Abstract
Hypoxia-inducible factors (HIFs) are heterodimeric transcription factors that mediate the adaptive response of mammalian cells and tissues to changes in tissue oxygenation. In the present study, we show an age-dependent decline in cortical HIF-1α accumulation and activation of HIF target genes in response to hypoxia. This inducible response is significantly attenuated in the cerebral cortex of 18-mo-old Fischer 344 rat yet virtually absent in the cerebral cortex of 24-mo-old Fischer 344 rat. This attenuated HIF-1α response had no effect on mRNA upregulation of HIF-independent genes in the aged cortex. We have provided evidence that this absent HIF-1α response is directly correlated with an increase in the expression of the HIF regulatory enzyme, prolyl 4-hydroxylase (PHD). In addition, our study shows that cortical HIF-2α expression in senescent normoxic controls is also significantly greater than that of younger normoxic controls, despite no difference in HIF-2α mRNA levels. The posttranslational regulation of HIF-2α under normoxic conditions seems to be attenuated in the aged rat brain, which is an in vivo demonstration of differential regulation of HIF-1α and HIF-2α.
Keywords: aging, hypoxia, hypoxia inducible factor-1, hypoxia inducible factor-2
the key sensor or sensors of low oxygen is a family of 2-oxoglutarate and iron-dependent enzymes called prolyl-4-hydroxylases, which regulate the activity of hypoxia-inducible factors (HIFs). HIF is a heterodimeric transcription factor that mediates the adaptive response of mammalian cells to hypoxia. The two HIF subunits, α (∼120 kDa) and β (91–94 kDa), are members of the bHLH/PAS family of transcription factors and are both needed for DNA binding and activation of target genes that contain hypoxic response elements (HREs) (18, 27, 38–42, 45). There are three distinct HIF-α subunit homologues (HIF-1α, HIF-2α/EPAS-1, and HIF-3α) and three HIF-β homologues [HIF-1β/aryl hydrocarbon receptor nuclear translocator (ARNT1), ARNT2, and ARNT3] (8, 9). HIF-1α and 1β (ARNT1) are universally expressed in all human and rodent tissues, whereas HIF-2α, HIF-3α, ARNT2, and ARNT3 show a more restricted pattern of expression (1, 14, 39, 42, 43). The expression of the α subunit is regulated by oxygen levels, whereas the β subunit is constitutively expressed (9, 38–42, 45). During normoxia, the HIF-α subunit is expressed and rapidly hydroxylated at two conserved proline residues in an oxygen-dependent manner. This posttranslational modification of the α subunit is mediated by the EGLN (egl nine homolog of C. elegans) family of HIF prolyl 4-hydroxylases (PHDs 1, 2, and 3) (7, 8, 9, 17, 18, 26, 28). Activity of these enzymes requires molecular oxygen (O2), Fe2+, ascorbate, and 2-oxoglutarate as cofactors (7, 17, 41). The hydroxylated prolines are recognized by the product of the Von Hippel-Lindau tumor suppressor gene that targets the HIF-α protein for ubiquitination and subsequent proteosomal degradation (9, 18, 24, 26, 36, 40–42). During hypoxia, oxygen becomes limiting, and prolyl hydroxylase activity is diminished, leading to the accumulation of nonhydroxylated HIF-α, which allows for translocation to the nucleus and dimerization with HIF-β. HIF-1α is the most extensively characterized α subunit in various in vivo and in vitro models. HIF-1 is also widely expressed in the hypoxic and ischemic brain; its expression has been described in neurons, glia, and endothelial cells (6, 12, 13). HIF-2α expression is also induced in the hypoxic brain, primarily in astrocytes and endothelial cells (14). There are more than 100 HIF target genes that have been identified, which play roles in physiological processes, including vasomotor control, angiogenesis, erythropoiesis, iron metabolism, cell proliferation/death, and energy metabolism (1, 2, 10, 12, 14, 31, 38–42, 43, 45).
In a preliminary study, we made the observation that the hypoxia-induced HIF-1α accumulation in the cerebral cortex of 24-mo-old Fischer 344 (F344) rats was virtually absent and significantly less than that of younger rats (6 mo) (31). Chemical induction of cortical HIF-1α accumulation, through cobalt chloride administration, was intact in the old F344 rats and similar to that of the younger F344 rats. This raised the possibility of diminished oxygen sensitivity in the senescent rat brain. In this study, we examined hypoxia-induced HIF-1α accumulation and HIF-1 activity in the rodent brain as a function of age in greater detail to determine whether the observed decline in accumulation and subsequent HIF-1 activation could be detected at younger ages. We have also examined cortical PHD expression in the younger and aged rats to see whether the HIF-1α decline paralleled an increase in PHD expression. We found that the decline in cortical HIF-1α accumulation and activation of HRE target genes in response to hypoxia were significantly attenuated in the 18-mo-old rats and virtually absent in the 24-mo-old rats. There was significantly higher PHD expression in 24-mo-old control F344 rats vs. younger control rats (6 mo). The results also show that cortical HIF-2α expression in 24-mo-old normoxic control rats was significantly greater than that of younger normoxic controls despite no difference in HIF-2α mRNA levels. The posttranslational regulation of HIF-2α under normoxic conditions seems to be attenuated in the aged rat brain, which is an in vivo demonstration of differential regulation of HIF-1α and HIF-2α in the aged rat brain.
