected by Zip3 elimination; studies are underway to determine what specific cellular functions are regulated by Zip3-modulated Zn pools.
In conclusion, our results demonstrate a role for Zip3 in mammary gland Zn biology and implicate Zn reuptake from the secreted milk pool in regulating specific cellular functions, such as apoptogenesis. The fact that mammary gland function did not appear to be significantly impaired may speak to the plasticity of this unique secretory tissue. Importantly, the primary localization of Zip3 to the luminal membrane of the mammary epithelial cell in combination with direct functional evidence that Zn reuptake from the secreted milk pool is reduced and results in increased milk Zn concentration, provides an explanation as to why we previously observed a limited phenotype in Zip3-null offspring (2, 3), and provides direct evidence that Zip3 does not play a major role in Zn acquisition from maternal circulation. However, expansion of our initial study indicates that Zn status was clearly, yet subtly compromised, illustrating the importance of characterizing the role of Zip3 in cellular and physiological functions essential in Zn redistribution and retention.
Perspectives and Significance
Our previous studies indicate that Zip3 plays a role in Zn uptake into mammary cells (13) but, curiously, no demonstrable phenotype is observed in offspring suckled from Zip3-null mice unless maintained on a Zn-deficient diet (3). This contradiction led us to explore the function of Zip3 in mammary gland Zn uptake directly in Zip3-null mice as dogma suggests that this reflects the redundancy of Zn importing protein function. Our data provide evidence to the contrary, and indicate that the intracellular and luminal membrane orientation of Zip3 and not compensatory-increased Zn uptake facilitated by another Zn importer, directly explains the lack of robust phenotype in Zip3-null mice. Importantly, our data assist in elucidating the complex integration and function of Zn transporting mechanisms in specific tissues and provide some interesting clues as to the role of this Zn transporter in biology. This study highlights the fact that profound changes in tissue morphology can occur despite the tight regulation of total tissue Zn content and provides important evidence that subtle changes in specific intracellular Zn pools play a critical role in regulating cellular function.
GRANTS
This work was supported by intramural grants (to S. L. Kelleher and B. Lönnerdal) and National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-59369 (to G. K. Andrews).
Acknowledgments
We thank Xiaogu Du and Jose Zambra for excellent technical assistance in the Lönnerdal lab, Young Ah Seo and Vanessa Velasquez for excellent technical assistance in the Kelleher lab, and Jim Geiser for excellent technical assistance in the Andrews lab.
Present address: J. Dufner-Beattie, Apath LLC, St. Louis, MO.
REFERENCES
- 1.Desouki M, Geradts J, Milon B, Franklin RB, Costello LC. hZip2 and hZip3 zinc transporters are down regulated in human prostate adenocarcinomatous glands. Mol Cancer 6: 37–43, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dufner-Beattie J, Huang Z, Geiser J, Xu W, Andrews GK. Mouse ZIP1 and ZIP3 genes together are essential for adaptation to dietary zinc deficiency during pregnancy. Genesis 44: 239–251, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Dufner-Beattie J, Huang ZL, Geiser J, Xu W, Andrews GK. Generation and characterization of mice lacking the zinc uptake transporter ZIP3. Mol Cell Biol 25: 5607–5615, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dufner-Beattie J, Kuo YM, Gitschier J, Andrews GK. The adaptive response to dietary zinc in mice involves the differential cellular localization and zinc-regulation of the zinc transporters ZIP4 and ZIP5. J Biol Chem 279: 49082–49090, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Dufner-Beattie J, Langmade SJ, Wang F, Eide D, Andrews GK. Structure, function and regulation of a subfamily of mouse zinc transporter genes. J Biol Chem 278: 50142–50150, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Eide DJ The SLC39 family of metal ion transporters. In: The ABC of Solute Carriers, edited by Hediger MA. New York: Springer-Verlag, 2003.
