Abstract
Proton nuclear magnetic resonance (1H-NMR) spectroscopy of plasma provides a global metabolic profiling method that shows promise for clinical diagnostics. However, cross-sectional studies are complicated by a lack of understanding of intraindividual variation, and this limits experimental design and interpretation of data. The present study determined the diurnal variation detected by 1H NMR spectroscopy of human plasma. Data reduction methods revealed three time-of-day metabolic patterns, which were associated with morning, afternoon, and night. Major discriminatory regions for these time-of-day patterns included the various kinds of lipid signals (-CH2- and -CH2OCOR), and the region between 3 and 4 ppm heavily overlapped with amino acids that had α-CH and α-CH2. The phasing and duration of time-of-day patterns were variable among individuals, apparently because of individual difference in food processing/digestion and absorption and clearance of macronutrient energy sources (fat, protein, carbohydrate). The times of day that were most consistent among individuals, and therefore most useful for cross-sectional studies, were fasting morning (0830–0930), postprandial afternoon (1430–1630), and nighttime samples (0430–0530). Importantly, the integrated picture of metabolism provided by 1H-NMR spectroscopy of plasma suggests that this approach is suitable to study complex regulatory processes, including eating patterns/eating disorders, upper gastrointestinal functions (gastric emptying, pancreatic, biliary functions), and absorption/clearance of macronutrients. Hence, 1H-NMR spectroscopy of plasma could provide a global metabolic tolerance test to assess complex processes involved in disease, including eating disorders and the range of physiological processes causing dysregulation of energy homeostasis.
Keywords: metabolomics, diurnal variation, eating disorders, gastrointestinal regulation
global metabolic profiling coupled with bioinformatic methods offers an approach to study the integration of macronutrient energy metabolism and can be useful to detect disease, toxicity, and nutritional deficiency (11, 23, 30, 36). A variety of metabolic profiling methods are available, including gas chromatography-mass spectrometry, liquid chromatography-mass spectrometry, and NMR spectroscopy (10, 31, 39). In view of its capability to handle multiple specimens in a high-throughput, semiautomated system, 1H-NMR may offer unique insight into the in vivo metabolism of macronutrients (1, 2, 9). Dysfunction in macronutrient metabolism, i.e., carbohydrate, fat, protein, and alcohol, is relevant to obesity, metabolic syndrome, diabetes, cardiovascular disease and other common pathological conditions (19, 25).
Pioneering studies by Nicholson and coworkers (3, 24, 27, 35) established the utility of 1H NMR spectroscopy for analyses of biofluids. In these applications, statistical pattern recognition techniques were used for data reduction and analysis (23, 26, 29, 41, 43), thereby allowing extraction of metabolic information from the complexity of the information-rich data (4, 9, 14). Although much of this research addressed metabolic variations induced by toxicological exposures (18, 24, 32), early studies with human plasma demonstrated that 1H-NMR spectroscopy can also be used to discriminate individuals with cardiovascular disease (4, 8, 40). In the latter study, differences were apparent in the intensities of proton signals associated with lipids, establishing the utility of NMR spectroscopy for study of physiological variations of lipids (1, 15, 37). In addition to lipids, which represent a very complex mixture, more than 75 individual metabolites have been identified by 1H-NMR spectroscopy of human plasma (1, 12, 17, 34). Most of these are related to macronutrient metabolism and include glucose, amino acids, and intermediates of energy metabolism. Regulation of these intermediates in plasma represents an important homeostatic control, and dysregulation of macronutrient energy metabolism has been implicated in aging (22) and a number of obesity-related disease processes (20, 38, 39).
As a basis to improve utility of 1H-NMR spectroscopy for cross-sectional comparisons, we designed a study to evaluate the magnitude of time-dependent intraindividual variation in human plasma. We collected plasma samples hourly over a 24-h period from 10 healthy individuals given standardized meals at timed intervals in the controlled environment of the Emory University Hospital General Clinical Research Center (GCRC). In view of previously observed differences in diurnal variation due to age (7), we selected 10 subjects (five males, five females) representing a considerable range in age (22–83 yr). 1H-NMR spectra were recorded for the resulting 250 samples, and the spectra were analyzed by data reduction methods to examine variations according to age, sex, body mass index (BMI), and time of day. Principal component analysis (PCA) and k-means clustering analysis showed separation of three time-of-day patterns (classes), which were determined by signals of the regions, including lipids, glyceryl signal of lipids and amino acids. Hour-to-hour classifications of spectra from individuals were variable, suggesting utility for individual assessment of complex gastrointestinal physiology related to eating.
MATERIALS AND METHODS
Human subjects.
The procedures followed were in accordance with the ethical standards and approval of the Emory University Institutional Review Board (IRB 382-2000). The sample size consisted of 10 healthy, nonsmoking subjects, as determined by power analysis considering one-sample t-test (power 0.80 and α=0.05). Subjects were studied in two age groups with five subjects each (22–45 yr and 75–83 yr) and also in two groups by sex. Furthermore, the effect of BMI was studied between 18.5–24.9 and 25–32.6 (kg/m2). Following informed consent, subjects were screened in the Emory GCRC, with a complete medical history and physical examination, urinalysis, blood chemistry profile, and complete blood count to rule out acute/chronic illnesses (see Appendix 1 in the online version of this article). Eligible subjects were admitted for a 26-h overnight stay in the GCRC within 3 wk of screening.
Study procedures.
Blood was drawn hourly for 24 h (from 0830 to 0830) into EDTA-containing tubes at the Emory Hospital GCRC. Blood samples were spun at 3,000 g for 15 min at 4°C to remove blood cells, and plasma samples were transferred to individual vials for storage at −70° C until time of analysis. Subjects were given standardized, nutritionally balanced meals to provide, over a 24-h period, energy intake at estimated basal energy expenditure (derived from the Harris Benedict equation) +30% and protein intake at 0.8 g/kg per day. Water was provided ad libitum. Meals were provided as a percentage of total energy intake at breakfast at 0930 (30%), lunch at 1330 (30%), dinner at 1730 (30%), and an evening snack at 2130 (10%), just following the timed blood draw for that hour. Subjects were allotted 45 min to complete their meals and 15 min to complete the snack. Activity was limited to walking in the GCRC.
1H-NMR spectroscopy.
Plasma samples were thawed, and 600 μl was mixed with 66 μl of deuterium oxide (D2O) containing TMS [3-(trimethylsilyl)-1-propanesulfonic acid sodium salt (C6H15NaO3SSi, 1% wt/wt)]. Plasma samples for pH ranged from 7.4 to 7.6. 1H NMR spectra were measured at 600 MHz on a Varian INOVA 600 spectrometer with water presaturation at 25°C. Spectra were measured with 64 scans into 16,384 data points over a spectral width of 6,600.7 Hz. Acquisition time was 2.55 s (d1 = 0, pulse = 5 μs, presaturation = 1 s, acquisition = 1.5 s) with line broadening 0.3 Hz. To check the reproducibility of the NMR analysis, identical plasma samples were run at multiple time points after thawing (0, 1.5, 3, 4, and 6 h). The correlation coefficients of these spectra relative to the zero time spectrum were 0.96, 0.93, 0.97, 0.97, respectively.