MATERIALS AND METHODS
Exposure to hypobaric hypoxia.
Male F344 rats aged 3–24 mo were ordered from the National Institute of Aging. Animal housing and handling met Institutional Animal Care and Use Committee standards. The age-grouped rats were subjected to 72 h of hypoxia in hypobaric chambers maintained at 380 Torr (0.5 atm) for the first 24 h (equivalent to 10% normobaric oxygen) and 290 Torr (0.4 atm) for the last 48 h (equivalent to 8% normobaric oxygen). The custom-made steel chambers [Wright chambers (50)], which had a clear Plexiglas door, were connected to a vacuum source that reduced the internal atmospheric pressure. Each experimental group of rats had an aged-matched control group, which was kept outside the hypobaric chambers but in the same location. After the 3-day hypoxic exposure, experimental and control rats were briefly anesthetized and decapitated. The brains were immediately removed and frozen in liquid nitrogen. The tissues were stored at −80 C until further processing.
Northern blot analysis.
Total RNA was extracted from frozen brain cortex using an RNA extraction kit (RNAgents; Promega, Madison, WI). Equal samples (10 μg) of total RNA were electrophoresed in 1% agarose-formaldehyde gels and transferred to nylon membranes (Millipore, Billerica, MA). The membrane was then UV cross-linked, prehybridized with Quickhyb solution (Stratagene, La Jolla, CA) containing salmon testes DNA (0.1 mg/ml) and then hybridized overnight (68°C) with random prime-labeled probes. Specific cDNA probes for cyclooxygenase-2 (COX-2) (1.156-kb EcoRI fragment from 5′-end of mouse cDNA), Egr-1 (BglII fragment of mouse full-length cDNA), HIF-2α (PstI-HindIII fragment of HIF-2 cDNA), and 18S were purchased from Invitrogen Life Technologies (Rockville, MD). HIF-1α and β-actin cDNAs were generated by RT-PCR using the Access RT-PCR system kit (Promega) with the following primers: sense 5′AGTCAGCAACGTGGAAGG-3′ and antisense 5′-GGGAGAAAATCAAGTCGT-3′ for HIF-1α; and sense 5′TGTTCGGAGTGGAGCAG-3′and antisense 5′-AACCCTAAGGCCAACCGTGAA-3′ for β-actin. Labeling of the probes was performed using [32P]-dCTP and a random-primed DNA labeling kit (Invitrogen Life Technologies). The blots were washed twice with 2× SSC, 0.1% SDS, once with 0.1× SSC, 0.1% SDS and exposed to radiographic film and analyzed by PhosphorImager.
Real-time PCR analysis.
Total RNA was extracted from frozen brain cortex using an RNA extraction kit (RNAgents; Promega, Madison, WI). Complementary DNA was synthesized from 2 μg of total RNA using the Superscript III system with an oligo-dT primer (Invitrogen, Carlsbad, CA). Real-time PCR analysis was performed with 0.5 μl of the final cDNA synthesis mix using commercially produced rat-specific Taq-Man-based gene expression assays (Applied Biosystems, Foster City, CA). The following assays were used: carbonic anhydrase 9 (CAIX, cat. no. Rn01764729_m1), erythropoietin (Epo; cat. no. Rn00566529_m1), glucose transporter 1 (Glut-1; cat. no. Rn01417099_m1), prolyl 4-hydroxylase 1 (PHD 1; cat. no. Rn01476583_m1), prolyl 4-hydroxylase 2 (PHD 2; cat. no. Rn00710295_m1), prolyl 4-hydroxylase 3 (PHD 3; cat. no. Rn00571341_m1), and vascular endothelial growth factor (VEGF; cat. no. Rn00582935_m1). The PCR reactions were performed in an ABI 7500 real-time PCR thermocycler (Applied Biosystems). All reactions were performed in duplicate using β-actin as an endogenous control. Experiments were independently repeated at least three times.
Preparation of whole cell lysates.
Frozen brain cortical samples were dissected in a dish on dry ice and homogenized by standard procedures. A stainless-steel motorized homogenizer was used to homogenize in ice-cold lysis buffer (50 mM Tris·HCl, 150 mM NaCl, 5 mM EDTA, 1% NP-40; Boston Bioproducts, Boston, MA) containing EDTA-free protease inhibitor tablet (Complete Mini, Roche Diagnostics, Indianapolis, IN). Homogenized samples were kept on ice for an hour and then centrifuged at 14,000 g for 30 min. Supernatants were collected, aliquoted, and stored at −20 C. Protein concentrations of aliquots were determined by Bradford protein assay with BSA used as standards (Bio-Rad, Hercules, CA).
Western blot analysis.