- 7.Franklin RB, Feng P, Milon B, Desouki MM, Singh KK, Kajdacsy-Balla A, Bagasra O, Costello L. hZIP1 zinc uptake transporter down regulation and zinc depletion in prostate cancer. Mol Cancer 4: 32–37, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He L, Girijashanker K, Dalton TP, Reed J, Li H, Soleimani M, Nebert DW. ZIP8, member of the solute-carrier-39 (SLC39) metal-transporter family: Characterization of transporter properties. Mol Pharmacol 70: 171–180, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Ho LH, Ruffin RE, Murgia C, Li L, Krilis SA, Zalewski PD. Labile zinc and zinc transporter ZnT4 in mast cell granules: Role in regulation of caspase activation and NF-κB translocation. J Immunol 172: 7750–7760, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Kambe T, Geiser J, Lahner B, Salt DE, Andrews GK. Slc39a1 to 3 (subfamily II) Zip genes in mice have unique cell-specific functions during adaptation to zinc deficiency. Am J Physiol Regul Integr Comp Physiol 294: R1474–R1481, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelleher SL, Lönnerdal B. Marginal maternal Zn intake in rats alters mammary gland Cu transporter levels and milk Cu concentration and affects neonatal Cu metabolism. J Nutr 133: 2141–2148, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Kelleher SL, Lönnerdal B. Zinc transporters in the mammary gland respond to marginal zinc and vitamin A intake during lactation in rats. J Nutr 132: 3280–3285, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Kelleher SL, Lönnerdal B. Zip3 plays a major role in zinc uptake into mammary epithelial cells and is regulated by prolactin. Am J Physiol Cell Physiol 288: C1042–C1047, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Kelleher SL, Lönnerdal B. Zn transporter levels and localization change throughout lactation in rat mammary gland and are regulated by Zn in mammary cells. J Nutr 133: 3378–3385, 2003. [DOI] [PubMed] [Google Scholar]
- 15.King JC Enhanced zinc utilization during lactation may reduce maternal and infant zinc depletion. Am J Clin Nutr 75: 2–3, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Michalczyk A, Varigos G, Catto-Smith A, Blomeley RC, Ackland ML. Analysis of zinc transporter, hZnT4 (SIc30A4), gene expression in a mammary gland disorder leading to reduced zinc secretion into milk. Hum Genet 113: 202–210, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Michalczyk AA, Allen J, Blomeley RC, Ackland ML. Constitutive expression of hZnT4 zinc transporter in human breast epithelial cells. Biochem J 364: 105–113, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kagara N, Tanaka N, Noguchi S, Hirano T. Zinc and its transporter ZIP10 are involved in invasive behavior of breast cancer cells. Cancer Sci 98: 692–697, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neville MC Physiology of lactation. Clin Aspects Hum Milk Lactation 26: 251–279, 1999. [PubMed] [Google Scholar]
- 20.Palmiter RD, Huang L. Efflux and compartmentalizatin of zinc by members of the SLC30 family of solute carriers. In: The ABC of Solute Carriers, edited by Hediger MA. New York: Springer-Verlag, 2003.
- 21.Shen H, Qin H, Guo J. Concordant correlation of LIV-1 and E-cadherin expression in human breast cancer cell MCF-7. Mol Biol Rep 36: 653–659, 2009. [DOI] [PubMed] [Google Scholar]
- 22.Sun D, Zhang L, Wang Y, Wang X, Hu X, Cui Fa, Kong F. Regulation of zinc transporters by dietary zinc supplement in breast cancer. Mol Biol Rep 34: 241–247, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Tang Z, Sahu SN, Khadeer MA, Bai G, Franklin RB, Gupta A. Overexpression of the ZIP1 zinc transporter induces an osteogenic phenotype in mesenchymal stem cells. Bone 38: 181–198, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Taylor KM A distinct role in breast cancer for two LIV-1 family zinc transporters. Biochem Soc Trans 36: 1247–1251, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Taylor KM, Morgan HE, Smart K, Zahari NM, Pumford S, Ellis IO, Robertson JF, Nicholson RI. The emerging role of the LIV-1 subfamily of zinc transporters in breast cancer. Mo Med 13: 396–406, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor KM, Vichova P, Jordan N, Hiscox S, Hendley R, Nicholson RI. ZIP7-mediated intracellular zinc transport contributes to aberrant growth factor signaling in antihormone-resistant breast cancer cells. Endocrinology 149: 4912–4920, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Wang F, Dufner-Beattie J, Kim BE, Petris MJ, Anderews GK, Eide DJ. Zinc-stimulated endocytosis controls activity of the mouse ZIP1 and ZIP3 zinc uptake transporters. J Biol Chem 279: 24631–24639, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Weaver BP, Dufner-Beattie J, Kambe T, Andrews GK. Novel zinc-responsive post-transcriptional mechanisms reciprocally regulate expression of the mouse Slc39a4 and Slc39a5 zinc transporters (Zip4 and Zip5). Biol Chem 388: 1301–1312, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]