Preprocessing of NMR spectra.
Preprocessing of spectral data included baseline correction, spectral alignment, elimination of uninformative spectral regions, and normalization relative to the internal standard. A polynomial regression (NUTS program, Acorn NMR, Livermore, CA) was used for baseline correction. A beam search algorithm in MATLAB (MathWorks, Natick, MA) (28), which enhances the computational efficiency of the genetic algorithm (16) was used for peak alignment. The water signal region (4.5–5 ppm) was eliminated because of variable suppression of the water signal. On the basis of published research from the Nicholson group (34) and our own statistical analyses, the spectral regions in 5.4–6.7 ppm and 0–0.1 ppm contained no significant metabolite signal and were eliminated. The spectral regions containing signal from EDTA and EDTA bound with endogenous plasma calcium and magnesium were also excluded from the analysis. Thus, the number of spectral data points was reduced to 8,873 from 16,384 with minimal loss of spectral information but with improvement of useful signal regions compared with nonuseful regions. Spectra were scaled to the signal of TMS for normalization. For some analyses, interindividual variations were minimized by averaging the intensity at each of the 8,873 frequencies for the 10 subjects at each of the 25 time points.
Principal component analysis.
Data reduction by PCA (14, 43) was performed using Pirouette software (Infometrix, Bothell, WA). Three-dimensional PCA score plots and loading plots were used to visualize relational patterns and discriminatory factors to classify the groups.
K-means clustering.
Because the PCA indicated that three groups could be identified, we employed the k-means clustering algorithm (13, 21) with k=3 (MATLAB, MathWorks) to associate individual time-of-day spectra with time-of-day clusters. Recent studies have shown that the relaxed solution of k-means clustering, specified by the cluster indicators, is given by the principal components (13, 46). Specifically, k-means clustering was applied to the set of data points in three-dimensional space (25 time points), using the number of clusters k (three clusters) and their initial location (seed points), to assign each data point to one of the three clusters by minimizing the mean squared Euclidean distance between data points and the seed points. The seed points were then replaced by the mean of the currently assigned clusters, and this procedure was repeated with updated seed points until the assignments did not change.
Statistical analyses.
One-way ANOVA was used to test whether the intensities in the regions where metabolites were included by the loading plots of the PCA (13, 46) were significantly different for the individual time-of day samples. Pearson correlation coefficients showed that 0830 on day 2 was not significantly different from 0830 on day 1. A multiple testing procedure based on the concept of the False Discovery Rate [FDR; (6, 42)], using MATLAB (MathWorks), was used to test for spectral frequencies which contributed significantly to time-of-day classes. For the latter, we performed a two-sample t-test for each frequency between different time points, and because we had three time classes, we used three sets of pairwise tests: morning vs. afternoon, afternoon vs. night, and morning vs. night. A permutation method was used in which the labels (1–25; time points) on the metabolites were shuffled, and t-statistics were recalculated. This procedure was repeated K times to obtain a set of null statistics 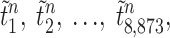 n = 1, 2, …, K. We set K = 200. The P value for the region i (for i = 1, 2, …, 8,873) was calculated as
n = 1, 2, …, K. We set K = 200. The P value for the region i (for i = 1, 2, …, 8,873) was calculated as
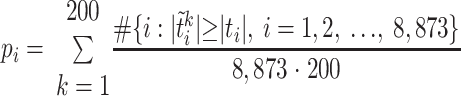 |
Once we obtained the P values for all test statistics (the regional intensities), we performed the FDR procedure that used ordered P values (6, 42) for the frequencies to find the significant regions containing important metabolites that discriminated between time-of-day classes. Significance levels were P < 0.05 for ANOVA and P < 0.1 for FDR.
RESULTS
PCA.
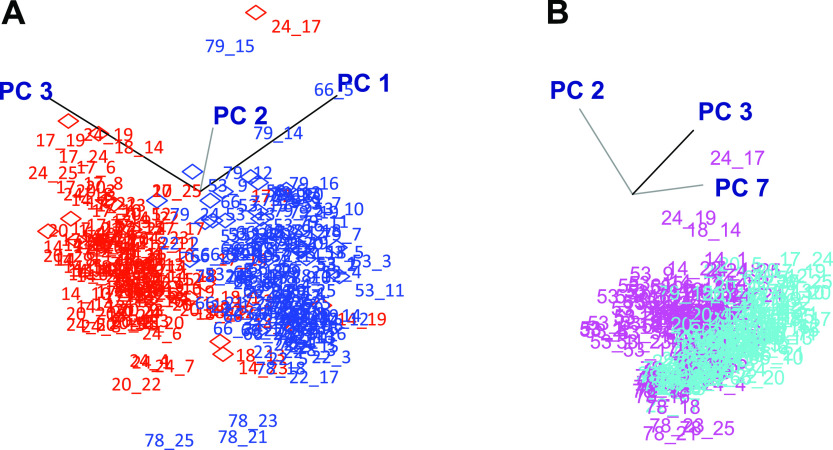
The first three principal components (PCs) of an unsupervised analysis explained 77% of total variation (see Appendix 2 in the online version of this article) and separated spectra into two age groups, 22–45 yr and 75–83 yr, as viewed in a three-dimensional (3-D) score plot (Fig. 1A). The first three PCs also showed some separation into male and female groups (see Appendix 3 in the online version of this article), but analysis by sex showed that PC1 did not contribute to this classification. Instead, males and females were separated by PC2, PC3, and PC7 (Fig. 1B). PC7, which represents 1.7% of the variability in the entire data (see Appendix 2 in the online version of this article), was important in this classification. Also, because of the large variation of BMI in this study, PCA was performed on the groups. PCA separated groups partially according to BMI (see Appendix 4 in the online version of this article). Considering the status of menopause in five female individuals, PCA categorized into premenopause and postmenopause groups (see Appendix 5 in the online version of this article). Unexpectedly, PCA did not classify the 250 1H-NMR spectra according to time of day using any combination of the first 9 PCs (data not shown). Thus, even though the present design used a controlled environment, a consistent day-night cycle, as well as standardized and timed meals, data reduction by PCA did not capture metabolic variation in a manner that could be classified according to time of day.
Fig. 1.