Whole cell lysates (25 and/or 50 μg of protein) were denatured in Laemmli buffer and electrophoresed on SDS-PAGE under reducing conditions. The proteins were transferred onto nitrocellulose membranes (Bio-Rad) by standard procedures. Membranes were blocked with 10% nonfat dry milk in Tris-buffered saline (TBS) containing 0.1% Tween 20 (Bio-Rad). After blocking, membranes were incubated in 3% BSA in TBS with 0.1% Tween 20, containing primary antibodies. The specific primary antibodies of interest were mouse monoclonal antibodies for HIF-1α (1:500; R&D Systems, Minneapolis, MN), HIF-2α (1:500; Novus Biologicals, Littleton, CO), PHD 1–3 (1:500), and goat polyclonal for β-actin (1:2,000; Santa Cruz Biotechnology, Santa Cruz, CA). After a series of washes in TBS-Tween at room temperature for 1 h, membranes were incubated with corresponding horseradish peroxidase-conjugated secondary antibodies for an hour. A final series of washes were done in TBS-Tween for at least another hour before enhanced chemiluminescence (SuperSignal) was used to visualize specific protein-antibody complexes.
Statistical analysis.
All results are shown as means ± SD, and in all cases, P < 0.05 was considered statistically significant. SigmaScan was used to quantify the densitometry of antigen-antibody complexes and normalized to that of β-actin (optical density ratios). Statistical analysis was carried out using SPSS software. Student's t-test was used to assess differences between two groups, such as control to hypoxic optical density ratios.
RESULTS
HIF-1α expression as a function of age.
Western blot analysis showed the characteristic hypoxia-inducible protein signal with an approximate molecular mass of 120 kDa in neuronal nuclear extract (positive control) and cortical samples of younger (3, 6, and 12 mo) F344 rats following 72 h of hypoxia (Fig. 1A). The signal detected with the HIF-1α antibody used in this study shows multiple bands that might correspond to various posttranslational modifications and ubiquitinated protein. This pattern is similar to HIF-1α Western blot results, previously published using the similar commercial antibodies (12–14, 19, 31). In a previous work, the specificity of the signal detected was confirmed by siRNA or genetic downregulation of HIF-1α in cells and brain tissue samples, respectively (14). In contrast, normoxic cortical samples from control rats of all ages showed negligible bands by comparison. Optical density ratios (relative to β-actin) show a statistically significant increase in HIF-1α expression (10- to 12-fold) levels in the younger rats following hypoxic exposure (Fig. 1B). The increase in HIF-1α was significantly reduced to two- to three-fold in the 18-mo-old rats and absent in the 24-mo-old rats following hypoxic exposure (Fig. 1B).
Fig. 1.
Attenuation of hypoxia-inducible factor-α (HIF-α) accumulation with advanced age. A: Western blot analysis of HIF-1α and β-actin, as a function of age, in cortical brain samples of rats exposed to normoxic (C) and hypoxic (H) conditions. Blot represents five control and five experimental rats ages 3, 6, 12, 18, and 24 mo of age. Nuclear extract from cortical neurons exposed to 1% oxygen was used as a positive control (+). B: optical density (OD) ratios of HIF-1α as a function of age. Each value represents the mean ± SD from at least three rats. *P < 0.05 compared with control (nonhypoxic) cortical samples. **P < 0.05 compared with OD value of hypoxic HIF-1α response in younger experimental groups.
HIF-1α mRNA expression as a function of age.
To determine whether the decreased HIF-1α protein expression in the aging brain during hypoxia was due to a reduction of HIF-1α steady-state mRNA levels, we performed Northern blot analysis of HIF-1α. As shown in Fig. 2, systemic hypoxia did not affect HIF-1α or HIF-1β mRNA levels in the cerebral cortex of young (3 and 6 mo) and old rats (24 mo). β-actin showed equal RNA loading for all samples.
Fig. 2.
HIF-1 mRNA expression intact in the aged rat brain. Expression of HIF-1 mRNA in cortical brain samples of young (3 and 6 mo) and old (24 mo) rats exposed to normoxia or hypoxia. Northern blots containing total RNA were hybridized to rat HIF-1α or HIF-1β cDNA probes. β-actin served as the sample-loading control. Data are representative of at least 3 experiments.
HIF-1 target genes.
To determine whether decreased levels of HIF-1α protein lead to a reduction in HIF transcriptional activity, we measured mRNA levels of well-defined HIF target genes using a previously published protocol. Specifically, we used cortical mRNA samples from 6- and 24-mo-old F344 rats to analyze the hypoxia-induced transcriptional activation of the following HIF-regulated genes: glucose transporter-1 (Glut-1), Epo, vascular endothelial growth factor (VEGF), and carbonic anhydrase IX (CAIX). RT-PCR analysis showed up to a three-fold increase in target gene mRNA following hypoxic exposure in 6-mo-old rats. This hypoxia-induced increase in mRNA was absent in 24-mo-old rats for all of the HIF-dependent targets analyzed (Fig. 3).
Fig. 3.
Attenuation of transcriptional activation of HIF-1 target genes in the aged rat brain. Relative mRNA levels of carbonic anhydrase 9 (CAIX; A), erythropoietin (Epo; B), glucose transporter-1 (Glut-1; C), and vascular endothelial growth factor (VEGF; D) in cortical brain samples of 6- and 24-mo-old rats. Results were expressed as fold induction compared with cortical samples of brains exposed to normoxia and normalized to β-actin mRNA. Each individual value represents the mean ± SD from three independent experiments from at least three different rats. *P < 0.05 compared with normoxia, **P < 0.05 compared with 6 mo of hypoxia.
HIF-1-independent target genes.
The decreased expression of HIF target gene mRNAs in aged animals is unlikely to be caused by a widespread reduction in transcriptional activity since Northern blot analysis shows upregulation of HIF-independent hypoxia-inducible genes in the aged rat brain (Fig. 4). Upregulation of COX-2 and Egr-1(early growth response protein 1, nuclear protein, and transcriptional regulator) was similar across all age groups subjected to systemic hypoxia. In addition, comparable mRNA levels of 18S RNA were observed in all age groups to show equal loading.
Fig. 4.
Intact mRNA upregulation of HIF-independent genes intact in the aged rat brain. Expression of Egr-1 and COX-2 mRNA in cortical brain samples of rats (ranging in age from 3 to 24 mo) exposed to normoxia or hypoxia. Northern blots containing total RNA were hybridized to rat Egr-1 and COX-2 cDNA probes. 18S RNA served as the sample-loading control. Data are representative of at least 3 experiments.
Prolyl hydroxylase expression.
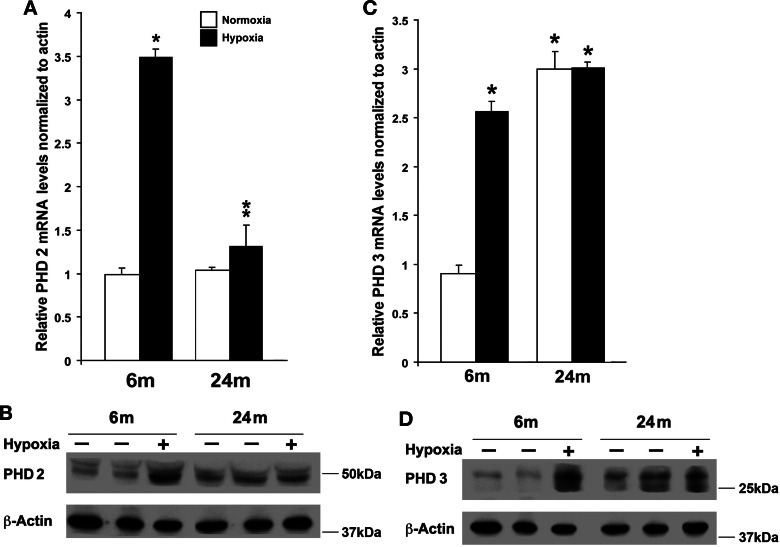
Northern blot analysis of HIF-1α mRNA levels indicated that the decline in hypoxia-induced accumulation of PHD during aging is unlikely because of decreased transcription of the HIF-1α gene and suggests that the deficit might reside at the level of posttranslational regulation of HIF-1α protein stability that occurs during hypoxia. We then decided to explore the possibility that the expression of prolyl hydroxylases is altered in the aging brain. Cortical mRNA expression of PHD 1, which is not known to contain an HRE and is nonresponsive to hypoxia (17), was approximately five times greater in 24-mo-old rats relative to 6-mo-old rats (Fig. 5A). There was also a significant increase in PHD 1 protein levels in older vs. younger normoxic control rats (Fig. 5B), which optical density ratios showed to be 2- to 3-fold higher (Fig. 5C). PHD 2 and PHD 3 do contain HREs and thus respond to HIF-1 activation (17). Hypoxic induction of PHD 2 and PHD 3 mRNA in cortical samples followed a similar pattern as the other more conventional HIF-1 target genes in the younger 6-mo-old rats, with up to a three-fold increase in mRNA levels (Fig. 6, A and B). This increase in PHD 2 message correlated with a significant increase in PHD 2 protein (Fig. 6B). This increase in PHD 2 mRNA and protein levels following hypoxia was also absent in the 24-mo-old rat brain. PHD 3 mRNA levels were up to three times higher in the older control rats relative to the younger controls and exhibited no further increase following hypoxia (Fig. 6C). The increase in PHD 3 message also correlated with a significant increase in PHD 3 protein (Fig. 6D). Thus, taken together, our results show that expression of PHDs is altered in the cerebral cortex of aging animals, suggesting a potential alteration in the oxygen-sensing machinery.
Fig. 5.
Increased expression of prolyl-4-hydroxylase 1 (PHD 1) in the aged rat brain. A: Relative mRNA levels of PHD 1 in cortical brain samples of 6- and 24-mo-old rats assessed by real-time RT-PCR analysis. Results were expressed as fold induction compared with cortical samples of brains exposed to normoxia and normalized to β-actin mRNA. Each individual value represents the mean ± SD from three independent experiments from at least three different rats. B: representative Western blot analysis of PHD 1 and β-actin expression in cortical samples of 6- and 24-mo-old control rats. C: optical density (OD) ratios of PHD 1 expression in cortical samples of 6- and 24-mo-old control rats. Each value represents the mean ± SD from at least three rats. *P < 0.05 compared with 6-mo-old cortical samples.
Fig. 6.
Induction of PHD 2 and PHD 3 in cortical brain of young and old rats. Relative mRNA levels of PHD 2 (A) and PHD 3 (C) in cortical brain samples of 6- and 24-mo-old rats assessed by real-time RT-PCR analysis. Results were expressed as fold induction compared with cortical samples of brains exposed to normoxia and normalized to β-actin mRNA. Each individual value represents the mean ± SD from three independent experiments from at least three different rats. *P < 0.05 compared with 6-mo-old normoxia. B and D: representative Western blots of PHD 2 (B) and PHD 3 (D) and β-actin expression in cortical samples of 6- and 24-mo-old rats exposed to normoxic (C) and hypoxic (H) conditions.