Separation of human plasma metabolic profiles by principal component analysis (PCA) according to age (A) and sex (B). Hourly plasma samples were collected over a 24-h period from 10 healthy individuals (five males and five females) with five subjects <46 yr (22–45 yr) and five subjects >46 yr (75–83 yr) and 1H-NMR spectra were recorded. For the 250 spectra, the first three principal components (PCs) represented 77% of the total variation, and PC7 represented 1.7% of the total variation (see Appendix 2 in the online version of this article). PC1 largely accounted for separation, according to age and did not contribute to separation according to sex (see Appendix 3 in the online version of this article). No separation according to time of day was apparent with any combination of PC1–PC9. A: three-dimensional score plot of PC1–PC3 revealed separation according to age (red, 22–45 yr; blue, 75–83 yr). B: three-dimensional score plot of PC2, PC3, and PC7 revealed separation according to sex (magenta, female; cyan, male).
Analysis of averaged spectra.
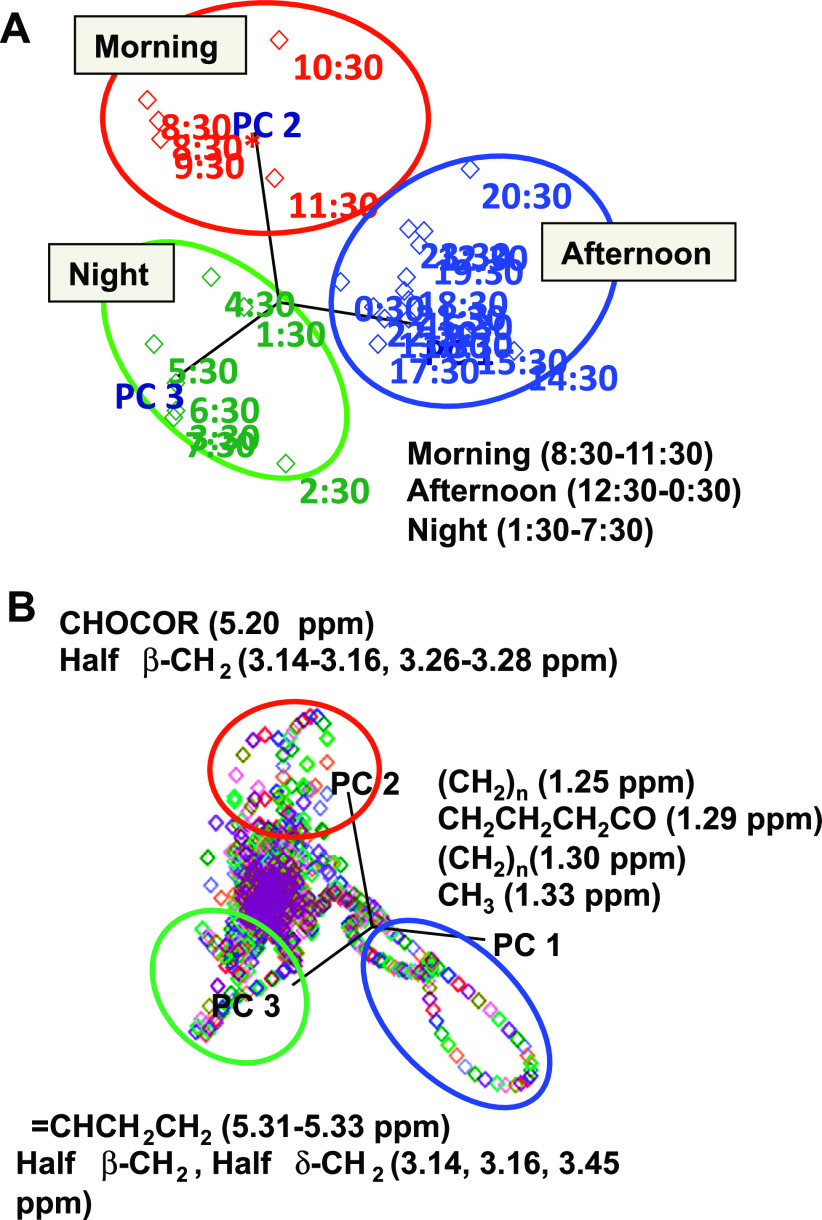
The lack of discriminatory power for meal patterns may be due to heterogeneity among participants. To minimize interindividual variation effects, spectra were averaged for the subjects for each sampling time, and PCA was performed on the 25 averaged spectra. The first 3 PC accounted for 78% of the time-of-day variation of the averaged spectra. A 3-D score plot of PC1 to PC3 showed that these new variables separated the serial time points with a trajectory that could be visualized as three different time-of-day classes (morning, afternoon, and night) (Fig. 2A). In this plot, the time-of-day variation was largely described by PC1. Importantly, the 0830 and 0930 time points, which were both fasting samples, appeared distinct from the night class and similar to the first two postprandial (1030, 1130) morning samples. An alternative way to consider these samples is that the 0830 and 0930 time points represent a unique morning class and that the 1030 and 1130 data represent transitions in the trajectory to the afternoon class. A critical issue with the discrimination of 0830 and 0930 as a separate class from the night class is that there was no obvious reason for a difference between these and the earlier 0630 and 0730 spectra. Thus, the data suggest that circadian variation, arousal, minimal physical activity, or influences of the research environment altered the global metabolic profile even in the fasted state. Consequently, sampling times at 0830–0930 would appear to minimize differences in the trajectory from night to morning and, at the same time, avoid early postprandial effects. Also, the discriminatory regions to categorize morning, afternoon, and night are illustrated in Fig. 2B. In the morning, the discriminatory signals had features such as CHOCOR and half β-CH2, including glyceryl of lipids and tyrosine. In the afternoon, the dominant features contained (CH2)n, CH3CH2CH2CO, CH2, and CH3, such as the molecules from LDL, VLDL, lipid, and lactate. In the night, the regions containing half β-CH2 of histidine and =CHCH2CH2 of unsaturated lipid were distinctly different from morning and afternoon.
Fig. 2.
Separation of human plasma metabolic profiles by PCA according to time of day. Hourly 1H-NMR spectra collected from 10 subjects over a 24-h period were averaged for each time of day to minimize interindividual effects. A: three dimensional score plot separated three time-of-day classes (morning, 0830–1130; afternoon, 1230–0030; night, 0130–0730). *Represents next morning. B: PCA loading plot showed major discriminatory signals from the afternoon, night, and morning including lipid (CH2CH2CH2CO) and lactate-(CH2-)n for afternoon, amino acids (half β-CH2) and lipid (-CHCH2CH2) for night, and amino acids half β-CH2, and glyceryl signal (CHOCOR) of lipids for morning, respectively.
K-means clustering analysis.