HIF-2α expression.
The surprising finding that expression of PHDs was altered in the aging brain opens the possibility that the expression of other HIF-α isoforms is also affected. In this regard, we studied the expression levels of the second most abundant HIF-α isoform HIF-2α that was previously shown to be expressed primarily by astrocytes (14). In the younger rats, there was a pronounced increase in HIF-2α expression following hypoxia, similar to that of HIF-1α. In contrast, baseline HIF-2α expression in cortical samples from older normoxic control rats was significantly greater than younger normoxic controls (Fig. 7, B and C), despite that there was no difference in cortical mRNA levels across all age groups (Fig. 7A). Moreover, there was no further increase in HIF-2α expression in the older rats following hypoxic exposure.
Fig. 7.
Increased normoxic HIF-2α expression in the aged rat brain. A: expression of HIF-2α mRNA in cortical brain samples of rats (ranging in age from 3 to 24 mo) exposed to normoxia or hypoxia. 18S RNA served as the sample-loading control. B: Western blot analysis of HIF-2α and β-actin in cortical brain samples of 6- and 24-mo-old rats exposed to normoxic (C) and hypoxic (H) conditions. C: optical density (OD) ratios of HIF-2α expression in cortical samples of 6- and 24-mo-old control rats. Each value represents the mean ± SD from at least three rats. *P < 0.05 compared with 6-mo-old cortical samples.
DISCUSSION
In this study, we explored one aspect of the oxygen-sensing and hypoxic-response mechanism that operates in mammalian cells, the HIF system. This pathway regulates homeostatic responses to stress conditions involving hypoxia, including wound healing and angiogenesis that are known to be altered as a function of age. We studied the HIF pathway in the brain of F344 rats, an established model of aging, in the context of systemic hypobaric hypoxia.
Attenuation of cortical HIF-1α and target gene activation as a function of age.
Our results have shown that there is an alteration of the HIF system as a function of age. We observed a progressive decline in cortical hypoxia-induced HIF-1α protein accumulation from 3 to 24 mo of age. This defect appears to be more dramatic at the later age groups, with the response being greatly diminished but still present in 18-mo-old F344 rats and completely absent in 24-mo-old F344 rats. The inability of the aging brain to trigger HIF-1α protein accumulation in response to hypoxia, as expected, led to decreased HIF-1 activation, resulting in a reduction of HIF target gene activation. Our data revealed that there were no differences in cortical mRNA levels of HIF-1α, so it is unlikely that there was a reduction in the rate of transcription of the HIF-1α gene or the half-life of the HIF-1α mRNA that could explain the findings. In addition, other HIF-independent hypoxia-inducible genes, such as COX-2 and Egr-1, were also upregulated at the mRNA level in response to hypoxia, indicating a defect restricted to the HIF pathway.
An alternative explanation for this reduced HIF response could be that the rate of HIF-1α protein synthesis was diminished in aging animals. Although it is difficult to measure the rate of protein synthesis in whole animals, we think that this is an unlikely event since HIF-1α protein accumulation could be induced by systemic administration of cobalt chloride in both young and old animals. Cobalt, a known chemical activator of HIF, interferes with HIF hydroxylation and subsequent degradation by directly and indirectly disrupting the reduced iron within the catalytic domain of prolyl hydroxylases (29, 37). This significantly reduces PHD activity, even in the presence of oxygen, and, consequently, a robust HIF-1α response occurs in vitro and in vivo. Taken together, these data suggest that the basic regulatory elements that control HIF-1α protein accumulation in cortical brain appear to remain intact in aging animals. The observation that chemical induction of HIF-1α protein accumulation is still possible in the aging brain suggests that oxygen sensing could be altered as a function of age.
Increased cortical PHD expression in the aged rat brain.
The attenuation of the hypoxia-induced HIF-1 response in aged rats appears to occur as a result of changes in the expression levels of PHD enzymes. PHD enzymes regulate the posttranslational stabilization of HIF-α protein and have been shown to exhibit tissue-specific expression patterns. (3, 15, 32). Our results showed that PHD 1 expression at the mRNA and protein levels was significantly increased in cortical brain of normoxic 24-mo-old rats. In addition, PHD 3 mRNA and protein levels were also increased in the normoxic aging cerebral cortex. In younger 6-mo-old rats, the mRNA and protein levels of PHD 3 were significantly upregulated by hypoxia, an observation that is in agreement with previous studies documenting this hypoxia-induced response (17, 44). Interestingly, hypoxia does not affect the baseline levels of PHD 3 that are already higher in normoxic old animals compared with young ones. In the case of PHD 2, considered the major regulator of HIF-1α in vitro (7), its mRNA and protein levels were significantly increased in the cortex of hypoxic 6-mo-old rats compared with normoxic age-matched controls. This observation is also in agreement with previous studies showing that PHD 2 expression is influenced by HIF activity (17, 44). In this regard, PHD 2 upregulation in hypoxic 24-mo-old animals was blunted further, confirming the reduced HIF activity observed in aging animals exposed to hypoxia.
Attenuation of normoxic HIF-2α degradation in aged cortical brain.
Surprisingly, HIF-2α baseline levels were increased in 24-mo-old rats compared with 6-mo-old rats. This result suggests that either the posttranslational regulation of HIF-2α under normoxic conditions is affected or that HIF-2α gene transcription is robustly increased during aging. Northern blot analysis showed that cortical HIF-2α mRNA levels were comparable between young and old animals, a similar finding to what we observed for HIF-1α mRNA levels. Therefore, we speculate that HIF-1α and HIF-2α are differentially regulated in the aged rat brain. A similar observation has been made in neuron-specific conditional HIF-1α knockout mice, which appear to also have elevated baseline expression of HIF-2α, perhaps as a compensatory response to HIF-1α downregulation. Although HIF-2α has a more restricted pattern of expression than HIF-1α, it is still surprising that the increased HIF-2α expression in the aged rats brain does not appear to increase message levels of key factors, such as VEGF and Epo. HIF-1 and HIF-2 appear to regulate the transcription of both analogous and discrete sets of target genes, but this may not necessarily translate to in vivo models (21, 22, 42, 47, 49). Recent in vitro reports have suggested that HIF-2α may not be transcriptionally active and that this may be due to a HIF-2α-specific transcriptional repressor (22).
Potential mechanisms and limitations of the study.
These observations could be due to metabolic changes and/or reactive oxygen species and the related oxidative cell injury that are associated with age. Exposure of cells to H2O2 diminishes accumulation of both HIF-1α and HIF-2α across a range of experimental conditions, including hypoxia and also chemical induction (48). The differential regulation of HIF-1α and HIF-2α could be due to cell type-specific differences or alterations in metabolism. The elevated HIF-2α accumulation in the aged normoxic rat brain could be due to some other posttranslational modification that prevents hydroxylation and/or degradation. Testing these potential mechanisms would be beyond the limits of an in vivo model. Although only cortical samples were examined in this study, HIF-1α accumulation and hypoxia-induced angiogenesis are widespread throughout the brain in multiple rodent strains (12, 31, 43, 46). Thus, the lack of an HIF-1α response in the aged cerebral cortex is likely a generalized observation. Other aged rodent strains may or may not respond in the exact same fashion, as is mentioned in Other related studies in the discussion. This study only focused on a particular FiO2, but it is possible that a greater hypoxic stimulus could potentially elicit a HIF-1α response in the aged F344 rats.
Other related studies.
Although the emphasis of this study is the rodent brain, similar occurrences of age-related changes in HIF-1α accumulation and/or activity have been shown in the literature using different models and/or tissues (19, 25). Frenkel-Denkberg et al. (19) have reported impaired multiorgan HIF-1 DNA binding activity in senescent mice (24 mo), despite finding no differences in HIF-1α expression relative to 3 mo-old mice (19). The study reported higher normoxic HIF-1α expression in the aged mouse brain and liver vs. younger mice. Following the hypoxic exposure, HIF-1α was actually greater in the aged liver samples. Hwang et al. (25) have reported contrary results showing significantly greater normoxic HIF-1 DNA binding activity in lung samples from older (20 mo) vs. younger (3 and 12 mo) rats. The greater DNA binding activity in the aged lung samples was not significantly increased in aged lung samples following 6 h of hypoxia. Both studies were relatively acute (30–90 min and 6 h of hypoxia, respectively) compared with ours, and their differing observations demonstrate potential model and tissue-specific differences in DNA binding studies.
Perspectives and Significance
HIF is largely responsible for activating many genes involved in mediating adaptive responses to hypoxia on a cellular and systemic level. It mediates oxygen-dependent expression of a number of genes involved in adaptive responses, such as angiogenesis, erythropoiesis, and energy metabolism (1, 2, 10, 12, 14, 31, 38–43, 45). The lack of an HIF-1 response and the lack of transcriptional activation of VEGF could limit the ability to increase microvascular density to match neuronal activity under chronic hypoxic conditions. Diminished HIF-1 could also potentially disrupt the continuous maintenance of capillary density in the aged rat brain, thus affecting plasticity-associated learning and neuronal survival. This scenario could increase the likelihood of pathological circumstances and possibly degenerative disease states. Signaling mechanisms that involve oxygen homeostasis, such as the HIF system, are also implicated in various models of ischemia. Therefore, limited HIF activation could diminish prosurvival responses during ischemic injury. Consequently, defects in HIF-1 signaling could make senescent animals more susceptible to detrimental effects of chronic hypoxic and ischemic stress. More insights into the mechanisms responsible for these and other associated phenomena might open the door for potential therapeutic manipulation.
In conclusion, our study has shown that cortical HIF-1α accumulation and HIF-1 activation is diminished with age. The decrease is directly correlated with increased PHD expression, with expression levels of PHDs 1 and 3 being elevated. In addition, our study suggests that cortical HIF-2α expression in old normoxic controls is also significantly greater than that of younger normoxic controls despite similar message levels. The posttranslational regulation of HIF-2α under normoxic conditions appears to be attenuated in the aged rat brain, demonstrating differential regulation of HIF-1α and HIF-2α in vivo.