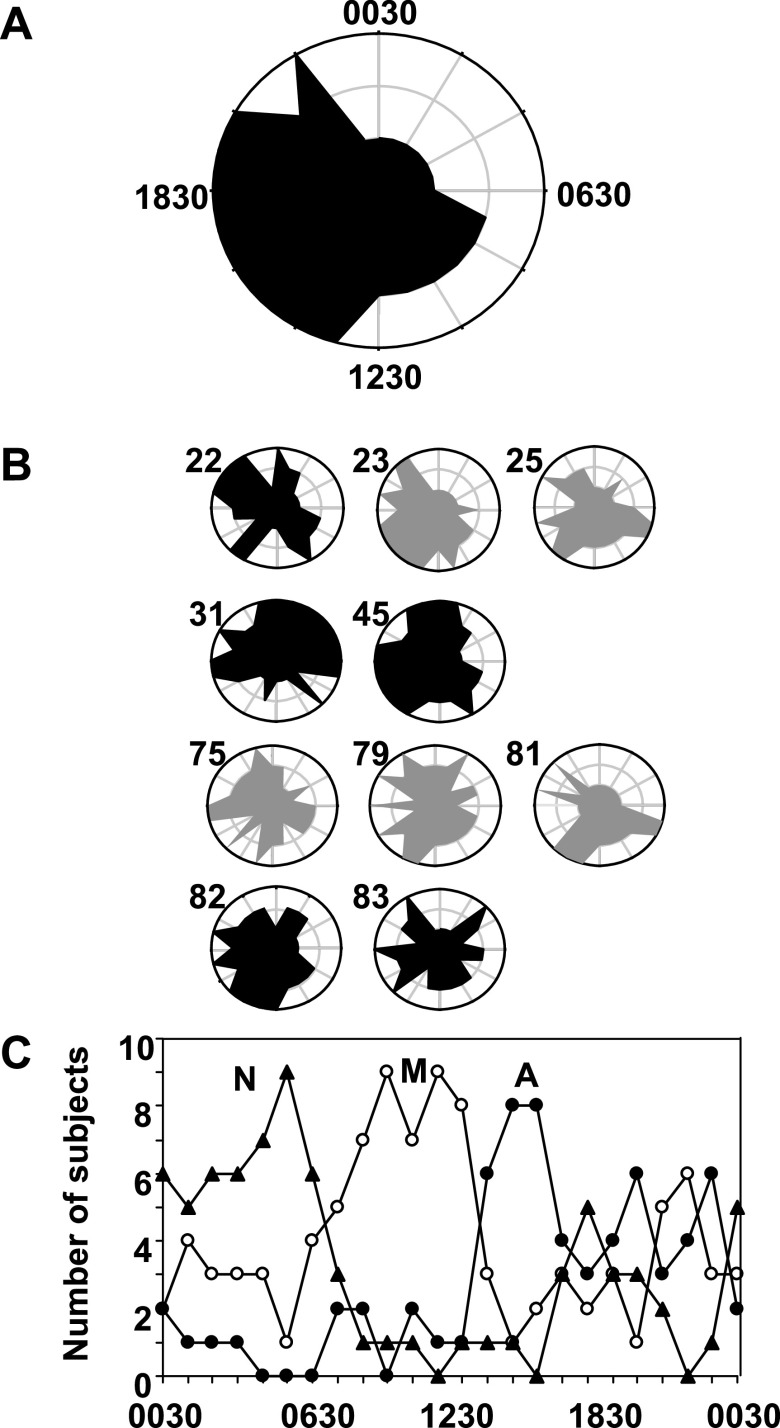
As an alternate approach to test the classification obtained by PCA, k-means clustering was performed on the averaged data for each of the 25 time points. Because the PCA analysis revealed natural groupings into three periods of the day, we set k = 3 to see whether this independent approach revealed the same classifications. Results are visualized as polar plots (Fig. 3A) where the two 0830 points were averaged and the angular axis was used to describe one 24-h day (15 degrees per hour). The radial axes (distances from the center) were used to visualize classification by k-means clustering with three radial distances, in which the shortest distance from the center was night (distance 1), the middle distance was morning (distance 2), and the greatest distance was afternoon (distance 3). The result (see Appendix 6 in the online version of the article) showed the same classification as the PCA, with the exception that 1230 was classified as morning rather than afternoon in detail, and 0730 was categorized in the morning rather than night. The main conclusion from consideration of results from both PCA and k-means clustering is that metabolic variations in human plasma associated with time of day can be separated into morning, afternoon, and night patterns when interindividual effects are minimized by averaging the spectra.
Fig. 3.
Time-dependent metabolic patterns in human plasma 1H-NMR spectra based on k-means clustering analysis. The k-means clustering algorithm was used to determine classification according to time of day, with k equal to 3 based upon the results of the PCA analysis (Fig. 2). A: hourly 1H-NMR spectra collected from 10 subjects over a 24-h period were averaged for each time of day to minimize interindividual effects. k-Means clustering of the averaged spectra revealed separation corresponding to night, morning, and afternoon classes of the PCA (see Appendix 3 in the online version of this article). These classifications are plotted on a 24-h polar plot, in which the radial distance 1 is night, 2 is morning, and 3 is afternoon. B: polar plots of k-means clustering analysis of individuals using the same criteria as for the average (A) revealed marked heterogeneity in time-of-day metabolic patterns of individuals. The age is given next to each plot. Black represents females, and gray represents males. C: frequency distribution of time-of-day classifications according to k-means clustering analysis shows that the most consistent classification is obtained for night at 0430–0530, morning at 0830–1230, and afternoon at 1430–1630.
Analysis of spectra from individual subjects.
To determine time-of-day variation within individuals, the same k-means clustering algorithm was applied to the 25 spectra of hourly plasma samples from individuals.
The polar plot for k-means clustering analysis of the individual spectra is shown as Fig. 3B along with corresponding plot for the average time-of-day classification for the 10 subjects (Fig. 3A). The plots show that individuals differed in metabolic pattern according to time-of-day classification; none of the subjects matched the average pattern. Samples taken at 0430 and 0530 were most frequently classified as night (Fig. 3C). Samples from 0830 through 1230 were most frequently classified as morning, and the most frequent afternoon classification was at 1430 to 1630 (Fig. 3C). Therefore, from the standpoint of the frequency of individual sample classifications matching the average classification, the best time points for global metabolic profiling are night, 0430–0530; morning, 0830–1230; and afternoon, 1430–1630.
To see how time-of-day varied in each subject, data were evaluated in terms of duration, start time, and mean time of the most contiguous afternoon class data (see Appendix 6 in the online version of this article). The afternoon class was selected because it represented the period expected to have the highest absorption of macronutrients. For the averaged data, the afternoon class began at 1330 and was centered at 1700 with 7-h duration (Fig. 3A). The individual plots showed a considerable variation in start times, ranging from 1130 to 1530, with a mean at 1345 (±4.3 h). The mean duration of the afternoon class varied from 1 to 12 h (mean ± SD, 7.2 ± 2.3 h), centered at 17:20 (±5.5 h). Thus, the phasing and duration of metabolic changes associated with the afternoon class differed among individuals, indicating that variation in the rate of assimilation of macronutrients from food could underlie the inability of PCA to reveal time-of-day variation when applied to spectra of all individual samples.
Variation of key signals by morning, afternoon and night classes.
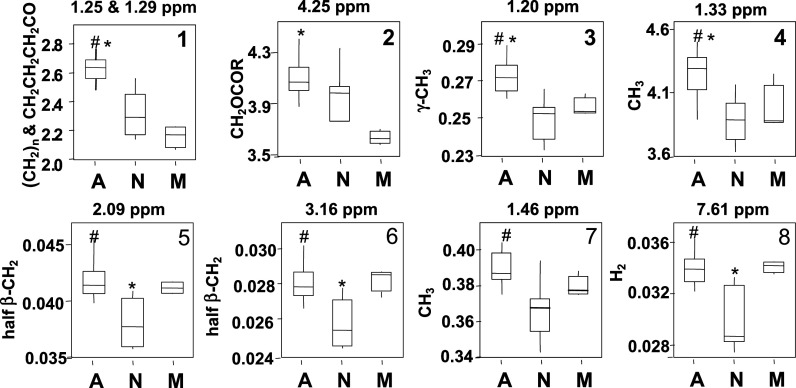
Although the overlap of NMR signals precludes identification of chemicals by the methods used, the loading plot of the PCA (Fig. 2B) showed that the discriminatory signals for the night class were in regions where amino acids and lipids contribute signal; for the morning class, they were in the region where the glyceryl signal of lipids and amino acids are found; and for the afternoon class, they were in the regions where lactate, VLDL, LDL, lipid, and amino acid signals are found. Univariate statistics were applied to discrete spectral regions to determine the variation by time of day. The intensities of metabolic signals were summed in the regions, then analyzed by one-way ANOVA according to night, morning, and afternoon (Fig. 4). The results showed that the VLDL plus LDL region in the afternoon was significantly different from other groups, with lowest values in the morning.
Fig. 4.
Box plots of significance for region containing molecule, which discriminated morning, afternoon, and night classes of 1H-NMR spectra of human plasma. The spectral regions identified in the PCA loading plot (Fig 2B) were summed to provide relative quantifications of the spectral regions corresponding to LDL plus VLDL, glyceryl signal of lipids, 3-hydroxybutyrate (3-OH-butyrate), glutamine and alanine, and 1-way ANOVA was performed according to time of day. 1, LDL plus VLDL; 2, glyceryl signal of lipids; 3, 3-hydroxybutyrate; 4, lactate; 5, glutamine; 6, tyrosine; 7, alanine; 8, 3-methylhistidine. A, afternoon; N, night, M, morning. #Significantly different from N (P < 0.05), *Significantly different from M (P < 0.05). Assignments from Ref 34.
Comparing the region containing lipid (Fig. 4) signal of morning to afternoon, there was 24% intensity change in the NMR spectrum. In the region where alanine is found, the intensity of afternoon was 8% higher than that of night. The region of glyceryl signal of lipids was also higher in the afternoon compared with the morning and night. The regions of glutamine, tyrosine, and 3-methylhistidine signals were significantly lower in the night than those intensities in the morning or afternoon (Fig. 4).
Patterns of time-of-day variation of individual metabolic signals.
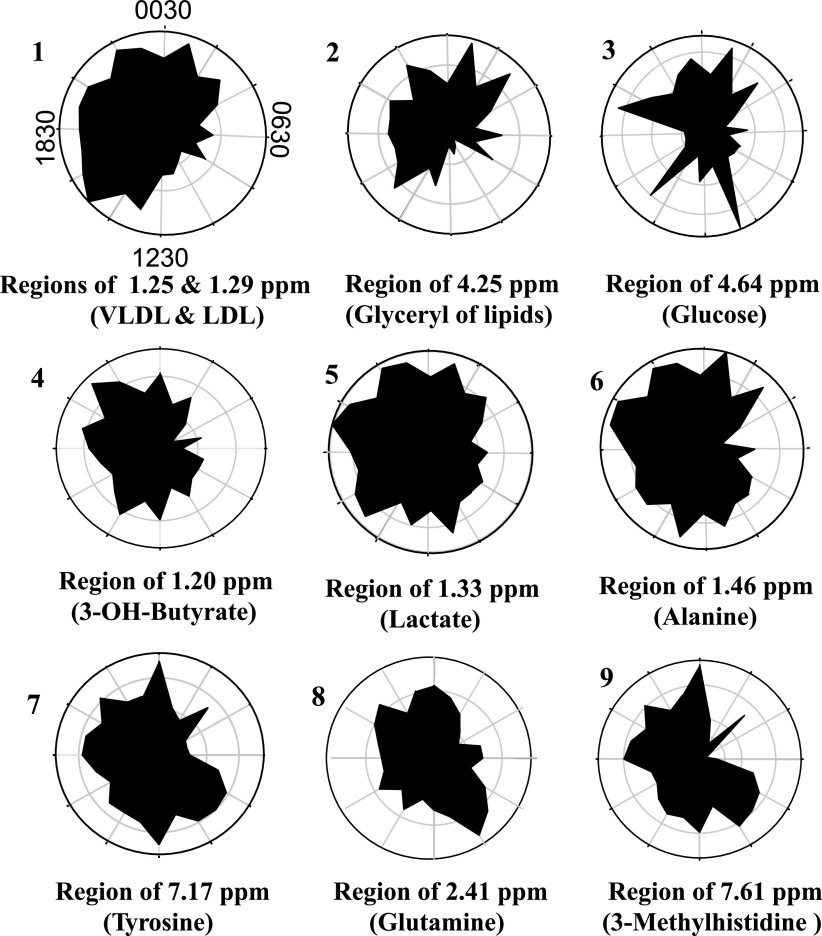
The hour-to-hour variation of regional signals with corresponding VLDL and LDL, glyceryl of lipids, 3-hydroxybutyrate, alanine, glutamine, tyrosine, lactate, and glucose, were plotted (Fig. 5) to visualize time-of-day changes. To normalize the data for comparison, the radial axes (distances from the center) were expressed as the percentage of individual intensity for the respective metabolic signal as a function of the angular axis (time of day). Plots of the time-dependent variations of these key metabolic signals showed that the patterns of VLDL and LDL, glyceryl signal of lipids, and alanine were consistent with the pattern of the time-dependent k-means clustering of averaged spectra (Fig. 3A). However, the plots revealed time-dependent shifts in the lipid signals relative to other signals. The time-dependent variation for the region of glucose was markedly different from the other metabolites, with maximal values in the samples 2 h after meal times (0930, 1330, 1730, and 2130).
Fig. 5.
Time-of-day variation in metabolic signals which discriminate 1H-NMR spectra of human plasma according to time of day. 1: regions of very low density lipoprotein and low-density lipoprotein regions were summed and expressed as VLDL and LDL in the upper left circle. The radial axis is a linear scale from minimum to maximum expressed on the angular axis as a function of time of day (24-h clock). The precise orientation is shifted by 0.5 h from a usual 24-h clock because the samples were taken on the half-hour, i.e., 0030 is labeled on top and 1230 is on the bottom. Other figures are similarly oriented and represent the regions, including glyceryl signal of lipids [glyceryl(lipid)] (2), glucose (3), 3-hydroxybutyrate (3-OH-butyrate) (4), lactate (5), alanine (6), tyrosine (7), glutamine (8) and 3-methylhistidine (9). For each plot, hourly values were summed, and each respective hourly value was expressed as a percentage of summed value.