GRANTS
This research was supported by the National Institutes of Health Grants R01-NS38632 and T32-GM007250.
REFERENCES
- 1.Acker T, Acker H. Cellular oxygen sensing need in CNS function: physiological and pathological implications. J Exp Biol 207: 3171–3188, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Acker T, Fandrey J, Acker H. The good, the bad and the ugly in oxygen-sensing: ROS, cytochromes and prolyl-hydroxylases. Cardiovasc Res 71: 195–207, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Appelhoff RJ, Tian YM, Raval RR, Turley H, Harris AL, Pugh CW, Ratcliffe PJ, Gleadle JM. Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J Biol Chem 279: 38458–38465, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Aprelikova O, Wood M, Tackett S, Chandramouli GV, Barrett JC. Role of ETS transcription factors in the hypoxia-inducible factor-2 target gene selection. Cancer Res 66: 5641–5647, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Baranova O, Miranda LF, Pichiule P, Dragatsis I, Johnson RS, Chavez JC. Neuron-specific inactivation of the hypoxia inducible factor 1-α increases brain injury in a mouse model of transient focal cerebral ischemia. J Neurosci 27: 6320–6332, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergeron M, Yu AY, Solway KE, Semenza GL, Sharp FR. Induction of hypoxia-inducible factor-1 (HIF-1) and its target genes following focal ischaemia in rat brain. Eur J Neurosci 11: 4159–4170, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Berra E, Benizri E, Ginouves A, Volmat V, Roux D, Pouyssegur J. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1α in normoxia. EMBO J 22: 4082–4090, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294: 1337–1340, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Bruick RK, McKnight SL. Transcription. Oxygen sensing gets a second wind. Science 295: 807–808, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Bunn HF, Poyton RO. Oxygen sensing and molecular adaptation to hypoxia. Physiol Rev 76: 839–885, 1996. [DOI] [PubMed] [Google Scholar]
- 11.Callapina M, Zhou J, Schmid T, Köhl R, Brüne B. NO restores HIF-1α hydroxylation during hypoxia: role of reactive oxygen species. Free Radic Biol Med 39: 925–936, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Chavez JC, Agani F, Pichiule P, LaManna JC. Expression of hypoxia-inducible factor-1alpha in the brain of rats during chronic hypoxia. J Appl Physiol 89: 1937–1942, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Chavez JC, LaManna JC. Activation of hypoxia-inducible factor-1 in the rat cerebral cortex after transient global ischemia: potential role of insulin-like growth factor-1. J Neurosci 22: 8922–8931, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chavez JC, Baranova O, Lin J, Pichiule P. The transcriptional activator hypoxia inducible factor 2 (HIF-2/EPAS-1) regulates the oxygen-dependent expression of erythropoietin in cortical astrocytes. J Neurosci 26: 9471–9481, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cioffi CL, Liu XQ, Kosinski PA, Garay M, Bowen BR. Differential regulation of HIF-1 alpha prolyl-4-hydroxylase genes by hypoxia in human cardiovascular cells. Biochem Biophys Res Commun 303: 947–953, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Elvert G, Kappel A, Heidenreich R, Englmeier U, Lanz S, Acker T, Rauter M, Plate K, Sieweke M, Breier G, Flamme I. Cooperative interaction of hypoxia-inducible factor-2α (HIF-2α) and Ets-1 in the transcriptional activation of vascular endothelial growth factor receptor-2 (Flk-1). J Biol Chem 278: 7520–7530, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen, E, Wilson, MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107: 43–54, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Freeman RS, Hasbani DM, Lipscomb EA, Straub JA, Xie L. SM-20, EGL-9, and the EGLN family of hypoxia-inducible factor prolyl hydroxylases. Mol Cells 16: 1–12, 2003. [PubMed] [Google Scholar]
- 19.Frenkel-Denkberg G, Gershon D, Levy AP. The function of hypoxia-inducible factor 1 (HIF-1) is impaired in senescent mice. FEBS Lett 462: 341–344, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Gerald D, Berra E, Frapart YM, Chan DA, Giaccia AJ, Mansuy D, Pouysségur J, Yaniv M, Mechta-Grigoriou F. JunD reduces tumor angiogenesis by protecting cells from oxidative stress. Cell 118: 781–794, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC. Differential roles of hypoxia-inducible factor 1α (HIF-1α) and HIF-2α in hypoxic gene regulation. Mol Cell Biol 23: 9361–9374, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu CJ, Iyer S, Sataur A, Covello KL, Chodosh LA, Simon MC. Differential regulation of the transcriptional activities of hypoxia-inducible factor 1 α (HIF-1α) and HIF-2α in stem cells. Mol Cell Biol 26: 3514–3526, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu CJ, Sataur A, Wang L, Chen H, Simon MC. The N-terminal transactivation domain confers target gene specificity of hypoxia-inducible factors HIF-1α and HIF-2α. Mol Biol Cell 18: 4528–4542, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci USA 95: 7987–7992, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hwang IS, Fung ML, Liong EC, Tipoe GL, Tang F. Age-related changes in adrenomedullin expression and hypoxia-inducible factor-1 activity in the rat lung and their responses to hypoxia. J Gerontol A Biol Sci Med Sci 62: 41–49, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG Jr. HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292: 464–468, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Iyer NV, Leung SW, Semenza GL. The human hypoxia-inducible factor 1α gene: HIF1A structure and evolutionary conservation. Genomics 52: 159–165, 1998. [DOI] [PubMed] [Google Scholar]