False discovery rate analysis of spectra.
As an independent statistical test for time-of-day effects, we used FDR to test for spectral regions with significant differences as a function of time of day; major results from the ordered P-value method controlling FDR are given in Table 1. The results showed that the regional signals such as lipid and lipoprotein (lipid, unsaturated lipid, LDL, and VLDL) varied significantly between all three time classes. Signals from spectral regions, including cholesterol, glyceryl moiety of lipid, and choline moiety of lipid differed in two of three comparisons. The spectral regions, including lactate, differed between morning and afternoon and also between afternoon and night. The regions containing amino acids such as Ala, His, Phe, and Tyr varied significantly between all three time classes. Signals associated with other amino acids (Gln, Gly, citrulline) differed in two of the comparisons, while Asp, Leu, Pro, Lys, and Ile differed in one of the comparisons. Some other signals from the regions of 3-hydroxybutyrate, acetoacetate, 3-methylhistidine, and formate also differed significantly in one or more comparison. Thus, the FDR results confirmed that the spectral regions for lipoproteins, amino acids, and lactate contribute to time-of-day variation in global metabolic profiles as detected by 1H-NMR spectroscopy and also show that time-dependent changes in signals from other macronutrient metabolites can be measured by this approach.
Table 1.
False discovery rate according to regional signals including metabolites differing between time-of-day patterns
| N-M | P Value | A-N | P Value | M-A | P Value |
|---|---|---|---|---|---|
| Glu | 0.0013 | VLDL | 0.00002 | LDL | 0.0000001 |
| 3-Methyl-His | 0.0014 | LDL | 0.00002 | VLDL | 0.000002 |
| His | 0.0017 | 3-OH-butyrate | 0.00007 | Cholesterol | 0.00004 |
| 1-MethylHis | 0.0023 | Tyr | 0.0001 | Unsat. lipid | 0.0001 |
| Formate | 0.0024 | 1-Methyl-His | 0.0001 | Lactate | 0.0002 |
| Tyr | 0.0024 | Phe | 0.0001 | Lipid | 0.0002 |
| Phe | 0.0048 | Lipid | 0.0002 | 3-OH-butyrate | 0.0011 |
| Trp | 0.0057 | His | 0.0003 | Asp | 0.0012 |
| Unsat lipid | 0.0059 | Ile | 0.0004 | Choline lipid | 0.0013 |
| 3-OH-butyrate | 0.0070 | 3-Methyl-His | 0.0006 | Glyceryl lipids | 0.0013 |
| Choline (lipid) | 0.0115 | Cholesterol | 0.0019 | Gln | 0.0017 |
| VLDL | 0.0142 | Glu | 0.0029 | Ala | 0.0026 |
| Ala | 0.0176 | Lactate | 0.0039 | Cholesterol | 0.0043 |
| Val | 0.0195 | Formate | 0.0050 | His | 0.0043 |
| Lipid | 0.0212 | Lys | 0.0052 | Citrulline | 0.0047 |
| LDL | 0.0228 | Ala | 0.0056 | Tyr | 0.0058 |
| Leu | 0.0086 | Acetoacetate | 0.0064 | ||
| Citrulline | 0.0086 | Creatinine | 0.0123 | ||
| Unsat lipid | 0.0090 | Phe | 0.0126 | ||
| Cholesterol | 0.0094 | Citrate | 0.0148 | ||
| Pro | 0.0095 | ||||
| Val | 0.0111 | ||||
| Acetoacetate | 0.0142 | ||||
| Gly | 0.0171 | ||||
| Glyceryl(lipids)0.0333 | |||||
| Tyr | 0.0333 | ||||
| HDL | 0.0354 | ||||
| Choline (lipid) | 0.0362 |
N-M, night vs. morning; A-N, afternoon vs. night; M-A, morning vs. afternoon.
DISCUSSION
Macronutrient intake is highly variable and probably contributes to age-related disease development in many ways (5, 20, 30, 44, 45). For instance, excess intake is associated with poor glucose homeostasis and increased generation of toxic reactive oxygen species (19, 25, 33). Eating behaviors and gastric emptying time can affect digestion, and disturbances in intestinal absorption of macronutrients can occur at multiple levels (e.g., absorption is affected by pancreatic, biliary, and intestinal mucosal function). Disposition of absorbed macronutrients can be altered by organ function and metabolic status (e.g., hepatic and renal function, underlying nutritional status). Although specific abnormalities associated with disease can be diagnosed by a range of tests (e.g., serum creatinine concentration, blood glucose), limited methods are available to detect more complex interactions that can affect health.
1H-NMR spectroscopy is a global metabolic profiling method that provides information on a range of macronutrients and can be performed within minutes with minimum sample processing. With appropriate standardization and analytical procedures, 1H-NMR spectroscopy could provide a useful complement to available diagnostic tests by providing information on multiple macronutrients and their interactions. However, application has been limited by the complexity and variability of the information contained within the spectra.
Here, we addressed the issue of time-of-day variability in 1H-NMR spectra of human plasma. We selected individuals with a considerable age range and an equal number of males and females to assure that variability due to age, sex and BMI were included in the overall analysis. Although EDTA contributed to the plasma spectra, this contribution was constant, and the regions of the signal were excluded so that EDTA did not interfere with interpretation. The results of the PCA surprisingly showed that samples collected hourly over 24 h classified according to age largely by PC1, classified according to sex by PC2, PC3, and PC7, and classified according to BMI by PC1, PC3 and PC5, However, PCA did not provide detectable classification in terms of time of day. When data were averaged to minimize interindividual effects, PCA and k-means clustering analyses revealed time-of-day classification. Importantly, application of the k-mean clustering procedure to spectra from individuals showed that considerable heterogeneity existed in the duration and phasing of the afternoon class, indicating substantial differences among individuals in the absorption and disposition of macronutrients. One-way ANOVA showed that increased signals from lipids and amino acids contributed to the afternoon classification. Thus, the data imply that considerable differences occur in absorption and clearance of lipids and amino acids due to differences in integrated functions of eating behavior, digestion, and metabolic functions.
The data also show that substantial variations can occur in association with age, sex, BMI, and perhaps other factors. Although more detailed studies are needed to understand specific associations, the results point to a need for consideration of each of these factors in experimental design. Specifically, if study populations are not adequately matched in distributions of age, sex, and BMI, metabolic variability detected by 1H-NMR spectroscopy could reflect differences in these parameters rather than other dietary or health parameters of interest.
The most consistent time-of-day classifications for individual spectra by k-means clustering analysis were obtained for 0430–0530 (night), 0830–1230 (morning), and 1430–1630 (afternoon) time points (Fig. 3C). Compared with the classification obtained by PCA of averaged samples, these time points are approximately in the middle of the time periods classified together. A transition in classification from night (0130–0730) to morning (0830–1230) occurred prior to eating (which was immediately after 0930 sample collection), and the first postprandial times (1030, 1130) classified separately from afternoon, which contained the other postprandial time points. These results are counter to the expectation that fasting 0830 and 0930 would be more similar to fasting 0630 and 0730 than to the plasma taken at 1 h and 2 h after eating. However, in the PCA, 1030 and 1130 points are on a trajectory from 0830 to 1230, which could indicate that circadian effects, arousal, or other environmental factors accounted for the separation of the 0830 and 0930 points from the night class.
The basis for the separation of night and morning classes will need further study, especially because fasting, morning sample collection is a standard procedure in clinical medicine. The results suggest that morning, fasting blood samples with some low activity level, e.g., as obtained for 0830–0930, may represent the best choice for cross-sectional studies using 1H-NMR spectroscopy of plasma in adult humans. Importantly, comparisons that would appear to be valid by usual criteria, e.g., between fasting samples collected from hospitalized subjects at 0630 to 0730 and fasting samples collected in a clinic at 0830–0930, may be confounded by factors causing the transition from night to morning classification. Additional research will be needed to address this point.
The marked variability among individuals in start time and duration of the afternoon class suggests that considerable information could be extracted from meal challenge studies that employ 1H-NMR spectroscopy of plasma. On the basis of the current analysis, such information could include the kinetics of appearance and removal of glucose, lactate, amino acids, and lipids, but methods to provide metabolite identification would be needed. With controlled meal composition and timing, tests could be developed to assess effects of meal composition and energy content on macronutrient metabolism in individual subjects in the clinical setting, thereby providing a global metabolic equivalent of a glucose tolerance test. Alternate forms of such a test could evaluate eating behaviors (e.g., “nibbling” vs. “gorging”), as well as integrated gastric emptying and digestive functions as components of eating disorders and obesity.
Overlap of signals from different chemicals represents a limitation of 1H-NMR spectroscopy; however, high-abundance metabolites, as well as metabolites without spectral overlap are readily detected. Up to 75 compounds have been identified in human plasma (34) but fully automated procedures for spectral analysis are not yet available. The current data suggest that a systematic development of 1H-NMR spectroscopy approaches with bioinformatics has potential for multiparameter monitoring of complex nutritional processes, especially using a meal challenge protocol.
Perspectives and Significance
The present study shows that interindividual variations were sufficiently large to prevent detection of time-of-day effects by PCA of individual spectra from 10 individuals. However, PCA of spectra averaged according to time of sampling showed three time-of-day patterns, morning, afternoon, and night. The major discriminatory regions included lipids, lactate, glyceryl signal of lipids, and amino acids. The start time and duration of the afternoon class varied among individuals, indicating that the integrated regulation of absorption and metabolism was variable among individuals. The results suggest that 1H-NMR spectroscopy of human plasma could be useful to provide a global metabolic tolerance test to assess individual fitness for macronutrient homeostasis following food challenge. To obtain the most useful metabolic information in the clinical setting, the time of collection should be considered in interpretation of data.
GRANTS
This study was supported by grants from the National Institutes of Health: Grants DK-066008, ES-012929, ES-011195, DK-55850, and General Clinical Research Center Grant RR00039.
Supplementary Material
Acknowledgments
The help of the nursing and laboratory staff of the Emory University Hospital GCRC is gratefully appreciated.
REFERENCES
- 1.Ala-Korpela M, Korhonen A, Keisala J, Horkko S, Korpi P, Ingman LP, Jokisaari J, Savolainen MJ, Kesaniemi YA. 1H NMR-based absolute quantitation of human lipoproteins and their lipid contents directly from plasma. J Lipid Res 35: 2292–2304, 1994. [PubMed] [Google Scholar]
- 2.Beckonert O, Keun HC, Ebbels TM, Bundy J, Holmes E, Lindon JC, Nicholson JK. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat Protoc 2: 2692–2703, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Beckwith-Hall BM, Brindle JT, Barton RH, Coen M, Holmes E, Nicholson JK, Antti H. Application of orthogonal signal correction to minimise the effects of physical and biological variation in high resolution 1H NMR spectra of biofluids. Analyst 127: 1283–1288, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Beger RD, Holland RD, Sun J, Schnackenberg LK, Moore PC, Dent CL, Devarajan P, Portilla D. Metabonomics of acute kidney injury in children after cardiac surgery. Pediatr Nephrol 23: 977–984, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Bell JD, Sadler PJ, Morris VC, Levander OA. Effect of aging and diet on proton NMR spectra of rat urine. Magn Reson Med 17: 414–422, 1991. [DOI] [PubMed] [Google Scholar]
- 6.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B B57: 289–300, 1995. [Google Scholar]
- 7.Blanco RA, Ziegler TR, Carlson BA, Cheng PY, Park Y, Cotsonis GA, Accardi CJ, Jones DP. Diurnal variation in glutathione and cysteine redox states in human plasma. Am J Clin Nutr 86: 1016–1023, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Brindle JT, Antti H, Holmes E, Tranter G, Nicholson JK, Bethell HW, Clarke S, Schofield PM, McKilligin E, Mosedale DE, Grainger DJ. Rapid and noninvasive diagnosis of the presence and severity of coronary heart disease using 1H-NMR-based metabonomics. Nat Med 8: 1439–1444, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Cho HW, Kim SB, Jeong MK, Park Y, Miller NG, Ziegler TR, Jones DP. Discovery of metabolite features for the modelling and analysis of high-resolution NMR spectra. Int J Data Min Bioinform 2: 176–192, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coen M, Holmes E, Lindon JC, Nicholson JK. NMR-based metabolic profiling and metabonomic approaches to problems in molecular toxicology. Chem Res Toxicol 21: 9–27, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Colatsky TJ, Sumner S. Metabolic profiling and biomarker discovery. Curr Opin Investig Drugs 4: 262–263, 2003. [PubMed] [Google Scholar]