- 28.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim Av Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292: 468–472, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Kaczmarek M, Cachau RE, Topol IA, Kasprzak KS, Ghio A, Salnikow K. Metal ions-stimulated iron oxidation in hydroxylases facilitates stabilization of HIF-1 α protein. Toxicol Sci 107: 394–403, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Köhl R, Zhou J, Brüne B. Reactive oxygen species attenuate nitric-oxide-mediated hypoxia-inducible factor-1α stabilization. Free Radic Biol Med 40: 1430–1442, 2006. [DOI] [PubMed] [Google Scholar]
- 31.LaManna JC, Chavez JC, Pichiule P. Structural and functional adaptation to hypoxia in the rat brain. J Exp Biol 207: 3163–3169, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Lieb ME, Menzies K, Moschella MC, Ni R, Taubman MB. Mammalian EGLN genes have distinct patterns of mRNA expression and regulation. Biochem Cell Biol 80: 421–426, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Marti HH Erythropoietin and the hypoxic brain. J Exp Biol 207: 3233–3242, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Rivard A, Fabre JE, Silver M, Chen D, Murohara T, Kearney M, Magner M, Asahara T, Isner JM. Age-dependent impairment of angiogenesis. Circulation 99: 111–120, 1999. [DOI] [PubMed] [Google Scholar]
- 35.Rivard A, Berthou-Soulie L, Principe N, Kearney M, Curry C, Branellec D, Semenza GL, Isner JM. Age-dependent defect in vascular endothelial growth factor expression is associated with reduced hypoxia-inducible factor 1 activity. J Biol Chem 275: 29643–29647, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Salceda S, Caro J. Hypoxia-inducible factor 1α (HIF-1α) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem 272: 22642–22647, 1997. [DOI] [PubMed] [Google Scholar]
- 37.Salnikow K, Donald SP, Bruick RK, Zhitkovich A, Phang JM, Kasprzak KS. Depletion of intracellular ascorbate by the carcinogenic metals nickel and cobalt results in the induction of hypoxic stress. J Biol Chem 279: 40337–40344, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Semenza GL, Agani F, Booth G, Forsythe J, Iyer N, Jiang BH, Leung S, Roe R, Wiener C, Yu A. Structural and functional analysis of hypoxia-inducible factor 1. Kidney Int 51: 553–555, 1997. [DOI] [PubMed] [Google Scholar]
- 39.Semenza GL Oxygen-regulated transcription factors and their role in pulmonary disease. Respir Res 1: 159–162, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Semenza GL HIF-1, O2, and the 3 PHDs: how animal cells signal hypoxia to the nucleus. Cell 107: 1–3, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Semenza GL HIF-1 and mechanisms of hypoxia sensing. Curr Opin Cell Biol 13: 167–171, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Semenza GL Life with oxygen. Science 318: 62–64, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Sharp FR, Bernaudin M. HIF1 and oxygen sensing in the brain. Nat Rev Neurosci 5: 437–448, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Stiehl DP, Wirthner R, Köditz J, Spielmann P, Camenisch G, Wenger RH. Increased prolyl 4-hydroxylase domain proteins compensate for decreased oxygen levels. Evidence for an autoregulatory oxygen-sensing system. J Biol Chem 281: 23482–23491, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA 92: 5510–5514, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ward NL, Moore E, Noon K, Spassil N, Keenan E, Ivanco TL, LaManna JC. Cerebral angiogenic factors, angiogenesis, and physiological response to chronic hypoxia differ among four commonly used mouse strains. J Appl Physiol 102: 1927–1935, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Warnecke C, Zaborowska Z, Kurreck J, Erdmann VA, Frei U, Wiesener M, Eckardt KU. Differentiating the functional role of hypoxia-inducible factor (HIF)-1α and HIF-2α (EPAS-1) by the use of RNA interference: erythropoietin is a HIF-2α target gene in Hep3B and Kelly cells. FASEB J 18: 1462–1464, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Wiesener MS, Turley H, Allen WE, Willam C, Eckardt KU, Talks KL, Wood SM, Gatter KC, Harris AL, Pugh CW, Ratcliffe PJ, Maxwell PH. Induction of endothelial PAS domain protein-1 by hypoxia: characterization and comparison with hypoxia-inducible factor-1α. Blood 92: 2260–2268, 1998. [PubMed] [Google Scholar]
- 49.Wiesener MS, Jürgensen JS, Rosenberger C, Scholze CK, Hörstrup JH, Warnecke C, Mandriota S, Bechmann I, Frei UA, Pugh CW, Ratcliffe PJ, Bachmann S, Maxwell PH, Eckardt KU. Widespread hypoxia-inducible expression of HIF-2α in distinct cell populations of different organs. FASEB J 17: 271–273, 2003. [DOI] [PubMed] [Google Scholar]
- 50.Wright BM Aparatus for exposing animals to reduced atmospheric pressure for long periods. Br J Haematol 10: 75–77, 1964. [DOI] [PubMed] [Google Scholar]