- 12.de Graaf RA, Behar KL. Quantitative 1H NMR spectroscopy of blood plasma metabolites. Anal Chem 75: 2100–2104, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Ding C, He X. K-means clustering via principal component analysis. Proc Intl Conf Machine Learning 69: 225–232, 2004. [Google Scholar]
- 14.Eriksson L, Antti H, Gottfries J, Holmes E, Johansson E, Lindgren F, Long I, Lundstedt T, Trygg J, Wold S. Using chemometrics for navigating in the large data sets of genomics, proteomics, and metabonomics (gpm). Anal Bioanal Chem 380: 419–429, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Fearnside JF, Dumas ME, Rothwell AR, Wilder SP, Cloarec O, Toye A, Blancher C, Holmes E, Tatoud R, Barton RH, Scott J, Nicholson JK, Gauguier D. Phylometabonomic patterns of adaptation to high fat diet feeding in inbred mice. PLoS ONE 3: e1668, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forshed J, Schuppe-Koistinen I, Jacobbson SP. Peak alignment of NMR signals by means of a genetic algorithm. Anal Chim Acta 487: 189–199, 2003. [Google Scholar]
- 17.Foxall PJ, Spraul M, Farrant RD, Lindon LC, Neild GH, Nicholson JK. 750 MHz 1H-NMR spectroscopy of human blood plasma. J Pharm Biomed Anal 11: 267–276, 1993. [DOI] [PubMed] [Google Scholar]
- 18.Gartland KP, Sanins SM, Nicholson JK, Sweatman BC, Beddell CR, Lindon JC. Pattern recognition analysis of high resolution 1H NMR spectra of urine. A nonlinear mapping approach to the classification of toxicological data. NMR Biomed 3: 166–172, 1990. [DOI] [PubMed] [Google Scholar]
- 19.Go YM, Jones DP. Redox compartmentalization in eukaryotic cells. Biochim Biophys Acta 1780: 1273–1290, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffin JL The Cinderella story of metabolic profiling: does metabolomics get to go to the functional genomics ball? Philos Trans R Soc Lond B Biol Sci 361: 147–161, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hand DJ, Krzanowski WJ. Optimising k-means clustering results with standard software packages. Comp Stat Data Analysis 49: 969–973, 2005. [Google Scholar]
- 22.Hansen JM, Go YM, Jones DP. Nuclear and mitochondrial compartmentation of oxidative stress and redox signaling. Annu Rev Pharmacol Toxicol 46: 215–234, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Holmes E, Antti H. Chemometric contributions to the evolution of metabonomics: mathematical solutions to characterising and interpreting complex biological NMR spectra. Analyst 127: 1549–1557, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Holmes E, Nicholls AW, Lindon JC, Connor SC, Connelly JC, Haselden JN, Damment SJ, Spraul M, Neidig P, Nicholson JK. Chemometric models for toxicity classification based on NMR spectra of biofluids. Chem Res Toxicol 13: 471–478, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Jones DP Radical-free biology of oxidative stress. Am J Physiol Cell Physiol 295: C849–C868, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kemsley EK, Le Gall G, Dainty JR, Watson AD, Harvey LJ, Tapp HS, Colquhoun IJ. Multivariate techniques and their application in nutrition: a metabolomics case study. Br J Nutr 98: 1–14, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Keun HC, Ebbels TM, Bollard ME, Beckonert O, Antti H, Holmes E, Lindon JC, Nicholson JK. Geometric trajectory analysis of metabolic responses to toxicity can define treatment specific profiles. Chem Res Toxicol 17: 579–587, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Lee GC, Woodruff DL. Beam search for peak alignment of NMR signals. Anal Chem Acta 513: 413–416, 2004. [Google Scholar]
- 29.Lindon JC, Holmes E, Bollard ME, Stanley EG, Nicholson JK. Metabonomics technologies and their applications in physiological monitoring, drug safety assessment and disease diagnosis. Biomarkers 9: 1–31, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Lindon JC, Holmes E, Nicholson JK. Metabonomics in pharmaceutical R&D. FEBS J 274: 1140–1151, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Lindon JC, Holmes E, Nicholson JK. Metabonomics techniques and applications to pharmaceutical research & development. Pharm Res 23: 1075–1088, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Lindon JC, Keun HC, Ebbels TM, Pearce JM, Holmes E, Nicholson JK. The Consortium for Metabonomic Toxicology (COMET): aims, activities, and achievements. Pharmacogenomics 6: 691–699, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Luo M, Fernandez-Estivariz C, Jones DP, Accardi CR, Alteheld B, Bazargan N, Hao L, Griffith DP, Blumberg JB, Galloway JR, Ziegler TR. Depletion of plasma antioxidants in surgical intensive care unit patients requiring parenteral feeding: effects of parenteral nutrition with or without alanyl-glutamine dipeptide supplementation. Nutrition 24: 37–44, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicholson JK, Foxall PJ, Spraul M, Farrant RD, Lindon JC. 750 MHz 1H and 1H-13C NMR spectroscopy of human blood plasma. Anal Chem 67: 793–811, 1995. [DOI] [PubMed] [Google Scholar]
- 35.Nicholson JK, O'Flynn MP, Sadler PJ, Macleod AF, Juul SM, Sonksen PH. Proton-nuclear-magnetic-resonance studies of serum, plasma and urine from fasting normal and diabetic subjects. Biochem J 217: 365–375, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noguchi Y, Sakai R, Kimura T. Metabolomics and its potential for assessment of adequacy and safety of amino acid intake. J Nutr 133: 2097S–2100S, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Otvos JD, Jeyarajah EJ, Bennett DW, Krauss RM. Development of a proton nuclear magnetic resonance spectroscopic method for determining plasma lipoprotein concentrations and subspecies distributions from a single, rapid measurement. Clin Chem 38: 1632–1638, 1992. [PubMed] [Google Scholar]
- 38.Ramsey JJ, Harper ME, Weindruch R. Restriction of energy intake, energy expenditure, and aging. Free Radic Biol Med 29: 946–968, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Schnackenberg LK, Beger RD. Monitoring the health to disease continuum with global metabolic profiling and systems biology. Pharmacogenomics 7: 1077–1086, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Serkova N, Fuller TF, Klawitter J, Freise CE, Niemann CU. H-NMR-based metabolic signatures of mild and severe ischemia/reperfusion injury in rat kidney transplants. Kidney Int 67: 1142–1151, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Serkova NJ, Niemann CU. Pattern recognition and biomarker validation using quantitative 1H-NMR-based metabolomics. Expert Rev Mol Diagn 6: 717–731, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Storey JD A direct approach to false discovery rates. J R Statist Soc B 64: 479–498, 2002. [Google Scholar]
- 43.Trygg J, Holmes E, Lundstedt T. Chemometrics in metabonomics. J Proteome Res 6: 469–479, 2007. [DOI] [PubMed] [Google Scholar]
- 44.van Ommen B, Stierum R. Nutrigenomics: exploiting systems biology in the nutrition and health arena. Curr Opin Biotechnol 13: 517–521, 2002. [DOI] [PubMed] [Google Scholar]
- 45.Zeisel SH, Freake HC, Bauman DE, Bier DM, Burrin DG, German JB, Klein S, Marquis GS, Milner JA, Pelto GH, Rasmussen KM. The nutritional phenotype in the age of metabolomics. J Nutr 135: 1613–1616, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zha H, Ding C, Gu M, He X, Simon HD. Spectral relaxation for K-means clustering. Neur Inf Proc Syst 14: 1057–1064, 2001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.